Abstract
During the maturation of Xenopus oocytes, Cdc6 expression is necessary to establish replication competence to support early embryonic DNA replication. However, Cdc6 is expressed before the completion of MI at a time when its function as a replication factor is not required, suggesting additional roles for Cdc6 in meiosis. Confocal immunofluorescence microscopy revealed that Cdc6 protein was distributed around the spindle precursor at the time of germinal vesicle breakdown (GVBD) and localized to the margin of the nascent spindle early in prometaphase. Cdc6 subsequently localized to spindle poles in late prometaphase, where it remained until metaphase arrest. Microinjection of antisense oligonucleotides specific for Cdc6 mRNA disrupted spindle assembly, resulting in defects, including delayed spindle assembly, misoriented and unattached anaphase spindles, monasters, multiple spindles, microtubule aggregates associated with condensed chromosomes, or the absence of recognizable spindle-like structures, depending on the level of residual Cdc6 expression. Furthermore, Cdc6 co-localized with γ-tubulin in centrosomes during interphase in all somatic cells analyzed and associated with spindle poles in mitotic COS cells. Our data suggest a role for Cdc6 in spindle formation in addition to its role as a DNA replication factor.
Key words: Cdc6, spindle assembly, Xenopus, oocytes, pre-RC proteins
Introduction
Meiosis is a reductional division that generates haploid gametes from diploid germ cells. This reduction in chromosome number is achieved by one round of replication followed by two successive divisions.1–3 During oocyte formation, this process is not only discontinuous, but also lacks checkpoints. Such characteristics pose challenges to produce healthy functional oocytes without genomic defects. The lack of checkpoints, which allows oocytes with misaligned chromosomes to proceed through meiosis, certainly contributes to the high occurrence of improper chromosome segregation in human females, resulting in genetic disorders and spontaneous abortions. However, insights into the mechanisms that drive the meiotic cell cycle would be helpful to further the understanding of such problems.
To form a functional oocyte that can support early embryonic cell cycles after fertilization, three tasks must be achieved during meiosis: (1) the establishment and maintenance of replication competence; (2) the inhibition of DNA replication between successive meiotic divisions and (3) the achievement of proper chromosome segregation.
The establishment and maintenance of replication competence during oocytes maturation has been well studied over the past two decades. These studies have confirmed the fluctuation in replication competence during meiosis and its dependence on the expression of the pre-replication complex (pre-RC) protein Cdc6.4–7 In G2 arrest, when oocytes lack replication competence, Cdc6 protein is absent. As soon as oocytes re-enter meiosis, Cdc6 protein synthesis is resumed, leading to the restoration of replication competence, intriguingly before the completion of MI.5–7
While replication competence is acquired early during the meiotic process, it must be repressed during the remainder of meiosis (especially between the two meiotic divisions),8–10 as it is only necessary after fertilization to support the early embryonic cell cycle. The early expression of Cdc6, at a time when its function as a replication factor is not required, suggests that Cdc6 might possess additional meiotic roles such as being involved in meiotic division or chromosome segregation. Several results support this hypothesis. First, when Cdc6 is overexpressed in CHO cells, it localizes to the entire spindle apparatus.11 Second, mouse oocytes lacking Cdc6 showed abnormal spindle formation.12 Third, several replication factors have other roles during the cell cycle besides their involvement in DNA synthesis.13–16 For instance, ORC subunits and geminin have been implicated in chromosome segregation, cytokinesis and centrosome duplication.17–22
For this report, we used confocal immunofluorescence microscopy to examine the distribution of Cdc6 during the maturation of Xenopus oocytes. Cdc6 protein is diffusely distributed around the spindle precursor and localized to the poles of both MI and MII spindles. Furthermore, we used an antisense oligonucleotide approach to inhibit Cdc6 expression and studied its effect on spindle formation. Lower level of Cdc6 expression, disrupted spindle assembly, resulting in abnormal spindle phenotypes such as delayed spindle assembly, misoriented and unattached anaphase spindles, monasters, multiple spindles, microtubule aggregates associated with condensed chromosomes, and the absence of any spindle-like structure. These phenotypes suggest that during meiosis, Cdc6 is required for spindle assembly and may also play a role in cytokinesis and extrusion of the first polar body. Finally, we also examined the distribution of Cdc6 in a variety of cultured mammalian cells, observing that Cdc6 co-localizes with γ-tubulin in centrosomes during interphase and associates with spindle poles during mitosis in COS 7 cells. Our data suggest that, in addition to its role as a DNA replication factor, Cdc6 plays an important role in spindle assembly or organization.
Results
Cdc6 protein is associated with meiotic spindle poles during maturation.
To test if endogenous Cdc6 protein is involved in meiotic spindle formation we used confocal immunofluorescence microscopy to examine its distribution during meiotic maturation of Xenopus oocytes. Stage VI oocytes that underwent maturation after progesterone treatment were fixed in formaldehydeglutaraldehyde-taxol (FGT) at various times. Depending on the course of maturation oocytes were bisected either transversely or longitudinally to observe MI and MII spindles.
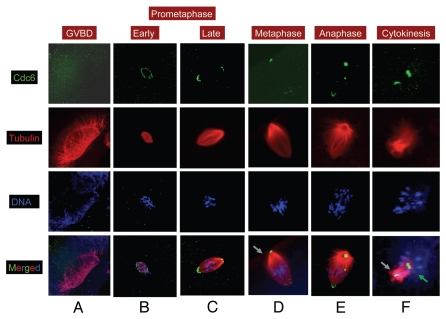
At the onset of GVBD, Xenopus oocytes assemble a novel MTOC transient microtubule array (MTOC-TMA) that serves as the precursor for the first meiotic spindle23 (Fig. 1A, red). Staining with anti-Cdc6 was diffusely distributed around this spindle precursor, but did not localize to the microtubules of the MTOC-TMA itself (Fig. 1A, green). In deeper sections a faint Cdc6 staining was also found at the base of the MTOC-TMA (data not shown). In early prometaphase (between 0 and 15 min post-GVBD), barrel-shaped microtubule arrays were observed to surround condensed chromosomes (Fig. 1B, red and blue). Cdc6 protein was present along the entire outer margin of these early spindles (Fig. 1B, green). Between 30 and 60 min post-GVBD, tubulin staining revealed the presence of organized late prometaphase spindles (Fig. 1C, red) containing condensed chromosomes (Fig. 1C, blue). At this stage, Cdc6 protein localized predominantly to the spindle poles, forming a cap-like structure at both ends. The signal for Cdc6 was higher at the animal end of the spindle than at the vegetal end (Fig. 1C, green). This differential Cdc6 staining between the animal and vegetal spindle poles was prominent at prometaphase, metaphase, anaphase and telophase of meiosis I, indicating that this was not an artifact related to spindle orientation. Between 60 and 90 min post-GVBD, we noticed spindles (Fig. 1D, red) with chromosomes aligned at the metaphase plate (Fig. 1D, blue). While at this time Cdc6 continued to localize to spindle poles (Fig. 1D, green), the cap-like structure faded, and Cdc6 stained a locus with high intensity at the animal spindle pole close to the spindle attachment site, suggesting its involvement in spindle attachment to the animal cortex. In contrast, Cdc6 staining was weaker and more diffuse at the spindle pole facing the vegetal cortex. Later, between 105 and 120 min after maturation, tubulin staining revealed the presence of elongated anaphase spindles with one set of chromosomes migrating toward each pole (Fig. 1E, red and blue). During this time, Cdc6 localization at the spindle poles was similar to that observed in metaphase spindles (Fig. 1E, green). During telophase/cytokinesis (from 120 to 135 min, Fig. 1F), one spindle pole attached to the animal cortex and one set of chromosomes along with Cdc6 was extruded as the first polar body (Fig. 1F, grey arrow). The other spindle pole, facing the vegetal cortex (Fig. 1F, green arrow) retained a diffuse Cdc6 staining within a mass of tubulin associated to the other set of chromosomes that will serve as a precursor for the assembly of the second meiotic spindle.
Figure 1.
Cdc6 is localized to spindle poles in MI. Stage VI oocytes were treated with progesterone to induce maturation, and samples were collected at various time and processed for immunofluorescence. Oocytes were stained for α tubulin (red), Cdc6 (green) and DNA (blue). Grey arrow in (D) indicates the animal cortex. Grey arrow in panel f indicates the first polar body and green arrow indicates the second meiotic spindle precursor. Image scale: 140 µm (A), 80 µm (B), 40–50 µm (CµF).
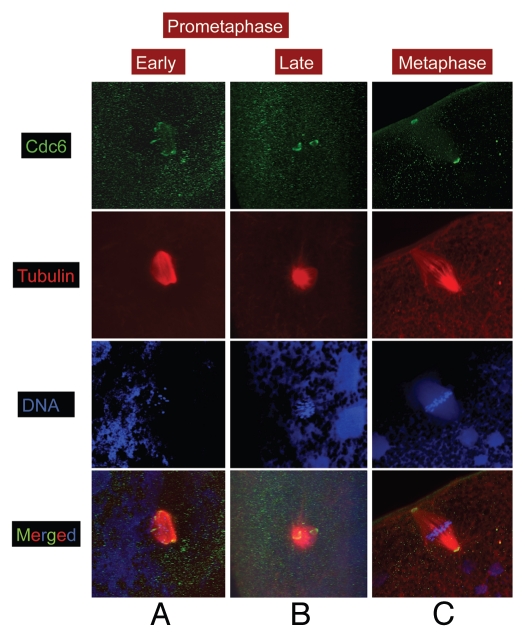
Cdc6 localization during meiosis II was similar to that observed during meiosis I. Cdc6 stained the outer margins of the spindles in early prometaphase II (Fig. 2A, green) and both spindle poles from late prometaphase II through metaphase (Fig. 2B and C, respectively). The majority of the metaphase II spindles we observed also displayed a stronger Cdc6 staining at the pole that was attached to the animal cortex (Fig. S1). Overall the localization of Cdc6 to spindles and spindle poles during MI and MII suggested that Cdc6 might play roles in meiotic spindle assembly and attachment to the animal cortex.
Figure 2.
Cdc6 localizes to spindle poles in MII. Stage VI oocytes were treated with progesterone to induce maturation, and samples were collected at various time and processed for immunofluorescence. Samples were stained for α tubulin (red), Cdc6 (green) and DNA (blue). Image scale: 90 µm.
Inhibition of Cdc6 function disrupts meiotic spindle assembly.
To test more directly if Cdc6 protein is required for spindle assembly, we microinjected oocytes with oligonucleotides specific for Cdc6 mRNA and anti-Cdc6 antibodies to deplete both Cdc6 mRNA and protein prior to inducing oocyte maturation with progesterone. The Cdc6 antisense oligonucleotides used in our study has previously been shown to specifically knockdown Cdc6 mRNA and thus Cdc6 protein in Xenopus oocytes.6 The specificity of Cdc6 antisense oligonucleotides to inhibit Cdc6 protein expression during maturation was further confirmed by protein gel blot analysis (Fig. S2). Oocytes were first injected with 70 to 90 ng of Cdc6 antisense or non-specific control oligonucleotides and spindle structure was studied throughout maturation. This amount of antisense oligonucleotide was chosen as it was slightly above the one that resulted in about 95% inhibition of Cdc6 expression as determined by protein gel blot analysis (Fig. S2).
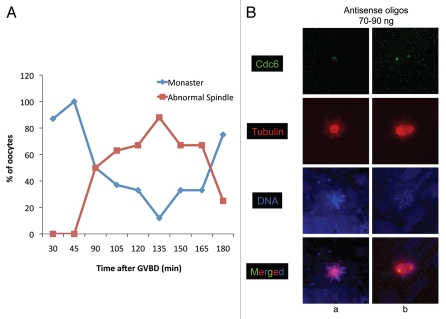
In early MI (0–45 min after GVBD), more than 80% of oocytes injected with antisense oligonucleotides had hollow appearing microtubule monasters with attached chromosomes (Fig. 3A and Ba). In late MI and early MII (90 to 160 min after GVBD), 30 to 40% of the oocytes still had monasters while the rest exhibited spindles which had an abnormal orientation or a delayed development (Fig. 3A). For example between 90–105 min after GVBD, oocytes injected with antisense had transversely oriented prometaphase like spindles while control samples had perpendicularly oriented spindles attached to the animal cortex (data not shown). By the time control oocytes reached MI cytokinesis and ejected their first polar body (between 120–135 min post-GVBD), the spindles of oocytes injected with antisense oligonucleotides were still oriented transverse to the animal-vegetal axis or aligned with the animal-vegetal axis but unattached to the animal cortex (Fig. 3A and Bb). First polar bodies were never found associated with these misoriented or unattached spindles.
Figure 3.
Inhibition of Cdc6 during maturation leads to delayed spindle assembly. Stage VI oocytes were injected with 70–90 ng of Cdc6 antisense or control non-specific oligonucleotides and treated with progesterone to induce maturation, and samples were fixed at various times and stained for α tubulin (red), Cdc6 (green) and DNA (blue). (A) Plot represents the % of oocytes with monasters or abnormal spindles (delayed spindles with abnormal or normal morphology) at indicated time during maturation. [B(a)] Monaster formation in Cdc6 antisense injected oocyte during MI (refer to Fig. 1C for control/normal spindle at this time). [B(b)] An unattached anaphase/telophase spindle seen at 135 min post-GVBD (refer to Fig. 1E and F for control/normal spindle phenotype at this time). Image scale: 70 µm.
All oocytes injected with 70 to 90 ng antisense oligonucleotides displayed weak Cdc6 staining indicating that this amount of oligonucleotides was unable to completely block Cdc6 expression during maturation, despite the fact that the protein was undetectable by protein gel blot analysis (Fig. S2). The small amount of Cdc6 expressed was found in the center of the hollow microtubule monasters or at the poles of abnormal spindles. The spindle defects observed when Cdc6 expression is significantly reduced suggested that Cdc6 is required for proper spindle assembly, rotation and attachment to the animal cortex during maturation of Xenopus oocytes.
In an effort to tighten the inhibition of Cdc6 synthesis during maturation, we increased the amount of antisense or non-specific control oligonucleotides (ranging from 110–210 ng) injected into stage VI oocytes along with a constant amount of Cdc6 or control IgG antibody. Injected oocytes were then induced to enter maturation and fixed in FGT during MI (mostly between 30–60 min post-GVBD).
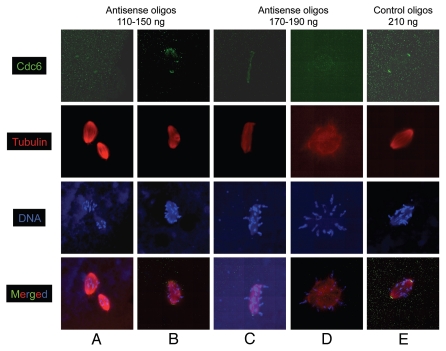
More than 95% of the oocytes injected with 110 ng to 170 ng of antisense oligonucleotide exhibited abnormal MI spindles. On the other hand, all the non-specific oligonucleotide-injected oocytes had normal MI spindles. Cdc6 antisense-injected oocytes displayed an assortment of defects such as the presence of multiple, tripolar or tetrapolar spindles as well as fused or elongated/distorted spindles (Fig. 4A–C). While the residual Cdc6 protein expressed in the antisense injected oocytes was found at the pole of the abnormal spindles or MTOC, it was insufficient to promote normal spindle assembly. For instance, when oocytes had two spindles, both showed very weak Cdc6 protein staining at their poles in comparison to control spindles (compare Fig. 4A and E). In addition, while these spindles were associated with condensed chromosomes they were of unequal size (Fig. 4A). When we observed tripolar spindles, weak Cdc6 staining was associated with the two major poles as well as the minor pole (Fig. 4B). When the spindles were either distorted or elongated, Cdc6 stained weakly the center of the spindle mass and ran as a “streak” from one end of the spindle to the other (Fig. 4C). In addition a tiny bright Cdc6 foci appeared to be associated with the poles of this distorted spindle. This structure may represent a large pseudolinear MTOC with Cdc6 in the middle. In this range of oligonucleotide concentrations (110 to 170 ng) we also found a few tetrapolar or fused spindles with condensed chromosomes (data not shown). Later on during meiosis I, none of these abnormal spindles exhibited proper orientation or attachment to the cortex and no polar bodies were found (data not shown). At a higher range (between 190 ng and 210 ng) of antisense oligonucleotides, instead of a spindle, we noticed a round tubulin mass associated with condensed chromosomes that had a negligible amount of Cdc6 protein in its center (Fig. 4D). This tubulin structure or very early/primitive MTOC was incapable of spindle assembly. When the antisense concentration was increased further (above 210 ng), we were unable to detect any tubulin array associated with condensed chromosomes or Cdc6 staining, while control oocytes displayed normal spindles confirming that the observed spindle defects are not an artifact of high amount of oligonucleotide injection (Fig. 4E). Altogether, our results support the idea that Cdc6 is required for meiotic spindle assembly and therefore chromosome segregation.
Figure 4.
Inhibition of Cdc6 during maturation causes abnormal spindle assembly. Stage VI oocytes were injected with various concentrations of Cdc6 antisense or control non-specific oligonucleotides, treated with progesterone to induce maturation and samples were collected during early MI (between 30–60 min post-GVBD) and stained for α tubulin (red), Cdc6 (green) and DNA (blue). (A and B) At 110–150 ng of antisense injection, multiple spindles, tripolar spindles were noted. (C and D) At 170–190 ng of antisense injection, elongated, distorted spindle-like structure or tubulin mass attached with condensed chromosomes were seen. (E) Control non-specific oligonucleotides injected (210 ng) oocytes had normal prometaphase MI spindle. Image scale: 65–70 µm.
Cdc6 co-localizes with γ-tubulin at interphase centrosomes of mammalian cells.
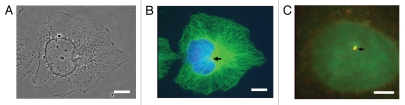
The phenotypes observed in this study (formation of multiple spindles and defective spindle assembly) are often attributed to centrosomal defects during mitosis. As oocytes utilize acentriolar centrosome for spindle assembly, we were prompted to check the association of Cdc6 with interphase centrosomes, mitotic poles and spindles during somatic cell division. To this end, we fixed cells from a variety of established cell lines in either paraformaldehyde or cold methanol and immunostained them for Cdc6, along with either α or γ-tubulin. In all cell lines examined, Cdc6 is localized at the centrosome during interphase (Figs. 5 and S3). In some cells Cdc6 was represented by one punctum and in other cells by two, while γ-tubulin always appeared as two punctae, reflecting the mother-daughter pair of centrioles. In all cases, Cdc6 overlapped with at least one of the two γ-tubulin punctae (Figs. 5 and S3).
Figure 5.
Cdc6 co-localizes with γ-tubulin at interphase centrosomes of mammalian cells. Affinity-purified antibodies to Cdc6 protein stain puncta at the perinuclear focus of microtubule staining (the centrosome) in a variety of mammalian cell lines, including HeLa cells [shown here in (B)]. At either lower (B) or higher (C) magnification, the colocalization of Cdc6 with one (or sometimes both) puncta of centrosomes (stained with antibodies to gamma tubulin) is observed. Cdc6 stained red; γ-tubulin (C) or α-tubulin (B) stained green, and DNA stained blue with Hoechst. (A) is the phase contrast image of HeLa cells. Scale bars in (A and B = 5 micron) and (C = 1 micron).
Although in COS-7 cells Cdc6 co-stained consistently with γ-tubulin at mitotic poles (Fig. S4), this was not the case in the other cell lines tested. For these other cell types, staining was variably strong or weak, sometimes staining multiple punctae and at other times staining homogeneously weakly.
Discussion
Cdc6 is a component of the pre-replication complex whose assembly is required for initiation of DNA replication in eukaryotes. Here we show that during Xenopus oocyte maturation, Cdc6 is also required for the proper assembly and orientation of the meiotic spindles as well as their attachment to the oocyte cortex. Decreased Cdc6 expression resulted in abnormal or lack of spindle, which impacted the cytokinesis process.
In Xenopus, like other animals, female meiosis is characterized by two consecutive asymmetric cell divisions and the presence of meiotic spindles that do not contain centrioles at their poles. The assembly and organization of the meiotic spindles is a complex process that includes distinct steps and varies slightly among species.24,25 In Xenopus oocytes, MI spindle assembly first requires the formation and compaction of the MTOC-TMA that gathers and transports the condensed chromosomes to the animal cortex. The MTOC-TMA then serves as a precursor for the bipolar spindle, which assembles and elongates during prometaphase such that its axis is transverse to the animal-vegetal axis of the oocytes. Finally, during late prometaphase, the spindle starts to rotate, aligning its axis with the animal-vegetal axis. Complete spindle rotation is achieved by the time of metaphase and is followed by the attachment of one spindle pole to the animal cortex in preparation for the first polar body expulsion. A similar assembly process is repeated during meiosis II.23
The localization of Cdc6 during oocytes maturation is reminiscent of the one previously observed for centrosomal proteins26–30 and in particular for NuMA, a nuclear protein that accumulates at the spindle poles during mitosis and meiosis.31 Like NuMA, Cdc6 was found associated to the MTOC-TMA that is considered the meiotic counterpart to the mitotic centrosome. Both proteins are first localized around the compacted MTOC-TMA and then redistributed to the poles of the first meiotic spindle. Various evidences indicate that NuMA is required during meiotic maturation and that its accurate translocation to the pole is not only dependent on cytoplasmic dynein, but is critical to the establishment of functional spindle poles.31 Similarities between NuMA and Cdc6 localization and the fact that decreased Cdc6 expression results in improper spindle assembly leads us to believe that Cdc6 may participate with NuMA in the establishment and maintenance of the spindle poles during meiosis.
A number of studies in different organisms reveal the presence of an evolutionary conserved mechanism in which the polarized localization of NuMA to the cell cortex is required for mitotic spindle positioning and asymmetric cell division observed during development.32 Furthermore, a recent study in C. elegans established a role for CDK-1 as an inhibitor of meiotic spindle rotation and proposed a model in which Cdk-1 inhibits cytoplasmic dynein activity by preventing its interaction with LIN-5, a NuMA ortholog.33 While the preferred localization of a subset of NuMA to the meiotic spindle attached to the animal cortex is unknown, our results identify Cdc6 as the first molecular marker that displays such asymmetric pole localization at the time of spindle rotation and attachment to the oocyte's animal cortex. Our data also indicate that the requirement of Cdc6 for spindle rotation and attachment is the most sensitive to Cdc6 depletion. Indeed, misoriented and unattached spindles were observed in oocytes injected with the lowest amounts of Cdc6 antisense oligonucleotides. Similar phenotypic defects involving delayed spindle assembly, monasters and transversely oriented anaphase or telophase spindles were previously observed in oocytes undergoing maturation in the presence of cytochalasin B, an inhibitor of actin polymerization.34 While Cdc6 may interact with F-actin at the spindle attachment site, we believe that Cdc6 localization to the spindle pole and its requirement for the proper orientation of the meiotic spindle is most likely to depend on a direct or indirect association with NuMA and thereby the activity of cytoplasmic dynein, a minus end directed microtubule motor. In this regard, it is interesting to note that the requirement of Cdc6 for cytokinesis during the mitotic cell cycle has been linked to its ability to inhibit CDK-1 activity.35,36 As CDK-1 prevents dynein-dependent spindle rotation during meiosis in C. elegans, one could imagine that Cdc6's role at the spindle pole is to locally regulate CDK-1 activity and promote dynein dependent rotation and attachment of the spindle leading to the successful expulsion of the polar body.
More severe defects in spindle assembly required the injection of higher amount of Cdc6 antisense oligonucleotides. These defects included the presence of more than one spindle, tripolar spindles, monasters or linear MTOC-TMA. At the highest concentration of Cdc6 antisense oligonucleotides, oocytes had no spindles or microtubule structure associated to chromosomes. Similar phenotypes were observed when antibodies against NuMA or cytoplasmic dynein were injected into oocytes during maturation.27 The presence of more than one bipolar spindle in Cdc6-depleted samples in MI may have resulted from the splitting of the MTOC-TMA.27 Further, monasters associated with chromosomes perhaps represent a very early or deformed MTOCTMA, which is incapable of spindle assembly.27 Together, our data clearly indicate the necessity of Cdc6 for the proper assembly and maintenance of MTOC during meiosis.
In somatic cells, many centrosomal proteins localize to spindle poles during mitosis. While we were able to reveal the presence of Cdc6 at centrosomes in several cell lines, our results for mitotic cells were more equivocal. Yim and Erikson reported that Cdc6 localizes to spindle poles in HeLa cells.35 Our results for this cell line varied, with Cdc6 staining mitotic poles both weakly and occasionally. This discrepancy may stem from different antibodies used or differing experimental conditions.
Many proteins that form the components of mitotic centrosomes are also constituents of MTOC-TMA formed in meiosis. We show that Cdc6 is yet another such protein and this finding strengthens the idea that the MTOC-TMA is a functional substitute for centrosomes in acentrosomal cells like oocytes. Further, major regulatory mechanisms that ensure precision of replication and chromosome segregation during cell division are similar between mitosis and meiosis. Therefore it is possible that during mitosis, Cdc6 localized at the centrosomes may be required for the maintenance and proper duplication of centrosome to prevent multipolar spindle formation and aberrant chromosome segregation.
Besides Cdc6, several other replication initiation factors like MCMs, ORCs and geminin are associated with centrosomes and play a role in chromosome segregation during mitosis.13,17–22,36–38 Depletion of some of these factors have resulted in mitotic abnormalities like multinucleated cells or fused cells, suggesting an effect on cytokinesis. In addition, ORC and geminin-depleted cells also showed abnormal centrosome duplication and conversely, their overexpression prevented centrosome duplication in the presence of hydroxyuridine. Therefore, pre-RC proteins may be involved in restricting centrosome duplication to only once per cell cycle similar to DNA replication. In light of these findings, certainly it will be worth understanding if other pre-RC components like MCMs, ORCs, Cdt1 and Geminin are also associated with either MTOC-TMA or spindle poles during meiosis. Such a study will shed light on mechanisms that facilitate centrosomal and acentrosomal mediated spindle assembly.
In conclusion, this study has revealed yet another important function for a DNA replication initiation factor, Cdc6, in chromosome segregation. This finding along with other reports support the idea that replication factors not only ensure that the genetic information is precisely duplicated only once per cell cycle, but are equally important to ensure that the duplicated information is precisely passed on to the next generation, thus making them indispensible for both S and M phases of the cell cycle. In mitosis, such a function is necessary to maintain proper functioning of the organism and to prevent serious diseases like cancer, while in meiosis, passing on the precise copy and number of the blueprint is necessary for species propagation.
Materials and Methods
Xenopus oocyte isolation and microinjection.
Stage VI oocytes were obtained from Xenopus ovary fragments treated with 2 mg/ml collagenase to remove follicle cells, as previously described in reference 5. At the region between animal and vegetal hemispheres, stage VI oocytes were microinjected with a cocktail of Cdc6 antibodies and Cdc6 antisense oligonucleotides. For control samples, a cocktail of nonspecific oligonucleotides and rabbit IgGs were injected. After injection oocytes were incubated for 4 h in Modified Barth's Saline [MBS: 88 mM NaCl, 1 mM KCl, 0.91 mM CaCl2, 0.33 mM Ca(N03)2, 0.82 mM MgS04, 2.4 mM NaHCO3, 10 mM Hepes, pH 7.5]. The phosphorothioate Cdc6 antisense 5′-C*T *T* TGC GAG ACT GCT TGG G*T *G* G-3′ and control/non specific oligonucleotide sequences 5′-C*C *T* CTT ACC TCA GTT ACA A*T *T* T-3′ were slightly modified based on the sequences described previously in reference 6 and 39. Rabbit-Cdc6 antibodies were elicited and purified as described previously in reference 5. Control rabbit IgGs were purchased from Santa Cruz Biotechnology.
Confocal immunofluorescence microscopy.
After microinjection, oocytes were induced to undergo meiotic maturation with progesterone. Samples were collected at regular intervals and association of Cdc6 with the spindle was studied by confocal immnunofluorescence microscopy. Oocytes were prepared for confocal microscopy as described previously in reference 40. Briefly, oocytes were fixed at room temperature in formaldehydeglutaraldehyde-taxol (FGT) buffer (80 mM K pipes, 1 mM MgCl2, 5 mM EGTA, 0.2% Triton X-100 (W/V), 3.7% paraformaldehyde (W/V), 0.25% glutaraldehyde (W/V) and 0.5 µM taxol) followed by an overnight postfixation in 100% methanol at room temperature. For Cdc6 staining, affinity-purified, polyclonal Cdc6 antibodies were used at 1:50 dilution. Spindles were stained with monoclonal antibodies to tyrosinated α-tubulin (clone YL1/2; Accurate Chemical and Scientific Corp.) at 1:100 dilution. Chromosomes were stained with TO-PRO-3 (Molecular Probes Inc.). Images were collected using BioRad 700M upright laser confocal microscope. Post-image processing was performed using the LSM software.
Immunofluorescence of mammalian cell lines.
A variety of mammalian cell lines, including COS-7 (epithelial), HeLa (epithelial), 3T3 (fibroblast) and H9C2 (undifferentiated myoblast), were analyzed for the presence and localization of Cdc6 protein in both interphase and mitotic cells. Cells were grown in DMEM plus 10% (V/V) FBS on glass coverslips for several days and then fixed in either freshly made formaldehyde [4% W/V in PHEM buffer (0.06 M PIPES, 0.025 M HEPES, pH 6.95, 10 mM EGTA, 2 mM MgSO4)] or ice-cold methanol containing 5 mM EGTA. After detergent extraction of the formaldehydefixed cells, cells on coverslips were blocked for 45 min (37°C) in PBS containing 5% (V/V) normal goat serum. Cells were then immunostained for Cdc6 protein using the affinity-purified, polyclonal antibodies named above and monoclonal antibodies to either γ-tubulin (clone GTU-88) or the microtubule array (clone B5-1-2). Cells were counterstained for DNA with Hoechst 33,342. Images were recorded using an Axiovert 135 TV microscope (Carl Zeiss, Inc.) and a Photometrics Sensys camera (Roper Scientific).
Acknowledgements
This work was supported by a National Institutes of Health grant to M.C. Y.N. gratefully acknowledges the partial funding provided by the Graduate School of Biomedical Sciences for travel to the University of Utah. Y.N. is thankful to Mr. Richard Bliss and Dr. Edward King for the confocal microscope training. Y.N. and M.C. thank the Department of Physiology, Texas Tech University Health Sciences Center, Lubbock and, Dr. Strahlendorf, Dr. Martinez, Dr. Escobar, Dr. Altenberg and Ms. Velvet Finckbone for providing access to the confocal microscope facility. We also thank Dr. Clinton Macdonald for providing access to his laboratory to perform some of the experiments and Dr. Hitesh Bagaria and Dr. Curt Pfarr for helpful discussions and critical reading of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Sutton WS. On the morphology of the chromosome group in Brachystola Magna. Biol Bull. 1902;4:24–39. doi: 10.2307/1535510. [DOI] [Google Scholar]
- 2.Sutton WS. The chromosomes in heredity. Biological Bulletin. Marine Biological Laboratory. 1903;4:231–251. doi: 10.2307/1535741. [DOI] [Google Scholar]
- 3.Farmer JB, Moore JES. On the maiotic phase (reduction divisions) in animals and plants. Q J Microsc Sci. 1905;48:489–557. [Google Scholar]
- 4.Gurdon JB. On the origin and persistence of a cytoplasmic state inducing nuclear DNA synthesis in frogs' eggs. Proc Natl Acad Sci USA. 1967;58:545–552. doi: 10.1073/pnas.58.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitmire E, Khan B, Coué M. Cdc6 synthesis regulates replication competence in Xenopus oocytes. Nature. 2002;419:722–725. doi: 10.1038/nature01032. [DOI] [PubMed] [Google Scholar]
- 6.Lemaître JM, Bocquet S, Méchali M. Competence to replicate in the unfertilized egg is conferred by Cdc6 during meiotic maturation. Nature. 2002;419:718–722. doi: 10.1038/nature01046. [DOI] [PubMed] [Google Scholar]
- 7.Lemaître JM, Bocquet S, Terret ME, Namdar M, Aït-Ahmed O, Kearsey S, et al. The regulation of competence to replicate in meiosis by Cdc6 is conserved during evolution. Mol Reprod Dev. 2004;69:94–100. doi: 10.1002/mrd.20153. [DOI] [PubMed] [Google Scholar]
- 8.Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, et al. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwabuchi M, Ohsumi K, Yamamoto TM, Sawada W, Kishimoto T. Residual Cdc2 activity remaining at meiosis I exit is essential for meiotic M-M transition in Xenopus oocyte extracts. EMBO J. 2000;19:4513–4523. doi: 10.1093/emboj/19.17.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohsumi K, Sawada W, Kishimoto T. Meiosis-specific cell cycle regulation in maturing Xenopus oocytes. J Cell Sci. 1994;107:3005–3013. doi: 10.1242/jcs.107.11.3005. [DOI] [PubMed] [Google Scholar]
- 11.Illenye S, Heintz NH. Functional analysis of bacterial artificial chromosomes in mammalian cells: mouse Cdc6 is associated with the mitotic spindle apparatus. Genomics. 2004;83:66–75. doi: 10.1016/S0888-7543(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 12.Anger M, Stein P, Schultz RM. CDC6 requirement for spindle formation during maturation of mouse oocytes. Biol Reprod. 2005;72:188–194. doi: 10.1095/biolreprod.104.035451. [DOI] [PubMed] [Google Scholar]
- 13.Knockleby J, Lee H. Same partners, different dance: involvement of DNA replication proteins in centrosome regulation. Cell Cycle. 2010;9:4487–4491. doi: 10.4161/cc.9.22.14047. [DOI] [PubMed] [Google Scholar]
- 14.Lau E, Zhu C, Abraham RT, Jiang W. The functional role of Cdc6 in S-G2/M in mammalian cells. EMBO Rep. 2006;7:425–430. doi: 10.1038/sj.embor.7400624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boronat S, Campbell JL. Linking mitosis with S-phase: Cdc6 at play. Cell Cycle. 2008;7:597–601. doi: 10.4161/cc.7.5.5519. [DOI] [PubMed] [Google Scholar]
- 16.Lunn CL, Chrivia JC, Baldassare JJ. Activation of Cdk2/Cyclin E complexes is dependent on the origin of replication licensing factor Cdc6 in mammalian cells. Cell Cycle. 2010;9:4533–4541. doi: 10.4161/cc.9.22.13789. [DOI] [PubMed] [Google Scholar]
- 17.Hemerly AS, Prasanth SG, Siddiqui K, Stillman B. Orc1 controls centriole and centrosome copy number in human cells. Science. 2009;323:789–793. doi: 10.1126/science.1166745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasanth SG, Prasanth KV, Siddiqui K, Spector DL, Stillman B. Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance. EMBO J. 2004;23:2651–2663. doi: 10.1038/sj.emboj.7600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasanth SG, Prasanth KV, Stillman B. Orc6 involved in DNA replication, chromosome segregation and cytokinesis. Science. 2002;297:1026–1031. doi: 10.1126/science.1072802. [DOI] [PubMed] [Google Scholar]
- 20.Lu F, Lan R, Zhang H, Jiang Q, Zhang C. Geminin is partially localized to the centrosome and plays a role in proper centrosome duplication. Biol Cell. 2009;101:273–285. doi: 10.1042/BC20080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana KE, Gonzalez MA, Guarguaglini G, Nigg EA, Laskey RA. Depletion of licensing inhibitor geminin causes centrosome overduplication and mitotic defects. EMBO Rep. 2005;6:1052–1057. doi: 10.1038/sj.embor.7400527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tachibana KE, Nigg EA. Geminin regulates multiple steps of the chromosome inheritance cycle. Cell Cycle. 2006;5:151–154. doi: 10.4161/cc.5.2.2363. [DOI] [PubMed] [Google Scholar]
- 23.Gard DL. Microtubule organization during maturation of Xenopus oocytes: assembly and rotation of the meiotic spindles. Dev Biol. 1992;151:516–530. doi: 10.1016/0012-1606(92)90190-R. [DOI] [PubMed] [Google Scholar]
- 24.Ai JS, Li M, Schatten H, Sun QY. Regulatory Mechanism of Spindle Movements during Oocyte Meiotic Division. Asian-australas J Anim Sci. 2009;22:1477–1486. [Google Scholar]
- 25.Fabritius AS, Ellefson ML, McNally FJ. Nuclear and spindle positioning during oocyte meiosis. Curr Opin Cell Biol. 2011;23:78–84. doi: 10.1016/j.ceb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha BJ, Error B, Gard DL. XMAP230 is required for the assembly and organization of acetylated microtubules and spindles in Xenopus oocytes and eggs. J Cell Sci. 1998;111:2315–2327. doi: 10.1242/jcs.111.16.2315. [DOI] [PubMed] [Google Scholar]
- 27.Becker BE, Romney SJ, Gard DL. XMAP215, XKCM1, NuMA and cytoplasmic dynein are required for the assembly and organization of the transient microtubule array during the maturation of Xenopus oocytes. Dev Biol. 2003;261:488–505. doi: 10.1016/S0012-1606(03)00330-0. [DOI] [PubMed] [Google Scholar]
- 28.Kotani T, Yamashita M. Behavior of delta-tubulin during spindle formation in Xenopus oocytes: requirement of cytoplasmic dynein-dependent translocation. Zygote. 2005;13:219–226. doi: 10.1017/S0967199405003321. [DOI] [PubMed] [Google Scholar]
- 29.Fant X, Merdes A, Haren L. Cell and molecular biology of spindle poles and NuMA. Int Rev Cytol. 2004;238:1–57. doi: 10.1016/S0074-7696(04)38001-0. [DOI] [PubMed] [Google Scholar]
- 30.Gueth-Hallonet C, Antony C, Aghion J, Santa-Maria A, Lajoie-Mazenc I, Wright M, et al. gamma-Tubulin is present in acentriolar MTOCs during early mouse development. J Cell Sci. 1993;105:157–166. doi: 10.1242/jcs.105.1.157. [DOI] [PubMed] [Google Scholar]
- 31.Sun QY, Schatten H. Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein. Front Biosci. 2006;11:1137–1146. doi: 10.2741/1868. [DOI] [PubMed] [Google Scholar]
- 32.Radulescu AE, Cleveland DW. NuMA after 30 years: the matrix revisited. Trends Cell Biol. 2010;20:214–222. doi: 10.1016/j. tcb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellefson ML, McNally FJ. CDK-1 inhibits meiotic spindle shortening and dynein-dependent spindle rotation in C. elegans. J Cell Biol. 2011;193:1229–1244. doi: 10.1083/jcb.201104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gard DL, Cha BJ, Roeder AD. F-actin is required for spindle anchoring and rotation in Xenopus oocytes: a re-examination of the effects of cytochalasin B on oocyte maturation. Zygote. 1995;3:17–26. doi: 10.1017/S0967199400002331. [DOI] [PubMed] [Google Scholar]
- 35.Yim H, Erikson RL. Cell division cycle 6, a mitotic substrate of polo-like kinase 1, regulates chromosomal segregation mediated by cyclin-dependent kinase 1 and separase. Proc Natl Acad Sci USA. 2010;107:19742–19747. doi: 10.1073/pnas.1013557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yim H, Erikson RL. Regulation of the final stage of mitosis by components of the pre-replicative complex and a polo kinase. Cell Cycle. 2011;10:1374–1377. doi: 10.4161/cc.10.9.15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuermer A, Hoehn K, Faul T, Auth T, Brand N, Kneissl M, et al. Mouse pre-replicative complex proteins colocalise and interact with the centrosome. Eur J Cell Biol. 2007;86:37–50. doi: 10.1016/j.ejcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Pflumm MF, Botchan MR. Orc mutants arrest in metaphase with abnormally condensed chromosomes. Development. 2001;128:1697–1707. doi: 10.1242/dev.128.9.1697. [DOI] [PubMed] [Google Scholar]
- 39.McGarry TJ. Geminin deficiency causes a Chk1-dependent G2 arrest in Xenopus. Mol Biol Cell. 2002;13:3662–3671. doi: 10.1091/mbc.E02-04-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker BE, Gard DL. Visualization of the cytoskeleton in Xenopus oocytes and eggs by confocal immunofluorescence microscopy. Methods Mol Biol. 2006;322:69–86. doi: 10.1007/978-1-59745-000-3_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.