Abstract
Sex chromosome tetrasomy and pentasomy conditions occur in 1:18 000–1:100 000 male births. While often compared with 47,XXY/Klinefelter syndrome because of shared features including tall stature and hypergonadotropic hypogonadism, 48,XXYY, 48,XXXY and 49,XXXXY syndromes are associated with additional physical findings, congenital malformations, medical problems and psychological features. While the spectrum of cognitive abilities extends much higher than originally described, developmental delays, cognitive impairments and behavioural disorders are common and require strong treatment plans. Future research should focus on genotype–phenotype relationships and the development of evidence-based treatments.
Conclusion
The more complex physical, medical and psychological phenotypes of 48,XXYY, 48,XXXY and 49,XXXXY syndromes make distinction from 47,XXY important; however, all of these conditions share features of hypergonadotropic hypogonadism and the need for increased awareness, biomedical research and the development of evidence-based treatments.
Keywords: Hypogonadism, Klinefelter syndrome, XXXXY syndrome, XXXY syndrome, XXYY syndrome
INTRODUCTION
48,XXYY, 48,XXXY and 49,XXXXY syndromes are rare sex chromosome aneuploidy conditions that are characterized by the presence of two or more extra X and Y chromosomes in males. The presence of one or more additional X chromosome(s) above the typical 46,XY in males leads to testicular dysgenesis and hypergonadotropic hypogonadism, and thus, 48,XXYY, 48,XXXY and 49,XXXXY are often considered ‘variants’ of Klinefelter syndrome (47,XXY) because of these shared features. However, the increased risks for congenital malformations, additional medical problems and more complex psychological involvement in 48,XXYY, 48,XXXY and 49,XXXXY make distinction from 47,XXY important for these patients (1,2).
48,XXYY syndrome is the most common of these three syndromes and is estimated to occur in 1:18 000–1:40 000 male births (3). The incidence of 48,XXXY is estimated to be 1:50 000, while 49,XXXXY occurs in 1:85 000–1:100 000 male births (4). In contrast, 47,XXY (Klinefelter syndrome) is the most common chromosomal abnormality in humans, with newborn screening studies showing an incidence rate of approximately oen in 650 males (5,6).
The current review is based on published literature about these three sex chromosome tetrasomy and pentasomy disorders. The objective of this study is to review and compare the physical features, associated medical problems, neurodevelopmental and psychological features of these rare genetic conditions. We also aim to compare and contrast these conditions to 47,XXY and to describe why distinctions from 47,XXY are important in the understanding and treatment of males with 48,XXYY, 48,XXXY and 49,XXXXY.
CLINICAL FEATURES
Physical features
47,XXY (Klinefelter syndrome) is associated with tall stature, with studies reporting a mean adult height ranging from 179 to 188 cm (7,8). Males with both 48,XXYY and 48,XXXY also present with tall stature, while mean stature in 49,XXXXY is below average. In 48,XXYY syndrome, a cross-sectional study of 95 males age 1–55 showed mean birthweight and birth length in the average range; however, boys aged 10 or younger ranged significantly in height compared with typical peers, from short stature to heights in the upper percentiles (mean Z-score/standard deviation score +0.21 ± 1.45, range −4.5 to +2.8). In the 11- to 19-year-old age group, mean height was around the 75th percentile (Z-score +1.06 ± 1.17) and was above the 95th percentile in those 20 years and older (Z-score +2.18 ± 1.08), with a mean adult height 192.4 cm or 6 feet 3 inches (2). Thus, tall stature became more significant starting in adolescence. In 48,XXXY, less published data are available, although tall stature is frequently described to be similar to 47,XXY and 48,XXYY. The mean height in a study that included nine patients with 48,XXXY was at the 93rd percentile (Z-score −2.0 to +3.2) (8). In contrast, stature in males with 49,XXXXY syndrome is usually below average. Two recent studies including small sample sizes (n = 10, n = 13) have reported mean stature in 49,XXXXY at the 7th to 33rd percentile (Z-scores −0.47, −1.8), although both included mostly prepubertal males (8,9). The specific explanation for the short stature in 49,XXXXY compared with 47,XXY, 48,XXXY and 48,XXXY is hypothesized to be because of the influence of extreme overdosage of sex chromosome genes in the pentasomy condition affecting multiple organ sites and growth pathways; however, the specific explanation is unknown (8) (see Table 2 for comparisons).
Table 2.
Comparison of male sex chromosome aneuploidy disorders
| 47,XXY | 48,XXYY | 48,XXXY | 49,XXXXY | |
|---|---|---|---|---|
| Prevalence | 1:650–1:1000 males (5,6) | 1:18 000–1:40 000 males (3) | 1:17 000–1:50 000 males (4,5) | 1:85 000–1:100 000 males (4,5) |
| % of expected cases diagnosed in lifetime | 25–35% (42) | Unknown | Unknown | Unknown |
| Age at diagnosis | 6–10% diagnosed in prenatal period <5% identified prior to age 10 (42) |
2% diagnosed in prenatal period >70% identified prior to age 10 Mean age at diagnosis: 6.8 years (2) |
No data | No prenatal cases described 100% identified prior to age 10 Mean age at diagnosis: 4 months (9) |
| Stature | Tall stature Mean adult height 179–188 cm (5 feet 11 in feet to 6 feet 2 inches) (7,8) |
Taller than XXY Mean adult height 193 cm (6 feet 4 inches) (2,8) |
Tall stature Mean adult height 190 cm (6 feet 3 inches) (8) |
Short stature Limited data available (8,9) |
| Congenital malformations | Clinodactyly common Congenital malformations 18% (Inguinal Hernia, Cleft palate – occult, submucous) (7) |
Clinodactyly common (70%) Congenital malformations 56% (Inguinal Hernia, Cardiac, Radioulnar synostosis, Cleft palate, Clubfoot, Renal dysplasia) (2,10,27) |
Limited data Clinodactyly common Radioulnar synostosis, Inguinal hernia Likely similar to 48,XXYY and 49,XXXXY (7,10) |
Congenital malformations 50–100% (Radioulnar synostosis, Hip dysplasia, Genitourinary malformations, Cleft palate, Inguinal hernia, Clubfoot, Cardiac) (7,9,10) |
| Hypergonadotropic hypogonadism | Yes | Yes | Yes | Yes |
| Developmental and cognitive | Speech and motor delays (40–75%) 50–75% learning disabilities Intellectual disability rare Mean FSIQ 89–102 Verbal IQ < Performance IQ (7,26) |
Speech and motor delays (75–92%) 100% learning disabilities Intellectual disability 26% Mean FSIQ 70–80 Verbal IQ < Performance IQ (2,10,22) |
Speech and motor delays (>50%) 100% learning disabilities Intellectual disability (>50%) Mean FSIQ 40–75 Verbal IQ < Performance IQ (10,22) |
Speech and motor delays (100%) 100% learning disabilities Intellectual disability (>95%) Mean FSIQ 20–60 Verbal IQ < Performance IQ (9,10,22,30) |
| Parent of origin of extra chromosomes | 50% Maternal 50% Paternal (43) |
100% Paternal (44–47) | 50% Maternal 50% Paternal (47) |
100% Maternal (47) |
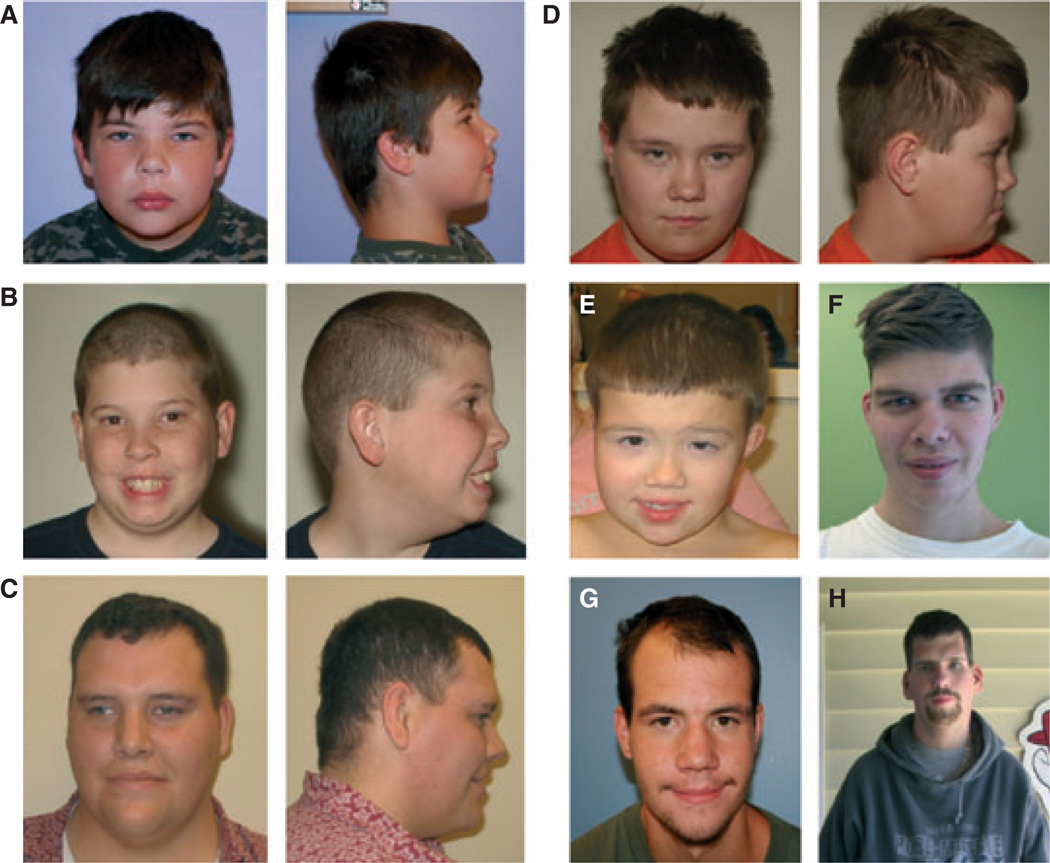
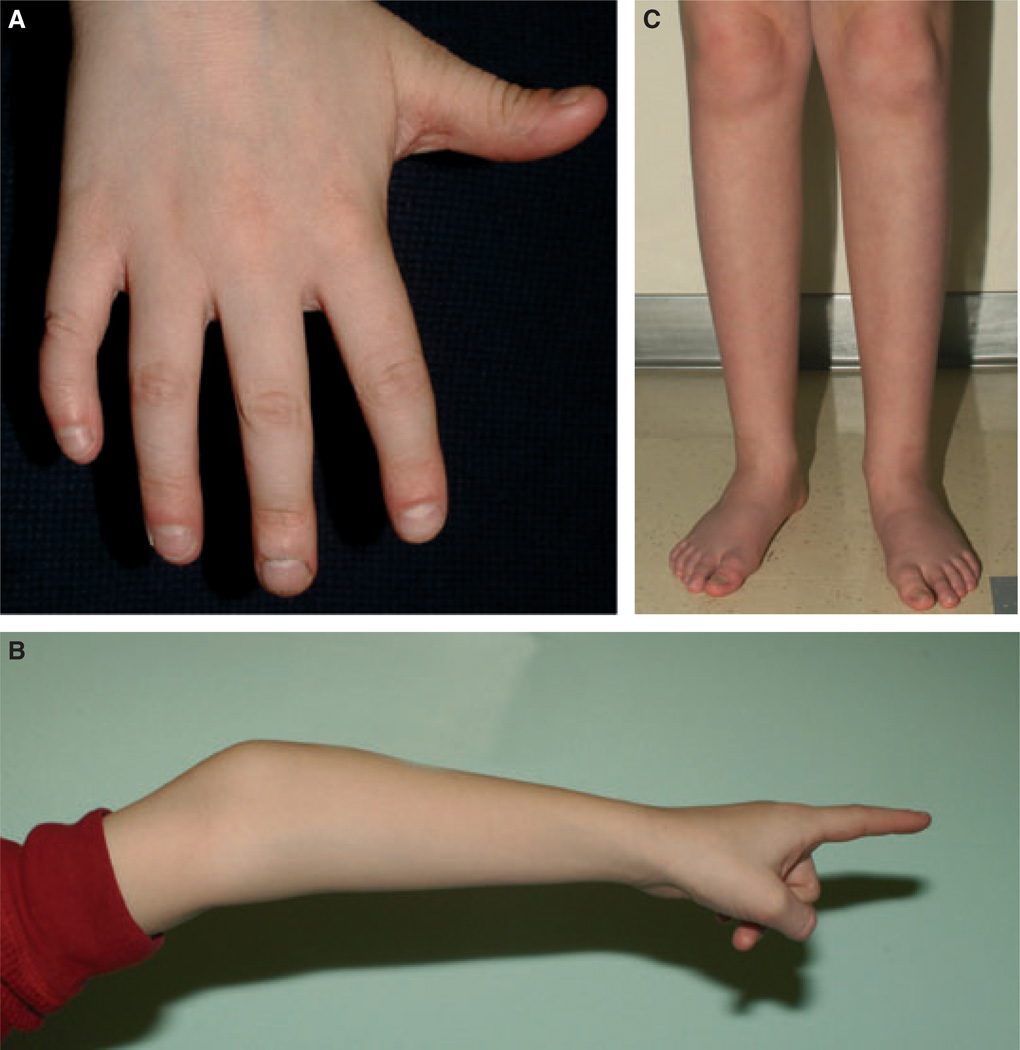
The degree of facial dysmorphism in all three syndromes is variable and often subtle, although dysmorphic features are typically more distinct in 49,XXXXY compared with 48,XXYY and 48,XXXY. Across all three conditions, common findings include hypertelorism, epicanthal folds, up-slanting palpebral fissures, hooded eyelids, fifth-digit clinodactyly, short nail beds (often with significant nail biting), pes planus, joint hyperextensibility and prominent elbows with cubitus varus (narrowed carrying angle) as shown in Figs 1 and 2. Musculoskeletal examination often shows limited supination and pronation because of radioulnar synostosis or congenital elbow dislocation (1,2,9,10). Significant dental problems with frequent caries, thin enamel, malocclusion, taurodontism and need for multiple dental procedures and orthodontia are very common (2,11,12). While all of the findings above have also been described in 47,XXY, the dysmorphic features and physical findings are more likely to occur in 48,XXYY, 48,XXXY and 49,XXXXY and are often distinct when present.
Figure 1.
Facial features in 48,XXYY, 48,XXXY and 49,XXXXY syndromes. Photograph of facial features in children and adults with XXYY (A, B, D, G, H), XXXY (D, F) and XXXXY (E) syndromes. Note features of hypertelorism (A, D, E), epicanthal folds (A, E, G), narrow palpebral fissures (A, C, E, F), strabismus (C, E), flattened occiput (A, C, D) and low frontal hairline (A, B, E, F). Note the sparse facial hair (C, F, G), prominent brow (C, F, G) and narrow shoulders (H) in the adults.
Figure 2.
Photograph of Physical features in XXYY, XXXY and XXXXY including: (A) Fifth-digit clinodactyly (and nail biting), (B) prominent elbows with hyperextensibility and (C) lower extremities with low muscle bulk in the calves, flat feet and mild pronation at the ankles.
For all three conditions, testicular size is typically in the average to low average range in the prepubertal period; however, microorchidism occurs in almost all cases of 48,XXYY, 48,XXXY and 49,XXXXY in adolescence and adulthood, with adult testicular volumes typically 1–4 mL (2,9). As in 47,XXY, mean phallus length is decreased. In 48,XXYY, mean phallus length from a sample of 44 males was at the 24th percentile (2). In a sample of 20 males with 49,XXXXY, phallus length was below the 10th percentile in all patients (9). Beyond single case reports, data are not available for 48,XXXY; however, microphallus is described in case reports, (10) and it is predicted that mean phallus length would be decreased as in the other conditions.
There is considerable variation in body habitus. While some individuals with 48,XXYY, 48,XXXY and 49,XXXXY share the narrow shoulders, gynaecomastia and eunuchoid body habitus classically described in Klinefelter syndrome, (7,13) this phenotype is not universal. Adolescents and adults with all three conditions vary from being underweight to obese, and only approximately 30% have gynaecomastia which is usually mild.
Other medical problems
Other medical problems and congenital malformations associated with 48,XXYY, 48,XXXY and 49,XXXXY syndrome are listed in Table 1. Overall, congenital malformations are more common in 49,XXXXY compared with 48,XXYY and 48,XXXY; however, they are above the rate seen in 47,XXY and in the general population for all three conditions. Congenital malformations including congenital heart defects, radioulnar synostosis, cleft palate (submucosal or occult), hip dysplasia, kidney dysplasia, clubfoot and inguinal hernia and/or cryptorchidism are identified in the first few years of life, often before a diagnosis of the sex chromosome aneuploidy is established. Seizure disorders (10–15%), strabismus, constipation and recurrent otitis are common in childhood in all three conditions (2,7,9,10,14). Data on the medical problems in adulthood are more limited, with most of the literature based on paediatric patients. However, based on studies and case reports that include adolescents and adults, other medical problems emerge and increase in adolescence and young adulthood such as scoliosis, tremor, deep vein thrombosis, pulmonary embolism and Type II diabetes (2,15–18). Increased mortality from non-Hodgkin lymphoma has been identified for 48,XXYY in a large epidemiologic study (19).
Table 1.
Medical problems associated with XXYY, XXXY and XXXXY syndromes*
| Hypotonia in childhood |
| Hypergonadotropic hypogonadism |
| Dental problems |
| Asthma/Reactive airway disease |
| Food/Environmental allergies |
| Cardiac malformations |
| Intention and postural tremor |
| Radioulnar synostosis/Congenital elbow dislocation |
| Congenital hip dysplasia |
| Pes planus/Flat feet |
| Clubfoot |
| Inguinal hernia/Undescended testicles |
| Deep vein thrombosis/Pulmonary embolism |
| Hypothyroidism |
| Type II diabetes |
| Scoliosis |
| Seizure disorder |
| Strabismus |
| Recurrent otitis with tympanostomy tubes |
| Gastroesophageal reflux |
| Constipation |
| Obstructive sleep apnoea |
| Osteoporosis |
Males with XXYY, XXXY and XXXXY are at increased risk for items on this list. Not all patients have all of these findings.
Hypogonadism and fertility
Because of the presence of the additional X chromosomes, testicular dysfunction becomes most apparent during adolescence, when testicular fibrosis and hypergonadotropic hypogonadism (testosterone deficiency) develop in most males with 48,XXYY, 48,XXXY and 49,XXXXY syndromes. While longitudinal studies of hormonal changes in these syndromes have not been completed, the boys seem to follow the same pattern as in 47,XXY which has been more extensively studied (see Wikstrom 2008 for review) (20). In childhood, levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and total testosterone are normal in the prepubescent range. FSH and LH rise in early puberty followed by a spontaneous increase in total testosterone in most patients that then plateaus in the low-normal range or decreases. Testicular histology in adults with 48,XXYY, 48,XXXY and 49,XXXXY shows the same hyalinization and fibrosis of the seminiferous tubules, loss of germ cells and Leydig cell hyperplasia as in XXY, with subsequent testosterone deficiency and infertility (21) (see appendix).
Current recommendations include initiation of testosterone therapy following practice standards established for 47,XXY (2,7). However, these recommendations vary considerably between sources, from those suggesting initiation of therapy at 11–12 years of age, (22,23) starting in mid-adolescence (24) or using elevated LH and/or low testosterone levels to determine timing for the initiation of therapy (17,25). Thus, additional studies are necessary to determine appropriate timing of testosterone therapy with respect to pubertal changes, bone density, motor development and psychological development. In all three conditions, additional research is needed to determine whether there are any differences or additional considerations compared with 47,XXY.
Developmental/psychological features
There is a large spectrum of involvement in the developmental and psychological features of 48,XXYY, 48,XXXY and 49,XXXXY syndromes, with some individuals much more affected than others. As with the medical features, males with 49,XXXXY are generally more affected compared with 48,XXYY and 48,XXXY; however, recent reports show a much broader spectrum and less significant impairments than originally described (9). Developmental delays are common in infancy and early childhood, with speech delays (especially in expressive language) present in almost all patients. In 48,XXYY, motor delays and associated hypotonia were present in 75%, with average age of independent ambulation achieved at 18 months (2). In contrast, a recent study in 49,XXXXY found motor delays and hypotonia in 100%, with independent ambulation at a mean of 25.5 months (9). Similar data for 48,XXXY have not been published.
Cognitive involvement is almost universal; however, again 48,XXYY and 48,XXXY are typically less affected compared with 49,XXXXY. It is estimated that cognitive abilities decrease by 10–15 IQ points for each additional X chromosome (22). A cognitive profile that includes strengths in visual perceptual, nonverbal cognitive skills and weaknesses in verbal skills has been well described in 47,XXY (26). This ‘split’ between verbal and nonverbal skills is also found in 48,XXYY, 48,XXXY and 49,XXXXY and is often even more significant in the sex chromosome tetrasomy and pentasomy conditions where the extra sex chromosomes have been shown to have a more significant effect on neurodevelopment related to language and verbal skills compared with nonverbal domains (2,9,26).
In 48,XXYY, the cognitive deficits range from milder language-based learning disabilities (reading disability, dyslexia) to mild intellectual disability in 30% (mild mental retardation) (2,22). For 48,XXXY, there is a lack of research in large cohorts; however, most reports describe cognitive abilities in the mild intellectual disability range, with full-scale IQ scores reported to range from 20 to 78 (10,22). A recent study including cognitive testing of 13 children with 49,XXXXY 2–7 years of age reports a mean nonverbal IQ of 87.1. This result cannot be easily compared with other studies reporting full-scale IQ in 48,XXYY, 48,XXXY or 49,XXXXY because it does not include assessment of verbal skills, which are universally affected across all three conditions, and most severe in 49,XXXXY. The finding of a low average nonverbal IQ is important however, because it highlights an area of relative strength in the cognitive profile of 49,XXXXY syndrome. While the spectrum of cognitive abilities spreads much higher than originally described for all three syndromes, cognitive impairments (especially in the verbal domain) commonly affect behaviour and academic, social and occupational capabilities (2,27).
Adaptive functioning is a term that describes the abilities of an individual to demonstrate daily living skills in domains such as self-care, social skills, community living, safety, functional use of academic skills (reading maps, calculating change from purchases), personal responsibility and occupational skills. Adaptive functioning abilities can be measured by a variety of standardized assessments where parents or caregivers provide information about the individual’s abilities across different domains, and standardized scores are generated which classify how an individual is functioning in daily life in comparison with age expectations. In typical populations, measures of adaptive functioning correlate with cognitive ability (IQ), and findings of adaptive functioning scores in the disability range are part of the criteria necessary for the diagnosis of intellectual disability. Adaptive functioning scores are also often used for eligibility for disability support services and analysis of the subdomains of adaptive functioning allows identification of individual areas of strength and weakness. Visootsak et al. compared results of an adaptive functioning assessment (Vineland Adaptive Behavior Scales) between a group of 13 males with 48,XXYY and 11 males with 48,XXXY and 49,XXXXY. The mean standardized scores for adaptive functioning were in the disability range in both groups. The 48,XXYY group had significantly higher scores in the domains of daily living skills and communication compared with the 48,XXXY/49,XXXXY group, however no significant differences in the social skills domain (22). Adaptive functioning skills were further explored in a cohort of 47 males with 48,XXYY syndrome and were found to be significantly lower than IQ in most cases, with a mean adaptive functioning score of 68.9 which falls in the disability range. These findings indicate that overall daily functioning is often more impaired than would be expected based on cognitive (IQ) scores in males with 48,XXYY (2). While the factors involved in the discrepancy between cognitive abilities and adaptive functioning deficits are not fully understood, these deficits contribute to the disability seen in most individuals with these conditions and prevent many individuals from achieving academic and occupational success.
In addition to cognitive and learning problems, other neurodevelopmental and psychological disorders are significant components of the phenotype of 48,XXYY, 48,XXXY and 49,XXXXY syndromes and are typically more severe and/or complex when compared with 47,XXY. Developmental dyspraxia contributes to the early language and motor deficits (9,28). Attention deficit hyperactivity disorder (ADHD) is present in over 70% of males with 48,XXYY, with symptoms of inattention, distractibility, poor organizational skills, hyperactivity and/or impulsivity affecting daily functioning (29). This is significantly higher than the 35–45% rate of ADHD in 47,XXY. The prevalence of ADHD has not yet been directly studied in 48,XXXY or 49,XXXXY, although reports include descriptions of various ADHD symptoms including difficulties with hyperactivity and behavioural regulation (22,30). Evaluation and diagnosis of ADHD also become more complex in groups with more significant cognitive impairments.
Social development can also be affected in these conditions, likely related to a combination of factors including language and cognitive deficits. The results of the Visootsak et al. study that identified adaptive functioning deficits in the social skills and communication domains raised questions as to whether there may also be elevated rates of autism spectrum disorders (22). In 48,XXYY, the prevalence of autism spectrum disorders ranges from 28% to 45%, with most cases on the mild range of the autism spectrum (pervasive developmental disorder) showing difficulties with social skills and reciprocal social interactions (29,31). Autism symptoms have not been studied in 48,XXXY. Gropman et al. (9) screened seven children with 49,XXXXY using the Gilliam Autism Rating Scale screening questionnaire; there were no concerns for autism identified in this sample.
Other emotional symptoms including emotional immaturity, anxiety symptoms, obsessive-compulsive behaviours, impulsivity, behavioural dysregulation and tic disorders are more commonly seen in these conditions compared with 47,XXY and with the general population (2,22). Psychotic disorders have been described in a few patients with 48,XXYY, (32,33), and while psychosis is uncommon, these cases are important to note because similar symptoms of psychosis are reported in XXY, (34,35) which has been proposed as a genetic model population for schizophrenia (36,37).
Psychopharmacologic medications to manage behavioural and psychiatric symptoms are effective in the sex chromosome tetrasomy and pentasomy patients and are important to consider in children and adolescents presenting with moderate to severe behavioural or emotional symptoms in conjunction with behavioural therapies (2,33). In our 2008 study, a striking 36% of adult males with 48,XXYY had previous hospitalization(s) for behavioural or psychiatric concerns, calling attention to the severity of psychological problems in some patients and the need for aggressive treatments when psychological or behavioural concerns arise (2).
Interplay between medical and psychological factors
Consideration of the psychological phenotypes of 48,XXYY, 48,XXXY and 49,XXXXY syndromes is important for clinicians treating these patients, because these psychological factors affect compliance with treatments. For example, decisions regarding formulation/delivery of testosterone therapy require consideration of background psychological problems in the patient. In some adolescents, significant anxiety around needles or medical visits makes the topical preparations ideal. However, patients with more significant cognitive deficits, poor self-care skills or ADHD (and their parents/caretakers) may prefer injectable testosterone to improve compliance with treatment.
Research and clinical experience across all three disorders also demonstrates that the behavioural phenotype in 48,XXYY, 48,XXXY and 49,XXXXY often affects decisions about the use and timing of testosterone therapy for hypogonadism. For example, in our study that included 22 adult males with 48,XXYY, all were hypogonadal on laboratory testing, but only 64% (14/22) were on testosterone therapy because of concerns about worsening behaviours in the untreated patients (2). Six of the eight untreated patients had significant health consequences such as osteoporosis, and four had been on testosterone therapy at some point in the past without negative effects. There have been two case reports of improvements in aggressive behaviour after the initiation of testosterone in 48,XXYY in the literature (38,39). However, Lee (32) described one case of a 31-year-old male with 48,XXYY and schizophrenia, with worsening of behaviour after initiating testosterone treatment. Our clinical experience supports either an improvement or no significant change in behaviours following the initiation of testosterone in these patients. However, as in 47,XXY, fewer behavioural difficulties may result by timing the initiation of therapy in early puberty when testosterone deficiency develops and beginning with low doses that are gradually increased, as opposed to initiating therapy in young adulthood at adult doses after prolonged hypogonadism. Changes in testosterone dosage should not occur at the same time as changes in psychopharmacologic medication, so that behavioural effects of the individual changes can be determined.
While there is a lot of new interest in strategies to evaluate for viable sperm in the ejaculate of early pubertal boys with 47,XXY, (20,40,41) the lower cognitive abilities, social deficits and poor expressive language abilities in the tetrasomy and pentasomy disorders make ethical considerations around consent even more important to consider than in 47,XXY. Whereas some adult males with 48,XXYY and 48,XXXY may live independently and be capable of supporting a child, the percentage is considerably lower compared with 47,XXY where most individuals achieve independence. In the early pubertal period when these procedures are encouraged, a large percentage of parents with children with 48,XXYY, 48,XXXY and 49,XXXXY are putting great effort into helping their son receive appropriate supports in the education system or addressing a variety of behavioural and/or emotional difficulties. Thus, efforts to pursue cryopreservation of sperm at this age are likely to be much lower in families of male children with sex chromosome tetrasomy and pentasomy because of the poorer prognosis in adulthood. However, each adolescent should be evaluated independently to determine whether attempts at cryopreservation are appropriate for that individual.
DIAGNOSIS OF 48,XXYY, 48,XXXY AND 49,XXXXY SYNDROMES
47,XXY, 48,XXYY, 48,XXXY and 49,XXXXY syndromes are identified by standard karyotype (chromosome analysis) or chromosomal microarray showing the presence of extra X and/or Y chromosomes. 47,XXY is currently significantly underdiagnosed by medical professionals. Based on comparisons between the number of cases identified by medical professionals through genetic testing and the known incidence from newborn screening studies of approximately 1:650 male births, currently only approximately 25% of individuals with 47,XXY are ascertained in their lifetime (6,42). Similar studies are not available for 48,XXYY, 48,XXXY and 49,XXXXY because of the decreased incidence of these conditions; however, because there is an increased likelihood of dysmorphic features, congenital anomalies and more severe developmental delays, it is assumed that a larger percentage of individuals with the tetrasomy and pentasomy conditions are identified in their lifetime.
In a study of 95 males with 48,XXYY, the mean age of diagnosis with 48,XXYY syndrome was 7.7 years, compared with a mean age of diagnosis of 4 months of age in study including 16 males with 49,XXXXY (2,9). This much younger age of diagnosis in 49,XXXXY compared with 48,XXYY is likely related to the more significant facial dysmorphisms, hypotonia and congenital malformations in 49,XXXXY which are more likely to lead to genetic testing by health care providers. In 48,XXXY, case reports describe age at diagnosis ranging from the neonatal period to adulthood. In contrast to 47,XXY, where evaluation for infertility leads to diagnosis in about 15–20% of identified cases, (42) we could find no reports of nonmosaic 48,XXYY, 48,XXXY or 49,XXXXY identified through evaluation for infertility. This difference is likely because of the more significant cognitive and behavioural features compared with 47,XXY.
AETIOLOGY AND GENETIC FACTORS
Most cases of 48,XXYY syndrome are thought to result from the fertilization of a normal female oocyte (Xm), with an aneuploid sperm (XpYpYp) produced through nondisjunction events in both meiosis I and meiosis II of spermatogenesis. Alternatively, postzygotic nondisjunction may occur during mitosis, as demonstrated in 8% of individuals with 47,XXY and 14% of individuals with 47,XXX, resulting in XmXmYpYp (43). To date, parental origin studies have been conducted directly in 11 known cases of 48,XXYY, and all cases demonstrated the additional X (and Y) chromosome to be paternal in origin (44–47).
In 48,XXXY, parent of origin has been reported in five cases. In two of these cases, the origin was successive non-disjunction in formation of the sperm (XpXpYp) fertilizing a normal female oocyte (Xm). The other three cases showed XmXmXmYp, indicated double nondisjunction events during oogenesis (47).
49,XXXXY results from nondisjunction of the X chromosome during both meiosis I and meiosis II. Thus, an aneuploid oocyte (Xm Xm Xm Xm) is fertilized with a normal male sperm (Yp). This finding has been consistent in 20 reported cases (47).
Some studies in 47,XXY have suggested that parent of origin influences the phenotype through a proposed imprinting mechanism (48,49). Given the limited number of direct studies in 48,XXYY, 48,XXXY and 49,XXXXY, additional studies on how parental origin and epigenetic factors affect the phenotype are needed.
DISCUSSION AND FUTURE DIRECTIONS
Overall, recent studies in 48,XXYY, 48,XXXY and 49,XXXXY have identified a much larger spectrum of cognitive abilities than described in the original literature, where nearly all subjects had intellectual disability.
While many authors, including our group, have emphasized the factors that distinguish 48,XXYY, 48,XXXY and 49,XXXXY from 47,XXY/Klinefelter syndrome, recognition of these three disorders as entirely distinct from 47,XXY Klinefelter syndrome has both positive and negative implications (see Table 2 for comparisons). The most important reasons for distinction are related to recognition of the associated medical problems that are more common in the sex chromosome tetrasomy and pentasomy conditions and which often require additional evaluations, interventions and therapies. Also, while there is a spectrum of cognitive and behavioural symptoms in 47,XXY, those in 48,XXYY, 48,XXXY and 49,XXXXY are often more severe and require additional interventions and community supports. For example, in some states, 47,XXY is not a qualifying diagnosis for early intervention services because of the presence of normal early milestones in some 47,XXY infants and toddlers. In 48,XXYY, 48,XXXY and 49,XXXXY, early speech and motor delays are almost universal, and early intervention therapies are extremely important and warranted. Similarly, in adolescence and adulthood, the degree of disability and complexity of psychological symptoms are more significant in 48,XXYY, 48,XXXY and 49,XXXXY, and comparisons to adults with 47,XXY/Klinefelter syndrome (most of whom do not require adult disability services) hinder access to appropriate supports.
However, some factors make comparison to 47,XXY/Klinefelter syndrome important. First, 47,XXY is the most common chromosomal abnormality in males, with a prevalence of 1:650 male births. While research on 47,XXY is still extremely limited in comparison with other genetic and endocrinologic disorders with lower prevalence rates, there has been renewed interest in 47,XXY over the past 5 years, with research progressing in understanding cognitive features, (50,51) neuroimaging correlates, (52,53) advances in fertility research, (40,54) efficient and sensitive high-volume diagnostic techniques, (6,55) genotype–phenotype relationships (56,57) and associated medical conditions (17,19,41,58). Findings in all of these research areas are also pertinent to 48,XXYY, 48,XXXY and 49,XXXXY syndromes, and clinical treatments and additional research directions in these conditions will likely grow from findings in 47,XXY. If the syndromes are viewed as distinct conditions, they will be less likely to be included in new research projects, and research on the rarer disorders will be more difficult to fund and execute because of the lower sample sizes. Similarly, while 47,XXY/Klinefelter syndrome is the most common chromosomal abnormality in males, there is very low professional and public awareness and ongoing misperceptions of males with 47,XXY that are slowly improving with efforts of the national advocacy groups, the interesting new research in 47,XXY and increasing rates of genetic testing in medical practice. A complete distinction of 48,XXYY, 48,XXXY and 49,XXXXY would not be of benefit to these rarer conditions or to 47,XXY as it could lead to additional public confusion and duplication of advocacy efforts. Thus, education and advocacy about sex chromosome aneuploidy conditions as a group should be coordinated to be most effective.
Finally, the development of hypergonadotropic hypogonadism occurs in all aneuploidy conditions where males have one or more extra X chromosome(s), and the general evaluation and treatment protocols for testosterone replacement therapy do not currently differ based on the specific genetic diagnosis. Although more research is needed to determine whether there should be any differences in the approach to testosterone replacement therapies for 48,XXYY, 48,XXXY and 49,XXXXY compared with classic 47,XXY, currently the treatment approaches are the same as 47,XXY and classifying the conditions as entirely distinct from one another may lead to confusion or undertreatment of hypogonadism in 48,XXYY, 48,XXXY and 49,XXXXY.
The phenotype of all sex chromosome aneuploidy disorders is proposed to result from gene dosage effects, in which genes on the additional X and/or Y chromosomes that are not X-inactivated are therefore expressed at higher levels than in typical 46, XY males. For example, homologous genes in the pseudoautosomal regions of the sex chromosomes are expressed from both the X&Y chromosomes, and thus, in 48,XXYY, 48,XXXY and 49,XXXXY, these genes would be expressed twofold to threefold in comparison with a 46,XY male. Microarray studies in 47,XXY have identified genes that are differentially expressed compared with 46,XY, (59) and differentially expressed genes were correlated with verbal cognitive skills in one study (56). This study was small (six XY compared with 11 XXY subjects) and has yet to be replicated. While subjects with 48,XXYY, 48,XXXY and 49,XXXXY are slightly more difficult to locate in large numbers, we propose that the sex chromosome tetrasomy and pentasomy conditions may be a better comparison group for these initial genotype–phenotype studies because a 2.0-fold (for 48,XXYY and 48,XXXY) and 2.5-fold (for 49,XXXXY) increase in gene expression would be expected compared with 1.5-fold in 47,XXY. Also, the more significant cognitive and behavioural differences between 48,XXYY, 48,XXXY, and 49,XXXXY and 46,XY may prove more valuable in determining candidate genes involved in the psychological and behavioural phenotype. Studies that compare psychological features in 47,XXY to 46,XY often find inconsistent differences between groups, likely due to the significant overlap in the phenotypes, small sample sizes and confounding variables such as socioeconomic status and other environmental factors that impact cognitive outcomes. However, 48,XXYY, 48,XXXY and 49,XXXXY have much less overlap, so the differences between groups are easier to identify.
Many new techniques have been developed that can inexpensively and accurately screen large populations for sex chromosome aneuploidy (6,55). While universal newborn screening for these conditions remains controversial, newborn screening studies for research purposes would help to increase ascertainment of these rarer conditions so that prospective studies in unselected samples could be conducted. Consequently, interventions to address the developmental delays and learning problems could be studied in a controlled manner to determine whether earlier identification and interventions clearly lead to improved outcome.
Now that more comprehensive descriptions of the medical and psychological features of 48,XXYY and 49,XXXXY syndromes have been published, the next steps include identifying genes and gene pathways that lead to these features, as well as developing specific treatments and interventions. The recent surge in research in 47,XXY will provide important background applicable to the 48,XXYY, 48,XXXY and 49,XXXXY and prompt researchers in 47,XXY to value the utility and clinical importance of including the tetrasomy and pentasomy aneuploidies in their studies. We anticipate that with a new interest in copy number variations as a mechanism of variation and pathology in the general population, the sex chromosome aneuploidy disorders will benefit from related strategies developed and further interest in genomic sciences.
Clinical research over the next 5 years should focus on understanding factors that lead to the most significant medical problems in 48,XXYY, 48,XXXY and 49,XXXXY and developing evidence-based clinical care guidelines that are readily available to practitioners. It will be important to determine whether there are differences in the timing and progression of hypogonadism in 48,XXYY, 48,XXXY and 49,XXXXY compared with 47,XXY and to better understand the psychological and behavioural effects of testosterone therapy so that appropriate recommendations are made for each disorder. Increasing awareness and ascertainment of all sex chromosome aneuploidy disorders are important, and burgeoning molecular screening methods will facilitate achieving these goals.
ACKNOWLEDGEMENTS
FINANCIAL DISCLOSURE
We acknowledge funding from the Bonfils-Stanton Foundation, the Weckbaugh Foundation, The XXYY Project, The Children’s Hospital Research Institute, and the University of Colorado CCTSI and IDDRC. Informed consent from patients and their parents was obtained for publication of photographs.
APPENDIX: DISCUSSION FOLLOWING NICOLE TARTAGLIA’S PRESENTATION
Phenotypic characteristics in 48,XXYY and 48,XXXY men
Anders Juul (Copenhagen, Denmark)
The 48,XXYY, 48,XXXY and 49,XXXXY patients all have a tendency to develop deep venous thrombosis (DVT) with 20% of adults over the age of 20 years being affected. What is the mechanism of this and does it influence our decision to treat with testosterone?
Nicole Tartaglia
I do not think there is any research investigating mechanisms of DVT in XXYY specifically. It is my understanding that imbalances in oestrogen and testosterone lead to increased risk of DVT, and that maintaining normal testosterone levels reduces the risk of DVT. These patients were often untreated or undertreated with testosterone replacement. Another risk factor may be a tendency for this group to be less active physically due to low energy levels and motor coordination difficulties. Patients with KS (XXY) are also at increased risk for DVT.
References
- 1.Peet J, Weaver D, Vance G. 49,XXXXY: a distinct phenotype. Three new cases and review. J Med Genet. 1998;35:420–424. doi: 10.1136/jmg.35.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tartaglia N, Davis S, Hench A, Nimishakavi S, Beauregard R, Reynolds A, et al. A new look at XXYY syndrome: medical and psychological features. Am J Med Genet A. 2008;146A:1509–1522. doi: 10.1002/ajmg.a.32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen K, Nielsen J, Jacobsen P, Rolle T. The 48,XXYY syndrome. J Ment Defic Res. 1978;22:197–205. [PubMed] [Google Scholar]
- 4.Kleczkowska A, Fryns JP, Van den Berghe H. X-chromosome polysomy in the male. The Leuven experience 1966–1987. Hum Genet. 1988;80:16–22. doi: 10.1007/BF00451449. [DOI] [PubMed] [Google Scholar]
- 5.Evans JA, de von Flindt R, Greenberg C, Ramsay S, Hamerton JL. A cytogenetic survey of 14,069 newborn infants. IV. Further follow-up on the children with sex chromosome anomalies. Birth Defects Orig Artic Ser. 1982;18:169–184. [PubMed] [Google Scholar]
- 6.Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85:503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visootsak J, Graham JM., Jr Klinefelter syndrome and other sex chromosome aneuploidies. Orphanet J Rare Dis. 2006;1:42. doi: 10.1186/1750-1172-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottesen AM, Aksglaede L, Garn I, Tartaglia N, Tassone F, Gravholt CH, et al. Increased number of sex chromosomes affects height in a nonlinear fashion: a study of 305 patients with sex chromosome aneuploidy. Am J Med Genet A. 2010;152A:1206–1212. doi: 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gropman A, Rogol A, Fennoy I, Sadeghin T, Sinn S, Jameson R, et al. Clinical variability and novel neurodevelopmental findings in 49,XXXXY syndrome. Am J Med Genet Part A. 2010;152A:1523–1530. doi: 10.1002/ajmg.a.33307. [DOI] [PubMed] [Google Scholar]
- 10.Linden MG, Bender BG, Robinson A. Sex chromosome tetrasomy and pentasomy. Pediatrics. 1995;96:672–682. [PubMed] [Google Scholar]
- 11.Demirhan O. Clinical findings and phenotype in a toddler with 48,XXYY syndrome. Am J Med Genet. 2003;119A:393–394. doi: 10.1002/ajmg.a.20015. [DOI] [PubMed] [Google Scholar]
- 12.Joseph M. Endodontic treatment in three taurodontic teeth associated with 48,XXXY Klinefelter syndrome: a review and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:670–677. doi: 10.1016/j.tripleo.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Klinefelter H, Reifenstein E, Albright F. Syndrome characterized by gynecomastia aspermatogenesis without A-leydigism and increased excretion of follicle-stimulating hormone. J Clin Endocrinol Metab. 1942;2:615–627. [Google Scholar]
- 14.Simsek P, Utine G, Alikasifoglu A, Alanay Y, Boduroglu K, Kandemir N. Rare sex chromosome aneuploidies: 49,XXXXY and 48,XXXY syndromes. Turk J Ped. 2009;51:294–297. [PubMed] [Google Scholar]
- 15.Zelante L, Piemontese MR, Francioli G, Calvano S. Two 48,XXYY patients: clinical, cytogenetic and molecular aspects. Ann Genet. 2003;46:479–481. doi: 10.1016/s0003-3995(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 16.Tartaglia N, Borodyanskya M, Hall D. Tremor in XXYY syndrome. Mov Disord. 2009;24:2001–2007. doi: 10.1002/mds.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bojesen A, Gravholt CH. Klinefelter syndrome in clinical practice. Nat Clin Pract Urol. 2007;4:192–204. doi: 10.1038/ncpuro0775. [DOI] [PubMed] [Google Scholar]
- 18.Dubois S, Illouz F, Pinson L, Bonneau D, Rohmer V, Guichet A. Endocrine function in a 48,XXYY adult with type 2 diabetes: case report with a review of the literature. Ann Endocrinol (Paris) 2007;68:384–388. doi: 10.1016/j.ando.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst. 2005;97:1204–1210. doi: 10.1093/jnci/dji240. [DOI] [PubMed] [Google Scholar]
- 20.Wikstrom AM, Dunkel L. Testicular function in Klinefelter syndrome. Horm Res. 2008;69:317–326. doi: 10.1159/000117387. [DOI] [PubMed] [Google Scholar]
- 21.Leisti JT, Aula PP, Hjelt LH. Two cases of (Prepubertal) Klinefelter’s syndrome with XXYY sex chromosomes. Ann Hum Genet. 1964;28:71–76. doi: 10.1111/j.1469-1809.1964.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 22.Visootsak J, Rosner B, Dykens E, Tartaglia N, Graham JM., Jr Behavioral phenotype of sex chromosome aneuploidies: 48,XXYY, 48,XXXY, and 49,XXXXY. Am J Med Genet A. 2007;143:1198–1203. doi: 10.1002/ajmg.a.31746. [DOI] [PubMed] [Google Scholar]
- 23.Jones K, editor. Smith’s recognizable patterns of human malformation. 6th ed. Philadelphia: Elsevier; 2006. [Google Scholar]
- 24.Simpson JL, Swerdloff RS, Samango-Sprouse CA, Rogol A. Klinefelter syndrome. In: Suzanne BC, Judith EA, editors. Management of genetic syndromes. 2nd ed. NJ: John Wiley & Sons; 2005. [Google Scholar]
- 25.Richmond E, Rogol A. Male pubertal development and the role of androgen therapy. Nat Clin Pract Endocrinol Metab. 2007;3:338–344. doi: 10.1038/ncpendmet0450. [DOI] [PubMed] [Google Scholar]
- 26.Rovet J, Netley C, Keenan M, Bailey J, Stewart D. The psychoeducational profile of boys with Klinefelter syndrome. J Learn Disabil. 1996;29:180–196. doi: 10.1177/002221949602900208. [DOI] [PubMed] [Google Scholar]
- 27.Borgaonkar D, Mules E, Char F. Do the 48,XXYY males have a characteristic phenotype? Clin Genet. 1970;1:272–293. [Google Scholar]
- 28.Samango-Sprouse C, Rogol A. XXY the hidden disability and a prototype for an infantile presentation of developmental dyspraxia (IDD) Infants Young Child. 2002;15:11–18. [Google Scholar]
- 29.Tartaglia N, Davis S, Hansen R, Hagerman R. Abstract: attention deficit hyperactivity disorder and autism spectrum disorders in males with XXY, XYY, and XXYY syndromes. J Intel Dis Res. 2006;50:787. [Google Scholar]
- 30.Lomelino CA, Reiss AL. 49,XXXXY syndrome: behavioural and developmental profiles. J Med Genet. 1991;28:609–612. doi: 10.1136/jmg.28.9.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jha P, Sheth D, Ghaziuddin M. Autism spectrum disorder and Klinefelter syndrome. Eur Child Adolesc Psychiatr. 2007;16:305–308. doi: 10.1007/s00787-007-0601-8. [DOI] [PubMed] [Google Scholar]
- 32.Lee JW. An XXYY male with schizophrenia. Aust N Z J Psychiatr. 1996;30:553–556. doi: 10.3109/00048679609065032. [DOI] [PubMed] [Google Scholar]
- 33.Dervaux A, Artiges E. Olanzapine for violent schizophrenia and Klinefelter syndrome. Am J Psychiatr. 2002;159:493–494. doi: 10.1176/appi.ajp.159.3.493-a. [DOI] [PubMed] [Google Scholar]
- 34.van Rijn S, Aleman A, Swaab H, Kahn R. Klinefelter’s syndrome (karyotype 47,XXY) and schizophrenia-spectrum pathology. Br J Psychiatr. 2006;189:459–460. doi: 10.1192/bjp.bp.105.008961. [DOI] [PubMed] [Google Scholar]
- 35.DeLisi LE, Friedrich U, Wahlstrom J, Boccio-Smith A, Forsman A, Eklund K, et al. Schizophrenia and sex chromosome anomalies. Schizophr Bull. 1994;20:495–505. doi: 10.1093/schbul/20.3.495. [DOI] [PubMed] [Google Scholar]
- 36.DeLisi LE, Maurizio AM, Svetina C, Ardekani B, Szulc K, Nierenberg J, et al. Klinefelter’s syndrome (XXY) as a genetic model for psychotic disorders. Am J Med Genet B. 2005;135B:15–23. doi: 10.1002/ajmg.b.30163. [DOI] [PubMed] [Google Scholar]
- 37.Crow TJ. Sex chromosomes and psychosis. The case for a pseudoautosomal locus. Br J Psychiatr. 1988;153:675–683. doi: 10.1192/bjp.153.5.675. [DOI] [PubMed] [Google Scholar]
- 38.Heuser I, Hartmann A, Oertel H. Androgen replacement in a 48, XXYY-male patient. Arch Gen Psychiatr. 1999;56:194–195. doi: 10.1001/archpsyc.56.2.194. [DOI] [PubMed] [Google Scholar]
- 39.Sourial N, Fenton F. Testosterone treatment of an XXYY male presenting with aggression: a case report. Can J Psychiatr. 1988;33:846–850. doi: 10.1177/070674378803300912. [DOI] [PubMed] [Google Scholar]
- 40.Paduch DA, Bolyakov A, Cohen P, Travis A. Reproduction in men with Klinefelter syndrome: the past, the present, and the future. Sem Repro Med. 2009;27:137–148. doi: 10.1055/s-0029-1202302. [DOI] [PubMed] [Google Scholar]
- 41.Paduch DA, Fine RG, Bolyakov A, Kiper J. New concepts in Klinefelter syndrome. Curr Opin Urol. 2008;18:621–627. doi: 10.1097/MOU.0b013e32831367c7. [DOI] [PubMed] [Google Scholar]
- 42.Abramsky L, Chapple J. 47,XXY (Klinefelter Syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17:363–368. doi: 10.1002/(sici)1097-0223(199704)17:4<363::aid-pd79>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Hassold TJ, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet. 2007;16:R203–R208. doi: 10.1093/hmg/ddm243. [DOI] [PubMed] [Google Scholar]
- 44.Rinaldi A, Archidiacono N, Rocchi M, Filippi G. Additional pedigree supporting the frequent origin of XXYY from consecutive meiotic non-disjunction in paternal gametogenesis. J Med Genet. 1979;16:225–226. doi: 10.1136/jmg.16.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leal CA, Belmont JW, Nachtman R, Cantu JM, Medina C. Parental origin of the extra chromosomes in polysomy X. Hum Genet. 1994;94:423–426. doi: 10.1007/BF00201605. [DOI] [PubMed] [Google Scholar]
- 46.Iitsuka Y, Bock A, Nguyen DD, Samango-Sprouse CA, Simpson JL, Bischoff FZ. Evidence of skewed X-chromosome inactivation in 47,XXY and 48,XXYY Klinefelter patients. Am J Med Genet. 2001;98:25–31. [PubMed] [Google Scholar]
- 47.Lorda-Sanchez I, Binkert F, Hinkel KG, Moser H, Rosenkranz W, Maechler M, et al. Uniparental origin of sex chromosome polysomies. Hum Hered. 1992;42:193–197. doi: 10.1159/000154066. [DOI] [PubMed] [Google Scholar]
- 48.Wikstrom AM, Painter JN, Raivio T, Aittomaki K, Dunkel L. Genetic features of the X chromosome affect pubertal development and testicular degeneration in adolescent boys with Klinefelter syndrome. Clin Endocrinol (Oxf) 2006;65:92–97. doi: 10.1111/j.1365-2265.2006.02554.x. [DOI] [PubMed] [Google Scholar]
- 49.Stemkens D, Roza T, Verrij L, Swaab H, Van Werkhoven MK, Alizadeh BZ, et al. Is there an influence of X-chromosomal imprinting on the phenotype in Klinefelter syndrome? A clinical and molecular genetic study of 61 cases. Clin Genet. 2006;70:43–48. doi: 10.1111/j.1399-0004.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 50.van Rijn S, Swaab H, Aleman A, Kahn R. X Chromosomal effects on social cognitive processing and emotion regulation: a study with Klinefelter men (47,XXY) Schizophrenia Res. 2006;84:194–203. doi: 10.1016/j.schres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 51.Ross JL, Roeltgen D, Stefanatos G, Benecke R, Zeger M, Kushner H, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146A:708–719. doi: 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]
- 52.Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, et al. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:e232–e240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- 53.Itti E, Gaw Gonzalo IT, Pawlikowska-Haddal A, Boone KB, Mlikotic A, Itti L, et al. The structural brain correlates of cognitive deficits in adults with Klinefelter’s syndrome. J Clin Endocrinol Metab. 2006;91:1423–1427. doi: 10.1210/jc.2005-1596. [DOI] [PubMed] [Google Scholar]
- 54.Handelsman DJ. Update in andrology. J Clin Endocrinol Metab. 2007;92:4505–4511. doi: 10.1210/jc.2007-1431. [DOI] [PubMed] [Google Scholar]
- 55.Ottesen AM, Garn ID, Aksglaede L, Juul A, Rajpert-DeMeyts E. A simple screening method for detection of Klinefelter syndrome and other X-chromosome aneuploidies based on copy number of the androgen receptor gene. Mol Hum Reprod. 2007;13:745–750. doi: 10.1093/molehr/gam053. [DOI] [PubMed] [Google Scholar]
- 56.Vawter MP, Harvey PD, DeLisi LE. Dysregulation of X-linked gene expression in Klinefelter’s syndrome and association with verbal cognition. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:728–734. doi: 10.1002/ajmg.b.30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. X-chromosome inactivation patterns and androgen receptor functionality influence phenotype and social characteristics as well as pharmacogenetics of testosterone therapy in Klinefelter patients. J Clin Endocrinol Metab. 2004;89:6208–6217. doi: 10.1210/jc.2004-1424. [DOI] [PubMed] [Google Scholar]
- 58.Rovensky J. Rheumatic diseases and Klinefelter’s syndrome. Autoimmun Rev. 2006;6:33–36. doi: 10.1016/j.autrev.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Geschwind DH, Gregg J, Boone K, Karrim J, Pawlikowska-Haddal A, Rao E, et al. Klinefelter’s syndrome as a model of anomalous cerebral laterality: testing gene dosage in the X chromosome pseudoautosomal region using a DNA microarray. Dev Genet. 1998;23:215–229. doi: 10.1002/(SICI)1520-6408(1998)23:3<215::AID-DVG7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]