Abstract
Plants are one of the most fascinating and important groups of organisms living on Earth. They serve as the conduit of energy into the biosphere, provide food, and shape our environment. If we want to make headway in understanding how these essential organisms function and build the foundation for a more sustainable future, then we need to apply the most advanced technologies available to the study of plant life. In 2009, a committee of the National Academy highlighted the “understanding of plant growth” as one of the big challenges for society and part of a new era which they termed “new biology.” The aim of this article is to identify how new technologies can and will transform plant science to address the challenges of new biology. We assess where we stand today regarding current technologies, with an emphasis on molecular and imaging technologies, and we try to address questions about where we may go in the future and whether we can get an idea of what is at and beyond the horizon.
We believe that the major three challenges for humankind in the 21st century are food, energy, and the environment, including climate change and environmental degradation due to pollution and habitat loss. In a word: sustainability. Plant life plays an essential role in all three of these sustainability challenges. All of our food and the majority of our energy are produced by photosynthetic plants. Plants are major players in determining our climate, and agricultural expansion is a major factor in habitat encroachment and pollution of waterways by fertilizer application and runoff. Furthermore, these issues are not independent; as the climate changes, additional challenges are placed on plant performance and, thus, food supply and habitat. Research on plants is needed to provide solutions to these major challenges.
The fundamental biology of plants is similar to our own; they use the same genetic code, share many homologous genes, and even many regulatory mechanisms, basic biochemical pathways, and fundamental processes in cell biology. However, their form and lifestyle are fundamentally different. Plants can reach individual life spans of up to 5000 years; they can obtain adequate nutrition from the air and soil and survive adverse environmental conditions and attacks from pests, pathogens, and herbivores, despite remaining rooted in one spot for their lifetime. Plants are master chemists and can defend themselves with an incredible arsenal of chemicals. Many plants do not have a determinate body plan; a single genome is capable of producing an enormous range of size and form. Thus, plants are also valuable basic research objects since we can learn fundamental principles that are shared with humans and at the same time learn how different wiring can create such fundamental differences in form, biochemistry, and function.
While some of the richest ideas in the life sciences have been developed without application of specialized technology (the theory of natural selection and evolution stands out as a prime example), technology is more often a primary driver of new understanding. Two essential roles for new technology can be identified. First, advances in technology provide the means to test hypotheses formulated from less complete or less precise information. Second, technology provides new information to generate fresh hypotheses. Certainly the most influential advance in modern biology has been uncovering the molecular basis of inheritance and biological information transfer, namely, the discovery and structure of DNA and how this molecular information is transcribed into RNA and translated into proteins. This advance depended critically on technologies such as x-ray diffraction to test hypotheses generated from chemical analysis and, in turn, uncovered vast new fields of information that continue to revolutionize the course of biological research. The “central dogma” is also a prime example of how advances made possible by technology itself engenders powerful new technologies, accelerating the cycle of discovery. It is noteworthy that important new technologies can arise from pure curiosity-driven research, and these technologies can revolutionize areas far outside the original area of inquiry. Thermally stable DNA polymerases stand out as a prominent example.
In looking forward, we see the essential questions concerning technology and plant research to be threefold: (1) What existing technology do we need that is not currently being applied (Table 1)? (2) What new technology can be developed that we can readily envision (Table 2)? (3) What technology would we like to have but do not know how to create?
Table 1.
Types of Technologies Needed for the Future
| Technology | References |
| Gene replacement technology (as in yeast or mice, for example) | Hardy et al. (2010) |
| Artificial chromosomes and respective transformation technology | Chan et al. (2010); Gibson et al. (2008, 2010) |
| Tissue-representative cell lines as available for animal or human research | Ohashi-Ito and Fukuda (2010) |
| Challenges of cyberinfrastructure and data handling | Barga et al. (2011) |
| Reverse genetics tools that can be efficiently applied to plants beyond models | |
| Plant phenomics: high throughput that mimic real world, not pot, limited automatic greenhouses) | Plant accelerators, www.plantaccelerator.org.au; Biosphere 2, www.b2science.org |
| Tools for genomic engineering | Bogdanove and Voytas (2011) |
| Four-dimensional imaging at the super-resolution level | |
| Multimodal imaging | |
| Crystal structure of all (plant membrane) proteins | |
| Biochemical and/or structural function of all proteins | |
| Diagnostics of health; diagnostics for pathogens | |
| Methods to manipulate gene and protein function with high resolution in space and time and in combination (cellular and subcellular scales) | |
| High-throughput methods for identifying ligands and substrates | |
| Methods for systematic determination of the localization of all proteins | |
| Biosensors for plant hormones and signaling intermediates | |
| Biosensors for all key metabolites | |
| Tools to take cell-level biology and physiology to the environment | |
| Methods to image cell wall organization and molecular rearrangements | |
| Precise small molecule inhibitors for all proteins | |
| Tools for rapid increases or decreases in protein activity/amount | |
| Field-scale imaging to measure plant performance over time from individuals in a population | Asner and Martin (2011) |
| Remote (satellite or airplane) sensing of photosynthetic efficiency, nutritional status, and water status | Bramley et al. (2009) |
| Methods for imaging deep in tissues or in the soil |
Table 2.
What Can We Dream Up?
| Technology | References |
| A virtual plant to test hypotheses | http://virtualplant.bio.nyu.edu/cgi-bin/vpweb/; http://virtualplant.ru.ac.za/Main/virtual_Cover.htm; www.life.illinois.edu/plantbio/cell/; www-sop.inria.fr/virtualplants/wiki/doku.php?id=home; www.biologie.uni-hamburg.de/b-online/virtualplants/ipivp.html |
| “Plantized” yeast and animal cells as models | Assmann et al. (1985); Quintero et al. (2002); Wu et al. (2011) |
| Nanobots that can take multiplexed measurements in the cell and report results to us or travel to parts of the plant that we can’t access and take measurements | Ashley (2001); Hamilton and Gerngross (2007); Rodríguez-Escudero et al. (2005) |
| The plant “tricorder:” portable devices to scan a plant or plants in the field to obtain detailed physiological information. Can be arrayed to collect high-throughput population data. Similarly, a device that can fingerprint plant material to instantaneously identify the species. | Wah Chang’s tricorder: www.herocomm.com/BeginHere/CreatorsStory2.htm; Waters (2011) |
| Genetic vehicles for transferring complex traits or even complete pathways to new contexts for plants | Chan et al. (2010) |
The third question is important because it helps us to set goals and helps us to recognize more easily the potential application of new developments in more distant fields of research. This exercise is also fun (we like to think of it as the Star Trek question): What would be really useful if it indeed existed? The creators of Star Trek and other science fiction imagined a variety of useful future technologies, one of which, the handheld communicator, has come to fruition as the cell phone, much as originally imagined. While warp drive and the transporter are not likely around the corner, the idea of these technologies continues to inspire the imagination.
Over the past decade, plant biology has matured and is homing in on many of the classic great questions: mechanisms of cell and tissue development, the interplay of hormones, mechanisms of cell wall construction and growth, the structure and function of signaling and metabolic networks, and mechanisms of environmental perception and adaptation. Highlights include the insight that protein degradation is central to hormone signaling and perception, the discovery of regulatory small RNAs, the identification of ion transporters as receptors, the discovery of flowering signals, the delineation of regulatory networks for hormones, the molecular basis for guard cell movement, discovery of genetic control networks for development, and breakthroughs in applications such as submergence tolerant rice (Oryza sativa; Xu et al., 2006; Leung, 2008). A series of editorials in the new journal Frontiers in Plant Physiology provide some recent perspectives regarding grand challenges for different fields of plant science (Frommer, 2010; Jorgensen, 2010; Deal, 2011; Gilroy, 2011; Heazlewood, 2011; Huber, 2011; Körner, 2011; Murphy, 2011; Sinha, 2011; Von Wirén, 2011; Zwieniecki and Dumais, 2011), and a group in the UK recently published a catalog of 100 questions that are considered major challenges for the future of plant sciences (Grierson et al., 2011). Application of the best of current technologies and development of new technologies are needed to meet the grand challenges in plant science. In this Perspective, we consider the utility of novel molecular and imaging technologies, two areas of recent and significant technology innovation. In addition to the discussion here, the reader is referred to a document developed by the National Academy of Sciences of the US entitled “A New Biology for the 21st Century” (Committee on a New Biology for the 21st Century, 2009). A central feature of this document is a thorough analysis of the Grand Challenges of Plant Science to develop ideas regarding advances through technology.
THE NEW OMICS TECHNOLOGIES: CATALOGING TECHNOLOGIES AND BEYOND
The early years of molecular genetics made rapid strides, especially in revealing genes and pathways important for plant development; essential cellular functions, such as transporters; and components of important biochemical pathways, such as photosynthesis and lipid biosynthesis. Progress accelerated severalfold when advances in sequencing technology, aided soon thereafter by advances in computational technology, opened the door to whole-genome sequencing forever changed the possibilities for discovery.
A Revolution of DNA Sequencing Technologies
Thirty years ago we were thrilled when we could analyze DNA sequence 75 bp at a time using Maxam and Gilbert reactions (Maxam and Gilbert, 1977), or we could study the expression of a gene using RNA gel blots (Alwine et al., 1977). At the same time, the first solid-phase synthesis of oligonucleotides became available. DNA sequencing technology is undergoing continual change, with next-generation sequencing (NGS) achieving ever-increasing throughput and concomitant reductions in cost (Metzker, 2010). NGS started with massively parallel signature sequencing, Polony, and 454 platforms and now comprises Illumina (Solexa), SOLiD, ion conductor, DNA nanoball, Helioscope single-molecule, single-molecule real time, single-molecule real-time RNA polymerase, nanopore, and Visigen sequencing platforms. It is estimated that the cost of DNA sequencing drops at a faster rate than the cost of computer data processing (Figure 1). The decoding of the human genome, completed by a public consortium of universities in 2003, cost in excess of $500 million. What was a grand challenge a few years ago, namely, the “$1000 human genome,” is now in sight (Mardis, 2006). Today, both oligonucleotide synthesis and DNA sequencing are performed by companies or large facilities (e.g., at the Beijing Genome Institute, which today has some 137 Illumina HiSeq2000 and 27 Applied Biosystems SOLiD 4.0 systems; www.genomics.cn/). Whole genomes can be sequenced in a day, and ambitious projects are making rapid progress in deciphering the genome sequence of thousands of Arabidopsis thaliana accessions (Cao et al., 2011; Ledford, 2011). We expect that the genomes of many crops will be deciphered in the next few years. In addition, NGS developed to survey microorganisms associated with plants, the microbiome (Siezen and Kleerebezem, 2011), will help us to understand the structure and dynamics of microbial communities as well as their role in plant biology (Bisseling et al., 2009).
Figure 1.
Trajectory of Cost Reductions in DNA Sequencing.
Data replotted from: Wetterstrand K.A. For DNA sequencing costs, data are from the National Human Genome Research Institute Initiative Large-Scale Genome Sequencing Program available at www.genome.gov/sequencingcosts (accessed February 4, 2011).
Molecular Cloning
Despite breakthrough advances, such as PCR, and cloning systems, such as Gateway (Hartley et al., 2000) and USER (Nour-Eldin et al., 2010), cloning is still cumbersome. Ambitious graduate students are often surprised that the way to a major breakthrough runs through frustrating valleys of cloning experiences (e.g., PCR errors or difficulties in generating complex constructs). Therefore, one of the big challenges is a quantum leap in methods to facilitate cloning and molecular construction. Significant cost reductions in DNA synthesis, currently in the range of ~$0.30/bp, will facilitate developments to overcome this roadblock. We hope that this technology will be complemented by comprehensive clone depositories for plants, like those available for yeast, Caenorhabditis elegans, and mouse. Examples include genome-wide small interfering RNA libraries for mouse and human and antibody libraries for almost all human proteins. While comprehensive protein data repositories are publicly available for some organisms, such as the subcellular localization of all yeast proteins, similar data sets for plant proteins are missing.
Another major challenge is the transfer of genes into plant genomes. While transformation of Arabidopsis is fast and simple, transformation of other species frequently requires special expertise and involves extended tissue culture phases that lead to somaclonal variation. Moreover, transformation technology based on Agrobacterium tumefaciens is limited by the size of the T-DNA and allows transfer of a limited number of genes at a time. At present, stacking of traits in plants is limited to a handful of constructs, while synthetic biology in bacteria is now at a stage in which complete chromosomes can be synthesized and introduced into bacterial hosts to replace the genetic information (Gibson et al., 2008, 2010). The generation of plant minichromosomes (Birchler et al., 2008) combined with the ability to synthesize artificial chromosomes promises to overcome many of the limitations, providing us with the potential to create breakthroughs in synthetic biology for the plant field in the future. One of the remaining big challenges for the plant field is the development of efficient transformation technologies for introducing these large synthetic DNAs into plant genomes and, more importantly, the knowledge of the biology needed to assemble useful information and circuitry on these chromosomes.
At the same time, adding genes in random positions of the genome is far less powerful compared with gene replacement strategies, which are common in yeast and mouse. Nonhomologous recombination has limited our ability to use recombination-based gene replacement strategies; thus a major challenge in the plant filed is targeted gene replacement (i.e., the ability to replace an endogenous gene with a mutated version).
Beyond Cataloging
Large-scale sequence analysis technology has opened a wide range of opportunities beyond genome sequencing, including genome-wide analysis of the transcriptome (the set of RNA transcripts present in an organism, organ, tissue, or even cell type at a certain stage of development or in response to environmental conditions), the methylome (DNA methlyation landscape; Pelizzola and Ecker, 2011), the translatome (RNAs bound to polysomes; Mustroph et al., 2009), and transcription factor binding sites on specific chromosomes using chromatin immunoprecipitation (ChIPSeq; Ferrier et al., 2011). It is now even possible to map the position of ribosomes to mRNA at the genome scale using NGS (Ingolia, 2010; Ingolia et al., 2011). Transcript analysis is further facilitated by high-throughput quantitative RT-PCR and Fluidigm’s microfluidic expression analysis (Moltzahn et al., 2011). The breakthrough in identifying small RNAs as key regulators has led to cataloging of all small RNAs using NGS approaches. A major gap in this area is that all methods focus on measuring steady state levels of RNAs. In most organisms, including plants, RNA turnover is still poorly understood. Also here, the new sequencing technologies will help us systematically to identify the RNA degradome (Endres et al., 2011; Zheng et al., 2011).
For all of these genome-scale lines of inquiry, NGS technologies are replacing array-based technologies, such as microarrays or tiling arrays, not only due to lower cost and higher throughput, but also because the technology allows for quantitative analyses (e.g., counting of transcripts). Studies suggest that genome-wide RNA-seq is more sensitive and has a much larger quantitative range than microarray analysis and is at least comparable to quantitative RT-PCR. Nature Genetics has dedicated a website to collect the recent publications that continue to revolutionize this field (www.nature.com/nrg/series/nextgeneration/index.html).
With these new technologies comes a new set of challenges, such as optimizing library construction, amplification strategies, read length bias, and, importantly, data analysis and storage (Pop and Salzberg, 2008; Ozsolak and Milos, 2011). However, these technologies provide low resolution with respect to cells, and expression typically has been analyzed principally at the whole-plant or organ level. In recent years, considerable progress has been made in the field of cell-specific gene expression (Wee and Dinneny, 2010), a critical set of information for multicellular organisms. Progress was initiated by fluorescence-activated plant cell sorting (Brady et al., 2007), and this is being supplemented by methods that rely on tagging and affinity-purifying nuclear ribosome-associated RNAs using INTACT (Deal and Henikoff, 2011) or translatome-based methods (Mustroph et al., 2009). The latter two approaches achieve cell specificity by driving the affinity tag from cell-specific promoters.
Robotic technologies also play an increasing role in cataloging technologies, most prominently for the use of yeast two-hybrid technologies to map the interactome of plants (Lalonde et al., 2010; Dreze et al., 2011). NGS-based methods may soon replace the need for robotics here as well. Microfluidics may also play a role in molecular cataloging, as, for example, the microfluidic chip for high-throughput analysis of in vitro kinetics of protein interactions (Bates and Quake, 2009). Molecular interactions can also be measured in high-throughput platforms using label-free plasmon resonance energy imaging (Lausted et al., 2011). This technology may prove crucial since it will take us from a qualitative perspective of protein interactions to quantitative data sets.
While cataloging technologies expand our knowledge dramatically, a major gap remains regarding understanding the function of most cataloged genes, their regulation, and their interactions in networks. For example, a huge number of proteins in the best-studied plant, Arabidopsis, have no assigned biochemical activity. Moreover, while some proteins have putative functions assigned to them, these are based only on sequence homology (i.e., we do not know what their substrates or ligands are nor what specific pathways they mediate). Examples include the hundreds of receptor-like kinases and ~1000 F-box proteins. Importantly, we still lack an understanding of most cellular and subcellular processes in the multicellular plant at high temporal and spatial resolution. We have the tools to synthesize and engineer whole chromosomes in microorganisms, and soon we will have this capability in plants and can begin to construct minichromosomes. The biggest challenge will be determining how to engineer these chromosomes and build their regulatory circuits efficiently. If we consider plants as complex machines, similar to automobile engineering, we need a complete understanding of the underlying principles and the interplay of parts to engineer plants at will. This will require careful dissection of gene and protein functions combined with cellular and organismal physiology, systems biology, and computational modeling.
Progress in Mass Spectrometry Technology
Over the past few decades, massive progress has been made in mass spectrometry instrumentation and analysis tools, and wide distribution of this instrumentation has provided access to the technology to most plant scientists (McLafferty, 2011). Specific instruments have been developed for many different applications, ranging from small molecules to large protein complexes. Currently, the sensitivity of select instruments reaches the zeptomolar range (nanoelectrospray ionization; Shen et al., 2004). Some instruments provide extraordinary mass accuracy; Fourier transform ion cyclotron resonance mass spectrometry is an important and powerful tool in direct mass spectrometry analyses due to its ultrahigh resolution (>1,000,000) and mass accuracy (<1 ppm). High mass resolution is useful in empirical formula calculations and compound identification (Lei et al., 2011). These advances have fueled the development of genome-wide analysis of ion profiles (ionomics; Salt et al., 2008), metabolite profiles (metabolomics; Giavalisco et al., 2009), protein composition and relative abundance (proteomics; Heazlewood, 2011), the analysis of posttranslational protein modifications (phosphoproteomics; Ytterberg and Jensen, 2010), as well as analysis of other types of modifications, such as methylation, O-glycosylation, etc., and even protein interactions (interactomics; Gavin et al., 2011).These data sets will help us evaluate the relative contribution of transcriptional and posttranslational networks to the regulation of gene function. Ionomics and metabolomics today are largely defined by mass spectrometric analyses and can provide information down to the cellular level (Mach, 2008; Benfey, 2011; Conn et al., 2011). While these technologies are limited regarding subcellular resolution, techniques such as nonaqueous fractionation, in which cellular processes are arrested by rapid freezing and addition of organic solvents and organellar fractions are subsequently purified on density gradients, provide insights into the subcellular metabolome, ionome, or proteome (Krüger et al., 2011).
These mass spectrometry technologies are providing us with a molecular inventory and allow us systematically to identify important levels of posttranslational regulatory networks. Improvements in quantification will be critical for comparative analyses. Data sets derived using this rapidly progressing technology will be key to systems biology approaches that attempt to develop quantitative models of cells and organisms (Lee et al., 2010). With the increased availability of genome sequences, proteomics will be feasible and efficient in species other than Arabidopsis.
The New Genetics
For Arabidopsis, the production of extensive T-DNA insertion line collections has dramatically facilitated reverse genetics approaches. Multiple insertions are available for a large proportion of protein-encoding genes, and the specific mutation that is causative for an altered phenotype identified by forward genetic screens often can be identified within weeks. In Arabidopsis, it is now possible to use NGS to identify mutations in an F2 mutant population through next-generation mapping (Schneeberger et al., 2009; Austin et al., 2011). Similarly, the application of a combination of deletion generation by fast neutron bombardment with NGS promises to expand reverse genetics to many other plant species, thus significantly enhancing functional analyses in crops and other species (M.K. Barton, personal communication). Targeted induced local lesions in genomes (TILLING) has been another valuable tool for mutagenesis, and refinements such as ecotilling and deletion-TILLING provide novel means for high-throughput genotyping (Kurowska et al., 2011).
In parallel, advances in quantitative trait locus mapping, specifically association mapping, promise major advances for model systems and crop plants (Poland et al., 2011). The new genetics includes the methodology of chemical genetics, which expands our ability to detect phenotypes by directly targeting protein function through the use of small molecules that bind directly to proteins and alter protein function, thus circumventing problems with redundancy and lethality. Identification of the targeted protein can be pursued by interaction assays or sensitized genetic screens. This approach has been deployed with great success to gain major new insights into hormone signaling (Melcher et al., 2009; Nishimura et al., 2009; Park et al., 2009).
The New Structural Biology
Progress in expression systems, throughput, and analytical tools has lead to a dramatic increase in structural solutions of proteins. This holds true for membrane and soluble proteins. Structures that have been obtained recently for hormone receptors and recent progress in solving structures of transporters in the presence and absence of ligands have begun to provide us with snapshots of the molecular processes of enzyme and transporter activities (Krishnamurthy et al., 2009; Weyand et al., 2011). As yet, there is not a project to determine structures for plant proteins on a large scale, but such an endeavor would have great value in advancing our understanding of biochemical mechanisms and in looking at diversity and similarity in the proteome through the perspective of protein structure.
THE NEW CELL BIOLOGY AND IN VIVO BIOCHEMISTRY: IMAGING AND CELL BIOLOGICAL TECHNOLOGIES
Imaging is a principal means of assessing phenotype and function and an essential part of the modern biological tool kit. Operating over a range of spatial scales, spanning from tissues to single molecules, imaging provides the advantages of being able to observe and measure phenotype and function at cellular and even molecular resolution and to reveal biological dynamics in living tissue. These are capabilities that biochemical techniques rarely are able to achieve. And while physiological techniques such as microelectrode-based measurement have single-cell and even single-channel resolution, they are limited in their range of application and do not allow for efficient observation of variance over cellular scales.
Visible Light Imaging: Probe Development
In recent years, parallel advances in fluorescence-based microscopy methods and probes have revolutionized cell biology. The demonstration that intrinsically fluorescent proteins from jellyfish could be fused to other sequences and expressed and imaged in other species opened the floodgates to a new generation of experimentation and tool building. The fact that these probes are genetically encoded provides the possibility to tag target proteins with genetic specificity, a level not previously possible with antibodies. In addition, genetic encoding enables noninvasive introduction of probes at similar levels and spatio-temporal control as the native molecules. Noninvasive introduction is especially valuable in plant cells, whose rigid cell wall makes microinjection difficult and often presents challenges for the uptake and specific localization of probes introduced from the external solution (Fehr et al., 2004). It is now common practice to tag proteins of interest with fluorescent proteins to probe their localization, behavior, and possible interactions with other molecules in the cell.
Genetic encoding has also provided significant advantages for the development of new probes and has enabled the development of novel screens and tools. Improved and new probes have been developed by screening for naturally evolved variants and using molecular genetic techniques and high-throughput screening to evolve probes in the lab. Some key parameters that have been targeted are brightness, spectral output, pH sensitivity, oligomerization, and sophisticated properties such as photoconversion (Shaner et al., 2004, 2005; McKinney et al., 2009; Wu et al., 2011). Examples of novel screens and tools made possible by the genetic encoding of fluorescent probes include large-scale screens for protein localization (Cutler et al., 2000), which has been done effectively in yeast but remains to be done on a useful scale in plants, cell-type expression analysis based on fluorescence-activated cell sorting of disrupted and green fluorescent protein (GFP)–expressing cells (Birnbaum et al., 2003, 2005; Benfey, 2011), the ability to split probes into two components to use reconstitution of fluorescence as an assay for interaction of their tagged partners (Kerppola, 2008), and the creation of an ever growing array of novel cellular sensors (Shaner et al., 2005). Most of these sensors are genetically encoded and can thus be introduced into any cell or organism that is genetically transformable. These sensors can be based on changes in the expression or localization of a tagged and responsive protein (PH domain [Halet, 2005] or auxin probes [Ottenschlager et al., 2003]) or on a physical phenomenon called Förster resonance energy transfer (FRET) to probe changes in the spatial relationship of two compatible probes. FRET sensors are exemplified by small molecule sensors based on binding proteins that have different conformational states in the bound and unbound condition (ions, sugars, ATP, and signaling molecules, such as calcium or cyclic nucleotides) (Frommer et al., 2009). These genetically encoded sensors afford us with new possibilities to access new dimensions, such as millisecond time resolution and, importantly, cellular and subcellular resolution down to limited areas of the cell membrane (Okumoto et al., 2008). FRET can also be used to follow the activities of enzymes (such as proteases) in vivo, determine the phosphorylation state of a protein domain, measure membrane potential of subcellular membranes, and quantify tension between cytoskeletal proteins during growth (Frommer et al., 2009; Grashoff et al., 2010; Meng and Sachs, 2011; Zhou et al., 2011). Microfluidics has revolutionized single-cell analysis in yeast and animal systems (Bermejo et al., 2010, 2011b, 2011a). The recent development of a microfluidic multichannel device for root growth and imaging, the RootChip, now enables moderate throughput screening of mutants using the large spectrum of available FRET sensors in plants (Grossmann et al., 2011).
Of course, the jellyfish fluorescent proteins and relatives have not been the only active area for genetically based optical probe development. Binary probes have been created based on specific peptide motifs, and, recently, RNA motifs that interact with chemical fluors that are introduced to the cell have been developed. For example, the tetracysteine tags used for biarsenical labeling, termed FlAsH and ReAsH by Martin et al. (2005), offer the advantage of being considerably smaller than fluorescent proteins, whose large size can interfere with target protein function or assembly into complexes. However, they also can have high backgrounds due to off-target labeling. A recent report of specific RNA labeling using an evolved 60-base aptamer and a purpose-synthesized label is very exciting, with a variety of potential applications in plant biology (Paige et al., 2011).
Next-Generation Probes and Optogenetics
Plants have a wealth of light absorbing proteins, and these have been exploited to create useful new cell biological tools. For example, fluorescent tags have been created based the photoreceptors phytochrome and phototropin. As with tetracysteine tags, their primary advantage to date is their small size, but unlike tetracysteine tags, they do not create problems with off-target background. These proteins have been used not only to create probes for protein position, but also tools to alter protein function using light as a trigger (Leung et al., 2008; Levskaya et al., 2009; Wu et al., 2009) (Figure 2). These join a handful of other optically sensitive tools based on bacterial proteins, which have been used in exciting experiments to manipulate neuronal function through light sensate control of ion channels, a technique that has been dubbed optogenetics (Deisseroth, 2011). These light-based tools allow control of protein function with exquisite resolution in time and space and represent a bold and potentially revolutionary frontier in cell biology. While many of these tools are created with protein modules evolved in plants, we are not aware of their application in plant cells to date.
Figure 2.
Reversible Manipulation of Protein Function Using Targeted Illumination.
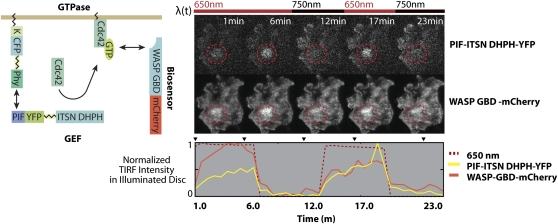
Fusion proteins featuring Phytochrome and its effector phytochrome interacting factor3 (PIF) allow for reversible control of protein recruitment to the plasma membrane at the spatial precision of optical resolution and on a time scale of seconds. Levskaya et al. (2009) showed that these tools could be used to manipulate cell shape by locally activating Rho-GTPase signaling through light-mediated recruitment of Rho effector proteins to the plasma membrane of mammalian cells. A fusion protein of cyan fluorescent protein (CFP) and Phytochrome B (PHY) is tethered to the plasma membrane, where activation by red light recruits phytochrome interacting factor3 fused to yellow fluorescent protein (YFP) and the catalytic domain of the RacGEF Tiam (ITSN DHPH). The concentration of the RacGEF domain at the membrane activates its target Rho GTPase Cdc42, which in turn recruits a sensor consisting of the GBD binding domain of the actin polymerization factor WASP fused to the mCherry fluorescent protein. (Reprinted by permission from Macmillan Publishers Ltd.: Nature [Levskaya et al., 2009; Figure 4], copyright 2009.)
We anticipate that the development of new tools and methods based on genetically encoded probes and photosensitive proteins will be a very active area of research in the next few years. As organisms that have evolved to use and respond sensitively to light cues, plants represent a potentially rich reservoir of raw material for the creation of new photosensitive tools to manipulate protein localization and function.
Visible Light Imaging: Instrumentation
Extraction of information from live-cell imaging is limited by several instrument-dependent factors, including the ability to detect the labeled structures above specimen background and instrument noise, the spatial resolution of the microscope, the time resolution of image acquisition, the stability of the label under sustained imaging, and damage to the specimen caused by free radical generation.
The development of highly quantum efficient back-thinned detectors with improved electronics and on-chip amplification in electron multiplying charge coupled devices (EMCCDs) and, more recently, scientific-grade complementary metal-oxide-semiconductor (CMOS) cameras have made significant advances in addressing most of the above limitations. With >90% quantum efficiency and reduction of dark current and read noise to levels approaching photon noise-limited performance into very low light regimes, EMCCD cameras have made single-molecule detection widely available while also allowing images to be captured at tens of millisecond intervals without significant read noise penalties. These rapid frame rates enable observation of molecular dynamics at shorter time scales and permit three-dimensional (3D) imaging at rates that were formerly the domain of two-dimensional (2D) imaging (Figure 3). In addition, more sensitive detection allows significantly lower excitation energies to be used, extending the life of probes and reducing cellular damage by free radical generation. High-end CMOS cameras are now being introduced that improve on EMCCD performance for a number of applications. Specifically, they provide large fields of view and can acquire images with high dynamic range at extremely fast frame rates with minimal read noise penalty. EMCCDs continue to have better quantum efficiency, but CMOS technology is catching up in signal-to-noise performance.
Figure 3.
Rapid 3D Time-Lapse Imaging.
Maximum projections made from spinning disk confocal stacks acquired from Arabidopsis leaf epidermal cells expressing GFP:a-tubulin 6 to label cortical microtubules. The images show the junction between two pavement cells. Twenty-five confocal sections were acquired every 5 s to generate 3D volumes ~7 μm in depth. (Image courtesy of D.W. Ehrhardt and Y. Fu, unpublished data.)
In thicker cells and specimens, background from the specimen needs to be reduced, so these cameras are often coupled with a means of optical sectioning, such as parallel scanning confocal imaging (spinning disk and its relatives). This combination has made possible extended dynamic imaging of single-protein complexes in plant cells (Paredez et al., 2006; Nakamura et al., 2010). Total internal reflection fluorescence (TIRF) is also commonly used in combination with EMCCD cameras. TIRF works by reflecting excitation light off of the interface between the cover glass and the specimen, creating an evanescent field above the interface that can usefully excite fluorescence in a plane ~150 nm thin, far thinner than optical sections created by confocal technologies. However, the volume that can be imaged is therefore confined to a single location in space: the thin plane just above the cover glass. While the depth of TIRF excitation is shallower than the typical plant cell wall, TIRF microscopes have nonetheless been used successfully to obtain high-quality images in the plant cell cortex (Konopka and Bednarek, 2008; Staiger et al., 2009). It remains unclear whether these images come from an evanescent field generated at the cell wall membrane boundary or from reducing the background fluorescence by low-angle illumination. TIRF and array scanning systems can be outfitted with devices to perform targeted photobleaching and photoactivation of probes, thus combining sensitive detection and extended fluorescence life with the ability to perform photomanipulation experiments.
While TIRF and spinning disk systems are coming into increasing use for rapid and sensitive detection of fluorescent probes with background rejection, significant improvements have also been made in point scanning microscopes. Conventional point scanning microscopes remain workhorses for quantitative 3D imaging and offer advantages when larger fields of view are required and greater out of focus light rejection is needed when working past the cover slip interface. These instruments are improved with every cycle of development, for example, by offering more sensitive detectors and modalities, such as spectral imaging, that are not commonly available on spinning disk and TIRF systems. Many imaging facilities find both array-scanning disk and point-scanning instruments to be valuable. Perhaps the most significant advance in point-scanning instruments (other advances will be discussed below) is the advent of multiphoton excitation, especially when combined with nondescanned detection. Multiphoton imaging has three potential advantages over single-photon imaging. First, the photon density required for excitation occurs only precisely at the scanned focal point; thus, no significant excitation occurs in the cell outside this very small volume. This greatly reduces photobleaching and cytotoxicity due to excitation of fluors outside the interrogated focal plane. Second, the long wavelengths used for excitation are scattered less as they pass through the specimen, permitting improved imaging in deeper tissue. Finally, since the location of emitted photons is determined by the position of the focal spot, spatial filtering with an aperture in the image path is not needed. In fact, all that matters is when photons are collected, not where they are collected. Thus, even highly scattered photons can be detected and used to create the image, significantly increasing the sensitivity of imaging. Multiphoton imaging has revolutionized in vivo imaging in brain tissue in animals and is being applied increasingly to plant tissues (Feijó and Moreno, 2004; Hamamura et al., 2011; Roppolo et al., 2011).
Optical sectioning can also be achieved computationally by systems that acquire images from multiple focal planes (deconvolution microscopes) or with multiple illumination patterns (structured illumination [SI]), coupled with processing by sophisticated algorithms. These systems are more sensitive than confocal microscopes because no light is thrown out by a physical filter, but unless coupled with arrays of processors, they do not provide resolved images in real time, limiting their use in applications that require immediate feedback to the observer. As computational power historically grows exponentially, it is anticipated that soon these systems will achieve real-time performance at reasonable cost. It is the author’s (D.W.E.) experience, however, that deconvolution of structures at or near highly refractile cell walls is particularly challenging. Furthermore, when imaging thick specimens, the captured volume can include substantial signal from outside of the volume, significantly reducing the signal to background.
Light sheet technology provides one of the newest means of obtaining optical sections using fluorescent probes. In light sheet imaging, excitation energy is provided at right angles to the imaging axis, typically by scanning a laser beam through an objective to produce a sheet of light at a defined plane through the specimen. Fluorophores above and below the plane of the light sheet are not excited, producing an optical section. Light sheet microscopy has been used successfully to create 3D images of developing mammalian, insect, and fish embryos while exposing them to levels light of two to three orders of magnitude below that of conventional and confocal imaging (Keller et al., 2008, 2010; Reynaud et al., 2008). It has been applied recently to Arabidopsis tissue to image both root development and subcellular events (Maizel et al., 2011; Sena et al., 2011) (Figure 4). While more limited in lateral resolution than confocal imaging, light sheet imaging has produced useful images of cytoskeletal organization and endosome dynamics at light levels that are near physiological (Maizel et al., 2011).
Figure 4.
Light Sheet–Based Imaging of Arabidopsis Seedlings.
(A) View of the central components of a digital scanned laser light sheet fluorescence microscope. The illumination system excites the fluorophores in a thin planar volume by rapidly scanning a micrometer-wide Gaussian laser beam inside the specimen. Fluorescence is collected at right angles to the illuminated plane by the detection system. The planar excitation volume and the focal plane of the detection system overlap. The intensity of the laser beam can be modulated in synchrony with the scanning process (SI). (Left figure by P. Theer.)
(B) Close-up of the sample chamber (boxed region in [A]). The root of the plant is growing on the surface of a Phytagel cylinder immersed in culture medium (half-strength Murashige and Skoog medium), while its leaves are in the air. The chamber is equipped with a perfusion system exchanging the whole chamber volume every 15 min and a sun-like lighting system covering the plant leaves from above.
(C) Side view of the two types of sample holder used for imaging. (Left) The root of the plant grows into a 0.5% Phytagel cylinder. For the image acquisition process, the Phytagel cylinder is extruded from the capillary, which is rigidified by an embedded carbon rod. (Right) The root grows through a plastic cone filled with 0.5% Phytagel maintained by a ring holder. In both designs, 2-d-old seedlings were transferred to the holders and further cultured in a tilted position such that the root grew toward the glass until the onset of imaging into the chambers indicated below. (Reprinted with permission from John Wiley and Sons, Ltd.: Plant Journal [Maizel et al., 2011; Figure 1], copyright 2011.)
Historically, intensity and color have been the most commonly used properties of fluorescence for biological detection. Fluorescence has a third property that can be exploited: its excitation lifetime. The lifetime of an excited electron is sensitive both to transitions leading to fluorescence and to nonradiative processes, such as quenching and energy transfer to another fluor. Thus, lifetime can be used to assess changes in the local environment of the fluor and energy transfer by FRET. Lifetime imaging can be performed on point scanning instruments in the time domain using pulsed lasers and multichannel time discrimination detectors, and in wide field, TIRF and array scanning instruments in the frequency domain using modulation of both the light source and the detector gain. Lifetime imaging has seen some application in plant biology (Immink et al., 2002; Russinova et al., 2004; Bhat et al., 2005), but its full potential has yet to be realized, in part because such instrumentation is not commonly accessible to plant research laboratories.
Instrument miniaturization and microendoscopic lenses based on fiber optics have revolutionized confocal imaging of the brain in free-running mice (Barretto et al., 2011). This technology may provide new ways to monitor processes deep inside a plant and perhaps in the soil. Miniaturized and lightweight instruments also may allow for portability and flexible positioning, bringing the microscope to the specimen and allowing confocal imaging to be deployed in intact adult plants and in the field.
Super-Resolution Microscopy
While advances in detectors and scanning technologies are pushing back the limits of detection, time, and low-light performance, an exciting array of new technologies is breaking the spatial resolution barrier set by diffraction to bring optical resolution down to meaningful molecular scales. Known by a variety of acronyms, these super-resolution methods are under active development and like all microscopy technologies, have individual strengths and weaknesses.
The stochastic methods characterized by photoactivated localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM) exploit state transitions of fluors to pinpoint individual probes in succession (Betzig et al., 2006; Rust et al., 2006; Hess et al., 2009) to achieve remarkably high resolutions (below 20 nm) but require time to build images by sequential rounds of individual fluor activation and centroid calculation. This time requirement limits application to dynamic structures and makes the method sensitive to specimen and instrument stability. PALM and STORM are limited by the availability of fluors than can be used, but that range is expanding as methods to exploit a wider range of state transitions are explored.
Stimulated emission depletion microscopy (STED) breaks the diffraction limit by scanning the specimen with an excitation spot of subdiffraction size (Hell and Wichmann, 1994; Rankin et al., 2011). This spot is created by selectively depopulating excited fluors at the spot edge using a second, donut-shaped beam. As the depopulation beam power is increased, the interrogation spot can be reduced to an arbitrarily small size; however, practical limitations on beam power and specimen integrity keep achievable resolutions above 5 nm and more realistically in the 20- to 40-nm range. As a point scanning method, STED images can also be built up more quickly than stochastic images, but STED can be limited by off-target consequences of the extraordinarily high beam power of the depopulation beam, and like PALM and STORM, STED is limited by the range of compatible fluors.
SI microscopy achieves super-resolution by exciting the specimen with patterned illumination at several angles and translocations. The superimposition of these images reveals high frequency information in a manner related to that seen with Moiré patterns (Gustafsson, 2005). Computational processing produces an image that is limited to double the diffraction-limited resolution. Therefore, SI is more limited in its resolving power than PALM or STED but can be used with any fluor and with low cost excitation instrumentation. Typically, nine raw images are acquired for one resolved image, so it is somewhat limited in time resolution compared with normal imaging due the need to collect and process multiple images. However, SI is being developed aggressively, with both 3D and video rate acquisition having been achieved (Gustafsson et al., 2008; Kner et al., 2009) in addition to dynamic imaging of whole living cells (Shao et al., 2011) (Figure 5).
Figure 5.
3D Super-Resolution Imaging of a Live Cell by SI Microscopy.
Maximum projection (A) and xz section (B) of a Hela cell stained with MitoTracker Green to label the mitochondria cell, as acquired with a 3D SI microscope. Images acquired with conventional epifluorescence are shown at left. Details of the SIM image are shown in (C) to (F). [Reprinted by permission from Macmillan Publishers Ltd.: Nature Methods [Shao et al., 2011; Figure 2], copyright 2011.)
Optical resolution typically is approximately threefold coarser along the z axis than it is in the xy-plane. Large gains in z resolution have been achieved in STORM imaging by creating point spread functions that are asymmetric on the z axis through use of a cylindrical lens in the optical path, thus permitting the z position of a point source to be estimated with greater precision. A related and more sophisticated approach has been taken by W.E. Moerner and colleagues using a reflective modulator to generate a double helix–shaped point spread function (Badieirostami et al., 2010; Lew et al., 2011). This method has brought axial resolution down well below 50 nm. Recent innovations in SI have also made super-resolution possible on the z axis.
To date there is only one publication applying any of these techniques to plant tissue (Fitzgibbon et al., 2010), in which proteins targeted to the endoplasmic reticulum and to plasmodesmata were imaged with 3D SI microscopy (3D-SIM) (Figure 6). Some challenges for super-resolution imaging in plant tissue (Gutierrez et al., 2010) include the limited depth of field offered by TIRF and pseudo-TIRF as is used for PALM and STORM imaging and degradation of the point spread function by scattering and spherical aberration with sample depth in 3D methods like 3D-SIM. The latter problem may be especially acute in plant cells because of the significant changes in refractive index between the cell wall and the cytosol. However, spherical aberration can be at least partially compensated for by adjusting the refractive index of the immersion oil and by using compensating optics between the lens and camera. The limitation of the useful focal plane for PALM and STORM imposed by TIRF may be addressed by other optical sectioning methods, such as spinning disk confocal and light sheet imaging, where probe signal and specimen background permits. While challenges remain, we expect super-resolution to be a technology of high impact that will see rapid development and increasing application.
Figure 6.
3D Super-Resolution Imaging of Plasmodesmata.
Simple and branched plasmodesmata viewed with confocal, wide-field, and 3D-SIM imaging.
(A) Conventional confocal image showing plasmodesmata containing MP-GFP (green) labeled with callose antibody (Alexa 594; red). Overlapping signals appear yellow (arrows), but individual plasmodesmata are not resolved. Bar = 5 μm.
(B) to (G) 3D-SIM images of simple plasmodesmata.
(B) Diagram of a single plasmodesmata pore, showing callose collars (red) separated from a single central cavity (green).
(C) to (G) 3D-SIM spatially resolves the callose collars of individual plasmodesmata from the central cavity. The corresponding wide-field images of the same pores pictured in (D) and (F) are shown in (E) and (G), respectively. A Y-shaped plasmodesmata configuration is shown in (D). An extension of the central cavity is seen in ([F]; arrowhead). W, Cell wall. Bars = 1 μm.
(H) A z series taken using 3D-SIM of a single plasmodesmata pore. The individual images are 125 nm apart. Note that two pores can be seen (arrows), each leading to a shared central cavity (arrowhead). Bar = 1 μm.
(Reprinted with permission from Fitzgibbon et al. [2010], Figure 1.)
Optical Tomography
Optical projection tomography (OPT) is a means of generating quantitative 3D images of relatively large specimens on the order of millimeters to centimeters (Sharpe et al., 2002). Essentially, it involves acquiring multiple transmitted light images of a transparent specimen as it is rotated through 360°. The imaging lens is focused into the specimen with a very deep depth of field, or focus is scanned at each rotation position. Differences in light absorption can be mapped by computational analysis, producing a 3D model. Similarly, the distribution of fluorescence can be mapped in multiple channels, permitting quantitative 3D mapping of florescent probes on a large spatial scale. OCT produces stunning images of mammalian embryos and has been applied to plant seedlings (Lee et al., 2006) (Figure 7). Significant application of OPT will be to provide quantitative morphometric data for models of growth and development. Another type of optical tomography, optical coherence tomography, is used to produce 3D images of specimens based on imaging of reflected light and has been used to make images in highly scattering samples, such as seeds (Hettinger et al., 2000; Reeves et al., 2002).
Figure 7.
OPT.
(A) OPT volume view of the first true leaves of an Arabidopsis seedling showing trichome cells on the adaxial leaf surface (cotyledons removed). The image was taken with fluorescence OPT (GFP1 filter). Bar = 285 μm.
(B) Part of an Arabidopsis silique imaged by fluorescence OPT (Texas Red filter). Bar = 42 μm.
(C) Clipping of an OPT volume of an Arabidopsis seedling to display vasculature. Vasculature is more autofluorescent than the surrounding tissue, making it appear brighter. Bar = 100 μm.
(D) An Arabidopsis silique (from [B]) clipped to reveal internal structure. A piece was removed using three clipping planes to show the seeds developing within. The removed piece is shown at left. Individual seeds were also dissected out using six clipping planes to display the heart-stage embryo (arrowhead) and endosperm within (two examples shown at right). Bar = 35 μm.
(Reprinted with permission from Lee et al. [2006], Figures 1 and 2.)
Array Tomography
Array tomography is a novel methodology developed by Micheva et al. (2010) that combines classical microscopy methods with modern high-throughput molecular probing, automated imaging, and volumetric image reconstruction to map the molecular architecture of tissues in three dimensions at high resolution. Tissue is fixed and embedded in a hydrophilic resin in preparation for thin sectioning as it would be for classic immunohistochemistry. However, ultrathin sectioning (50–200 nm) is automated, facilitating the construction of 2D arrays of continuous sections on optical cover slips. These arrays are probed with labeled antibodies to detect proteins of interest and are imaged at high resolution on an automated platform. Efficient stripping and reprobing allows up to 30 probes to be applied to the same sample. Computer-aided image stitching, registration, and volumetric reconstruction allow the distribution of these probes to be determined at diffraction-limited resolution and greater in three dimensions. This technique has produced stunning 3D maps of the mouse brain, revealing all synapses and neuronal connections. Yet to be applied in plants, it has enormous potential to provide atlases of the molecular architecture of cells and organs.
Image Processing
The advances described above are allowing us to collect high-resolution information along multiple axes of space, time, and wavelength. A growing challenge is how to analyze and manage these rapidly growing and complex data sets. Fortunately, digital storage is dropping rapidly in cost, and there is energetic development of software both in academia and in companies, but many challenges remain to be adequately addressed.
Among the frontiers in data analysis are improved methods to identify and measure desired features in multidimensional data sets. For example, in data sets with limited complexity, there are effective software tools for segmenting and tracking simple objects in three dimensions (Golgi bodies, for example). However, these tools quickly fail to perform well with increasing object density and morphological complexity. Tracking of polymer dynamics in three dimensions, for example, is still a challenge with commercial and open source tools. While measurements can be performed by hand in these more complex data sets, they are often subject to bias arising from sampling and researcher expectation, the need to select those images where features appear most visible, and the use of the human eye to make judgments. Automated and blind methods to accurately segment, measure, and track biological structures can help to reduce bias, allow for a much more detailed and precise picture of the biology, and open the door to new discoveries that are only possible by sampling and measuring large populations. Systems analyses involving modeling and simulation will depend critically on measurement quality and good information about distributions. Examples of advances in image segmentation and measurement in plant science are recently developed methods to segment cell volumes in roots and other tissues by applying customized seed filling techniques to 3D data sets acquired from confocal image stacks (Marcuzzo et al., 2009; Liu et al., 2011). Another set of standout methods are those to measure tissue growth from inherent fiducial markers in transmitted light or fluorescent images (van der Weele et al., 2003).
Electron Microscopy
Using extremely short wavelengths, electron microscopy (EM) provided an enormous advance in imaging, pushing our vision to the nanometer scale and below (Erni et al., 2009), thus providing our first views of cell structure at molecular resolution. While unparalleled for resolution, obtaining 3D information using EM has traditionally been challenging, and dynamic pictures are not possible. In addition, molecular information in EM has been limited as it typically requires secondary detection with decorated antibodies, which introduces uncertainties in determining the true location of target proteins due to nonspecific background and the bulky nature of gold particles and other particulate electron-dense tags. A recent breakthrough (Shu et al., 2011) promises the possibility of revolutionizing molecular labeling in EM using genetically encoded probes, providing some of the same advantages that GFP tagging affords while at the same time allowing for correlated imaging between the light and EM levels. Termed miniSOG for mini singlet oxygen generator, this new probe is both fluorescent and capable of generating an EM-detected signal. The EM signal is produced by polymerization of diaminobenzidine when miniSOG yields singlet oxygen upon illumination. This polymer, which is only produced efficiently in very close proximity to the source of singlet oxygen, can then be visualized by reaction with the electron-dense stain osmium. This breakthrough probe was engineered from the plant photoreceptor Phototropin 2. This advance represents an excellent example of how basic research in disparate organisms advances research across the board, including human biology.
A second area where EM has made significant advances is in taking imaging from 2D to 3D via EM tomography. Based on acquiring multiple images from different angles in thin sections and subsequent computer-aided reconstruction, EM tomography and EM cryotomography are yielding high-resolution views of 3D organization of essential cell structures, including cytoskeletal filaments, molecular motors, and cell walls in bacteria and other organisms (Tocheva et al., 2010). In plants, EM tomography has revealed the arrangement and morphology of essential components of the cytokinetic apparatus, such as microtubules and networks of fusing vesicles and membrane tubules (Tocheva et al., 2010), providing an important base for interpreting and guiding investigations into phragmoplast development using genetic, molecular, and light microscopy methods. It will be exciting to see how new genetically encoded EM tags can be combined with tomography to add molecular specificity to these 3D views of cellular organization.
Scanning Electron Microscopy
Scanning electron microscopy is a surface-based technique to reveal structure down to nanometer resolution. Advances in scanning electron microscopy include instruments that can operate in low vacuum, so-called environmental instruments, which permit imaging of fresh tissue with no special preparation. Scanning electron microscopy instruments can be outfitted with a variety of detectors, some of which can be used to analyze the molecular composition and crystallinity of the scanned surface. In plant biology, it is commonly used to produce projected images of organ and cell morphology, especially in genetic analysis. New developments in scanning electron microscopy include instruments that combine backscatter detection in scanning electron microscopy with a focused gallium ion beam to mill the surface of specimen blocks for imaging of sequential planes to create 3D image volumes (Merchan-Perez et al., 2009). This technique avoids the collection and handling of serial sections and allows for fully correlated volumetric information. Ion beam milling has also been used to mill frozen specimens to image interior features (Hayles et al., 2007) and to create thin sections for cryo electron tomography that avoid compression artifacts caused be sectioning of frozen material. These tomograms displayed features that were lost with more traditional preparation methods (Marko et al., 2007).
Atomic Force Microscopy and Multimode Imaging
Like scanning electron microscopy, atomic force microscopy (AFM) is a surface-based imaging technique. Using a probe tip on a scanning cantilever with fine positional feedback, AFM can map surface topology and molecular properties down to atomic resolution under ideal conditions. Applications in plants include imaging of cell wall structure, such as the arrangement of cellulose microfibrils adjacent to the plasma membrane (Kirby et al., 1996; Davies and Harris, 2003; Marga et al., 2005), and analysis of interactions among cell wall components in vitro (Cannon et al., 2008). AFM can also be used to probe the mechanical properties of surfaces. An exciting application of this capability in plant biology is a recent analysis of cell wall mechanics in the shoot apex (Milani et al., 2011; Peaucelle et al., 2011). AFM has also been applied to examine cell wall structure, especially cellulose microfibril arrangements at the inner surface of the cell wall. Perhaps the greatest excitement for biological application of AFM lies in the ability to functionalize the AFM tip by attaching molecules that can interact with the scanned surface with biological specificity. Notable examples of such studies are the mapping of peptidoglycan organization in the bacterial cell wall using a LysM probe (Andre et al., 2010) and mapping changes in the distribution of mechano-sensitive proteins in response to applied stress. Functionalized probes have also been used to measure molecular interactions, such as ligand-receptor binding, and the mechanical properties of proteins, such as matrix attachment proteins, at the single-molecule level on the surface of living cells (Muller and Dufrene, 2011). AFM technology is being developed at a rapid pace, with improvements in probe control and scanning speed and integration into platforms that allow multimodal imaging. The ability to combine fluorescence imaging with AFM enables many new experimental possibilities. An example is the application of polarized TIRF imaging with AFM to simultaneously monitor both lipid ordering via fluorescence polarization and lipid topology to study the molecular mechanism of peptide insertion into lipid bilayers (Oreopoulos and Yip, 2009). Multimodal functionalized AFM has the potential to open new doors to the study of plant cell wall structure and organization.
Laser Trapping
Laser traps, also known as optical tweezers, are a means to manipulate refractive objects about the size of bacteria mechanically. By functionalizing these objects, it is possible to perform sophisticated assays at a single-molecule level. However, unlike AFM, laser traps can also be applied inside living cells. Among the applications of laser traps have been analysis of protein motor function (Visscher et al., 1999), receptor ligand interactions (Kim et al., 2010), and nucleic acid polymerases (Abbondanzieri et al., 2005). Improved trapping technology has made it possible to resolve even single-molecule steps, yielding new insights in to the function of molecular machines. To date, there have been only a handful of applications in plant cells, including demonstrating physical interaction of both Golgi (Sparkes et al., 2009) and chloroplasts (Andersson et al., 2007) with the endoplasmic reticulum, and exploration of forces that position nuclei in root hairs (Ketelaar et al., 2002). We expect plant cell applications to grow, especially as there is a renewed interest in plant cell biomechanics.
Molecular Compositional Imaging
By applying spectroscopic methods in a fine-scale raster pattern, images of spectral information can be acquired to probe the distribution of molecules in cells and tissues. For example, images of mass spectrometry data can be created by scanning the specimen with an ionizing beam coupled with time-of-flight mass spectrometry. In secondary ion mass spectrometry imaging, an ion beam is employed to analyze the distribution of small molecules, such as metal ions and lipids. In matrix-assisted laser desorption ionization imaging, a laser beam is used to analyze larger molecules, such as peptides and proteins. Peptide imaging has been used to image signaling peptides in the plant cell wall (Sawa et al., 2006), lipid metabolites on the root surface (Jun et al., 2010), and epicuticular waxes in the cuticle (Cha et al., 2009). Infrared energy is used to image composition of biological specimens with Fourier transform IR microscopy. This technique is highly sensitive to slight changes in composition but produces complex spectra that require sophisticated data analysis to interpret. It has been used successfully in imaging of human disease (Miller and Dumas, 2010), and applications in plant biology include chemical composition of tissues and the rhizosphere (Heraud et al., 2007; Raab and Lipson, 2010). Recent advances include improvements in scanning speed using focal plane arrays (Heraud et al., 2007) and gains in resolution using multibeam super-resolution methods at synchrotrons (Nasse et al., 2010). Considerable effort has been applied to using Fourier transform IR for analysis of cell walls, but assigning changes in the observed spectra to changes in specific molecular species in the wall remains elusive (McCann et al., 2001). X-ray fluorescence imaging requires access to synchrotron radiation but creates images of elemental spectra from tissue without need of special preparation, thus allowing the distribution of metals to be visualized while avoiding artifacts due to tissue preparation techniques required for other scanning methods. It has been applied successfully to the study of metal homeostasis in plants (Punshon et al., 2009).
Population Scale/Remote Sensing
New technologies already in use include airplanes carrying light detection and ranging systems that identify biomass and potentially can determine species (Asner and Martin, 2011). Remote sensing technologies hold potential not only for monitoring of deforestation but specifically also for remote detection of plant properties in agricultural fields (e.g., remote monitoring of the nutrition status or occurrence of pathogen infection, which might be used to improve fertilization or pest management strategies with highly resolved local data).
THE NEW BIOINFORMATICS: KEY TO DATA HANDLING AND INTERPRETATION
The sheer mass of information generated by these technologies requires not only large databases, but novel ways of analyzing the data. A wide spectrum of Web resources has become key to handling molecular data, providing researchers with immense opportunities for data mining and hypothesis generation. These include The Arabidopsis Information Resource, the Bio-Array Resource for Plant Biology, the Salk Institute Genome Analysis Lab, the ARANET probabilistic functional gene network of Arabidopsis (Lee et al., 2010), and many others. New technologies are needed in the field of bioinformatics, exemplified by improvements in signal-to-noise filtering (Ideker et al., 2011).
Handling and access to image-based data are also subjects of intense discussion among plant cell biologists, in particular, how the growing volumes of image-based data might be leveraged better by the community. This question applies both to data that have already been at least partially described and cataloged, such as image databases of enhancer trap expression and protein localization, and to raw image data sets that support peer reviewed studies. In the former case, there are substantial issues regarding the security and searchability of these valuable community resources, and in the latter case there may be new insights waiting to be discovered in extant data sets. Of course, making use of such voluminous data sets depends on cost-effective storage, adequate annotation, and the availability of good search tools. For both molecular and imaging data, handling massive amounts of data will remain a challenge, especially in the light of shrinking budgets and massive progress in data production.
Systems Biology and Synthetic Biology
Arguably, the biggest challenge for new biology will be to move from data collection to modeling, specifically quantitative modeling, or from analyses to prediction. To be useful, models need to be of sufficient quality to generate testable hypotheses. Modeling is not particularly useful if it just confirms what is already known or if it is used to support one hypothesis while multiple alternative solutions are possible and the model only indicates that the hypothesis is one of the possible solutions.
Here, we define systems biology as a discipline that models processes, such as regulatory networks, and iteratively tests and improves these models to understand how cells carry out and regulate these processes. At a larger scale, it attempts to model the behavior of cells and organisms based on our current knowledge to better understand them. Ultimately, systems biology lays the basis for engineering organisms, a discipline defined as synthetic biology. Synthetic biology encompasses novel approaches developed in chemistry, namely, artificial cells constructed with nanostructured polymeric capsules or nanoreactors (van Dongen et al., 2010; Habibi et al., 2011), as well as attempts to redesign networks and pathways (e.g., to produce products in plants as factories) from the wildest dreams of building organisms from scratch. As a step in this direction, we are now able to synthesize complete chromosomes (Gibson et al., 2010). The key questions will be what to put on them and how to create the regulatory systems that will enable the gene products to work together efficiently. Regarding precise engineering of chromosomes, plant scientists recently made major steps: We are at the verge of being able to engineer chromosomes and to switch on and off genes at will based on the modular design of transcription activator-like effectors from pathogenic bacteria (Bogdanove and Voytas, 2011; Li et al., 2011).
SUMMARY AND OUTLOOK
Over the past decade, plant biology has matured and is homing in on many of the classic great questions: mechanisms of cell and tissue development, the interplay of hormones, mechanisms of cell wall construction and growth, the structure of signaling and metabolic networks, and mechanisms of environmental perception and adaptation. Highlights in advances include the insight that protein degradation is central to hormone signaling and perception, the identification of ion transporters as receptors, regulatory networks for hormones, nastic movements of guard cells, as well as breakthroughs in understanding embryo, guard cell, and root development. In many cases, the use of Arabidopsis as a model has helped to advance the science dramatically and has allowed us to lay the basis for understanding principle mechanisms conserved across species (McCourt and Benning, 2010).
Advances in technology are critical to future progress in the biological sciences. Some of the most highly cited articles are related to technology, for example, U.K. Laemmli’s protein gel electrophoresis system for proteins (Laemmli, 1970) and E.M. Southern’s famous article on detection of specific DNA fragments by electrophoresis and blotting (Southern, 1992). Distinguished scientists, such as George Church or Leroy Hood, built much of their careers on technology developments (Hood et al., 2004). It is obvious that technology opens up new horizons and speeds up discovery by orders of magnitude. However, new technologies alone cannot answer the questions we have; they can only contribute to new insights. It is also difficult to foresee what technologies can bring. We have seen many unexpected results, such as the finding of small RNAs as global regulators. Who would have predicted that we can make photosynthetic animals, as recently reported by Agapakis et al. (2011)?
It is even conceivable that new technologies may unravel what had been termed “new physics” as Schroedinger, Bohr, Delbrück, and Crick discussed in the context of the existence of complementarity in biology (McKaughan, 2005).
We certainly expect that new technologies will continue to revolutionize biological research. Plant science has not often been the driver of innovation but often enough has profited from developments made in other areas. This could be addressed and overcome by dedicated workshops and demonstrations, such as a recent meeting in the UK (Grierson et al., 2011), to accelerate technology development with an emphasis on plant-specific needs. One of the biggest challenges for the new plant sciences will be access to funding to support innovation and develop and use the new technologies. Today, access to NGS technology is limited, as is access to high-end proteomics and metabolomics facilities. Many of the instruments needed have a useful life span of only a few years and typically cost between $0.5 and $2 million. Funding for instrumentation is difficult to obtain through granting agencies; consequentially, progress is further slowed. If we estimate that funding for plant sciences is in the range of 1 to 2% of all of biology, it is not surprising that new technologies are seldom pioneered by and are only slowly adopted by plant scientists. This is in stark contrast with the importance of plant science for humankind. With this Perspective, we hope to encourage renewed interest in the fundamental importance and the promise of plant science research to address the major challenges faced by humankind in the 21st century.
Acknowledgments
We thank the National Science Foundation (Grants 1045185, 1052348, and 1021677) and the Department of Energy (Grant DE-FG02-04ER15542) for support to W.B.F. This is a modified version of a white paper generated for the Plant Science Research Summit in 2011 (http://my.aspb.org/resource/group/b1d27bb1-ad1a-49ae-9150-f9f931246ba3/greenpapers/newtechnologies_updated.pdf).
AUTHOR CONTRIBUTIONS
D.W.E. and W.B.F. contributed equally to the writing of this article.
References
- Abbondanzieri E.A., Greenleaf W.J., Shaevitz J.W., Landick R., Block S.M. (2005). Direct observation of base-pair stepping by RNA polymerase. Nature 438: 460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapakis C.M., Niederholtmeyer H., Noche R.R., Lieberman T.D., Megason S.G., Way J.C., Silver P.A. (2011). Towards a synthetic chloroplast. PLoS ONE 6: e18877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J.C., Kemp D.J., Stark G.R. (1977). Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. USA 74: 5350–5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M.X., Goksor M., Sandelius A.S. (2007). Membrane contact sites: physical attachment between chloroplasts and endoplasmic reticulum revealed by optical manipulation. Plant Signal. Behav. 2: 185–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre G., Kulakauskas S., Chapot-Chartier M.P., Navet B., Deghorain M., Bernard E., Hols P., Dufrene Y.F. (2010). Imaging the nanoscale organization of peptidoglycan in living Lactococcus lactis cells. Nat. Commun. 1: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley S. (2001). Nanobot construction crews. Sci. Am. 285: 84–85 [DOI] [PubMed] [Google Scholar]
- Asner G.P., Martin R.E. (2011). Canopy phylogenetic, chemical and spectral assembly in a lowland Amazonian forest. New Phytol. 189: 999–1012 [DOI] [PubMed] [Google Scholar]
- Austin R.S., Vidaurre D., Stamatiou G., Breit R., Provart N.J., Bonetta D., Zhang J., Fung P., Gong Y., Wang P.W., McCourt P., Guttman D.S. (2011). Next-generation mapping of Arabidopsis genes. Plant J. 67: 715–725 [DOI] [PubMed] [Google Scholar]
- Badieirostami M., Lew M.D., Thompson M.A., Moerner W.E. (2010). Three-dimensional localization precision of the double-helix point spread function versus astigmatism and biplane. Appl. Phys. Lett. 97: 161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barga R., Howe B., Beck D., Bowers S., Dobyns W., Haynes W., Higdon R., Howard C., Roth C., Stewart E., Welch D., Kolker E. (2011). Bioinformatics and data-intensive scientific discovery in the beginning of the 21st century. OMICS 15: 199–201 [DOI] [PubMed] [Google Scholar]
- Barretto R.P., Ko T.H., Jung J.C., Wang T.J., Capps G., Waters A.C., Ziv Y., Attardo A., Recht L., Schnitzer M.J. (2011). Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nat. Med. 17: 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S.R., Quake S.R. (2009). Highly parallel measurements of interaction kinetic constants with a microfabricated optomechanical device. Appl. Phys. Lett. 95: 73705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey P.N. (2011). Taking a developmental perspective on systems biology. Dev. Cell 21: 27–28 [DOI] [PubMed] [Google Scholar]
- Bermejo C., Haerizadeh F., Takanaga H., Chermak D., Frommer W.B. (2010). Dynamic analysis of cytosolic glucose and ATP levels in yeast using optical sensors. Biochem. J. 432: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo C., Haerizadeh F., Takanaga H., Chermak D., Frommer W.B. (2011a). Optical sensors for measuring dynamic changes in steady state levels of cytosolic glucose in yeast. Nat. Protoc. 6: 1806–1817 [DOI] [PubMed] [Google Scholar]
- Bermejo C., Ewald J., Lanquar V., Jones A., Frommer W.B. (2011b). In vivo biochemistry: Quantifying ion and metabolite levels in individual cells or cultures of yeast. Biochem. J. 438: 1–10 [DOI] [PubMed] [Google Scholar]
- Betzig E., Patterson G.H., Sougrat R., Lindwasser O.W., Olenych S., Bonifacino J.S., Davidson M.W., Lippincott-Schwartz J., Hess H.F. (2006). Imaging intracellular fluorescent proteins at nanometer resolution. Science 313: 1642–1645 [DOI] [PubMed] [Google Scholar]
- Bhat R.A., Miklis M., Schmelzer E., Schulze-Lefert P., Panstruga R. (2005). Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc. Natl. Acad. Sci. USA 102: 3135–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J.A., Yu W., Han F. (2008). Plant engineered minichromosomes and artificial chromosome platforms. Cytogenet. Genome Res. 120: 228–232 [DOI] [PubMed] [Google Scholar]
- Birnbaum K., Jung J.W., Wang J.Y., Lambert G.M., Hirst J.A., Galbraith D.W., Benfey P.N. (2005). Cell type-specific expression profiling in plants via cell sorting of protoplasts from fluorescent reporter lines. Nat. Methods 2: 615–619 [DOI] [PubMed] [Google Scholar]
- Birnbaum K., Shasha D.E., Wang J.Y., Jung J.W., Lambert G.M., Galbraith D.W., Benfey P.N. (2003). A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Bisseling T., Dangl J.L., Schulze-Lefert P. (2009). Next-generation communication. Science 324: 691. [DOI] [PubMed] [Google Scholar]
- Bogdanove A.J., Voytas D.F. (2011). TAL effectors: Customizable proteins for DNA targeting. Science 333: 1843–1846 [DOI] [PubMed] [Google Scholar]
- Brady S.M., Orlando D.A., Lee J.Y., Wang J.Y., Koch J., Dinneny J.R., Mace D., Ohler U., Benfey P.N. (2007). A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Bramley R.G.V. (2009). Lessons from nearly 20 years of Precision Agriculture research, development, and adoption as a guide to its appropriate application. Crop and Pasture Science 60: 197–217 [Google Scholar]
- Cannon M.C., Terneus K., Hall Q., Tan L., Wang Y., Wegenhart B.L., Chen L., Lamport D.T., Chen Y., Kieliszewski M.J. (2008). Self-assembly of the plant cell wall requires an extensin scaffold. Proc. Natl. Acad. Sci. USA 105: 2226–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., et al. (2011). Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 43: 956–963 [DOI] [PubMed] [Google Scholar]
- Cha S., Song Z., Nikolau B.J., Yeung E.S. (2009). Direct profiling and imaging of epicuticular waxes on Arabidopsis thaliana by laser desorption/ionization mass spectrometry using silver colloid as a matrix. Anal. Chem. 81: 2991–3000 [DOI] [PubMed] [Google Scholar]
- Chan S.W. (2010). Chromosome engineering: Power tools for plant genetics. TIBTECH 28: 605–610 [DOI] [PubMed] [Google Scholar]
- Committee on a New Biology for the 21st Century (2009). A New Biology for the 21st Century; National Research Council, Committee on a New Biology for the 21st Century: Ensuring the United States Leads the Coming Biology Revolution. (Washington, DC: The National Academies Press) [PubMed]
- Conn S.J., et al. (2011). Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23: 240–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.R., Ehrhardt D.W., Griffitts J.S., Somerville C.R. (2000). Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L.M., Harris P.J. (2003). Atomic force microscopy of microfibrils in primary cell walls. Planta 217: 283–289 [DOI] [PubMed] [Google Scholar]
- Deal R.B. (2011). Grand challenge: Accelerating discovery through technology development. Front. Plant Sci. 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal R.B., Henikoff S. (2011). The INTACT method for cell type-specific gene expression and chromatin profiling in Arabidopsis thaliana. Nat. Protoc. 6: 56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. (2011). Optogenetics. Nat. Methods 8: 26–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreze M., Carvunis A.R., Charloteaux B., Galli M., Pevzner S.J., Tasan M., Braun P., Vidal M. (2011). Evidence for network evolution in an Arabidopsis interactome map. Science 333: 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M.W., Cook R.T., Gregory B.D. (2011). A high-throughput sequencing-based methodology to identify all uncapped and cleaved RNA molecules in eukaryotic genomes. Methods Mol. Biol. 732: 209–223 [DOI] [PubMed] [Google Scholar]
- Erni R., Rossell M.D., Kisielowski C., Dahmen U. (2009). Atomic-resolution imaging with a sub-50-pm electron probe. Phys. Rev. Lett. 102: 096101. [DOI] [PubMed] [Google Scholar]
- Fehr M., Ehrhardt D.W., Lalonde S., Frommer W.B. (2004). Live imaging of glucose homeostasis in nuclei of COS-7 cells. J. Fluoresc. 14: 603–609 [DOI] [PubMed] [Google Scholar]
- Feijó J.A., Moreno N. (2004). Imaging plant cells by two-photon excitation. Protoplasma 223: 1–32 [DOI] [PubMed] [Google Scholar]
- Ferrier T., Matus J.T., Jin J., Riechmann J.L. (2011). Arabidopsis paves the way: Genomic and network analyses in crops. Curr. Opin. Biotechnol. 22: 260–270 [DOI] [PubMed] [Google Scholar]
- Fitzgibbon J., Bell K., King E., Oparka K. (2010). Super-resolution imaging of plasmodesmata using three-dimensional structured illumination microscopy. Plant Physiol. 153: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer W.B. (2010). Grand opportunities in physiology to address the grand challenges facing the planet. Front. Physiol. 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer W.B., Davidson M.W., Campbell R.E. (2009). Genetically encoded biosensors based on engineered fluorescent proteins. Chem. Soc. Rev. 38: 2833–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin A.C., Maeda K., Kuhner S. (2011). Recent advances in charting protein-protein interaction: Mass spectrometry-based approaches. Curr. Opin. Biotechnol. 22: 42–49 [DOI] [PubMed] [Google Scholar]
- Giavalisco P., Kohl K., Hummel J., Seiwert B., Willmitzer L. (2009). 13C isotope-labeled metabolomes allowing for improved compound annotation and relative quantification in liquid chromatography-mass spectrometry-based metabolomic research. Anal. Chem. 81: 6546–6551 [DOI] [PubMed] [Google Scholar]
- Gibson D.G., Smith H.O., Hutchison C.A., III, Venter J.C., Merryman C. (2010). Chemical synthesis of the mouse mitochondrial genome. Nat. Methods 7: 901–903 [DOI] [PubMed] [Google Scholar]
- Gibson D.G., et al. (2008). Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 319: 1215–1220 [DOI] [PubMed] [Google Scholar]
- Gilroy S. (2011). Plant cell biology: With grand challenges come great possibilities. Front. Plant Sci. 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C., Hoffman B.D., Brenner M.D., Zhou R., Parsons M., Yang M.T., McLean M.A., Sligar S.G., Chen C.S., Ha T., Schwartz M.A. (2010). Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466: 263–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson C.S., et al. (2011). One hundred important questions facing plant science research. New Phytol. 192: 6–12
- Grossmann G., Guo W.J., Ehrhardt D.W., Frommer W.B., Sit V.S., Quake S.R., Meier M. (2011). The RootChip: An integrated microfluidic chip for plant science. Plant Cell 23: 4234–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M.G. (2005). Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA 102: 13081–13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M.G., Shao L., Carlton P.M., Wang C.J., Golubovskaya I.N., Cande W.Z., Agard D.A., Sedat J.W. (2008). Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94: 4957–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R., Grossmann G., Frommer W.B., Ehrhardt D.W. (2010). Opportunities to explore plant membrane organization with super-resolution microscopy. Plant Physiol. 154: 463–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi N., Pastorino L., Soumetz F.C., Sbrana F., Raiteri R., Ruggiero C. (2011). Nanoengineered polymeric S-layers based capsules with targeting activity. Colloids Surf. B Biointerfaces 88: 366–372 [DOI] [PubMed]
- Halet G. (2005). Imaging phosphoinositide dynamics using GFP-tagged protein domains. Biol. Cell. 97: 501–518
- Hamamura Y., Saito C., Awai C., Kurihara D., Miyawaki A., Nakagawa T., Kanaoka M.M., Sasaki N., Nakano A., Berger F., Higashiyama T. (2011). Live-cell imaging reveals the dynamics of two sperm cells during double fertilization in Arabidopsis thaliana. Curr. Biol. 21: 497–502 [DOI] [PubMed] [Google Scholar]
- Hamilton S.R., Gerngross T.U. (2007). Glycosylation engineering in yeast: The advent of fully humanized yeast. Curr. Opin. Biotech. 18: 387–392 [DOI] [PubMed] [Google Scholar]
- Hardy S., Legagneux V., Audic Y., Paillard L. (2010). Reverse genetics in eukaryotes. Biol. Cell 102: 561–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J.L., Temple G.F., Brasch M.A. (2000). DNA cloning using in vitro site-specific recombination. Genome Res. 10: 1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayles M.F., Stokes D.J., Phifer D., Findlay K.C. (2007). A technique for improved focused ion beam milling of cryo-prepared life science specimens. J. Microsc. 226: 263–269 [DOI] [PubMed] [Google Scholar]
- Heazlewood J.L. (2011). Grand challenges in plant proteomics. Front. Plant Sci. 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell S.W., Wichmann J. (1994). Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19: 780–782 [DOI] [PubMed] [Google Scholar]
- Heraud P., Caine S., Sanson G., Gleadow R., Wood B.R., McNaughton D. (2007). Focal plane array infrared imaging: A new way to analyse leaf tissue. New Phytol. 173: 216–225 [DOI] [PubMed] [Google Scholar]
- Hess S.T., Gould T.J., Gunewardene M., Bewersdorf J., Mason M.D. (2009). Ultrahigh resolution imaging of biomolecules by fluorescence photoactivation localization microscopy. Methods Mol. Biol. 544: 483–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger J.W., de la Pena Mattozzi M., Myers W.R., Williams M.E., Reeves A., Parsons R.L., Haskell R.C., Petersen D.C., Wang R., Medford J.I. (2000). Optical coherence microscopy. A technology for rapid, in vivo, non-destructive visualization of plants and plant cells. Plant Physiol. 123: 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Heath J.R., Phelps M.E., Lin B. (2004). Systems biology and new technologies enable predictive and preventative medicine. Science 306: 640–643 [DOI] [PubMed] [Google Scholar]
- Huber S.C. (2011). Grand challenges in plant physiology: The underpinning of translational research. Front. Plant Sci. 2: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideker T., Dutkowski J., Hood L. (2011). Boosting signal-to-noise in complex biology: Prior knowledge is power. Cell 144: 860–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink R.G., Gadella T.W., Jr, Ferrario S., Busscher M., Angenent G.C. (2002). Analysis of MADS box protein-protein interactions in living plant cells. Proc. Natl. Acad. Sci. USA 99: 2416–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia N.T. (2010). Genome-wide translational profiling by ribosome footprinting. Methods Enzymol. 470: 119–142 [DOI] [PubMed] [Google Scholar]
- Ingolia N.T., Lareau L.F., Weissman J.S. (2011). Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 147: 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R.A. (2010). Of genes and genomes: Challenges for the twenty-first century. Front. Plant Sci. 1: 1
- Jun J.H., Song Z., Liu Z., Nikolau B.J., Yeung E.S., Lee Y.J. (2010). High-spatial and high-mass resolution imaging of surface metabolites of Arabidopsis thaliana by laser desorption-ionization mass spectrometry using colloidal silver. Anal. Chem. 82: 3255–3265 [DOI] [PubMed] [Google Scholar]
- Keller P.J., Schmidt A.D., Santella A., Khairy K., Bao Z., Wittbrodt J., Stelzer E.H. (2010). Fast, high-contrast imaging of animal development with scanned light sheet-based structured-illumination microscopy. Nat. Methods 7: 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P.J., Schmidt A.D., Wittbrodt J., Stelzer E.H. (2008). Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322: 1065–1069 [DOI] [PubMed] [Google Scholar]
- Kerppola T.K. (2008). Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys. 37: 465–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaar T., Faivre-Moskalenko C., Esseling J.J., de Ruijter N.C., Grierson C.S., Dogterom M., Emons A.M. (2002). Positioning of nuclei in Arabidopsis root hairs: An actin-regulated process of tip growth. Plant Cell 14: 2941–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zhang C.Z., Zhang X., Springer T.A. (2010). A mechanically stabilized receptor-ligand flex-bond important in the vasculature. Nature 466: 992–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A.R., Gunning A.P., Waldron K.W., Morris V.J., Ng A. (1996). Visualization of plant cell walls by atomic force microscopy. Biophys. J. 70: 1138–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kner P., Chhun B.B., Griffis E.R., Winoto L., Gustafsson M.G. (2009). Super-resolution video microscopy of live cells by structured illumination. Nat. Methods 6: 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka C.A., Bednarek S.Y. (2008). Variable-angle epifluorescence microscopy: A new way to look at protein dynamics in the plant cell cortex. Plant J. 53: 186–196 [DOI] [PubMed] [Google Scholar]
- Körner C. (2011). The grand challenges in functional plant ecology. Front. Plant Sci. 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H., Piscitelli C.L., Gouaux E. (2009). Unlocking the molecular secrets of sodium-coupled transporters. Nature 459: 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger S., Giavalisco P., Krall L., Steinhauser M.C., Büssis D., Usadel B., Flügge U.I., Fernie A.R., Willmitzer L., Steinhauser D. (2011). A topological map of the compartmentalized Arabidopsis thaliana leaf metabolome. PLoS ONE 6: e17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska M., Daszkowska-Golec A., Gruszka D., Marzec M., Szurman M., Szarejko I., Maluszynski M. (2011). TILLING: A shortcut in functional genomics. J. Appl. Genet. 52: 371–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lalonde S., et al. (2010). A membrane protein / signaling protein interaction network for Arabidopsis version AMPv2. Front. Physiol. 1: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausted C., Hu Z., Hood L. (2011). Label-free detection with surface plasmon resonance imaging. Methods Mol. Biol. 723: 321–333 [DOI] [PubMed] [Google Scholar]
- Ledford H. (2011). Halfway point for 1,001 genomes quest. Nature 477: 14. [DOI] [PubMed] [Google Scholar]
- Lee I., Ambaru B., Thakkar P., Marcotte E.M., Rhee S.Y. (2010). Rational association of genes with traits using a genome-scale gene network for Arabidopsis thaliana. Nat. Biotechnol. 28: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Avondo J., Morrison H., Blot L., Stark M., Sharpe J., Bangham A., Coen E. (2006). Visualizing plant development and gene expression in three dimensions using optical projection tomography. Plant Cell 18: 2145–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z., Huhman D.V., Sumner L.W. (2011). Mass spectrometry strategies in metabolomics. J. Biol. Chem. 286: 25435–25442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D.W., Otomo C., Chory J., Rosen M.K. (2008). Genetically encoded photoswitching of actin assembly through the Cdc42-WASP-Arp2/3 complex pathway. Proc. Natl. Acad. Sci. USA 105: 12797–12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H. (2008). Stressed genomics-bringing relief to rice fields. Curr. Opin. Plant Biol. 11: 201–208 [DOI] [PubMed] [Google Scholar]
- Levskaya A., Weiner O.D., Lim W.A., Voigt C.A. (2009). Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461: 997–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew M.D., Lee S.F., Badieirostami M., Moerner W.E. (2011). Corkscrew point spread function for far-field three-dimensional nanoscale localization of pointlike objects. Opt. Lett. 36: 202–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Huang S., Zhao X., Wright D.A., Carpenter S., Spalding M.H., Weeks D.P., Yang B. (2011). Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res. 39: 6315–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Chakraborty A., Singh D., Yadav R.K., Meenakshisundaram G., Reddy G.V., Roy-Chowdhury A. (2011). Adaptive cell segmentation and tracking for volumetric confocal microscopy images of a developing plant meristem. Mol. Plant 4: 922–931 [DOI] [PubMed] [Google Scholar]
- Mach J. (2008). Guard cell proteome reveals signals and surprises. Plant Cell 20: 3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel A., von Wangenheim D., Federici F., Haseloff J., Stelzer E.H. (2011). High-resolution live imaging of plant growth in near physiological bright conditions using light sheet fluorescence microscopy. Plant J. 68: 377–385 [DOI] [PubMed] [Google Scholar]
- Marcuzzo M., Quelhas P., Campilho A., Mendonca A.M. (2009). Automated Arabidopsis plant root cell segmentation based on SVM classification and region merging. Comput. Biol. Med. 39: 785–793 [DOI] [PubMed] [Google Scholar]
- Mardis E.R. (2006). Anticipating the 1,000 dollar genome. Genome Biol. 7: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marga F., Grandbois M., Cosgrove D.J., Baskin T.I. (2005). Cell wall extension results in the coordinate separation of parallel microfibrils: Evidence from scanning electron microscopy and atomic force microscopy. Plant J. 43: 181–190 [DOI] [PubMed] [Google Scholar]
- Marko M., Hsieh C., Schalek R., Frank J., Mannella C. (2007). Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat. Methods 4: 215–217 [DOI] [PubMed] [Google Scholar]
- Martin B.R., Giepmans B.N., Adams S.R., Tsien R.Y. (2005). Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 23: 1308–1314 [DOI] [PubMed] [Google Scholar]
- Maxam A.M., Gilbert W. (1977). A new method for sequencing DNA. Proc. Natl. Acad. Sci. USA 74: 560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann M.C., Bush M., Milioni D., Sado P., Stacey N.J., Catchpole G., Defernez M., Carpita N.C., Hofte H., Ulvskov P., Wilson R.H., Roberts K. (2001). Approaches to understanding the functional architecture of the plant cell wall. Phytochemistry 57: 811–821 [DOI] [PubMed] [Google Scholar]
- McCourt P., Benning C. (2010). Arabidopsis: A rich harvest 10 years after completion of the genome sequence. Plant J. 61: 905–908 [DOI] [PubMed] [Google Scholar]
- McKaughan D.J. (2005). The influence of Niels Bohr on Max Delbrück: revisiting the hopes inspired by “light and life”. Isis 96: 507–529 [DOI] [PubMed] [Google Scholar]
- McKinney S.A., Murphy C.S., Hazelwood K.L., Davidson M.W., Looger L.L. (2009). A bright and photostable photoconvertible fluorescent protein. Nat. Methods 6: 131–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLafferty F.W. (2011). A century of progress in molecular mass spectrometry. Annu. Rev. Anal. Chem. 4: 1–22 [DOI] [PubMed] [Google Scholar]
- Melcher K., et al. (2009). A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature 462: 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Sachs F. (2011). Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J. Cell Sci. 124: 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Perez A., Rodriguez J.R., Alonso-Nanclares L., Schertel A., Defelipe J. (2009). Counting synapses using FIB/SEM microscopy: A true revolution for ultrastructural volume reconstruction. Front. Neuroanat. 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker M.L. (2010). Sequencing technologies—The next generation. Nat. Rev. Genet. 11: 31–46 [DOI] [PubMed] [Google Scholar]
- Micheva K.D., Busse B., Weiler N.C., O’Rourke N., Smith S.J. (2010). Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron 68: 639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani P., Gholamirad M., Traas J., Arneodo A., Boudaoud A., Argoul F., Hamant O. (2011). In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J. 67: 1116–1123 [DOI] [PubMed] [Google Scholar]
- Miller L.M., Dumas P. (2010). From structure to cellular mechanism with infrared microspectroscopy. Curr. Opin. Struct. Biol. 20: 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltzahn F., Hunkapiller N., Mir A.A., Imbar T., Blelloch R. (2011). High throughput microRNA profiling: Optimized multiplex qRT-PCR at nanoliter scale on the fluidigm dynamic arrayTM IFCs. J. Vis. Exp. 54: 2552 [DOI] [PMC free article] [PubMed]
- Moore A. (2004). Waiter, there’s a nanobot in my martini! As nanotechnology gives birth to nanobiotechnology, definitions and perceptions are at risk of becoming mixed into an exotic cocktail. EMBO Rep. 5: 448–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D.J., Dufrene Y.F. (2011). Atomic force microscopy: A nanoscopic window on the cell surface. Trends Cell Biol. 21: 461–469 [DOI] [PubMed] [Google Scholar]
- Murphy A.S. (2011). Grand Challenge: Viewing transporter function in a pointillist landscape. Front. Plant Sci. 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A., Zanetti M.E., Jang C.J., Holtan H.E., Repetti P.P., Galbraith D.W., Girke T., Bailey-Serres J. (2009). Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Ehrhardt D.W., Hashimoto T. (2010). Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat. Cell Biol. 12: 1064–1070 [DOI] [PubMed] [Google Scholar]
- Nasse M.J., Mattson E.C., Reininger R., Kubala T., Janowski S., El-Bayyari Z., Hirschmugl C.J. (2010). Multi-beam synchrotron infrared chemical imaging with high spatial resolution: Beamline realization and first reports on image restoration. Nucl. Instrum. Methods Phys. Res. A 649: 172–176 [Google Scholar]
- Nishimura N., Hitomi K., Arvai A.S., Rambo R.P., Hitomi C., Cutler S.R., Schroeder J.I., Getzoff E.D. (2009). Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science 326: 1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour-Eldin H.H., Geu-Flores F., Halkier B.A. (2010). USER cloning and USER fusion: The ideal cloning techniques for small and big laboratories. Methods Mol. Biol. 643: 185–200 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K., Fukuda H. (2010). Transcriptional regulation of vascular cell fates. Curr. Opin. Plant Biol. 13: 670–676 [DOI] [PubMed] [Google Scholar]
- Okumoto S., Takanaga H., Frommer W.B. (2008). Quantitative imaging for discovery and assembly of the metabo-regulome. New Phytol. 180: 271–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreopoulos J., Yip C.M. (2009). Combinatorial microscopy for the study of protein-membrane interactions in supported lipid bilayers: Order parameter measurements by combined polarized TIRFM/AFM. J. Struct. Biol. 168: 21–36 [DOI] [PubMed] [Google Scholar]
- Ottenschlager I., Wolff P., Wolverton C., Bhalerao R.P., Sandberg G., Ishikawa H., Evans M., Palme K. (2003). Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsolak F., Milos P.M. (2011). RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 12: 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige J.S., Wu K.Y., Jaffrey S.R. (2011). RNA mimics of green fluorescent protein. Science 333: 642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez A.R., Somerville C.R., Ehrhardt D.W. (2006). Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Park S.Y., et al. (2009). Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A., Braybrook S.A., Le Guillou L., Bron E., Kuhlemeier C., Hofte H. (2011). Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Pelizzola M., Ecker J.R. (2011). The DNA methylome. FEBS Lett. 585: 1994–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland J.A., Bradbury P.J., Buckler E.S., Nelson R.J. (2011). Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc. Natl. Acad. Sci. USA 108: 6893–6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop M., Salzberg S.L. (2008). Bioinformatics challenges of new sequencing technology. Trends Genet. 24: 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punshon T., Guerinot M.L., Lanzirotti A. (2009). Using synchrotron X-ray fluorescence microprobes in the study of metal homeostasis in plants. Ann. Bot. (Lond.) 103: 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero F.J., Ohta M., Shi H., Zhu J.K., Pardo J.M. (2002). Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA 99: 9061–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab T.K., Lipson D.A. (2010). The Rhizosphere—A synchrotron-based view of nutrient flow in the root zone. Developments in Soil Science 34: 171–198 [Google Scholar]
- Rankin B.R., Moneron G., Wurm C.A., Nelson J.C., Walter A., Schwarzer D., Schroeder J., Colon-Ramos D.A., Hell S.W. (2011). Nanoscopy in a living multicellular organism expressing GFP. Biophys. J. 100: L63–L65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves A., Parsons R.L., Hettinger J.W., Medford J.I. (2002). In vivo three-dimensional imaging of plants with optical coherence microscopy. J. Microsc. 208: 177–189 [DOI] [PubMed] [Google Scholar]
- Reynaud E.G., Krzic U., Greger K., Stelzer E.H. (2008). Light sheet-based fluorescence microscopy: more dimensions, more photons, and less photodamage. HFSP J. 2: 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Escudero I., Roelants F.M., Thorner J., Nombela C., Molina M., Cid V.J. (2005). Reconstitution of the mammalian PI3K/PTEN/Akt pathway in yeast. Biochem. J. 390: 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo D., De Rybel B., Tendon V.D., Pfister A., Alassimone J., Vermeer J.E., Yamazaki M., Stierhof Y.D., Beeckman T., Geldner N. (2011). A novel protein family mediates Casparian strip formation in the endodermis. Nature 473: 380–383 [DOI] [PubMed] [Google Scholar]
- Russinova E., Borst J.W., Kwaaitaal M., Cano-Delgado A., Yin Y., Chory J., de Vries S.C. (2004). Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16: 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust M.J., Bates M., Zhuang X. (2006). Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3: 793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt D.E., Baxter I., Lahner B. (2008). Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol. 59: 709–733 [DOI] [PubMed] [Google Scholar]
- Sawa S., Kinoshita A., Nakanomyo I., Fukuda H. (2006). CLV3/ESR-related (CLE) peptides as intercellular signaling molecules in plants. Chem. Rec. 6: 303–310 [DOI] [PubMed] [Google Scholar]
- Schneeberger K., Ossowski S., Lanz C., Juul T., Petersen A.H., Nielsen K.L., Jørgensen J.E., Weigel D., Andersen S.U. (2009). SHOREmap: Simultaneous mapping and mutation identification by deep sequencing. Nat. Meth. 6: 550–551 [DOI] [PubMed] [Google Scholar]
- Sena G., Frentz Z., Birnbaum K.D., Leibler S. (2011). Quantitation of cellular dynamics in growing Arabidopsis roots with light sheet microscopy. PLoS ONE 6: e21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N.C., Campbell R.E., Steinbach P.A., Giepmans B.N., Palmer A.E., Tsien R.Y. (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22: 1567–1572 [DOI] [PubMed] [Google Scholar]
- Shaner N.C., Steinbach P.A., Tsien R.Y. (2005). A guide to choosing fluorescent proteins. Nat. Methods 2: 905–909 [DOI] [PubMed] [Google Scholar]
- Shao L., Kner P., Rego E.H., Gustafsson M.G. (2011). Super-resolution 3D microscopy of live whole cells using structured illumination. Nat. Methods 8: 1044–1046 [DOI] [PubMed] [Google Scholar]
- Sharpe J., Ahlgren U., Perry P., Hill B., Ross A., Hecksher-Sorensen J., Baldock R., Davidson D. (2002). Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296: 541–545 [DOI] [PubMed] [Google Scholar]
- Shen Y., Tolic N., Masselon C., Pasa-Tolic L., Camp D.G., II, Hixson K.K., Zhao R., Anderson G.A., Smith R.D. (2004). Ultrasensitive proteomics using high-efficiency on-line micro-SPE-nanoLC-nanoESI MS and MS/MS. Anal. Chem. 76: 144–154 [DOI] [PubMed] [Google Scholar]
- Shu X., Lev-Ram V., Deerinck T.J., Qi Y., Ramko E.B., Davidson M.W., Jin Y., Ellisman M.H., Tsien R.Y. (2011). A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 9: e1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen R.J., Kleerebezem M. (2011). The human gut microbiome: Are we our enterotypes? Microb. Biotechnol. 4: 550–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N.R. (2011). Plant developmental biology in the post-genomic era. Front. Plant Sci. 2: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E.M. (1992). Detection of specific sequences among DNA fragments separated by gel electrophoresis. 1975. Biotechnology 24: 122–139 [PubMed] [Google Scholar]
- Sparkes I.A., Ketelaar T., de Ruijter N.C., Hawes C. (2009). Grab a Golgi: Laser trapping of Golgi bodies reveals in vivo interactions with the endoplasmic reticulum. Traffic 10: 567–571 [DOI] [PubMed] [Google Scholar]
- Staiger C.J., Sheahan M.B., Khurana P., Wang X., McCurdy D.W., Blanchoin L. (2009). Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J. Cell Biol. 184: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocheva E.I., Li Z., Jensen G.J. (2010). Electron cryotomography. Cold Spring Harb. Perspect. Biol. 2: a003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weele C.M., Jiang H.S., Palaniappan K.K., Ivanov V.B., Palaniappan K., Baskin T.I. (2003). A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiol. 132: 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen S.F., Verdurmen W.P., Peters R.J., Nolte R.J., Brock R., van Hest J.C. (2010). Cellular integration of an enzyme-loaded polymersome nanoreactor. Angew. Chem. Int. Ed. Engl. 49: 7213–7216 [DOI] [PubMed] [Google Scholar]
- Visscher K., Schnitzer M.J., Block S.M. (1999). Single kinesin molecules studied with a molecular force clamp. Nature 400: 184–189 [DOI] [PubMed] [Google Scholar]
- Von Wirén N. (2011). Grand challenges in plant nutrition. Front. Plant Sci. 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters H. (2011). New $10 million X Prize launched for tricorder-style medical device. Nat. Med. 17: 754. [DOI] [PubMed] [Google Scholar]
- Wee C.W., Dinneny J.R. (2010). Tools for high-spatial and temporal-resolution analysis of environmental responses in plants. Biotechnol. Lett. 32: 1361–1371 [DOI] [PubMed] [Google Scholar]
- Weyand S., Shimamura T., Beckstein O., Sansom M.S., Iwata S., Henderson P.J., Cameron A.D. (2011). The alternating access mechanism of transport as observed in the sodium-hydantoin transporter Mhp1. J. Synchrotron Radiat. 18: 20–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Piatkevich K.D., Lionnet T., Singer R.H., Verkhusha V.V. (2011). Modern fluorescent proteins and imaging technologies to study gene expression, nuclear localization, and dynamics. Curr. Opin. Cell Biol. 23: 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.I., Frey D., Lungu O.I., Jaehrig A., Schlichting I., Kuhlman B., Hahn K.M. (2009). A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Xu X., Fukao T., Canlas P., Maghirang-Rodriguez R., Heuer S., Ismail A.M., Bailey-Serres J., Ronald P.C., Mackill D.J. (2006). Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Ytterberg A.J., Jensen O.N. (2010). Modification-specific proteomics in plant biology. J. Proteomics 73: 2249–2266 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Li Y.F., Sunkar R., Zhang W. (December 2, 2011). SeqTar: An effective method for identifying microRNA guided cleavage sites from degradome of polyadenylated transcripts in plants. Nucleic Acids Res. http://dx.doi.org/10.1093/nar/gkr1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Kozlov A.G., Roy R., Zhang J., Korolev S., Lohman T.M., Ha T. (2011). SSB functions as a sliding platform that migrates on DNA via reptation. Cell 146: 222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki M.A., Dumais J. (2011). Quantifying green life: Grand challenges in plant biophysics and modeling. Front. Plant Sci. 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]