Summary
Snail1 and ZEB1 are transcriptional repressors that drive tumor initiation and metastasis in animal models. Snail1 and ZEB1 are frequently coexpressed in tumor cell lines, suggesting that these factors may cooperate to promote tumor progression. However, coexpression of these transcriptional repressors in primary human cancer specimens has not been investigated. Previous studies assessed expression in primary breast cancers of Snail1 messenger RNA, which does not reflect Snail1 activity because Snail1 is subject to posttranslational modifications that inhibit its nuclear localization/activity. In the current study, using breast tumor cell lines of known Snail1 and ZEB1 expression status, we developed immunohistochemistry protocols for detecting nuclear Snail1 and nuclear ZEB1 proteins. Using these protocols, we assessed nuclear Snail1 and nuclear ZEB1 expressions in primary human breast cancers of varying subtypes (n = 78). Nuclear Snail1 and estrogen receptor α expression were inversely associated in primary breast cancers, and nuclear Snail1 was expressed in approximately 80% of triple-negative breast cancers (lacking estrogen receptor α, progesterone receptor, and human epidermal growth factor receptor 2 overexpression). In contrast, nuclear ZEB1 was expressed at a significantly lower frequency in these breast cancers. Notably, nuclear Snail1 protein was detected in 45% of ductal carcinoma in situ specimens (n = 29), raising the important possibility that nuclear Snail1 expression in early stage breast lesions may predict future development of invasive breast cancer. Collectively, our studies demonstrate frequent expression of nuclear Snail1, but not nuclear ZEB1, in invasive, triple-negative breast cancers as well as in intraductal carcinomas.
Keywords: Snail1, ZEB1, Breast cancer, Estrogen receptor, Ductal carcinoma in situ
1. Introduction
Snail1 and ZEB1 are E-box-binding transcriptional repressors that suppress transcription of epithelial genes (eg, E-cadherin, occludin) while inducing transcription of mesenchymal genes (eg, fibronectin). These transcriptional repressors promote the epithelial-mesenchymal transition, a cell program driving self-renewing activity [1,2], invasive behavior [3–6], tumor recurrence [7], and tumor metastasis in animal models [8,9]. Although the expression/activity of these transcriptional repressors in isolation has been assessed in tumor cells, it remains unclear if these factors cooperate to drive tumor cell invasive behavior.
The functions of Snail1 and ZEB1 seem redundant because they regulate transcription of a common set of genes [10]. Of note, Snail1 is required for ZEB1 expression during mesoderm formation in Drosophila embryonic development [11], and, in overexpression models, Snail1 drives ZEB1 transcription in tumor cell lines [12]. These findings suggest that Snail1 may be a central determinant of ZEB1 expression in tumor cells. However, Snail1 and ZEB1 coexpression in primary cancers has not been previously investigated.
Previous studies investigated the expression of Snail1 messenger RNA (mRNA) in primary breast cancers [7,13]. However, Snail mRNA expression does not predict nuclear Snail protein expression in these breast cancers because Snail1 is regulated by posttranslational modifications that influence its protein stability and nuclear localization [14,15]. Cell lines have been characterized that express both Snail1 mRNA and cytoplasmic Snail1 protein but lack nuclear Snail1 [14]. Importantly, these cell lines lack detectable Snail1 activity (eg, down-regulation of Snail1 target genes) [14]. These findings suggest that nuclear Snail1 protein is a better predictor of Snail1 transcriptional activity than Snail1 mRNA or Snail1 protein.
Snail1 protein expression has been reported previously in primary breast cancers. However, these studies did not attempt to distinguish between nuclear and cytoplasmic Snail1 [15–17]. Nuclear Snail1 protein expression has also been reported in invasive ductal breast cancers [18–20]. Considering that breast cancer is a heterogeneous disease, there exists a need to study nuclear Snail1 protein expression across the spectrum of human breast carcinomas. The current study assesses nuclear Snail1 and nuclear ZEB1 protein expression in human breast cancers of different subtypes. Our results indicate frequent expression of nuclear Snail1, but not nuclear ZEB1, in invasive estrogen receptor α (ERα) (−) breast cancers and in human ductal carcinoma in situ (DCIS) specimens. These findings raise the important possibility that Snail1 may be a prognostic marker in early stage breast lesions for future development of invasive breast cancer.
2. Methods
2.1. Breast tumor cell lines
MDA-MB-231 triple-negative breast tumor cells were obtained from the American Type Culture Collection (ATCC). The DKAT triple-negative breast cancer cell line was kindly provided by Victoria Seewaldt, MD. This cell line was derived from the malignant pleural effusion of a 35-year-old white woman who initially presented with a 4-cm ERα/progesterone receptor (PR) (−/−), human epidermal growth factor receptor 2(HER2)/Neu wild type, creatine kinase 5 (+), epidermal growth factor receptor (+) lymph node-negative breast cancer (T2N0M0). Ten months from initial diagnosis, metastasis to the lung, pleura, liver, and bone were observed. Twelve months from initial diagnosis, the woman died of rapid progression of disease. To generate the DKAT cell line, pleural fluid from this woman was centrifuged, cells were pelleted, and the pellet was resuspended in mammary epithelial cell growth medium supplemented with bovine pituitary extract, insulin, human recombinant epidermal growth factor, and hydrocortisone mammary epithelial growth medium (MEGM) (Lonza, Basel, Switzerland).
2.2. Primary breast cancer specimens
Snail1 and ZEB1 expression were assessed in human breast cancers (n = 59) from a breast cancer tissue microarray [21] as well as in triple-negative invasive ductal carcinoma (IDC) specimens (formalin-fixed, paraffin-embedded; n = 19) retrieved from the surgical pathology archives of Duke University Medical Center.
2.3. Snail1 immunohistochemistry
Formalin-fixed, paraffin-embedded sections were deparaffinized, blocked, boiled in pH 9.9 antigen retrieval buffer (Dako, Carpinteria, CA) at 100°C for 20 minutes, and incubated overnight at 4°C with a Snail1-specific monoclonal antibody (1:200) [22]. Snail1 was detected by indirect IHC using avidin-biotin peroxidase and the Catalyzed Signal Amplification System (Dako). Diaminobenzidine was used as a chromogen. Sections were counterstained with hematoxylin. Paraffin-embedded cell blocks of the DKAT and MDA-MB-231 cell lines served as external positive controls. Paraffin-embedded MCF-7 cells served as a negative external control. Negative control reactions used nonspecific mouse IgG in lieu of the Snail1 antibody.
2.4. Zeb1 IHC
Sections from formalin-fixed, paraffin embedded tissue culture cell blocks were heated in Tris (10 mmol/L)/EDTA (1 mmol/L EDTA) pH 9.0 for 20 minutes at 100°C and blocked with A/B blocking kit (Vector Labs, Burlingame, CA) followed by normal horse serum. Slides were incubated with a goat anti-ZEB1 antibody (E-20; Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1 μg/mL) overnight at 4°C followed by biotin-conjugated anti-goat IgG and horseradish peroxidase-ABC Elite complex (Vector Labs). Diaminobenzidine chromogen was used as a chromogen, and slides were counterstained with hematoxylin. MDA-MB-231 and MCF-7 cell blocks served as external positive and negative controls, respectively. Normal goat serum was used as a negative control reagent in lieu of the ZEB1 primary antibody.
2.5. Interpretation of Snail1 and ZEB1 immunostains
Intraductal and invasive carcinomas showing any degree of nuclear staining were scored as positive. For ZEB1, staining was often weak and/or focal. Stromal elements, especially endothelial cells, showed moderate to strong nuclear staining for Snail1 or ZEB1 in most cells and served as internal positive controls. For statistical analysis, cytoplasmic staining, which was observed in some neoplastic lesions, was disregarded.
2.6. Statistical methods
All pairwise associations with either nuclear Snail1 or ZEB1 were tested using Fisher exact method. Pearson χ2 test of proportions was used to test the proportion expressing ZEB1 protein in invasive lobular compared to invasive ductal breast cancers. All tests were 2 tailed, and P < .05 was considered statistically significant.
3. Results
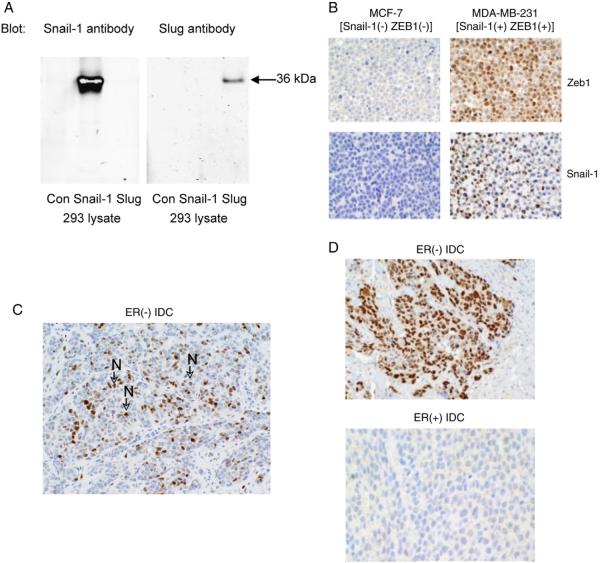
We used a previously characterized Snail1 antibody [22] to investigate the expression of nuclear Snail1 protein, representing the active form of this transcriptional repressor, in primary human breast cancers. This antibody is highly specific for Snail1 [22,23], and it has been used extensively to characterize Snail1 expression in tumors and embryonic samples by immunohistochemistry (IHC) [23–26]. Confirming previous findings [22], we demonstrate in Fig. 1A that this antibody reacts with Snail1 but not with the Snail1 relative, Slug. Using appropriate positive and negative control breast tumor cell lines, we developed an immunohistochemical staining protocol using this Snail1 antibody (Fig. 1B). Our studies using this protocol detected nuclear Snail1 protein in MDA-MB-231 (Fig. 1B) and DKAT (data not shown) breast tumor cell lines but not in nuclear Snail1-deficient MCF-7 breast tumor cells (Fig. 1B).
Fig. 1.
A, Protein lysates from 293 cells [Control (Con)], human Snail1-transfected 293 cells (Snail1), or human Slug-transfected 293 cells (Slug) were immunoblotted with a Snail1 [21] or Slug (Santa Cruz) antibody, + IRdye secondary antibody. Proteins were visualized using Odyssey Infrared Imaging. B, Formalin-fixed, paraffin-embedded MCF-7 and MDA-MB-231 cell pellets were stained with a ZEB1-specific or Snail1-specific antibody. C, Representative nuclear Snail1 staining of a formalin-fixed, paraffin-embedded ER (−) human ductal breast carcinoma. Nuclear-localized Snail1 is indicated by arrows labeled with an “N.” D, Representative Snail1 staining of an ER(−) and an ER (+) human ductal breast carcinoma. Note the expression of nuclear Snail1 in the ER (−) but not in the ER (+) specimen. All images were photographed at ×200 magnification.
Using our optimized Snail1 IHC protocol, we next investigated nuclear Snail1 protein expression in a breast cancer tissue microarray. Nuclear Snail1 protein was detected in tumor cells of a subset of invasive lobular and invasive ductal breast cancers (2/8 lobular and 12/51 ductal carcinoma specimens). In the invasive ductal breast cancers, we detected nuclear Snail1 frequently in ERα (−) but not in ERα (+) cancers (Fig. 1C and D). In fact, we observed a significant inverse association between ERα and nuclear Snail1 expression (nuclear Snail1 detected in 69% of ERα [−] ductal breast cancers versus in 8% of ERα [+] breast cancers; P < .0001) (Table 1A). Nuclear Snail1 expression was significantly associated with high-grade specimens (80% of grade 3 specimens versus 10% of grade 1 + 2 specimens; P < .0001) (Table 1B). Notably, nuclear Snail1 protein was detected in 75% (9/12 specimens) of triple-negative breast cancers, lacking ERα, progesterone receptor, and human epidermal growth factor receptor 2 overexpression.
Table 1A.
Inverse association between ERα and nuclear Snail1 expression in invasive ductal breast cancers (tissue microarray)
| No. of nuclear Snail1 (−) specimens | No. of nuclear Snail1 (+) specimens | |
|---|---|---|
| ERα (−) | 4 | 9 |
| ERα (+) | 35 | 3 |
P < .0001.
Table 1B.
Association between nuclear Snail1 and tumor grade in invasive ductal breast cancers (tissue microarray)
| No. of nuclear Snail1 (−) specimens | No. of nuclear Snail1 (+) specimens | |
|---|---|---|
| Low grade (grades 1 and 2) | 37 | 4 |
| High grade (grade 3) | 2 | 8 |
P < .0001.
Using a previously characterized ZEB1 antibody [9,27], we next developed an IHC protocol for detecting nuclear ZEB1 protein expression in breast tumor cell lines and in primary breast cancer specimens. As controls, we showed that this IHC protocol detects nuclear ZEB1 in MDA-MB-231 cells (ZEB1 [+]), but not in MCF-7 cells (ZEB1 [−]) (Fig. 1B). Nuclear ZEB1 protein was observed more frequently in invasive lobular breast cancers (38%, or 3/8 specimens) than in invasive ductal breast cancers (8%, or 4/51 specimens), P = .07. In contrast with our Snail1 data, no association was observed between nuclear ZEB1 expression and ERα status (Table 2A) or tumor grade (Table 2B). Furthermore, nuclear ZEB1 protein expression in tumor cells of primary invasive ductal breast cancers lacked significant association with nuclear Snail1 expression in these specimens (Table 2C).
Table 2A.
Lack of an association between nuclear ZEB1 and ERα expression in invasive ductal breast cancers (tissue microarray)
| # Nuclear ZEB1(−) Specimens | # Nuclear ZEB1(+) Specimens | |
|---|---|---|
| ERα(−) | 11 | 2 |
| ERα(+) | 36 | 2 |
p=0.27
Table 2B.
Lack of an association between nuclear ZEB1 and tumor grade in invasive ductal breast cancers (tissue microarray)
| # Nuclear ZEB1(−) Specimens | # Nuclear ZEB1(+) Specimens | |
|---|---|---|
| Low grade (grades 1 & 2) | 39 | 2 |
| High grade (grade 3) | 8 | 2 |
p=0.17
Table 2C.
Lack of an association between nuclear Snail-1 and nuclear ZEB1 expression in invasive ductal breast cancers (tissue microarray)
| # Nuclear Snail-1(−) Specimens | # Nuclear Snail-1(+) Specimens | |
|---|---|---|
| # Nuclear ZEB1(−) Specimens | 36 | 3 |
| # Nuclear ZEB1(+) Specimens | 11 | 1 |
p=1.00
Similar to the results obtained using the tissue microarray, we detected nuclear Snail1 protein in tumor cells of 84% of archival triple-negative breast cancers (16/19 specimens), whereas nuclear ZEB1 was expressed in tumor cells of 16% of triple-negative cancers (3/19 specimens) (Table 3). Due to the low frequency of ZEB1 expression in triple-negative breast carcinomas (2/3 ZEB1 [+] cases also expressed Snail1, whereas the third case was not evaluable for Snail1 expression), we were not able to assess an association between nuclear ZEB1 and Snail1 expression in this breast cancer subtype. Notably, both nuclear Snail1 and nuclear ZEB1 were detected in stromal cells associated with all triple-negative breast cancers (Table 3).
Table 3.
Nuclear Snail1 and nuclear ZEB1 expression in archival triple-negative cancers
| Parameter | Total no. of specimens | No. of positive specimens | % of positive specimens |
|---|---|---|---|
| Nuclear Snail1 (+) tumor cells | 19 | 16 | 84 |
| Nuclear Snail1 (+) stromal cells | 19 | 19 | 100 |
| Nuclear ZEB1(+) tumor cells | 19 | 3 | 16 |
| Nuclear ZEB1(+) stromal cells | 19 | 19 | 100 |
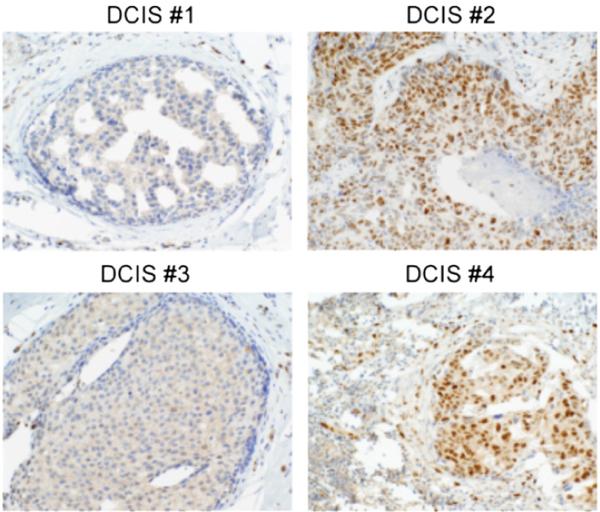
Considering that Snail1 and ZEB1 transcriptional repressors have been implicated in invasive tumor behavior [10], we sought to determine if these factors are expressed in preinvasive breast lesions. Nuclear Snail1 was detected in 45% of DCIS specimens (13/29 lesions) (Fig. 2), whereas nuclear ZEB1 was not detected in any DCIS specimens (data not shown). Nuclear Snail1 was inversely associated with ERα expression in these DCIS specimens (Table 4A), and this association was statistically significant (P = .014). Although nuclear Snail1 was detected more frequently in high-grade DCIS than in low-grade DCIS, the association between nuclear Snail1 expression and DCIS grade did not quite reach statistical significance (P = .054; Table 4B).
Fig. 2.
Nuclear Snail1 protein expression was assessed in 29 archival human DCIS specimens (see Table 4) using our optimized Snail1 IHC protocol. Nuclear Snail1 protein was detected in 13 of 29 specimens. Two representative nuclear Snail1 (−) specimens (no. 1, no. 3) and 2 nuclear Snail1 (+) specimens (no. 2, no. 4) are shown. Note nuclear staining in stromal cells in all 4 cases. Images photographed at ×400 magnification.
Table 4A.
Inverse Association between nuclear Snail-1 and ERα protein expression in intraductal carcinomas (DCIS)
| Variable | # Nuclear Snail-1(−) Lesions | # Nuclear Snail-1(+) Lesions |
|---|---|---|
| ERα(−) | 2 | 7 |
| ERα(+) | 15 | 5 |
p=0.014
Table 4B.
Association between nuclear Snail-1 expression and DCIS grade
| Variable | # Nuclear Snail-1(−) Specimens | # Nuclear Snail-1(+) Specimens |
|---|---|---|
| Low grade | 10 | 2 |
| High grade | 7 | 10 |
p=0.054
4. Discussion
Previous studies of Snail1 expression in breast cancer investigated Snail1 mRNA [7,13], which does not predict Snail1 activity because Snail1 stability/nuclear localization is regulated by Snail1 posttranslational modifications [14,15]. In the current work, we investigated expression of nuclear Snail1 protein in primary breast cancers and premalignant breast lesions using a highly specific Snail1 antibody. We detected nuclear Snail1 protein frequently in ERα (−) but not in ERα (+) invasive breast cancers. This finding is in agreement with previous reports [13,20,28,29] and likely reflects the ability of: (1) ERα to suppress Snail1 expression [29] and (2) Snail1 to suppress ERα transcription [28].
Similar to results reported for Snail1 mRNA [13], we observed an association between nuclear Snail1 protein expression and breast tumor grade. However, our work produced several results that differ from previous data obtained studying Snail1 mRNA. Although Snail1 mRNA was observed in 47% of human IDC specimens [13], we detected nuclear Snail1 protein in only 24% of IDC (Table 1A). Similar to other investigators [20], we detected nuclear Snail1 protein in a subset of lobular cancers (2/8 specimens), which have previously been reported to lack detectable Snail1 mRNA [13]. This result suggests that Snail1 mRNA detection by in situ hybridization is less sensitive than nuclear Snail1 protein detection by IHC. Collectively, our findings suggest that Snail1 mRNA expression in primary tumors does not predict expression of nuclear Snail1 protein.
Based on the previous demonstration that Snail1 drives ZEB1 transcription [12] and that Snail1 and ZEB1 are frequently coexpressed in tumor cell lines [12], we investigated the expression of these transcriptional repressors in primary breast cancers. We detected nuclear ZEB1 expression in only 8% of invasive ductal breast carcinomas (Table 2). In contrast, previous studies reported ZEB1 expression in 38% of dedifferentiated invasive ductal breast carcinomas [30] and in 70% of ERα (−) PR (−) breast cancer tissues [31]. Our detection of nuclear ZEB1 in a smaller percentage of IDC than was reported in these studies may relate to the fact that (1) Aigner et al [30] scored carcinoma specimens for total cellular ZEB1, not nuclear ZEB1, and (2) Graham et al [31] did not distinguish between stromal and tumor cell ZEB1 staining. Our detection of nuclear ZEB1 most frequently in invasive lobular breast cancer corroborates previous findings [30]. Contrary to studies in tumor cell lines [12], we did not detect an association between nuclear ZEB1 and nuclear Snail1 expression in tumor cells of primary breast cancers (Table 2C). This result suggests that nuclear Snail1 is not a central determinant of ZEB1 expression in tumor cells of primary breast cancers.
We detected nuclear Snail1 and nuclear ZEB1 proteins in breast cancer–associated stromal cells (Table 3), raising the possibility that Snail1 drives ZEB1 expression in stromal cells associated with primary breast cancers. Expression of E-cadherin transcriptional repressors has previously been reported in tumor-associated stromal cells [16,22,24,30,31], and stromal Snail1 expression in colon cancer predicts a poor patient prognosis [24]. These findings underscore the need for further work investigating how stromal Snail1 and ZEB1 expression influence breast cancer pathogenesis.
The frequency of Snail1 and ZEB1 expression in preinvasive mammary neoplasia has not been previously investigated. Our studies are important in demonstrating nuclear Snail1 protein, but not nuclear ZEB1 protein, in 45% of DCIS specimens (Table 4). Similar to the results obtained in invasive ductal breast cancers (Table 1A), nuclear Snail1 expression was inversely associated with ERα expression (Table 4A). Nuclear Snail1 expression was also associated with high tumor grade in DCIS lesions, although this association did not quite reach statistical significance (Table 4B). This finding corroborates previous results demonstrating Snail1 expression in a limited number of comedo-type DCIS [13,20]. However, in the current study, nuclear Snail1 was also expressed in a fraction (16%) of low-grade DCIS specimens (Table 4B). Our demonstration that nuclear Snail1 is expressed in 45% of DCIS specimens establishes an important foundation for future studies examining if nuclear Snail1 expression in premalignant breast lesions predicts future development of invasive breast cancer. Furthermore, our detection of nuclear Snail1 in a subset of preinvasive breast lesions suggests that Snail1 as well as upstream regulators of Snail1 expression/activity may be logical therapeutic targets for suppressing development of invasive breast cancer.
Acknowledgments
This work was supported by Friends for an Earlier Breast Cancer Test (to R.E. Bachelder) and National Institutes of Health grant CA141223 (to R.E. Bachelder).
Footnotes
This work was supported by Friends for an Earlier Breast Cancer Test (to: R.E. Bachelder).
References
- [1].Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wellner U, Schubert J, Burk UC, et al. The EMT activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting micro-RNAs. Nat Cell Biol. 2009;11:1487–95. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- [3].Yokoyama K, Kamata N, Fujimoto R, et al. Increased invasion and matrix metalloproteinase-2 expression by Snail induced mesenchymal transition in squamous cell carcinomas. Int J Oncol. 2003;22:891–8. [PubMed] [Google Scholar]
- [4].Olmeda D, Jorda M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene. 2007;26:1862–74. doi: 10.1038/sj.onc.1209997. [DOI] [PubMed] [Google Scholar]
- [5].De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F, Berx G. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 2005;65:6237–44. doi: 10.1158/0008-5472.CAN-04-3545. [DOI] [PubMed] [Google Scholar]
- [6].Whiteman EL, Liu CJ, Fearon ER, Margolis B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene. 2008;27:3875–9. doi: 10.1038/onc.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moody SE, Perez D, Pan TC, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- [8].Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDAMB-231 cells. Cancer Res. 2007;67:11721–31. doi: 10.1158/0008-5472.CAN-07-2318. [DOI] [PubMed] [Google Scholar]
- [9].Spaderna S, Schmalhofer O, Wahlbuhl M, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–44. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- [10].Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- [11].Lai ZC, Rushton E, Bate M, Rubin GM. Loss of function of the Drosophila zfh-1 gene results in abnormal development of mesodermally derived tissues. Proc Natl Acad Sci U S A. 1993;90:4122–6. doi: 10.1073/pnas.90.9.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guaita S, Puig I, Franci C, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem. 2002;277:39209–16. doi: 10.1074/jbc.M206400200. [DOI] [PubMed] [Google Scholar]
- [13].Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- [14].Dominguez D, Montserrat-Sentis B, Virgos-Soler A, et al. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol Cell Biol. 2003;23:5078–89. doi: 10.1128/MCB.23.14.5078-5089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- [16].Come C, Magnino F, Bibeau F, et al. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- [17].Logullo AF, Nonogaki S, Pasini FS, Osorio CA, Soares FA, Brentani MM. Concomitant expression of epithelial-mesenchymal transition biomarkers in breast ductal carcinoma: association with progression. Oncol Rep. 2010;23:313–20. [PubMed] [Google Scholar]
- [18].Becker KF, Rosivatz E, Blechschmidt K, Kremmer E, Sarbia M, Hofler H. Analysis of the E-cadherin repressor Snail in primary human cancers. Cells Tissues Organs. 2007;185:204–12. doi: 10.1159/000101321. [DOI] [PubMed] [Google Scholar]
- [19].Vincent T, Neve EP, Johnson JR, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–50. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lundgren K, Nordenskjold B, Landberg G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer. 2009;101:1769–81. doi: 10.1038/sj.bjc.6605369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heller G, Geradts J, Ziegler B, et al. Downregulation of TSLC1 and DAL-1 expression occurs frequently in breast cancer. Breast Cancer Res Treat. 2007;103:283–91. doi: 10.1007/s10549-006-9377-7. [DOI] [PubMed] [Google Scholar]
- [22].Franci C, Takkunen M, Dave N, et al. Expression of Snail protein in tumor-stroma interface. Oncogene. 2006;25:5134–44. doi: 10.1038/sj.onc.1209519. [DOI] [PubMed] [Google Scholar]
- [23].Escriva M, Peiro S, Herranz N, et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol. 2008;28:1528–40. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Franci C, Gallen M, Alameda F, et al. Snail1 protein in the stroma as a new putative prognosis marker for colon tumours. PLoS One. 2009;4:e5595. doi: 10.1371/journal.pone.0005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Herranz N, Pasini D, Diaz VM, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–81. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peinado H, Moreno-Bueno G, Hardisson D, et al. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–50. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- [27].Bracken CP, Gregory PA, Kolesnikoff N, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–54. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- [28].Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha. Mol Endocrinol. 2007;21:2907–18. doi: 10.1210/me.2007-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–19. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- [30].Aigner K, Dampier B, Descovich L, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–88. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Graham TR, Yacoub R, Taliaferro-Smith L, et al. Reciprocal regulation of ZEB1 and AR in triple negative breast cancer cells. Breast Cancer Res Treat. 123:139–147. doi: 10.1007/s10549-009-0623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]