Abstract
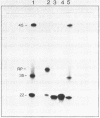
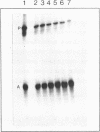
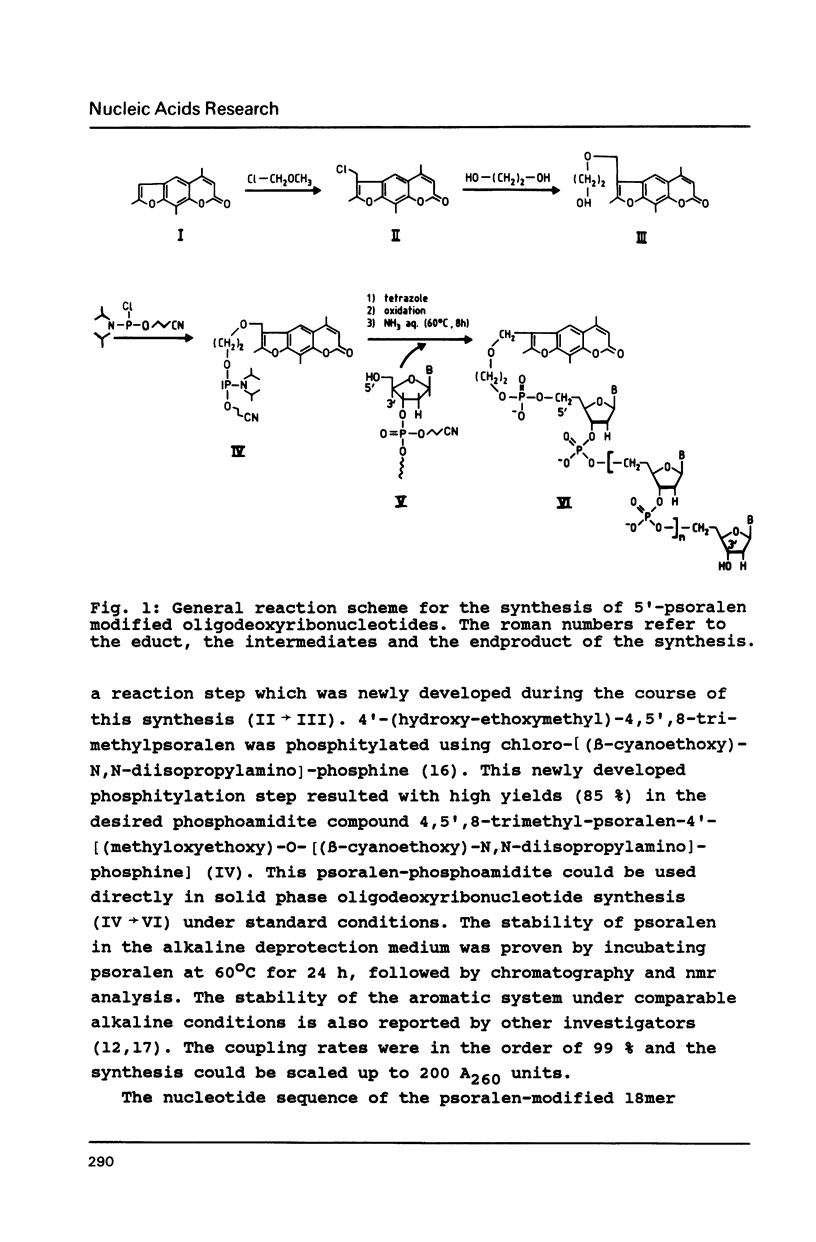
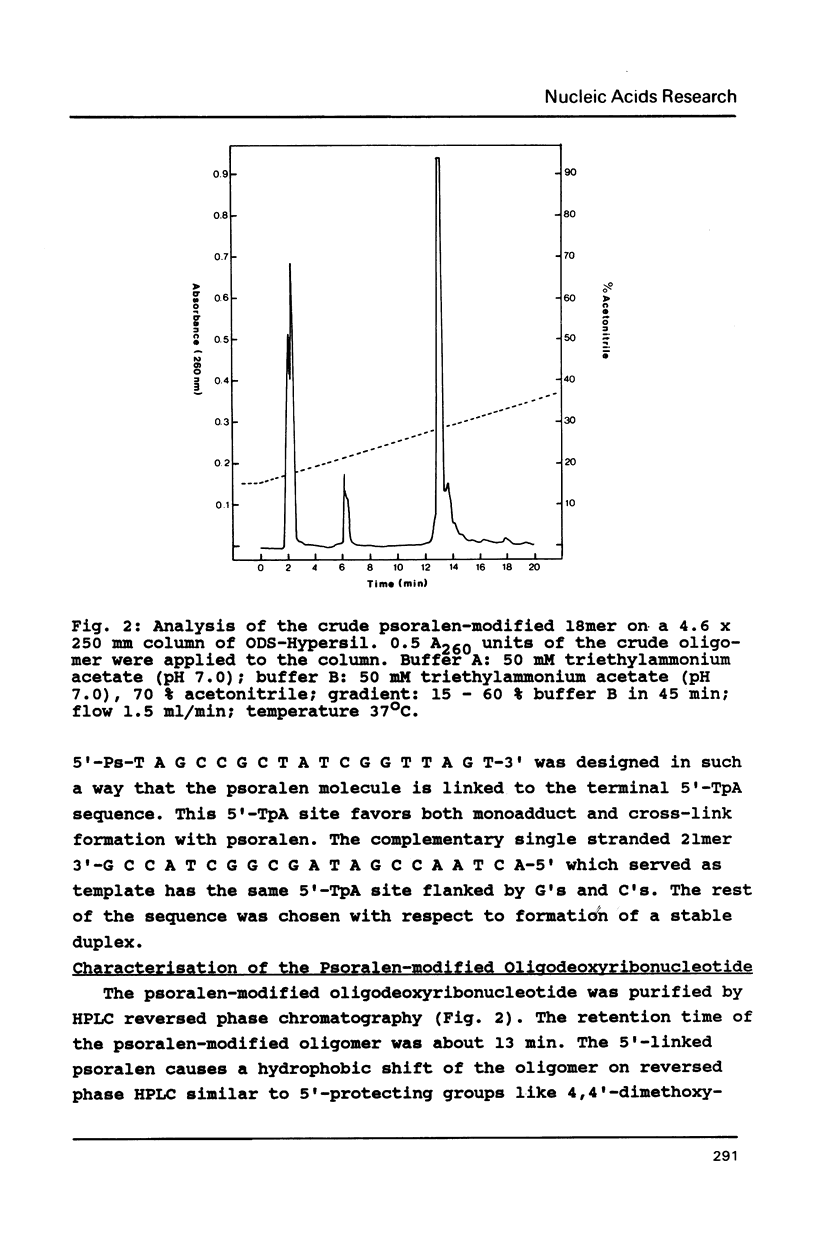
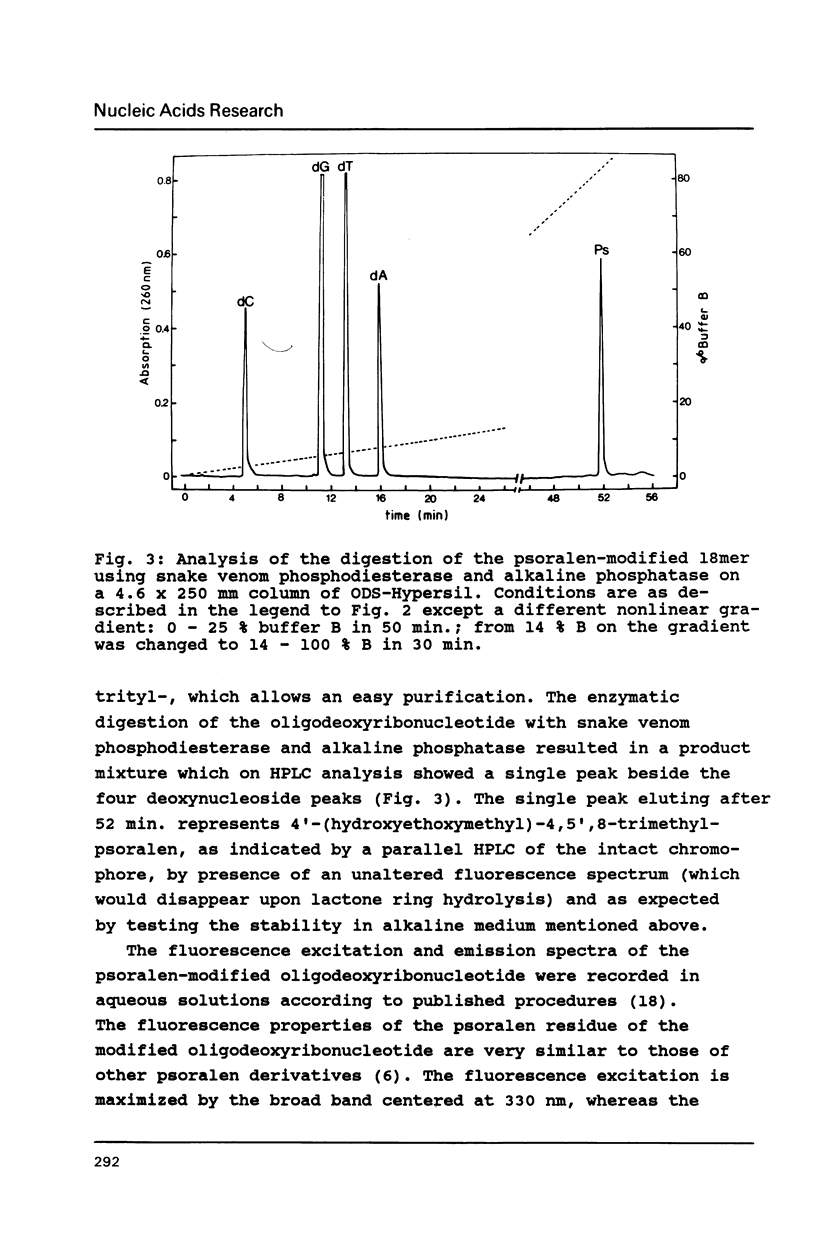
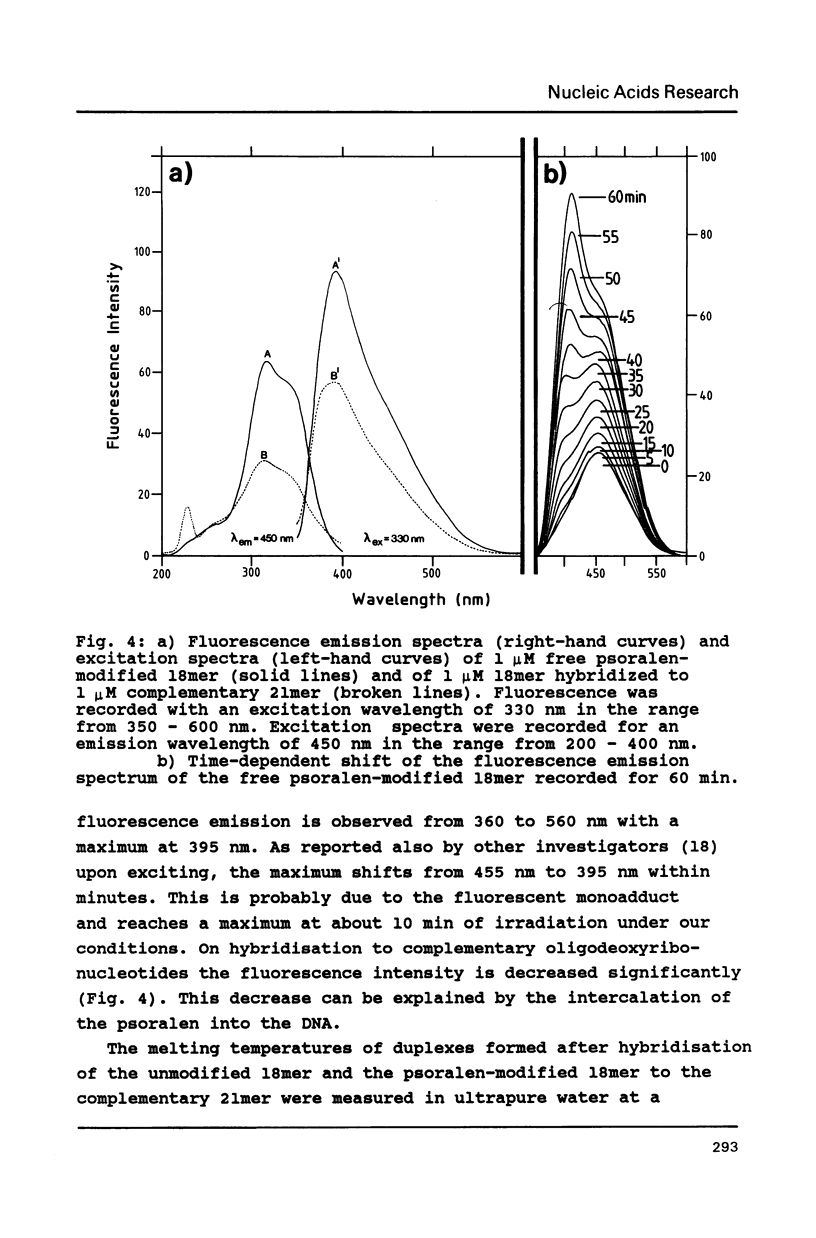
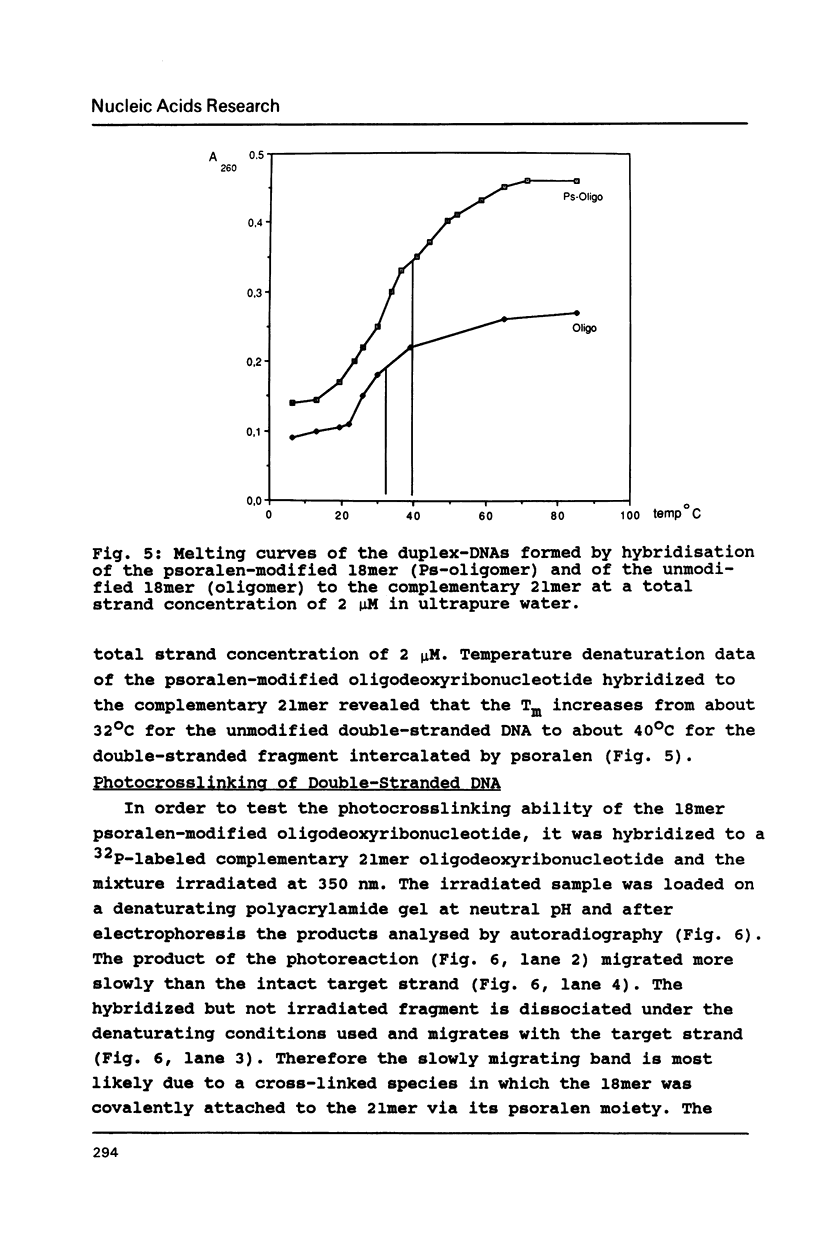
The psoralen derivative 4,5',8-trimethylpsoralen was covalently linked to the 5'-terminus of an 18mer oligodeoxyribonucleotide in the course of solid phase synthesis using phosphoroamidite chemistry. The derivative was introduced as a phosphitylation compound in the last cycle of the oligomer synthesis. The reagent was prepared by 4'-chloromethylation of 4,5',8-trimethylpsoralen, introduction of a linker by ethanediol and phosphitylation with chloro-[(beta-cyanoethoxy)-N,N-diisopropylamino]-phosphine. After oxydation and deprotection the 5'-psoralen modified oligodeoxyribonucleotide was characterised by HPLC. Hybridisation of the psoralen-modified oligomer to a complementary single stranded 21mer followed by irradiation at 350 nm revealed a photo-cross-linked double-stranded DNA fragment analysed on denaturing polyacrylamide gels. The cross-link could be reversed upon irradiation at 254nm.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Plon S. E., Wang J. C. The use of psoralen-modified DNA to probe the mechanism of enhancer action. Cell. 1986 May 23;45(4):567–574. doi: 10.1016/0092-8674(86)90288-6. [DOI] [PubMed] [Google Scholar]
- Gamper H., Piette J., Hearst J. E. Efficient formation of a crosslinkable HMT monoadduct at the Kpn I recognition site. Photochem Photobiol. 1984 Jul;40(1):29–34. doi: 10.1111/j.1751-1097.1984.tb04549.x. [DOI] [PubMed] [Google Scholar]
- Garrett-Wheeler E., Lockard R. E., Kumar A. Mapping of psoralen cross-linked nucleotides in RNA. Nucleic Acids Res. 1984 Apr 11;12(7):3405–3423. doi: 10.1093/nar/12.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Hearst J. E. Psoralen photochemistry. Annu Rev Biophys Bioeng. 1981;10:69–86. doi: 10.1146/annurev.bb.10.060181.000441. [DOI] [PubMed] [Google Scholar]
- Hélène C., Montenay-Garestier T., Saison T., Takasugi M., Toulmé J. J., Asseline U., Lancelot G., Maurizot J. C., Toulmé F., Thuong N. T. Oligodeoxynucleotides covalently linked to intercalating agents: a new class of gene regulatory substances. Biochimie. 1985 Jul-Aug;67(7-8):777–783. doi: 10.1016/s0300-9084(85)80167-x. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Hearst J. E. Characterization of the photoreaction between DNA and aminomethyl-trimethylpsoralen using absorption and fluorescence spectroscopy. Photochem Photobiol. 1981 Jun;33(6):785–791. doi: 10.1111/j.1751-1097.1981.tb05493.x. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Murakami A., Blake K. R., Lin S. B., Miller P. S. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with single-stranded DNA. Biochemistry. 1988 May 3;27(9):3197–3203. doi: 10.1021/bi00409a011. [DOI] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Aurelian L., Blake K. R., Murakami A., Reddy M. P., Spitz S. A., Ts'o P. O. Control of ribonucleic acid function by oligonucleoside methylphosphonates. Biochimie. 1985 Jul-Aug;67(7-8):769–776. doi: 10.1016/s0300-9084(85)80166-8. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E. Syntheses of rRNA, 5.8S, 5S and tRNA are inhibited equally by 8-methoxypsoralen phototreatment of Tetrahymena thermophila. Nucleic Acids Res. 1987 Feb 11;15(3):921–932. doi: 10.1093/nar/15.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckler S., Graves B., Kanne D., Rapoport H., Hearst J. E., Kim S. H. Structure of a psoralen-thymine monoadduct formed in photoreaction with DNA. J Mol Biol. 1982 Nov 25;162(1):157–172. doi: 10.1016/0022-2836(82)90166-8. [DOI] [PubMed] [Google Scholar]
- Sage E., Moustacchi E. Sequence context effects on 8-methoxypsoralen photobinding to defined DNA fragments. Biochemistry. 1987 Jun 16;26(12):3307–3314. doi: 10.1021/bi00386a010. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Hearst J. E. The effects of covalent additions of a psoralen on transcription by E. coli RNA polymerase. Nucleic Acids Res. 1987 Sep 11;15(17):6843–6854. doi: 10.1093/nar/15.17.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. B., Hearst J. E. Wavelength dependence for the photoreactions of DNA-psoralen monoadducts. 1. Photoreversal of monoadducts. Biochemistry. 1987 Jun 30;26(13):3786–3792. doi: 10.1021/bi00387a008. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Hearst J. E. Wavelength dependence for the photoreactions of DNA-psoralen monoadducts. 2. Photo-cross-linking of monoadducts. Biochemistry. 1987 Jun 30;26(13):3792–3798. doi: 10.1021/bi00387a009. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S., Noller H. F. Identification of sites of 4'-(hydroxymethyl)-4,5',8-trimethylpsoralen cross-linking in Escherichia coli 23S ribosomal ribonucleic acid. Biochemistry. 1983 Aug 16;22(17):4159–4164. doi: 10.1021/bi00286a026. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Jones B. K., Chu C. T. Photoreactivities and thermal properties of psoralen cross-links. Biochemistry. 1988 May 3;27(9):3204–3210. doi: 10.1021/bi00409a012. [DOI] [PubMed] [Google Scholar]
- Zhen W. P., Buchardt O., Nielsen H., Nielsen P. E. Site specificity of psoralen-DNA interstrand cross-linking determined by nuclease Bal31 digestion. Biochemistry. 1986 Oct 21;25(21):6598–6603. doi: 10.1021/bi00369a039. [DOI] [PubMed] [Google Scholar]