Abstract
Oligoribonucleotides containing 2',5'-phosphodiester linkages have been synthesized on a solid support by the 'silyl-phosphoramidite' method. The stability of complexes formed between these oligonucleotides and complementary 3',5'-RNA strands have been studied using oligoadenylates and a variety of oligonucleotides of mixed base sequences including phosphorothioate backbones. In many cases, particularly for 2',5'-linked adenylates, the UV melting profiles are quite sharp and exhibit large hyperchromic changes. Substituting a few 3',5'-linkages with the 2',5'-linkage within an oligomer lowers the Tm of the complex and the degree of destabilization depends on the neighboring residues and neighboring linkages. The 2',5'-linked oligoribonucleotides prepared in this study exhibited remarkable selectivity for complementary single stranded RNA over DNA. For example, in 0.01 M phosphate buffer--0.10 M NaCl (pH 7.0), no association was observed between 2',5'-r(CCC UCU CCC UUC U) and its Watson-Crick DNA complement 3',5'-d(AGAAGGGAGAGGG). However, 2',5'-r(CCC UCU CCC UUC U) with its RNA complement 3',5'-r(AGAAGGGAGAGGG) forms a duplex which melts at 40 degrees C. The decamer 2',5'-r(Ap)9A forms a complex with both poly dT and poly rU but the complex [2',5'-r(Ap)9A]:[poly dT] is unstable (Tm, -1 degree C) and is seen only at high salt concentrations. In view of their unnatural character and remarkable selectivity for single stranded RNA, 2',5'-oligo-RNAs and their derivatives may find use as selective inhibitors of viral mRNA translation, and as affinity ligands for the purification of cellular RNA.
Full text
PDF
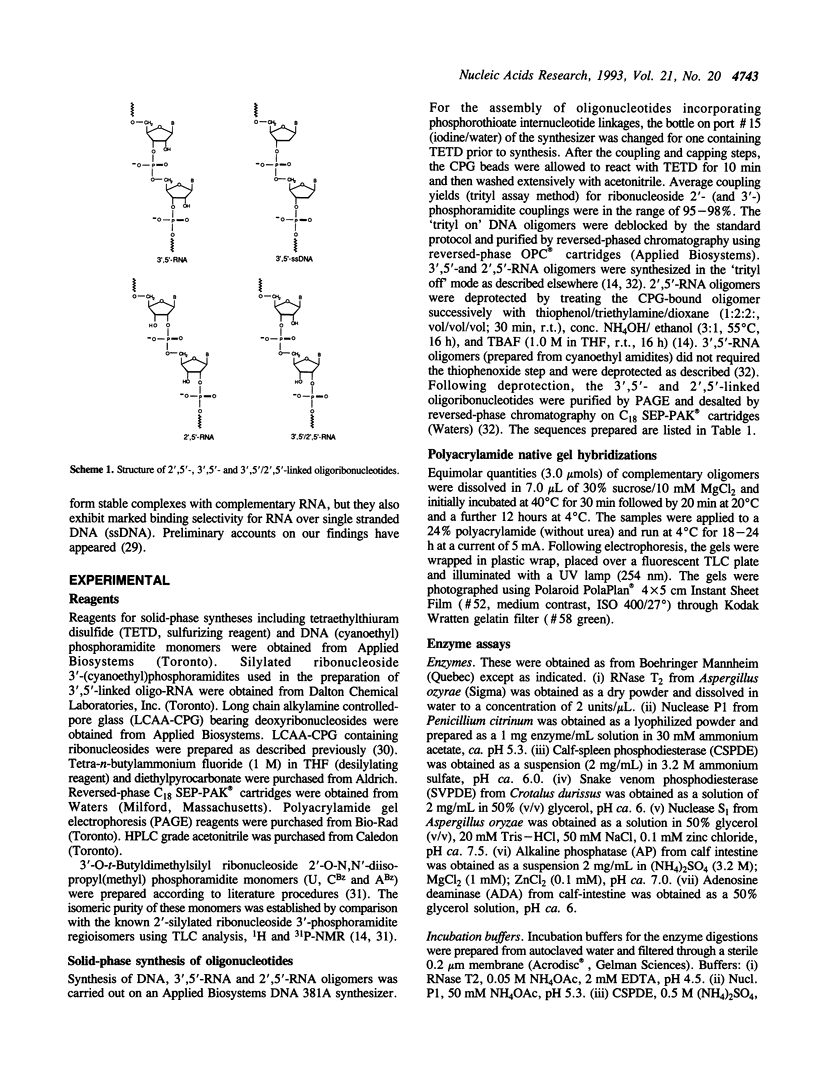

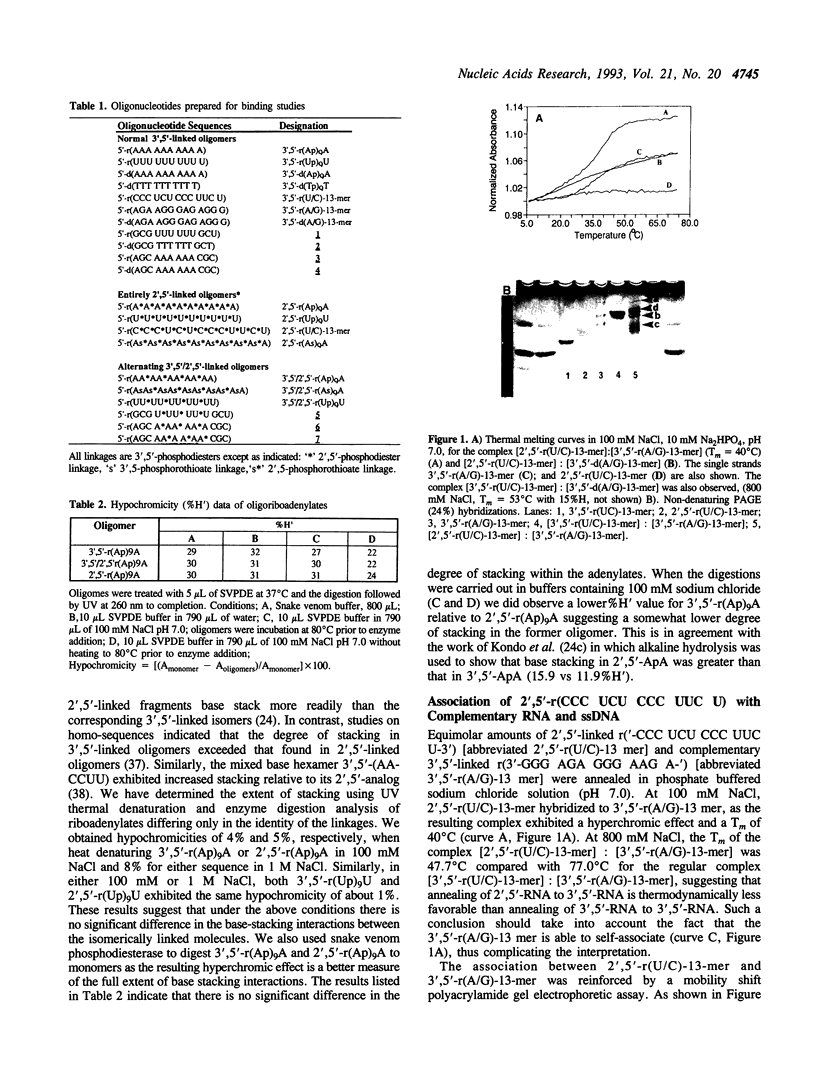
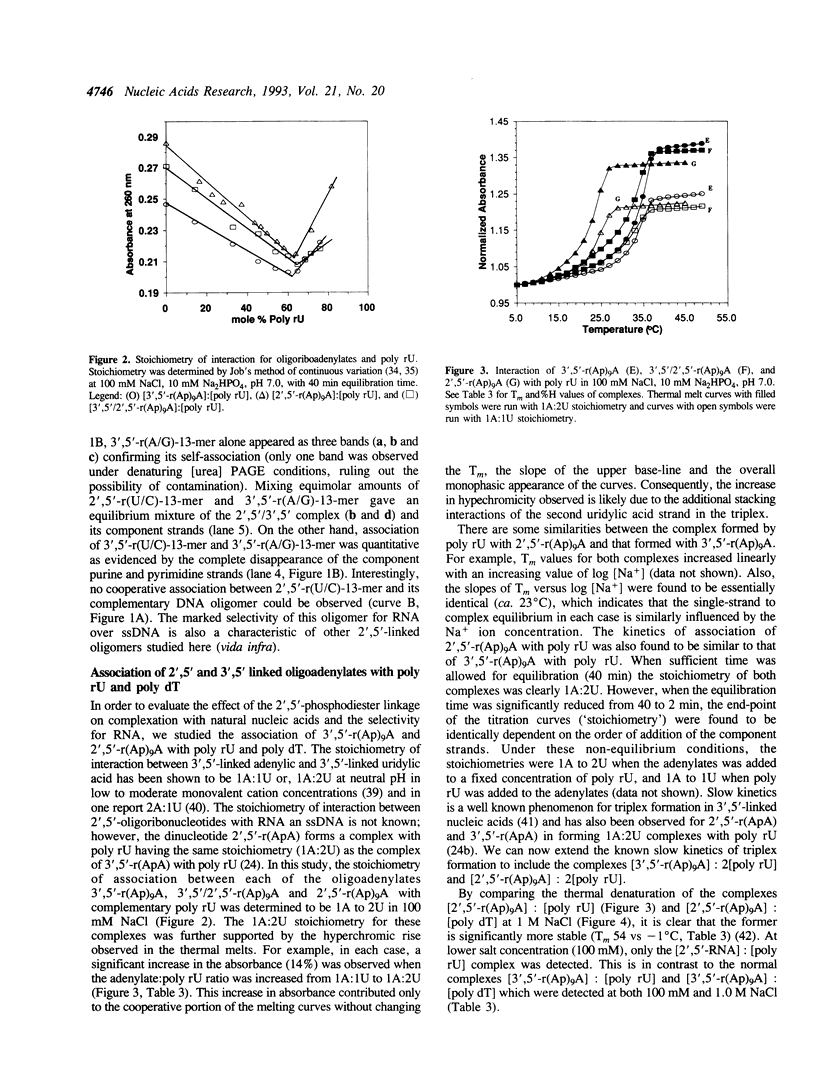

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Tang J. Y., Sun D., Sarin P. S., Zamecnik P. C. Synthesis and anti-HIV activity of oligoribonucleotides and their phosphorothioate analogs. Ann N Y Acad Sci. 1992 Oct 28;660:2–10. doi: 10.1111/j.1749-6632.1992.tb21052.x. [DOI] [PubMed] [Google Scholar]
- Blake R. D., Fresco J. R. Polynucleotides. VII. Spectrophotometric study of the kinetics of formation of the two-stranded helical complex resulting from the interaction of polyriboadenylate and polyribouridylate. J Mol Biol. 1966 Aug;19(1):145–160. doi: 10.1016/s0022-2836(66)80057-8. [DOI] [PubMed] [Google Scholar]
- Blake R. D., Massoulié J., Fresco J. R. Polynucleotides. 8. A spectral approach to the equilibria between polyriboadenylate and polyribouridylate and their complexes. J Mol Biol. 1967 Dec 14;30(2):291–308. [PubMed] [Google Scholar]
- Brahms J., Maurizot J. C., Michelson A. M. Conformational stability of dinucleotides in solution. J Mol Biol. 1967 May 14;25(3):481–495. doi: 10.1016/0022-2836(67)90200-8. [DOI] [PubMed] [Google Scholar]
- Brahms J., Michelson A. M., Van Holde K. E. Adenylate oligomers in single- and double-strand conformation. J Mol Biol. 1966 Feb;15(2):467–488. doi: 10.1016/s0022-2836(66)80122-5. [DOI] [PubMed] [Google Scholar]
- Bratty J., Wu T. F., Nicoghosian K., Ogilvie K. K., Perreault J. P., Keith G., Cedergren R. Characterization of a chemically synthesized RNA having the sequence of the yeast initiator tRNA(Met). FEBS Lett. 1990 Aug 20;269(1):60–64. doi: 10.1016/0014-5793(90)81118-8. [DOI] [PubMed] [Google Scholar]
- Broitman S. L., Im D. D., Fresco J. R. Formation of the triple-stranded polynucleotide helix, poly(A.A.U). Proc Natl Acad Sci U S A. 1987 Aug;84(15):5120–5124. doi: 10.1073/pnas.84.15.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco M. L., Garbesi A., Arcamone F., Maschera B., Palù G. Control of the expression of an early gene of SV40 with natural and modified oligonucleotides. Nucleic Acids Symp Ser. 1991;(24):274–274. [PubMed] [Google Scholar]
- Cook P. D. Medicinal chemistry of antisense oligonucleotides--future opportunities. Anticancer Drug Des. 1991 Dec;6(6):585–607. [PubMed] [Google Scholar]
- Cormier J. F., Ogilvie K. K. Synthesis of hexanucleotide analogues containing diisopropylsilyl internucleotide linkages. Nucleic Acids Res. 1988 May 25;16(10):4583–4594. doi: 10.1093/nar/16.10.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damha M. J., Ganeshan K., Hudson R. H., Zabarylo S. V. Solid-phase synthesis of branched oligoribonucleotides related to messenger RNA splicing intermediates. Nucleic Acids Res. 1992 Dec 25;20(24):6565–6573. doi: 10.1093/nar/20.24.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damha M. J., Giannaris P. A., Khan N. 2'-5'-linked oligonucleotides form stable complexes with complementary RNA and DNA. Nucleic Acids Symp Ser. 1991;(24):290–290. [PubMed] [Google Scholar]
- Damha M. J., Giannaris P. A., Zabarylo S. V. An improved procedure for derivatization of controlled-pore glass beads for solid-phase oligonucleotide synthesis. Nucleic Acids Res. 1990 Jul 11;18(13):3813–3821. doi: 10.1093/nar/18.13.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damha M. J., Ogilvie K. K. Oligoribonucleotide synthesis. The silyl-phosphoramidite method. Methods Mol Biol. 1993;20:81–114. doi: 10.1385/0-89603-281-7:81. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G., RICH A. Studies on the formation of two- and three-stranded polyribonucleotides. Biochim Biophys Acta. 1957 Dec;26(3):457–468. doi: 10.1016/0006-3002(57)90091-4. [DOI] [PubMed] [Google Scholar]
- FRESCO J. R. Helical arrangements in synthetic and natural polynucleotides. Trans N Y Acad Sci. 1959 Jun;21:653–658. doi: 10.1111/j.2164-0947.1959.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Froehler B., Ng P., Matteucci M. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 1988 Jun 10;16(11):4831–4839. doi: 10.1093/nar/16.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparutto D., Livache T., Bazin H., Duplaa A. M., Guy A., Khorlin A., Molko D., Roget A., Téoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases. Nucleic Acids Res. 1992 Oct 11;20(19):5159–5166. doi: 10.1093/nar/20.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V., Ghadage R. S., Ganesh K. N. Influence of internucleotide phosphate linkage on relative base stacking in 3'-5' and 2'-5' RNA: a circular dichroic spectroscopic study of RNA hexamer AACCUU. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1251–1257. doi: 10.1016/s0006-291x(05)81330-0. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kawai S. H., Wang D., Giannaris P. A., Damha M. J., Just G. Solid-phase synthesis and hybridization properties of DNA containing sulfide-linked dinucleosides. Nucleic Acids Res. 1993 Mar 25;21(6):1473–1479. doi: 10.1093/nar/21.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzek R., He L., Turner D. H. Association of 2'-5' oligoribonucleotides. Nucleic Acids Res. 1992 Apr 11;20(7):1685–1690. doi: 10.1093/nar/20.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N. S., Holmes H. M., Stempel L. M., Ts'o O. P. Influence of the phosphodiester linkage (3'-5', 2'-5', and 5'-5') on the conformation of dinucleoside monophosphate. Biochemistry. 1970 Sep 1;9(18):3479–3498. doi: 10.1021/bi00820a002. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Sproat B., Ryder U., Hamm J. Probing the structure and function of U2 snRNP with antisense oligonucleotides made of 2'-OMe RNA. Cell. 1989 Jul 28;58(2):383–390. doi: 10.1016/0092-8674(89)90852-0. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Zhang G. R., Sun D. K., Ikeuchi T., Sarin P. S. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Monny C. Polynucleotides. X. Oligonucleotides and their association with polynucleotides. Biochim Biophys Acta. 1967 Nov 21;149(1):107–126. doi: 10.1016/0005-2787(67)90695-8. [DOI] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Thenet S., Bertrand J. R., Paoletti J., Malvy C., Paoletti C. alpha-DNA II. Synthesis of unnatural alpha-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their substrate activities for nucleases. Nucleic Acids Res. 1987 Apr 24;15(8):3421–3437. doi: 10.1093/nar/15.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault J. P., Wu T. F., Cousineau B., Ogilvie K. K., Cedergren R. Mixed deoxyribo- and ribo-oligonucleotides with catalytic activity. Nature. 1990 Apr 5;344(6266):565–567. doi: 10.1038/344565a0. [DOI] [PubMed] [Google Scholar]
- Pieken W. A., Olsen D. B., Benseler F., Aurup H., Eckstein F. Kinetic characterization of ribonuclease-resistant 2'-modified hammerhead ribozymes. Science. 1991 Jul 19;253(5017):314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- Pilch D. S., Levenson C., Shafer R. H. Structural analysis of the (dA)10.2(dT)10 triple helix. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1942–1946. doi: 10.1073/pnas.87.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi J. D., Tinoco I., Jr Absorbance melting curves of RNA. Methods Enzymol. 1989;180:304–325. doi: 10.1016/0076-6879(89)80108-9. [DOI] [PubMed] [Google Scholar]
- Stirchak E. P., Summerton J. E., Weller D. D. Uncharged stereoregular nucleic acid analogs: 2. Morpholino nucleoside oligomers with carbamate internucleoside linkages. Nucleic Acids Res. 1989 Aug 11;17(15):6129–6141. doi: 10.1093/nar/17.15.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund C., Földesi A., Yamakage S., Chattopadhyaya J. Synthesis of branched nona and deca-RNA modelling the lariat formed in pre-mRNA processing reaction (splicing). Nucleic Acids Symp Ser. 1991;(24):9–12. [PubMed] [Google Scholar]
- Talbot S. J., Goodman S., Bates S. R., Fishwick C. W., Stockley P. G. Use of synthetic oligoribonucleotides to probe RNA-protein interactions in the MS2 translational operator complex. Nucleic Acids Res. 1990 Jun 25;18(12):3521–3528. doi: 10.1093/nar/18.12.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa I., Tazawa S., Stempel L. M., Ts'o P. O. L'adenylyl-(3'-5')-L-adenosine and L-adenylyl-(2'-5')-L-adenosine. Biochemistry. 1970 Sep 1;9(18):3499–3514. doi: 10.1021/bi00820a003. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Maitra R. K., Lesiak K., Khamnei S., Zhou A., Silverman R. H. Targeting RNA for degradation with a (2'-5')oligoadenylate-antisense chimera. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1300–1304. doi: 10.1073/pnas.90.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts'o P. O., Kondo N. S., Schweizer M. P., Hollis D. P. Studies of the conformation and interaction in dinucleoside mono- and diphosphates by proton magnetic resonance. Biochemistry. 1969 Mar;8(3):997–1029. doi: 10.1021/bi00831a033. [DOI] [PubMed] [Google Scholar]
- Turner D. H., Sugimoto N., Freier S. M. RNA structure prediction. Annu Rev Biophys Biophys Chem. 1988;17:167–192. doi: 10.1146/annurev.bb.17.060188.001123. [DOI] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon G. Oligonucleotide analogues as potential chemotherapeutic agents. Pharm Res. 1988 Sep;5(9):539–549. doi: 10.1023/a:1015985728434. [DOI] [PubMed] [Google Scholar]