Abstract
The discovery of activating BRAF mutations in ~50% of all melanomas has proved to be a turning point in the therapeutic management of the disseminated disease. In this commentary we review the latest research delineating the role of mutant BRAF in melanoma initiation and progression and discuss the remarkable 10-year journey leading up to the recent FDA-approval of the small molecule BRAF inhibitor vemurafenib. We further outline the most recent findings on the mechanisms that underlie intrinsic and acquired BRAF inhibitor resistance and describe ongoing preclinical and clinical studies designed to delay or abrogate the onset of therapeutic escape. It is hoped that our evolving understanding of melanoma genetics and intracellular signaling coupled with a growing armamentarium of signal transduction inhibitors will lead to significant improvements in the level and durability of therapeutic response in metastatic melanoma.
Keywords: melanoma, BRAF, vemurafenib, GSK2118436, resistance
Introduction
Disseminated melanoma has a dismal prognosis and is almost completely resistant to therapeutic modalities such as chemotherapy and radiotherapy. The 2002 discovery of activating mutations in the serine/threonine kinase BRAF (v-raf murine sarcoma viral oncogene homolog B1) in approximately 50% of all melanomas kick-started a targeted therapy “arms race” which in under 10 years led to the FDA-approval of the BRAF inhibitor vemurafenib1,2. Together, these studies have set a new benchmark for the rapid development of genome-specific anti-cancer agents. In this commentary we will review the latest developments in our understanding of the role of BRAF signaling in melanoma pathogenesis and will discuss the preclinical and clinical development of small molecule inhibitors of the BRAF/MEK/ERK signaling pathway. We will further outline the challenges that remain in the effective clinical translation of BRAF/MEK/ERK inhibitors with a particular emphasis upon emerging strategies for the management of BRAF inhibitor resistance in the clinic.
The biology of mutated BRAF and the role of the MEK/ERK signaling pathway in melanoma development and progression
BRAF is a member of the Raf family of serine threonine kinases (ARAF, BRAF and CRAF), which are part of the Ras/Raf/MEK/ERK mitogen activated protein kinase (MAPK) signal transduction cascade. The MAPK pathway is a key mediator of growth signaling that links cell surface growth factor receptors (such a receptor tyrosine kinases; RTKs) to the increased transcription of genes required for cell cycle entry. Although mutations in BRAF have been described at a number of sites (see3 for a comprehensive list), the majority, which account for >80%, result in the substitution of valine to glutamic acid (the BRAF V600E mutation)1,4. Acquisition of the V600E mutation destabilizes the inactive conformation of the BRAF kinase shifting the equilibrium to the active state5. Of the other BRAF mutations identified in melanoma, V600K, V600D/R are also common and represent 16% and 3% of all BRAF mutations, respectively6. In addition to melanoma, BRAF mutations are also common in many other cancers including papillary thyroid carcinoma, ovarian carcinoma and colorectal carcinoma7.
Despite the well established role of BRAF mutations in cancer, equivalent activating mutations in either ARAF or CRAF are extremely rare7. The reasons behind this are still subject to debate but seem to be a consequence of the relative ease of BRAF activation (with only Ras-mediated membrane recruitment required) compared to the more complex process of ARAF and CRAF activation (which involves a number of priming phosphorylation events at multiple sites by Src and other as yet unidentified kinases7).
V600E-mutated BRAF is a bona fide melanoma oncogene, with its introduction leading to the malignant transformation of immortalized human melanocytes both in vitro and in vivo8. In transgenic mouse models the conditional, melanocyte-specific expression of BRAF V600E leads to spontaneous melanoma formation, but only in conjunction with the inactivation of the tumor suppressor phosphatase and tensin homolog (PTEN)9. This, together with data showing that introduction of mutant BRAF alone into primary human melanocytes leads to senescence, suggests that although mutated BRAF may be an initiating factor in melanomagenesis, other co-operating events are also required10.
Although BRAF mutations are not ultraviolet (UV) radiation signature mutations, they have a tendency to occur on UV-exposed skin sites and are more prevalent in individuals with a poor tanning response11. There is also evidence that intermittent, rather than chronic sun-exposure is predictive for BRAF mutational status with BRAF mutant melanoma patients tending to be younger in age (<55 years old) with a lower cumulative UV exposure12. BRAF mutational status is also of prognostic value and is associated with inferior survival in the metastatic setting (8.5 months in BRAF wild-type vs 5.7 months for BRAF mutant melanoma)13.
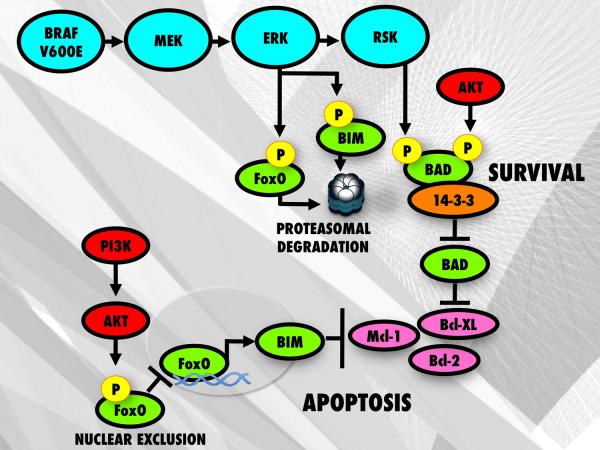
Much of the transforming activity of mutant BRAF is mediated through activation of the RAF/MEK/ERK pathway1. Signaling through the MAPK pathway drives the growth of melanoma cells through the upregulation of cyclin D1 expression and by downregulating the cell cycle inhibitor p27KIP1. It also serves to increase melanoma cell survival by regulating the expression and function of a number of pro and anti-apoptotic proteins, such as BIM, BMF, BAD and Mcl-114–17 (Figure 1). Inhibition of BRAF or MEK signaling using either small molecule inhibitors or siRNA knockdown increases the expression of the pro-apoptotic BH3-only protein BIM which induces apoptosis by binding to and antagonizing the activity of the pro-survival proteins Bcl-2, Bcl-w, Bcl-XL and Mcl-118,19. BIM is known to exist as three spliceforms BIM-EL (extra long), BIM-L (long), and BIM-S (short), with BIM-S being the most cytotoxic isoform19. The BRAF/MEK/ERK pathway regulates BIM expression through phosphorylation at Ser69, leading to its proteasomal degradation and by differentially regulating BIM splicing18,20. Survival of melanoma cells is also controlled in part by the anti-apoptotic protein, Mcl-1, whose stability can also be regulated through the BRAF/MEK/ERK pathway15 (Figure 1). A number of recent studies have further suggested a role for increased BMF (Bcl-2 modifying factor) expression in mediating the apoptotic response of melanoma cells treated with inhibitors of BRAF and MEK14,21.
Figure 1. BRAF V600E regulation of pro-apoptotic proteins promotes cell survival.
Inhibition of BRAF prevents proteasomal degradation of FOXO3a and BIM by blocking MEK/ERK mediated phosphorylation of FOXO3a at Ser294, Ser344 and Ser425 as well as phosphorylation of BIM at Ser69. However, in some melanomas with constitutive PI3K/AKT activity, increased AKT signaling leads to phosphorylation and nuclear exclusion of FOXO3a resulting in decreased expression of BIM. Both the MAPK pathway and AKT pathways can also regulate the activity of BAD through direct phosphorylation.
Constitutive activity in the BRAF/MEK/ERK pathway in melanoma drives the invasive and motile behavior of melanoma cells through the re-organization of the cytoskeleton, the activation of the cells' migratory machinery and the upregulation of matrix metalloproteinase expression22,23. Mechanistically, mutated BRAF downregulates the expression of the cyclic GMP phosphodiesterase PDE5A, which facilitates melanoma cell invasion by increasing intracellular levels of cGMP, leading to the release of cytosolic calcium and the phosphorylation of myosin light chain 224. Mutant BRAF also increased motility through direct effects upon the cytoskeleton. In this instance, BRAF signaling directly regulates the expression of RND3/RhoE/Rho8, a protein that mediates the crosstalk between the RAF/MEK/ERK and Rho/Rock/LIMK/Cofilin pathways25,26. Depletion of RND3 using either siRNA or following BRAF/MEK inhibition led to an increase in focal adhesion stability and an inhibition of melanoma cell invasion in 3D cell culture models25.
There is also evidence that oncogenic BRAF regulates the interaction of melanoma cells with the host microenvironment, particularly cells of the immune system. Inhibition of BRAF or MEK signaling in melanoma cells upregulates the expression of highly immunogenic differentiation antigens, leading to enhanced T-cell recognition27–29. The apparent ability of BRAF/MEK inhibitors to enhance the immune cell recognition of melanoma may offer a rationale for the future combination of BRAF inhibitors with immunotherapies such as ipilimumab30.
The preclinical development of BRAF, MEK and ERK inhibitors in melanoma
At the time of the initial discovery of activating BRAF mutations in melanoma, selective BRAF kinase inhibitors had not been developed. One drug that was available at the time was the multi-kinase inhibitor sorafenib (Nexxavar ®)31. Although this was primarily a CRAF inhibitor, it did have some activity against mutant BRAF and was enthusiastically embraced as the first BRAF specific agent. Despite some activity in cell culture and the ability to stabilize disease in human melanoma xenograft models, sorafenib was ultimately found to have little activity in human melanoma patients, even when selected for their BRAF mutational status32,33.
The latest generation of BRAF inhibitors offers a significant improvement over sorafenib in terms of potency against mutant BRAF and far fewer off-target effects. Compounds that have been evaluated preclinically include AZ628, XL281, GDC-0879, SB590885, GSK2118436 (GSK2118436) and vemurafenib (PLX4032, and its analogue PLX4720)34–38 (Figure 2). Of these, vemurafenib and GSK2118436 have been the most extensively studied both preclinically and clinically. Vemurafenib is an ATP-competitive RAF inhibitor (WT BRAF IC50, 100nM; mutant BRAF IC50, 31 nM; CRAF IC50, 48 nM) that selectively inhibits the phospho-ERK expression and growth of melanoma cell lines harboring BRAF V600E and V600K mutations35,39. In cell line models, vemurafenib induced both a G1-phase cell cycle arrest and an apoptotic response that was well correlated with increased BIM expression as well as the regression of established human BRAF V600E mutant melanoma xenografts40,41.
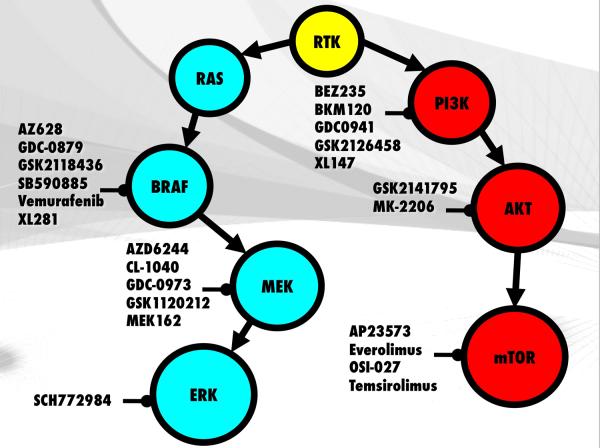
Figure 2.
Targeted kinase inhibitors of the MAPK and PI3K/AKT/mTOR pathways. Several compounds such as AZ628, dabrafenib, GDC-0879, SB90885 and XL281 are being evaluated for clinical use with vemurafenib being the only currently FDA approved BRAF inhibitor. Compounds that target the MAPK cascade such as the MEK inhibitor GSK1120212 and the ERK inhibitor SCH772984 as well as PI3K, AKT and mTOR inhibitors are also being developed as single and combined agents for the treatment of BRAF mutant melanomas.
One unexpected finding was that small molecule BRAF inhibitors activated MAPK signaling pathway in melanoma cells that were BRAF wild-type42–45. In vitro, the paradoxical activation of MAPK signaling arose in BRAF wild-type cell lines that harbored either an oncogenic Ras mutation or had upstream constitutive RTK activity (such as in HER2)42,44,46. The paradoxical activation of Raf that occurred following BRAF inhibition was found to increase proliferation and also enhanced the invasion of the melanoma cells through increased FAK activity as well as increasing the survival of NRAS mutated melanoma cell lines by increasing Mcl-1 expression45. Nearly all BRAF inhibitors evaluated to date, including sorafenib, vemurafenib, GSK2118436 and XL281 have been associated with the development of proliferative skin lesions most frequently identified as squamous cell carcinomas of the keratoacanthoma (KA) type36,47,48. These lesions, although rapidly growing, can be managed through surgical excision and do not recur. There is growing evidence that KAs arise as a result of the paradoxical activation of MAPK signaling following BRAF inhibition. Sequencing of a large cohort of KAs from patients that had spontaneous KAs, were immunosuppresed or were undergoing RAF inhibitor therapy demonstrated a significant enrichment for Ras mutations (21% vs 3%) in those individuals undergoing RAF inhibitor therapy49,50. These and other observations have prompted the development of a new generation of BRAF inhibitors, so-called “paradox breakers” (typified by PLX-Pb4) that apparently prevent the paradoxical activation of MAPK signaling. Although published data is currently lacking on these new drugs, it is hoped that their improved selectivity profile may prevent the development of KA and delay the time to resistance.
MEK and ERK inhibitors
A large body of preclinical work has demonstrated that virtually all melanomas have high constitutive activity in the MAPK pathway. The reasons for this are numerous, and can include activating BRAF and NRAS mutations, autocrine stimulation of growth factor receptors, such as c-MET and FGFR151, activation through avβ3 integrin binding52, and increased Notch1 signaling53. Most early studies of MEK inhibition in melanoma preceded the discovery of BRAF mutations and instead focused upon the role of MAPK in suppressing differentiation (an observation that is now proving critical for the development of BRAF/immunotherapy combinations)54–56.
Preclinical studies have shown that inhibition of MEK in melanoma cells using inhibitors such as PD98059, PD0325901, CI-1040 and AZD6244 leads to a profound G1 phase cell cycle arrest57–59 (Figure 2). These cytostatic effects are associated with the inhibition of cyclin D1 expression, increased p27KIP1 expression and hypophosphorylation of the retinoblastoma protein57,59. In some sensitive BRAF mutant melanoma cell lines, MEK inhibition also induces apoptosis60. In general, the inhibition of MEK alone was not found to induce high levels of apoptosis and anti-tumor effects were enhanced following the dual inhibition of MEK and PI3K or by combining MEK inhibitors with chemotherapy drugs, such as paclitaxel58,61 (Figure 2).
Another class of drugs targeting the MAPK pathway currently under development are the ERK inhibitors, AEZS-131 (Æterna Zentaris) and SCH772984 (Merck/Schering Plough). Although little published data is currently available on these compounds they seem pharmacologically distinct from MEK inhibitors and they may be of some future utility in overcoming vemurafenib resistance.
Clinical development of BRAF and MEK inhibitors
The first RAF inhibitor to be developed clinically in melanoma was the pan-kinase inhibitor sorafenib31. In early clinical trials, single agent sorafenib showed little efficacy and was associated with a median progression-free survival (PFS) of 3 months and only 1 response out of 39 melanoma patients treated62. On the basis of phase II trials, where hints of sorafenib response were seen in combination with chemotherapy, large-scale phase III trials of sorafenib in combination with chemotherapy were initiated. The randomized studies showed variable results with low response rates and did not provide evidence that the clinical effects of sorafenib were mediated through RAF inhibition33. It now seems likely that any sorafenib activity observed in melanoma patients was independent of its effects upon BRAF and may instead have been a reflection of its off-target inhibitory effects on kinases such as VEGFR32,63. It is worth noting that the putative anti-angiogenic effects of sorafenib are the likely basis of the drug's efficacy in renal cell carcinoma64.
The clinical failure of sorafenib led some to question whether oncogenic BRAF was really a good therapeutic target in melanoma. These concerns were quickly dispelled by the development of vemurafenib, a more potent and selective BRAF inhibitor, with fewer off-target effects than sorafenib35. In the phase I clinical trial, approximately 80% of BRAF V600E mutated melanoma patients treated at the recommended phase II dose of vemurafenib (960 mg, PO BID) responded to therapy. In all cases, responses were associated with inhibition of intratumoral phospho-ERK, reduction of Ki67 positivity by immunohistochemistry and the inhibition of glucose uptake as measured by FDG-PET65. An update of the phase I data, presented at the 2011 Society of Melanoma Research meeting, demonstrated the 1-year survival to be ~50% and the 2-year survival to be 38%, with a median overall survival of 13.8 months66.
The phase II trial of vemurafenib (960mg BID) (BRaf In Melanoma: BRIM-2) enrolled 132 patients who had progressed on other therapies. In this cohort, there were 3 complete responses, 66 confirmed partial responses, and 39 patients with stable disease. Median OS has yet to be met and the medial PFS for this refractory population was 6.8 months67. The pivotal phase III trial (BRIM-3) randomized 675 untreated metastatic melanoma patients to either vemurafenib (960mg PO BID) or dacarbazine (the “standard of care” for metastatic melanoma). The study was terminated early in December 2010 because of a clear overall survival benefit seen to vemurafenib, with a hazard ration of 0.44 favoring vemurafenib over dacarbazine48. The overall response rate for vemurafenib was 48% and median PFS of 5.3 months as compared to 5% response rate and median PFS of 1.6 months for dacarbazine48. At six months, the Kaplan-Meier estimate of overall survival was 83% and 63% for vemurafenib and dacarbazine respectively. Correlative studies showed that a >90% reduction in intratumoral phospho-ERK was required for a clinical response to be seen68. Based upon the phase II trial data, vemurafenib was FDA-approved for unresectable metastatic BRAF mutant melanoma in late 2011.
BRAF V600E/K mutant melanoma patients treated with the BRAF inhibitor GSK2118436 showed similarly impressive responses36. A single-arm phase II study (BREAK-2) (n=92: 76 V600E mutant: 16 V600K mutant) showed objective response rates in the V600E cohort of 59% (95% CI 48–70%) with responses seen in 2 V600K patients. Median progression free survival was 27 and 20 weeks, respectively in the two mutation subsets. At this time GSK2118436 is currently undergoing phase III evaluation.
MEK inhibition
The identification of BRAF and NRAS mutations in significant numbers of melanoma patients and the observation that nearly all melanomas showed constitutive MAPK activity, led to the development of small molecule MEK inhibitors, such as PD0325901, selumetinib (AZD6244) and CI-1040 in unselected groups of melanoma patients69–71 (Figure 2). The early studies on PD0325901 showed limited evidence of activity, with 2 out of 27 patients having partial responses and a further 5 patients having stable disease. In the phase I trial of AZD6244 one BRAF mutant melanoma patient was noted to have a complete response to AZD6244 persisting beyond 15 months72. However, a subsequent large phase II trial of AZD6244 vs temozolomide in an unselected melanoma patient population showed no difference in PFS73. Overall these initial studies were disappointing and it was not clear whether these drugs were able to reliably inhibit the MAPK pathway at the doses used. The use of MEK inhibitors was further limited by diarrhea and visual disturbance with serious retinal vein thrombosis being reported in small numbers of patients.
Interest in the clinical development of MEK inhibitors in BRAF mutant melanoma was renewed by the development of GSK112012, an allosteric MEK inhibitor that potently inhibits MAPK signaling at clinically achievable doses74,75. At this time, GSK1120212 represents the most extensively studied MEK inhibitor in BRAF mutant melanoma and appears to have the best single agent activity of any MEK inhibitor evaluated thus far. In the phase II trial of 57 BRAF mutant melanoma patients previously treated with chemotherapy or immunotherapy, there were 2 complete responses, 17 partial responses, and 27 patients with stable disease, indicating an objective response rate of 33% and a disease control rate of 81%76. In the BRAF inhibitor naïve cohort, updated data showed the median duration of response to be 5.7 months, with a median PFS of 4 months76. Similar levels of response were seen in V600E or V600K mutated patients; however, there was minimal activity seen in the cohort of patients previously treated with a BRAF inhibitor. GSK1120212 is currently being evaluated in a Phase III trial (NCT01245062). It is worth noting that although GSK21120212 represents the best activity in its class, single-agent BRAF inhibition appears to show greater clinical efficacy.
Strategies to manage BRAF inhibitor resistance
Despite the impressive levels of tumor shrinkage observed in BRAF mutant melanoma patients treated with small molecule BRAF inhibitors, responses are typically short-lived (PFS: ~ 7 months) with resistance occurring in nearly every case48,77. The observation that ~50% of BRAF mutant melanoma patients on vemurafenib therapy did not meet the RECIST criteria for a response further suggests the existence of intrinsic BRAF inhibitor resistance48. Intrinsic resistance has been well documented in preclinical studies, with BRAF V600E mutant melanoma cell lines showing a wide range of IC50 values to vemurafenib and other BRAF inhibitors38,78–80. Although the reasons behind this are not yet clear, melanomas are known to harbor complex patterns of mutations and genomic amplifications, with alterations being reported in (among other things), MITF, AKT3, COT, cyclin D1, CDK2, CDK4 and the retinoblastoma protein79,81–85. How these multiple factors interact to convey intrinsic resistance to BRAF inhibitors is currently the focus of intense investigation.
Intrinsic resistance to targeted therapy agents, such as trastuzumab in breast cancer, often results from increased basal levels of AKT signaling associated with the loss of PTEN expression/function86. In this instance, inhibition of EGFR is associated with impaired apoptosis and an overall worse response86. Emerging evidence suggests that impaired PTEN function may also, in part, mediate intrinsic vemurafenib resistance in melanoma80,87 (Figure 3). This has been shown to result from increased AKT signaling when BRAF is inhibited, which limits the nuclear accumulation of FOXO3a, leading to a decrease in BIM-mediated apoptosis87 (Figure 1). A recent clinical analysis of patients receiving the BRAF inhibitor GSK2118436 support these preclinical studies and show that BRAF mutant melanoma patients with a concurrent PTEN dysfunction exhibit lower response rates than BRAF mutant melanoma patients that retain PTEN function88. Similar findings were also reported in BRAF mutant melanoma cell lines treated with the MEK inhibitor AZD6244, where increased IGF-I mediated AKT signaling limited the apoptotic response60. In both of these cases, the IGF-I-mediated effects upon AKT signaling and intrinsic apoptosis resistance were overcome following treatment with a MEK + an AKT or mTORC1/2 inhibitor or a BRAF inhibitor + an AKT inhibitor60,87 (Figure 3).
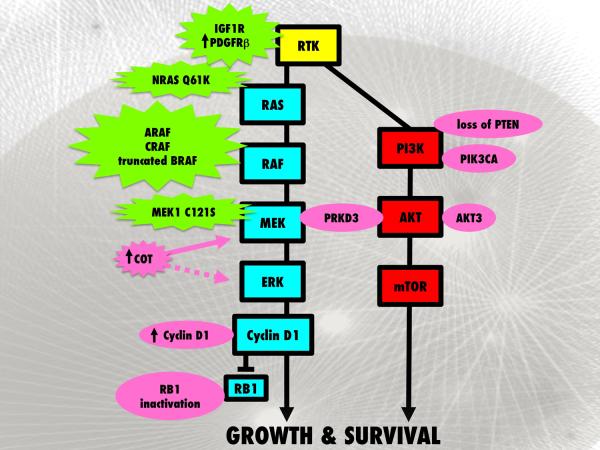
Figure 3. Known mechanisms of BRAF inhibitor resistance.
Multiple acquired (shown in green) and intrinsic (shown in pink) mechanisms of BRAF inhibitor resistance have been identified. Chronic BRAF inhibition can lead to activation of the MAPK pathway through acquisition of secondary NRAS Q61K mutations, RAF isoform (ARAF>BRAF>CRAF) signal switching, truncation of BRAF to a BRAF inhibitor refractory form, MEK1 C121S mutation as well as increased COT expression which has been linked to intrinsic as well as acquired resistance. RTK signaling via IGF1R and PDGFRb can also activate the MAPK and the PI3K/AKT pathways. Intrinsic mechanisms of resistance have been associated with amplification of cyclin D1 or RB1 inactivation allowing cells to continue through the cell cycle. Loss of PTEN and genetic alterations in PIK3CA and AKT3 are associated with de novo resistance by increasing PI3K/AKT activity while PRKD3 activation can increase both MAPK and PI3K/AKT signaling.
Inhibition of BRAF signaling in melanoma cell lines leads to the attenuation of MEK/ERK signaling, the inhibition of cyclin D1 expression and a G1-phase cell cycle arrest38. Work from our group identified a sub-set of BRAF mutant melanoma cell lines and tumor specimens with genomic amplification of cyclin D179. Melanoma cell lines with cyclin D1 amplification in concert with a BRAF mutation showed intrinsic resistance to BRAF inhibition and continued to enter the cell cycle when oncogenic BRAF was inhibited79. Deregulation of the G1 cell cycle checkpoint, as a result of mutational inactivation of the retinoblastoma protein (RB1), in conjunction with PTEN loss (found in 2/19 of BRAF V600E melanoma cell lines) also conveyed intrinsic resistance to inhibitors of BRAF and MEK80 (Figure 3).
Other recent studies identified protein kinase D3 (PRKD3) as being a potential mediator of intrinsic resistance to the RAF inhibitors RAF265 and vemurafenib89 (Figure 3). In this instance, the siRNA knockdown of PRKD3 reduced the IC50 of both RAF265 and vemurafenib in multiple melanoma cell lines and prevented the reactivation of MAPK signaling following drug treatment89. In BRAF V600E mutant cell lines lacking PTEN expression, PRKD3 activity further contributed to resistance through the reactivation of PI3K/AKT signaling following RAF inhibition89.
Acquired resistance to BRAF inhibitors occurs in the majority of patients treated90. Experimental studies suggest that minor populations of BRAF V600E-mutant melanoma cells exhibit drug tolerance and survive the initial treatment, with full resistance typically emerging after 3–6 months38,91,92. Unlike the resistance reported to other targeted therapies such as EGFR inhibitors in non-small cell lung cancer and imatinib resistance in gastrointestinal stromal tumors (GIST) and chronic myeloid leukemia (CML), resistance to BRAF inhibition is not associated with the acquisition of secondary (so called “gate-keeper”) mutations in the kinase that prevent drug binding93–95. Although in vitro studies identified threonine-529 as the gate-keeper residue site in BRAF, a large scale sequencing analysis of the gene at exon 13 (where Thr-529 lies) did not identify this mutation in specimens from melanoma patients failing vemurafenib therapy96,97. Instead, preclinical studies where BRAF inhibitor resistance was generated in vitro showed that acquired resistance was mediated through a diverse array of mechanisms including constitutive signaling in receptor tyrosine kinases (IGF1R and PDGFR-β), increased expression of the MAP kinase family member COT (MAP3K8, TPL-2), acquisition of mutations in NRAS and MEK1 and as the result of a BRAF truncations37,83,97–100 (Figure 3). These observations support earlier pre-clinical studies showing that exogenously added growth factors and cytokines were able to rescue melanoma cells from cell death following siRNA-induced knockdown of BRAF101,102. The relative importance and frequency of each of these proposed resistance mechanisms in melanoma patients failing therapy are not currently clear and still require extensive clinical validation.
Although a number of potential vemurafenib resistance mechanisms have been reported, nearly all rely upon a common set of signaling pathways. Preclinical studies have already shown that reactivation of MAPK signaling is commonly associated with vemurafenib resistance and that combined MEK + BRAF inhibition is effective at abrogating the resistance mediated by MEK1 mutations, COT overexpression, BRAF truncation and acquired Ras mutations38,83,99,100. In contrast, resistance mediated through increased IGF1R signaling appears to be overcome by dual MEK + PI3K inhibition and resistance mediated through increased PDGFR-β signaling can be reversed through the targeting of the mTOR/PI3K/AKT pathway98,103.
Clinical evaluation of BRAF inhibitor based combinations
The hypothesis that the recovery of MEK/ERK signaling underlies some of the resistance to BRAF inhibitors is currently being clinically validated in a phase I/II trial of the GlaxoSmithKline BRAF inhibitor GSK2118436 in combination with the MEK inhibitor GSK1120212 (NCT01072175) as well as a trial of vemurafenib in combination with MEK inhibitor GDC-0973 (NCT01271803). Early indications suggest these combinations may be effective, with the recent data presented at ASCO showing the GSK2118436 + GSK1120212 combination to be associated with an objective response rate of 77% (150mg GSK2118436, 1mg GSK1120212). Of interest, the combination was found to significantly reduce the incidence of keratoacanthoma (<1%, n=109), validating the role of rebound BRAF inhibitor mediated MEK/ERK signaling in the development of these tumors74.
The phase I/II expansion cohort of the GSK2118436/GSK1120212 combination is also being evaluated in BRAF mutant melanoma patients who progressed on a prior BRAF inhibitor. In this study, 26 patients with metastatic melanoma who progressed on either GSK2118436 or vemurafenib alone were enrolled. Six of the patients had also previously received a MEK inhibitor. The median time since discontinuing prior BRAF inhibitor therapy was 1.1 months. The objective response rate was 19% but 62% of patients had some degree of tumor regression. The median progression-free survival was 3.6 months. Though most responses were short-lived, a subset of PR and SD patients (n=7) were still on therapy beyond 30 weeks104. In contrast, single agent GSK112012 had little efficacy in patients who progressed on prior BRAF inhibitor therapy66.
Based upon preclinical observations demonstrating the dual requirement for the BRAF/MEK and PI3K/AKT signaling pathways in melanoma progression, and the likely role of enhanced PI3K signaling in BRAF inhibitor resistance, clinical trials have been initiated to examine the PI3K/mTOR inhibitor BEZ235 in combination with the MEK inhibitor MEK162 (NCT01337765)9,61,91,98,105. This trial is currently enrolling patients who are BRAF mutant and those who are NRAS mutant.
The suggestion that BRAF targeted agents may enhance the effects of immunotherapy led to the initiation of a phase I/II clinical trial to evaluate the efficacy of vemurafenib in combination with the anti-CTLA-4 antibody ipilimumab (NCT01400451).
Future perspectives
The recent years have seen incredible progress in our management of advanced melanoma. If progress continues as we expect, a future can be envisaged in which rationally designed BRAF inhibitor-based drug combinations may be able to significantly extend the life span of BRAF-mutant melanoma patients. The possible development of successful BRAF inhibitor/immune therapy based combinations offers the real possibility that very durable responses could be achieved. Although significant challenges remain as to the identification of good response biomarkers and strategies to overcome acquired drug resistance these remain exciting times for the melanoma community.
Acknowledgments
Source of Funding: Dr Keiran Smalley is currently receiving grants U54 CA143970-01 and R01 CA161107-01 from the National Institutes of Health, The Harry Lloyd Trust and the State of Florida (09BN-14).
Footnotes
Conflicts of Interest For the remaining authors none were declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med. 2011 doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematology/oncology clinics of North America. 2009;23:529–45. ix. doi: 10.1016/j.hoc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Smalley KS, Xiao M, Villanueva J, et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009;28:85–94. doi: 10.1038/onc.2008.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 7.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 8.Karasarides M, Chiloeches A, Hayward R, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–8. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 9.Dankort D, Curley DP, Cartlidge RA, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 11.Landi MT, Bauer J, Pfeiffer RM, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313:521–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 12.Viros A, Fridlyand J, Bauer J, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS medicine. 2008;5:e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–46. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 14.Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670–81. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–56. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–12. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 17.Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cartlidge RA, Thomas GR, Cagnol S, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–44. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 20.Jiang CC, Lai F, Tay KH, et al. Apoptosis of human melanoma cells induced by inhibition of B-RAF(V600E) involves preferential splicing of bim(S) Cell Death Dis. 2010;1:e69. doi: 10.1038/cddis.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanBrocklin MW, Verhaegen M, Soengas MS, Holmen SL. Mitogen-activated protein kinase inhibition induces translocation of Bmf to promote apoptosis in melanoma. Cancer Res. 2009;69:1985–94. doi: 10.1158/0008-5472.CAN-08-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods D, Cherwinski H, Venetsanakos E, et al. Induction of beta 3-integrin gene expression by sustained activation of the Ras-regulated Raf-MEK-extracellular signal-regulated kinase signaling pathway. Molecular and Cellular Biology. 2001;21:3192–205. doi: 10.1128/MCB.21.9.3192-3205.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard CA, Hayes L, Wojnowski L, Zimmer A, Marais RM, Norman JC. B-Raf acts via the ROCKII/LIMK/cofilin pathway to maintain actin stress fibers in fibroblasts. Mol Cell Biol. 2004;24:5937–52. doi: 10.1128/MCB.24.13.5937-5952.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arozarena I, Sanchez-Laorden B, Packer L, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–33. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein RM, Spofford LS, Abel EV, Ortiz A, Aplin AE. B-RAF regulation of Rnd3 participates in actin cytoskeletal and focal adhesion organization. Mol Biol Cell. 2008;19:498–508. doi: 10.1091/mbc.E07-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 28.Kono M, Dunn IS, Durda PJ, et al. Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol Cancer Res. 2006;4:779–92. doi: 10.1158/1541-7786.MCR-06-0077. [DOI] [PubMed] [Google Scholar]
- 29.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–6. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nature reviews Drug discovery. 2011;10:411–2. doi: 10.1038/nrd3463. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 32.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 33.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 34.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kefford R, Arkenau H, Brown MP, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. Journal of Clinical Oncology. 2010;28:8503. [Google Scholar]
- 37.Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–61. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paraiso KH, Fedorenko IV, Cantini LP, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubinstein JC, Sznol M, Pavlick AC, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 70:5518–27. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 41.Lee JT, Li L, Brafford PA, et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigm Cell Melanoma R. 2010;23:820–7. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene. 2010 doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph EW, Pratilas CA, Poulikakos PI, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–8. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz GK, Robertson S, Shen A, et al. A phase I study of XL281, a selective oral RAF kinase in patients with advanced solid tumors. Journal of Clinical Oncology. 2009;27:3513. [Google Scholar]
- 48.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kee D, Oberholzer PA, Dziunycz P, et al. Squamous cell tumors from RAF inhibitor treated patients have a distinct mutational profile supporting a mechanism of therapy induced tumorigenesis in Ras-primed cells. Pigm Cell Melanoma R. 2011;24:1019. [Google Scholar]
- 50.Oberholzer PA, Kee D, Dziunycz P, et al. RAS Mutations Are Associated With the Development of Cutaneous Squamous Cell Tumors in Patients Treated With RAF Inhibitors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 52.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Research. 2003;63:756–9. [PubMed] [Google Scholar]
- 53.Liu ZJ, Xiao M, Balint K, et al. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–90. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- 54.Smalley K, Eisen T. The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. Febs Letters. 2000;476:198–202. doi: 10.1016/s0014-5793(00)01726-9. [DOI] [PubMed] [Google Scholar]
- 55.Englaro W, Rezzonico R, Durand-Clement M, Lallemand D, Ortonne JP, Ballotti R. Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. The Journal of biological chemistry. 1995;270:24315–20. doi: 10.1074/jbc.270.41.24315. [DOI] [PubMed] [Google Scholar]
- 56.Englaro W, Bertolotto C, Busca R, et al. Inhibition of the mitogen-activated protein kinase pathway triggers B16 melanoma cell differentiation. The Journal of biological chemistry. 1998;273:9966–70. doi: 10.1074/jbc.273.16.9966. [DOI] [PubMed] [Google Scholar]
- 57.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haass NK, Sproesser K, Nguyen TK, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008;14:230–9. doi: 10.1158/1078-0432.CCR-07-1440. [DOI] [PubMed] [Google Scholar]
- 59.Smalley KS, Contractor R, Haass NK, et al. Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. Br J Cancer. 2007;96:445–9. doi: 10.1038/sj.bjc.6603596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gopal YN, Deng W, Woodman SE, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res. 2010;70:8736–47. doi: 10.1158/0008-5472.CAN-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 62.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jilaveanu L, Zito C, Lee SJ, et al. Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res. 2009;15:1076–85. doi: 10.1158/1078-0432.CCR-08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flaherty KT, Puzanov I. Building on a foundation of VEGF and mTOR targeted agents in renal cell carcinoma. Biochemical Pharmacology. 2010;80:638–46. doi: 10.1016/j.bcp.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smalley KS, Aplin AE, Flaherty KT, et al. Meeting report from the 2011 international melanoma congress; Tampa, Florida. Pigm Cell Melanoma R 2012;25:E1-E11. [DOI] [PubMed] [Google Scholar]
- 67.Hersey P, Smalley KS, Weeraratna A, et al. Meeting report from the 7th International Melanoma Congress; Sydney. Nov, 2010. Pigm Cell Melanoma R 2011;24:e1-15. [DOI] [PubMed] [Google Scholar]
- 68.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–93. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 70.Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–62. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 71.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banerji U, Camidge DR, Verheul HMW, et al. The First-in-Human Study of the Hydrogen Sulfate (Hyd-Sulfate) Capsule of the MEK1/2 Inhibitor AZD6244 (ARRY-142886): A Phase I Open-Label Multicenter Trial in Patients with Advanced Cancer. Clinical Cancer Research. 2010;16:1613–23. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 73.Kirkwood JM, Bastholt L, Robert C, et al. Phase II, Open-Label, Randomized Trial of the MEK 1/2 Inhibitor Selumetinib as Monotherapy versus Temozolomide in Patients with Advanced Melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Infante JR, Falchook GS, Lawrence DA, et al. Phase I/II Study of the oral MEK1/2 inhibitor GSK1120212 dosed in combination with the oral BRAF inhibitor GSK2118436. J Clin Oncol. 2011;29:CRA8503. [Google Scholar]
- 75.Gilmartin AG, Bleam MR, Groy A, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 76.Kim K, Lewis K, Pavlick AC, et al. A phase II study of the MEK1/MEK2 inhibitor GSK21120212 in metastatic BRAF V600E or K mutant cutaneous melanoma patients previously treated with or without a BRAF inhibitor. Pigm Cell Melanoma R. 2011;24:1021. [Google Scholar]
- 77.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tap WD, Gong KW, Dering J, et al. Pharmacodynamic characterization of the efficacy signals due to selective BRAF inhibition with PLX4032 in malignant melanoma. Neoplasia. 2010;12:637–49. doi: 10.1593/neo.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smalley KS, Lioni M, Palma MD, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7:2876–83. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xing F, Persaud Y, Pratilas CA, et al. Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene. 2011 doi: 10.1038/onc.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–22. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 82.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 83.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010 doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sauter ER, Yeo UC, von Stemm A, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62:3200–6. [PubMed] [Google Scholar]
- 85.Du J, Widlund HR, Horstmann MA, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565–76. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 86.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–85. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 87.Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Research. 2011;71:2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nathanson KL, Martin AM, Letrero R, et al. Tumor genetic analysis of patients with metastatic melanoma treated with the BRAF inhibitor GSK2118436. Journal of Clinical Oncology. 2011;29 doi: 10.1158/1078-0432.CCR-13-0827. abstr 8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen J, Shen Q, Labow M, Gaither LA. Protein kinase D3 sensitizes RAF inhibitor RAF265 in melanoma cells by preventing reactivation of MAPK signaling. Cancer Research. 2011;71:4280–91. doi: 10.1158/0008-5472.CAN-10-3761. [DOI] [PubMed] [Google Scholar]
- 90.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–9. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang CC, Lai F, Thorne RF, et al. MEK-Independent Survival of B-RAFV600E Melanoma Cells Selected for Resistance to Apoptosis Induced by the RAF Inhibitor PLX4720. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-2225. [DOI] [PubMed] [Google Scholar]
- 92.Basile KJ, Abel EV, Aplin AE. Adaptive upregulation of FOXD3 and resistance to PLX4032/4720-induced cell death in mutant B-RAF melanoma cells. Oncogene. 2011 doi: 10.1038/onc.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–7. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 94.Yauch RL, Dijkgraaf GJ, Alicke B, et al. Smoothened Mutation Confers Resistance to a Hedgehog Pathway Inhibitor in Medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duensing S, Duensing A. Targeted therapies of gastrointestinal stromal tumors (GIST)--the next frontiers. Biochem Pharmacol. 2010;80:575–83. doi: 10.1016/j.bcp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Whittaker S, Kirk R, Hayward R, et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Science translational medicine. 2010;2:35ra41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]
- 97.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagle N, Emery C, Berger MF, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3085–96. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–90. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Christensen C, Guldberg P. Growth factors rescue cutaneous melanoma cells from apoptosis induced by knockdown of mutated (V 600 E) B-RAF. Oncogene. 2005;24:6292–302. doi: 10.1038/sj.onc.1208758. [DOI] [PubMed] [Google Scholar]
- 102.Gray-Schopfer VC, Karasarides M, Hayward R, Marais R. Tumor necrosis factor-alpha blocks apoptosis in melanoma cells when BRAF signaling is inhibited. Cancer Res. 2007;67:122–9. doi: 10.1158/0008-5472.CAN-06-1880. [DOI] [PubMed] [Google Scholar]
- 103.Shi H, Kong X, Ribas A, Lo RS. Combinatorial Treatments That Overcome PDGFR{beta}-Driven Resistance of Melanoma Cells to V600EB-RAF Inhibition. Cancer Research. 2011;71:5067–74. doi: 10.1158/0008-5472.CAN-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flaherty KT, Infante JR, Falchook GS, et al. Phase I/II expansion cohort of BRAF inhibitor GSK2118436 + MEK inhibitor GSK21120212 in patients with BRAF mutant metastatic melanoma who progressed on a prior BRAF inhibitor. Pigm Cell Melanoma R. 2011;24:1022. [Google Scholar]
- 105.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600E BRaf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–39. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]