Abstract
Emotional arousal influences the consolidation of long-term memory. This review discusses experimental approaches and relevant findings that provide the foundation for current understanding of coordinated interactions between arousal activated peripheral hormones and the brain processes that modulate memory formation. Rewarding or aversive experiences release the stress hormones epinephrine (adrenalin) and glucocorticoids from the adrenal glands into the bloodstream. The effect of these hormones on memory consolidation depends upon binding of norepinephrine to beta-adrenergic receptors in the basolateral complex of the amygdala (BLA). Much evidence indicates that the stress hormones influence release of norepinephrine in the BLA through peripheral actions on the vagus nerve which stimulates, through polysynaptic connections, cells of the locus coeruleus to release norepinephrine. The BLA influences memory storage by actions on synapses, distributed throughout the brain, that are engaged in sensory and cognitive processing at the time of amygdala activation. The implications of the activation of these stress-activated memory processes are discussed in relation to stress-related memory disorders.
Keywords: stress, emotion, plasticity, modulation, synapse, amygdala, epinephrine, norepinephrine, vagus nerve
1. INTRODUCTION
“The usefulness of all the passions consists in their strengthening and prolonging in the soul thoughts which are good for it to conserve…”
Descartes, The Passions of the Soul (1647)
The profound effect of emotion on memory storage was recognized long before the days of Descartes. In fact, it was said that throwing a child into a river after witnessing an important event, such as a wedding or granting of land to a township, was a medieval method for encouraging a lasting memory of the occasion (McGaugh, 2003). Considerable attention has been devoted to understanding the impact of emotional arousal on brain systems that store new experiences into long-term memory. Emotional arousal serves an important role in memory processing by first initiating attentional (Revelle and Loftus, 1992; Walker, 1958) and metabolic (Ekman et al., 1983; Witvliet and Vrana, 1995) resources that permit organisms to adapt rapidly to environmental challenges. Physiological changes recruited to mobilize resources for immediate responses also serve a second crucial function. They modulate brain processes to ensure that significant experiences are stored effectively into long-term memory. Thus, emotional arousal serves an important adaptive role in initiating rapid responses to momentary fluctuations in environmental conditions, and in regulating neural representations of these changes. This review discusses experimental approaches and relevant findings that provide the foundation for current understanding of coordinated interactions between arousal activated peripheral hormones and brain processes that modulate memory formation.
2. EFFECTS OF AROUSAL ON PERIPHERAL HORMONES & CENTRAL NOREPINEPHRINE
Emotional arousal produced by aversive stressors or highly rewarding events results in the release of epinephrine (adrenalin) and glucocorticoids (cortisol; corticosterone in rats) from the adrenal glands (Roozendaal et al., 2009a). Drugs and other treatments that increase concentrations of epinephrine or glucocorticoids during, or following, learning enhance memory in rats and mice (Gold and van Buskirk, 1978; Introini-Collison et al., 1992; Jurado-Berbel et al., 2010; King and Williams, 2009; Liang et al., 1995; Roozendaal et al., 2006; Williams and McGaugh, 1993; Williams et al., 2000; Williams et al., 1998) as well as human subjects (Cahill and Alkire, 2003; Kuhlmann and Wolf, 2006; Moor et al., 2005; Zoladz et al., 2011). These peripherally acting hormones must, of course, interact with the central nervous system to modulate memory formation. Extensive evidence indicates that they influence memory consolidation by stimulating the release of norepinephrine in the amygdala. Experimental manipulations that disrupt amygdala functioning block the memory enhancement induced by systemic administration of epinephrine or corticosterone. Lesions of the amygdala (Liang et al., 1990; Roozendaal et al., 1996) or the major input and/or output pathways from the amygdala (Liang and McGaugh, 1983; Roozendaal and McGaugh, 1996) block epinephrine-induced memory enhancement. Further, drugs that block receptors that bind norepinephrine in the amygdala (Liang et al., 1986; Quirarte et al., 1997), or deplete norepinephrine concentrations (Liang et al., 1995), block the memory enhancing actions of epinephrine and corticosterone. Direct infusions of norepinephrine or beta-noradrenergic agonists into the basolateral complex of the amygdala (BLA) after training improve memory for many kinds of tasks (Ferry and McGaugh, 1999; Ferry et al., 1999; Hatfield and McGaugh, 1999; Huff et al., 2006; LaLumiere et al., 2003). Moreover, the memory deficits observed following adrenalectomy, which severely depletes peripheral concentrations of epinephrine and glucocorticoids (Borrell et al., 1983; Liang et al., 1986), are reversed by direct infusion of norepinephrine into the amygdala (Liang et al., 1986).
Training conditions that evoke emotional arousal (e.g. footshock stimulation), or direct injections of epinephrine or corticosterone in doses that facilitate memory, significantly increase norepinephrine release in the amygdala (Galvez et al., 1996; McReynolds et al., 2010; Williams et al., 2000; Williams et al., 1998). When rats were trained to avoid a footshock in a single trial inhibitory avoidance task, extracellular concentrations of norepinephrine in the amygdala increased to 300% above basal levels and norepinephrine levels were highly correlated with memory retention performance tested 24 hours later. Unexpectedly, norepinephrine levels remained elevated for two hours after inhibitory avoidance training (McIntyre et al., 2002). Studies using in vivo electrophysiological recordings also demonstrated that delivery of a footshock during inhibitory avoidance training significantly increased the firing rate of lateral/basolateral amygdala neurons in cats for two hours following training (Pelletier et al., 2005). These findings fit well with evidence that memory-modulating drugs can be effective when infused into the amygdala within hours after training (McGaugh and Roozendaal, 2009).
2.1 Arousal enhances human memory
Experiments using procedures modified from those first developed by Heuer and Reisberg (1990) have demonstrated that arousing stimuli are also well remembered by human subjects. The procedure consisted of presenting a series of pictures accompanying one of two stories. Identical pictures were presented to all participants, but the narrative describing visual scenes in the story was emotionally neutral for control subjects and emotionally arousing for the experimental group. The subjects were then given a surprise memory test several weeks later. Subjects in the experimental group recalled a significantly greater number of the slides relative to subjects that heard only the emotionally neutral story (Cahill et al., 1995).
In subsequent studies, control and experimental groups heard the same emotionally neutral narrative during the first and last phases of a three-part story. The two groups differed only in the middle phase of the study where an emotionally arousing narrative was presented to the experimental group. A common finding of these studies is that heart rate, blood pressure and other sympathetic aspects of arousal, such as the galvanic skin response, are significantly elevated following presentation of emotionally arousing information (Abercrombie et al., 2008; Anderson et al., 2006; Cahill and Alkire, 2003; Cahill et al., 1994; Critchley et al., 2002; Moor et al., 2005; Nielson et al., 2005). More importantly, the magnitude of indices of arousal reliably predicts the percentage of emotional, but not neutral, components of the story that are recalled during retention testing. Elevations in physiological arousal are significant predictors of later memory as emotion-induced improvements in memory are observed when the delay between initial learning and subsequent tests for retention with humans is as short as 30 min or even as long as one and a half months (Anderson et al., 2006; Cahill et al., 1994; Nielson et al., 2005; Quevedo et al., 2003; Steidl et al., 2011).
2.2 A role for epinephrine in human memory consolidation
Human studies combining behavioral and physiological measures have been instrumental in identifying some of the processes underlying memory enhancement induced by emotional arousal. The findings of such studies fit well with those obtained in animal experiments. For example, animal studies provided the initial evidence that exposure to emotionally arousing learning conditions elicits secretion of the adrenal hormone epinephrine (Mabry et al., 1995; McCarty and Gold, 1981) that, in turn, produces a host of sympathetic responses (Vincent et al., 1986; Vincent et al., 1983) by binding to beta-adrenergic receptors. Human studies reveal that exposure to pictures that evoke emotional arousal produce significant elevations in epinephrine secretion that leads to increased heart rate (Gerra et al., 1996). Epinephrine involvement in mediating the consequences of emotional arousal on memory is also indicated by findings of human studies using experimental procedures that increase the release of this adrenal hormone. For instance, many posttraining procedures that increase epinephrine release, including increases in muscle tension (Nielson and Jensen, 1994), application of cold press stressors, or direct administration of epinephrine (Cahill and Alkire, 2003; Moor et al., 2005) produce significant improvements in memory. Further, the memory enhancement produced by these treatments is blocked by drugs (such as propranolol) that prevent epinephrine from binding to beta-adrenergic receptors (Cahill et al., 1994; Hurlemann et al., 2005; van Stegeren, 2008; van Stegeren et al., 1998).
There is also evidence that emotional arousal induces norepinephrine release in human subjects (Segal and Cahill, 2009>). As in vivo assessment of norepinephrine activity in the human brain is not possible, salivary alpha-amylase (sAA) is used as a biological marker of endogenous norepinephrine activity. Levels of secretion of sAA correlate significantly with levels of norepinephrine measured in plasma (Chatterton et al., 1996). Segal and Cahill (2009) reported that sAA levels assessed shortly after presentation of emotionally arousing material correlated significantly (+0.72) with memory of material assessed on a surprise retention test one week later.
There is extensive evidence that in human subjects, as well as rats, emotional arousal effects on memory involve activation of the amygdala. Several studies using positron emission topography (PET) or functional magnetic imaging (fMRI) report that neuronal activity assessed in the amygdala at the time of encoding emotionally arousing stimuli correlates significantly with the strength of subsequent memory (Cahill et al., 1996; Canli et al., 2000; Hamann et al., 1999). The involvement of the amygdala in mediating these effects is further indicated by the evidence that a human subject with amygdala damage resulting from Urbach-Wiethe did not display enhanced recall of emotional information (Cahill et al., 1995; Markowitsch et al., 1994). Studies using functional brain imaging provide additional evidence concerning the involvement of the amygdala and norepinephrine release in emotionally enhanced memory. Studies by van Stegeren and colleagues (2006; 2005) using functional brain imaging while participants view emotionally arousing slides reveal concomitant increases in amygdala activity and sAA levels. These effects were significantly reduced by administration of the beta-adrenergic receptor antagonist propranolol (van Stegeren et al., 2006; van Stegeren et al., 2005). It is interesting to note that blocking beta-adrenergic receptors only suppresses activity in response to emotionally arousing, as opposed to neutral, visual slides (van Stegeren et al., 2005).
3. PATHWAYS FOR CONVEYING EFFECTS OF AROUSAL HORMONES ON THE BRAIN
Findings indicating that amygdala norepinephrine levels are sensitive to epinephrine secretion provide strong evidence suggesting that emotion-induced secretion of epinephrine facilitates memory by direct actions on the amygdala. However, this implication is complicated by evidence that, even in highly stressful situations, epinephrine does not pass from the peripheral circulation into the brain (Arai et al., 1981; Pluta et al., 1994). As such, an understanding of how emotional arousal affects memory will not be complete without identifying the neural mechanism(s) that permit this hormone to regulate norepinephrine in the brain.
3.1 Role of the vagus nerve
Despite the limited access of epinephrine to the central circulation, exogenous peripheral administration of this hormone modulates several functions in the brain that regulate arousal, cerebral auditory evoked responses, attention, cortical information processing and memory storage (Berntson et al., 2003; Dahlgren et al., 1980; Elwood et al., 1986; Gold and van Buskirk, 1978; Introini-Collison et al., 1992; Williams et al., 1998). Reports from anatomical, electrophysiological and pharmacological experiments suggest that epinephrine's actions on memory and in potentiating norepinephrine output in the amygdala may be initiated by the activation of peripheral vagal fibers that project to the brain. By tracing the course of human vagal fibers from an area below the diaphragm to the adrenals, Kollmann (1860) provided the first sign of a possible relationship between the adrenal gland, which releases epinephrine, and the vagus nerve. Using more sophisticated anatomical procedures, Teitelbaum (1933) and Coupland, Parker, Kesse, and Mohamed (1989) confirmed the finding that dorsal and ventral branches of the vagus nerve innervate the adrenal glands. Other studies show that electrical stimulation of the adrenal nerve evokes action potentials in the vagus nerve (Niijima, 1992). The possibility that ascending vagal fibers may have central stimulant effects mediated primarily by peripheral adrenergic mechanisms was originally postulated by Izquierdo et al. over 50 years ago (Izquierdo et al., 1959, 1960). As ascending fibers of the vagus contain adrenergic receptors in both rats (Schreurs et al., 1986) and humans (Lawrence et al., 1995), activated vagal fibers can convey input regarding changes in hormonal release to the brain.
An electrophysiological study provided more direct evidence that epinephrine's actions on the brain may be mediated by activating ascending fibers of the vagus nerve (Miyashita and Williams, 2006). This study assessed vagal activity with physiological recordings of the nerve in anesthetized rats given saline, epinephrine, the peripherally acting beta-adrenergic antagonist sotalol, or sotalol followed by epinephrine. Epinephrine produced a significant increase in vagal nerve firing relative to controls, and blockade of peripheral beta-adrenergic receptors with sotalol completely abolished the capacity for epinephrine to influence neural discharge along the vagus. These findings demonstrate that peripheral fibers of the vagus nerve are responsive to fluctuations in circulating levels of epinephrine, and also show that the excitatory actions of epinephrine on vagal neural discharge are mediated through influences on peripheral beta-adrenergic receptors.
3.2 Activation of the locus coeruleus
Seymour Kety suggested, four decades ago, that emotionally arousing experiences would activate the locus coeruleus (LC), producing release of norepinephrine in a variety of cortical and subcortical brain regions, to enhance synaptic plasticity and memory (1970). Supporting evidence has mounted since Kety's insightful prediction. Information regarding heightened activity in visceral sensory organs is transmitted by ascending vagal fibers to the nucleus of the solitary tract (NTS) located in the brainstem (Kalia and Sullivan, 1982; Sumal et al., 1983). In response to this activation, NTS neurons influence central noradrenergic activity through direct synapses on neurons in the LC (Van Bockstaele et al., 1999).
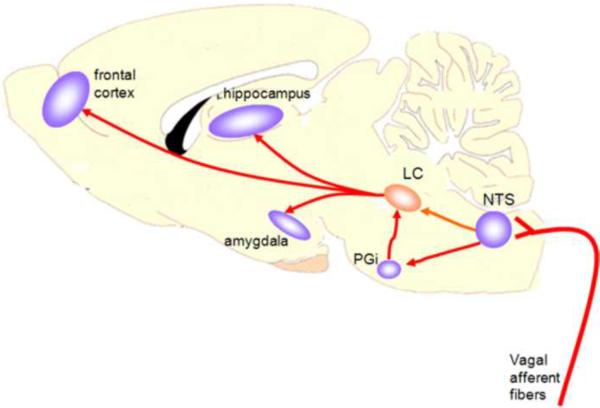
It is well established that the majority of noradrenergic terminals innervating the BLA originate in the LC (Asan, 1998; Fallon et al., 1978; Loughlin et al., 1986; Ricardo and Koh, 1978). Studies using in vivo microdialysis in behaving animals demonstrate that reversible inactivation of NTS neurons suppresses epinephrine-induced increases in amygdala concentrations (Williams et al., 1998). Other findings show that pharmacological blockade of the NTS also attenuates the improvement in memory produced by peripheral injection of epinephrine (Williams and McGaugh, 1993). Combined with evidence that intra-amygdala infusion of a beta-noradrenergic antagonist blocks memory enhancement produced by systemic administration of epinephrine (Liang et al., 1986), these studies imply that brainstem neurons in the NTS influence memory consolidation by regulating norepinephrine release in the amygdala. In a simplistic scheme, these findings indicate that a neural circuit involved in the physiological components of emotional arousal, including the vagus nerve, NTS, and LC, regulates norepinephrine release in the amygdala to modulate memory storage (Figure 1). Although the LC receives a diverse number of inputs from the brainstem NTS, parabrachial nuclei, paragigantocellular nucleus, prefrontal cortex, hypothalamus, insular cortex, and central amygdala, findings characterizing how these connections regulate norepinephrine release from LC neurons are scant (Cedarbaum and Aghajanian, 1978; Jodo and Aston-Jones, 1997; Lee et al., 2005; Luppi et al., 2006).
Figure 1. Schematic diagram depicting the contribution of the nucleus tractus solitarius (NTS) as both a recipient of peripheral inputs from the vagus nerve and a transmitter of these visceral signals to limbic structures that process memory after emotionally arousing events.
Feedback regarding fluctuations in peripheral visceral systems are conveyed to the brain via peripheral vagal fibers. The terminals of the vagus nerve synapse directly within the NTS. After activation by vagal afferents, NTS neurons convey information to structures that process memory such as the amygdala, hippocampus and frontal cortex via a polysynaptic pathway to the locus coeruleus (LC). Norepinephrine (NE) is known as one of the primary transmitters to mediate synaptic communication between these structures.
Clues to understanding how LC activity and the central release of norepinephrine are influenced by arousal are revealed by studies using a number of different approaches. For example, peripheral administration of epinephrine, in doses that affect memory, increases the firing rate of neurons in the LC (Holdefer and Jensen, 1987) and increases the number of NTS or LC neurons that express c-fos, an immediate early gene that serves as a marker for neuronal activation (Racotta et al., 1995). Afferent vagal fibers may mediate spontaneous activity in the LC, which is depressed when the vagus nerve is severed at the cervical level with vagotomy (Svensson and Thorén, 1979). Moreover, electrical stimulation of ascending vagal fibers produce significant burst firing in LC neurons (Dorr and Debonnel, 2006; Groves et al., 2005). Importantly, electrical stimulation of the vagus nerve immediately after training enhances long-term memory for inhibitory avoidance in rats (Clark et al., 1998), and verbal recognition in humans (Clark et al., 1999). Excitatory changes induced in LC neurons by vagal activation may explain this memory enhancement. Experiments using in vivo microdialysis to measure extracellular norepinephrine in the BLA of awake, behaving animals provide more direct evidence of functional interactions between the vagus nerve, LC and amygdala norepinephrine activity. For example, stimulation of the vagus nerve, at an intensity identical to that which improves memory in rodents, produces a long-lasting increase in norepinephrine output in the amygdala that persists for up to 180 min post-stimulation (Hassert et al., 2004). Since vagal input to the brain is limited almost exclusively to the NTS, these findings provide evidence that neuronal firing in the amygdala is regulated, at least in part, by input received from projection neurons originating in the NTS that influence LC functioning.
3.3 The NTS integrates effects of peripheral arousal on the brain
The amino acid glutamate is the primary neurotransmitter mediating synaptic communication between vagal afferents and neurons they synapse on in the NTS. Direct stimulation of ascending vagal fibers causes a significant increase in glutamate concentrations measured in the NTS (Allchin et al., 1994; Granata and Reis, 1983). A recent series of experiments demonstrated the importance of glutamate activation of NTS neurons in modulating memory in rats. Exposure to novel contexts produce heightened states of arousal and activation of the brain that facilitate memory storage (Okuda et al., 2004). Blocking glutamate receptors in the NTS with the AMPA receptor antagonist CNQX attenuates novelty induced enhancement in fear conditioning that occurs by conditioning animals in an unfamiliar context (King and Williams, 2009). AMPA and NMDA receptors are distributed throughout the NTS (de Paula et al., 2007; Vardhan et al., 1993a, b) and this study selectively targeted AMPA receptors with a dose of CNQX previously shown to suppress NTS neuronal firing in response to stimulation of the vagus nerve (Granata and Reis, 1983; Andresen and Yang, 1990).
As blocking beta-adrenergic receptors with sotalol produced a reduction in fear retention similar to that produced by CNQX infusions into the NTS, a subsequent experiment examined whether memory enhancement resulting from novelty-induced arousal may involve peripheral hormonal secretion. This study found that increasing peripheral sympathetic output with epinephrine injections in habituated subjects enhanced the marginal levels of fear conditioning retention exhibited by subjects that were habituated to the conditioning chamber. Moreover, and importantly, blocking NTS glutamatergic receptors with CNQX attenuated the enhancement in memory produced by epinephrine injections (King and Williams, 2009). This study extends our understanding of the consequences of arousal on cognitive processes by indicating that postsynaptic AMPA receptors in the NTS play an important role in transmitting the physiological changes from novelty-induced arousal on brain systems that encode memory. Other studies provide additional evidence that increased glutamate transmission in the NTS enhances memory for emotionally arousing experiences. For instance, posttraining microinjections of glutamate into the NTS, where its neurons synapse with vagal afferents, improves memory for inhibitory avoidance (Kerfoot et al., 2008; Kerfoot and Williams, 2011; Miyashita and Williams, 2002), and also produces significant and long-lasting increases in norepinephrine concentrations sampled from the amygdala (Miyashita and Williams, 2002).
4. THE AMYGDALA MODULATES MEMORY CONSOLIDATION BY INTERACTING WITH OTHER BRAIN REGIONS
As discussed above, extensive evidence indicates that activation of the amygdala by stress hormones modulates the consolidation of emotionally arousing memories. It does not follow, however, that memory should be impaired by the loss of the amygdala, or that the amygdala is the site of storage of emotionally arousing memories. Inactivation of the amygdala prior to training or retention does not impair performance on a spatial water maze task, or a spatial win-shift or win-stay, stimulus-response task in rats (McDonald and White, 1993; McDonald et al., 2010). The evidence that intra-amygdala infusions of d-amphetamine and other drugs can affect performance on tasks such as these (Packard et al., 1994; Packard and Gabriele, 2009) suggests that the amygdala is a modulatory structure; although it can influence memory, it is not necessary for maintaining or expressing memory. The evidence that memory for the aversive inhibitory avoidance task is not lost when the amygdala is inactivated or lesioned one day to one month after training (Bevilaqua et al., 1997; Izquierdo et al., 1997; Parent et al., 1995; Parent et al., 1994), indicates that the amygdala is not the final storage site for emotionally arousing memories.
Packard, Cahill, and McGaugh (1994) found compelling evidence that the amygdala modulates memory storage in efferent brain regions in rats. Posttraining infusions of d-amphetamine administered directly into the dorsal hippocampus enhanced long-term memory for a spatial version of a water maze task and infusions into the caudate nucleus enhanced memory for a stimulus-response version of the water maze task. Posttraining infusions of d-amphetamine into the amygdala enhanced long-term memory for both versions of the water maze task. Importantly, inactivation of the amygdala prior to retention testing did not block the memory enhancement induced by posttraining intra-amygdala d-amphetamine infusions (Table 1). These findings support the view that the amygdala is activated during times of arousal to facilitate the storage of information but it is not the site of memory storage (McGaugh, 2004; McGaugh et al., 1996). Consistent with this view, the amygdala shares extensive connections with cortical and subcortical regions implicated in memory storage processes (McDonald, 1991a, b; Petrovich et al., 2001). Packard and colleagues recently observed that anxiogenic drugs impair hippocampus-dependent spatial memory and enhance caudate nucleus-dependent stimulus-response memory, and the amygdala plays a critical role in this differential effect (Packard and Gabriele, 2009).
Table 1.
Amygdala modulation of hippocampus- and caudate nucleus-dependent memory tasks.
| /------------Memory retention-----------/ | ||
|---|---|---|
|
| ||
| Spatial task | Cued task | |
| Posttraining infusions | ||
| d-amphetamine, hippocampus | Enhanced | No effect |
| d-amphetamine, caudate nucleus | No effect | Enhanced |
| d-amphetamine, amygdala | Enhanced | Enhanced |
| Posttraining & Pretesting infusions | ||
| Post: d-amphetamine, amygdala | Enhanced | Enhanced |
| Pretest: lidocaine, amygdala | ||
Findings subsequent to Packard, Cahill and McGaugh's initial discovery revealed memory consolidation-related interactions of the amygdala with many brain regions, including the nucleus accumbens (LaLumiere et al., 2005; Setlow et al., 2000), insular cortex (Miranda and McGaugh, 2004), entorhinal cortex (Roesler et al., 2002), rostral anterior cingulate cortex (Malin et al., 2007), medial prefrontal cortex (Roozendaal et al., 2004), cerebellum (Zhu et al., 2011), primary visual cortex (Dringenberg et al., 2004), and primary auditory cortex (Chavez et al., 2009). One extensively studied interaction is that of the BLA with the dorsal hippocampus. In particular, activation of beta-adrenergic receptors in the BLA appears to be involved in the modulatory influence of intra-dorsal hippocampus glucocorticoid infusions. Posttraining infusions of a beta-adrenergic receptor antagonist into the BLA blocked the memory enhancing effects of a glucocorticoid-receptor agonist administered into the hippocampus after inhibitory avoidance training (Roozendaal et al., 1999). Electrophysiological findings complement these behavioral findings. Fear-conditioned stimuli elicit theta activity in both the amygdala (Paré et al., 2002) and the hippocampus (Moita et al., 2003), and it is synchronized between the two structures (Narayanan et al., 2007; Pape et al., 2005; Seidenbecher et al., 2003) suggesting that theta synchrony in the circuit plays a role in such learning (Lesting et al., 2011).
The concept of “synaptic tagging”, first described by Frey and Morris (1997), was based on the seminal finding that transient synaptic potentiation induced by weak stimulation could be made to persist as long-term potentiation (LTP) if another synapse of the same neuron was given stronger stimulation. Because protein synthesis is required for LTP and tight temporal association was necessary for the facilitating effect, these authors proposed that proteins synthesized by the strong stimulation aided in maintaining the potentiation of weakly stimulated, “tagged” synapses. This concept is appealing because it attempts to explain the long-lasting stabilization of specific synapses that might underlie long-term memory. The synaptic tagging hypothesis was further developed by groups studying memory and emotion. For example “behavioral tagging” describes an analogous process using behavioral stimulation. Evidence for behavioral tagging comes from studies indicating that exposure to a novel stimulus within hours of training can produce long-lasting memory of tasks that would otherwise not be remembered for the long term. This effect depends upon protein synthesis at the time of exposure to the novel stimulus (Ballarini et al., 2009). Likewise, the concept of “emotional tagging” was suggested by findings of electrophysiological studies linking hippocampal plasticity with emotional arousal, amygdala activation and memory enhancement (Bergado et al., 2011; Richter-Levin and Akirav, 2003). It suggests that synaptic activity in the hippocampus initiates long-term plasticity (and memory) only if it is associated with arousal-induced amygdala activity. In support of the concept, high frequency stimulation of the BLA facilitates population spike LTP in the dentate gyrus of the hippocampus (Ikegaya et al., 1995), and the potentiation depends on activation of BLA beta-adrenergic receptors (Ikegaya et al., 1997). Similarly, dentate gyrus LTP is enhanced when tentanic stimulation of the perforant path is accompanied by appetitive or aversive stimuli (Seidenbecher et al., 1997). Perhaps most importantly, stimulation of the amygdala can transform transient into long-lasting, protein synthesis-dependent plasticity in the hippocampus (Frey et al., 2001). Taken together, these results provide strong support for the “emotional tagging” theory.
4.1 Amygdala modulation of multiple memory systems in humans
Findings of human brain imaging and memory studies provide additional evidence that the amygdala and medial temporal lobe interact to consolidate memories of emotionally arousing material. The finding that patients with medial temporal lobe lesions do not show enhancement of emotional memory suggests that the connections between the amygdala and medial temporal lobe may be essential for amygdala-modulated enhancement of memory (Richardson et al., 2004). Kilpatrick and Cahill used structural equation modeling to identify candidate connections with the amygdala in human subjects during viewing of emotional or neutral films. Regions showing different functional connectivity under the two conditions were the parahippocampal gyrus and prefrontal cortex (Kilpatrick and Cahill, 2003). Results of neuroimaging investigations indicate that successful encoding of emotional material is associated with enhanced functional connectivity between the amygdala and the hippocampus (Dolcos et al., 2004; Hamann et al., 1999; Kensinger and Corkin, 2004; Murty et al., 2010; Murty et al., 2009; St Jacques et al., 2009). In addition, co-activation of the left inferior frontal gyrus, amygdala, and hippocampus during reappraisal of negative pictures was associated with performance on a memory test given to healthy subjects two weeks later (Hayes et al., 2010). Thus, the evidence from imaging experiments is highly consistent with that of animal experiments indicating that the influences of emotional activation on memory consolidation involves amygdala interactions with regions in the medial temporal lobe.
5. SYNAPTIC MECHANISMS OF AMYGDALA MODULATION OF LONG-TERM MEMORY
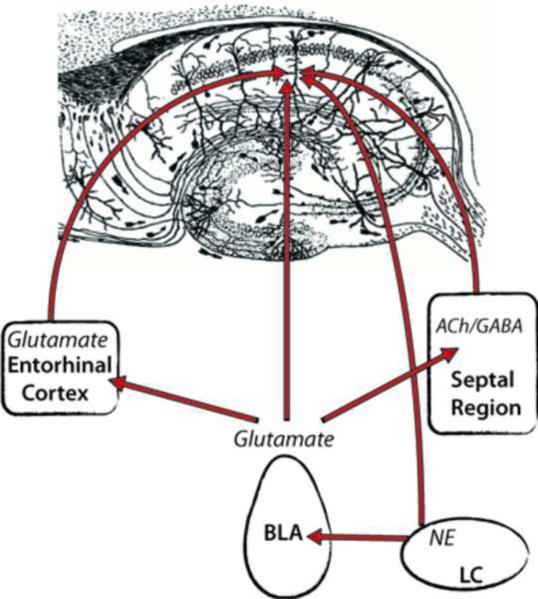
As discussed above, considerable evidence indicates that amygdala modulation of memory consolidation involves influences on other brain regions, but little is known about the synaptic mechanisms of this interaction. One way to observe the effects of BLA activity on synaptic activity elsewhere in the brain is to measure expression of immediate early genes (IEGs) that are rapidly induced in response to synaptic activity. The IEG Arc (also Arg 3.1) is of particular interest as it appears to be a specific marker of memory-related plasticity (Miyashita et al., 2008). It is expressed primarily in calcium/calmodulin protein kinase II-positive cells (Vazdarjanova et al., 2006) and it is present in the postsynaptic densities of recently activated synapses (Moga et al., 2004). Blockade of Arc protein expression with intra-cranial infusions of Arc antisense oligodioxinucleotides impairs long-term memory without affecting acquisition, and maintenance of LTP without affecting induction (Guzowski et al., 2000; Holloway and McIntyre, 2011; Ploski et al., 2008). Expression of Arc mRNA and its protein product has been examined in the hippocampus following training on emotionally arousing tasks and after posttraining intra-BLA administration of memory-modulating drugs (Huff et al., 2006; McIntyre et al., 2005; McReynolds et al., 2010). The findings indicate that, like exposure to a novel context (Guzowski et al., 2001; Miyashita et al., 2009; Vazdarjanova et al., 2006), training on aversive tasks increased expression of Arc mRNA in the dorsal hippocampus. Memory enhancing, posttraining infusions of the beta-adrenergic receptor agonist clenbuterol administered into the BLA resulted in increased expression of Arc protein in the dorsal hippocampus, but no increase in Arc mRNA above training-induced levels (2005). This result is notable because of evidence that Arc mRNA is transported to stimulated regions of dendrites where it can be translated in synapses (Steward and Schuman, 2001; Steward et al., 1998; Yin et al., 2002). In light of the finding that BLA stimulation can transform transient plasticity into protein synthesis-dependent long-term plasticity in the hippocampus (Bergado et al., 2003; Frey et al., 2001; Uzakov et al., 2005), these findings suggest that hippocampal activity produced by the novel context induces transcription and transport of Arc mRNA to stimulated synapses where it is more likely to be translated to protein if the BLA is stimulated subsequently by drug infusions, electrically, or by endogenous stress hormones (Figure 2).
Figure 2. Schematic diagram depicting a theoretical model of how the basolateral complex of the amygdala (BLA) modulates synaptic plasticity in the hippocampus.
Contextual/sensory input initiates transcription of the immediate early gene Arc in pyramidal cells of the CA1 region of the dorsal hippocampus. Arc mRNA is transported to the postsynaptic density of synapses stimulated by the novel context. In position to modify engaged synapses, Arc is either translated to protein, and can thus affect the strength of the synapse, or it is degraded. The coincident and long-lasting amygdala response to stress hormones contributes, directly or indirectly (through entorhinal cortex or septal region), to protein synthesis-dependent changes that underlie long-term plasticity and memory by influencing the translation or degredation of Arc, and possibly other plasticity-related proteins. (Hippocampus illustration, Cajal, 1911).
The amygdala also interacts with cortical areas during memory consolidation. For example, Malin and colleagues (2007) found that posttraining infusions of the cholinergic agonist oxotremorine into the rostral anterior cingulate cortex enhanced memory for inhibitory avoidance but lesions of the BLA blocked this memory enhancement. Lesions of the BLA alone did not impair memory retention. A similar result was found with infusions of the cyclic adenosine monophosphate analog 8-Bromo-cAMP administered into the entorhinal cortex immediately after inhibitory avoidance training; BLA lesions blocked the memory enhancement (Roesler et al., 2002). Electrophysiological findings also indicate that the BLA interacts with the entorhinal cortex. Neuronal activity in the BLA oscillates in phase with that in the entorhinal cortex (Paré and Gaudreau, 1996) and spontaneous discharges of BLA neurons induce short-latency “sharp potentials” in the entorhinal cortex (Paré et al., 1995). Further, BLA stimulation induces fast, low amplitude activity in neocortical electroencephalograms and electrocorticograms (Dringenberg and Vanderwolf, 1996). The influence of the BLA on cortical activity appears to depend on the cholinergic system as inactivation of the basal forebrain blocks cortical activation produced by amygdala stimulation (Dringenberg and Vanderwolf, 1996).
Two studies demonstrated that amygdala stimulation induces synaptic plasticity in primary sensory cortices. Dringenberg and colleagues found that early phase LTP could be converted to late phase LTP in thalamo-cortical synapses of the primary visual cortex when theta burst stimulation of the dorsal lateral geniculate was followed immediately by stimulation of the amygdala (Dringenberg et al., 2004). This effect was blocked by the centrally acting cholinergic antagonist scopolamine, but not by the peripherally acting cholinergic antagonist, methyl-scopolamine (Dringenberg et al., 2004). In the auditory cortex, multiunit extracellular recordings revealed receptive fields that respond preferentially to specific tone frequencies. These receptive fields can shift in response to training or stimulation of the nucleus basalis and this cortical plasticity, like that in the visual cortex, depends on activation of central muscarinic acetylcholine receptors (Weinberger and Bakin, 1998). In a recent study by Chavez and colleagues, pairing of BLA stimulation with a tone shifted the tuning of neurons in the primary auditory cortex (Chavez et al., 2009). These modifications of primary sensory cortex representations provide additional evidence of amygdala influences on synaptic plasticity mediating the modulation of storage of emotionally arousing memory.
The neuromodulators norepinephrine and acetylcholine arise from brainstem and basal forebrain regions that have widespread projections throughout the brain, and both are involved in memory consolidation and synaptic plasticity. When norepinephrine is released in the BLA by LC afferents, it is also released in other limbic and cortical regions (Loizou, 1969). Stimulation of the LC can initiate protein synthesis-dependent LTP in the dentate gyrus (Gelinas and Nguyen, 2005; Walling and Harley, 2004) and administration of a beta-adrenergic receptor agonist transforms early phase LTP to late phase LTP in the rat hippocampus in a manner that depends on dendritic protein synthesis (Gelinas and Nguyen, 2005). In a recent study, norepinephrine induced hippocampal LTP when paired with a mild electrical stimulation, but not when administered alone (Hu et al., 2007), indicating that synapses become potentiated when emotional arousal is coupled with an increase in norepinephrine availability. The observation that norepinephrine application enhances neuronal responses while reducing background spontaneous activity has led some to the conclusion that norepinephrine may increase the signal-to-noise ratio in the cortex and hippocampus (Sara, 1985; Segal and Bloom, 1976a, b; Woodward et al., 1979). A similar change in signal-to-noise ratio is seen in the auditory cortex (Foote et al., 1983) and the visual cortex (Kasamatsu and Heggelund, 1982) in response to sensory stimuli. It is therefore conceivable, that norepinephrine may be required in both the BLA and in cortical synapses, in synchrony, to effect change. Similarly, acetylcholine released by the basal forebrain is necessary for emotional arousal-induced memory enhancement and cortical plasticity. Lesions of corticopetal projections from the nucleus basalis magnocellularis (NBM) using 192 IgG-saporin block memory enhancement induced by intra-BLA infusions of norepinephrine (Power et al., 2002). Therefore, the BLA may modulate memory and cortical plasticity through its direct projections to the NBM and LC, or by coincident actions on the neocortex (Figure 2).
Thus far, only unidirectional effects of BLA stimulation on target brain regions have been discussed. However, bidirectional connections may also play a role in BLA-influenced memory consolidation. For example, findings of a recent study indicate that glucocorticoids affect memory consolidation through a bidirectional interaction of the medial prefrontal cortex (mPFC) and the BLA (Roozendaal et al., 2009b). It is well-established that systemic administration of the stress hormone corticosterone enhances long-term memory for emotionally arousing tasks, and this effect is critically dependent upon beta-adrenergic receptors in the BLA, despite the fact that corticosterone freely enters the brain and binds to central receptors (Roozendaal et al., 2009a). Glucocorticoid enhancement of memory involves projections from the BLA to other regions of the brain including the bed nucleus of the stria terminalis (Roozendaal and McGaugh, 1996), the nucleus accumbens (Setlow et al., 2000), and the hippocampus (McReynolds et al., 2010; Roozendaal and McGaugh, 1997). But mounting evidence indicates that the mPFC exerts an inhibitory influence on the BLA (Milad et al., 2004; Quirk and Gehlert, 2003; Rosenkranz et al., 2003). Stress and glucocorticoids can alter mPFC functioning, releasing the BLA from mPFC inhibitory control, thereby increasing BLA response readiness. In support of this idea, posttraining intra-mPFC infusions of the glucocorticoid receptor (GR) agonist RU 28362 enhanced memory for the inhibitory avoidance task and increased phosphorylation of the microtubule-associated protein-2 (MAP2) kinase Erk I/II in the BLA. Similarly, administration of the GR agonist into the BLA enhanced memory for the task and increased phosphorylation of Erk I/II in the mPFC. Interference with the Erk response through infusions of a MAP kinase (MEK) inhibitor into either the BLA or the mPFC blocked memory enhancement produced by intra-cranial infusions of the GR agonist, indicating that glucocorticoid effects are regulated by a bidirectional BLA-mPFC circuit (Roozendaal et al., 2009b).
6. CLINICAL RELEVANCE
“The usefulness of all the passions consists in their strengthening and prolonging in the soul thoughts which are good for it to conserve… and all the harm they can do consists in their strengthening and conserving these thoughts more than is necessary”
Descartes, the Passions of the Soul, 1647
The knowledge garnered from the studies described above may contribute to the development of treatments for individuals suffering from memory and anxiety disorders. Firstly, it informs research designed to determine the pathologies underlying the disorders. Secondly, recent studies have begun to use the information available to treat anxiety-based memory disorders using pharmacological therapy.
6.1 Understanding memory and anxiety
Individuals with age-associated memory impairment (Esler, Kaye, Thompson, Jennings, Cox, Turner, Lambert, & Seals, 1995) or those diagnosed with Alzheimer's disease (Umegaki, Ikari, Nakahata, Yoshimura, Endo, Yamamoto, & Iguchi, 2000; Borson, Barnes, Veith, Halter, & Raskind, 1989) show significantly blunted or reduced epinephrine responses at rest or in response to stress. Findings from the studies discussed above suggest that epinephrine released in the periphery during emotionally arousing experiences plays a critical role in modulating the conversion of new information into memory storage. Therefore, it is possible that the impairment in encoding new information into memory in these individuals may result, at least partly, from decreased availability of epinephrine to affect mnemonic processing.
The extensive evidence that stress hormones influence the consolidation of emotionally arousing memories is beginning to influence investigations of stress-related individual differences that may predispose certain individuals to posttraumatic stress disorder (PTSD). For example, a single-nucleotide polymorphism of the glucocorticoid receptor gene (Bcl1) is associated with hypersensitivity to glucocorticoids and lower circulating cortisol levels. A recent investigation found that cardiac surgery patients with a homozygous Bcl1 polymorphism had more anxiety and long-term traumatic memories of their experiences in the intensive care unit than heterozygous Bcl1 patients. Patients with the polymorphism also exhibited significantly higher PTSD symptom scores (Hauer et al., 2011). In an earlier study, de Quervain and colleagues found a functional polymorphism in a gene encoding the alpha-2B-adrenergic receptor (ADRA2B) that was associated with the strength of emotional memories in healthy humans, and traumatic memories in survivors of the Rwandan civil war (de Quervain et al., 2007). This group subsequently found that carriers of the genetic variant exhibited increased amygdala activation that was coupled with activation of the insular region of the cortex (Rasch et al., 2009). Both brain regions are hyperactive in PTSD (Liberzon and Martis, 2006). In a more recent study, subjects with PTSD showed exaggerated amygdala and hippocampal activity during encoding of stimuli that were rated as negative. Hippocampal activation was associated with better memory (Brohawn et al., 2010). These results are consistent with those of animal research indicating that glucocorticoids and the adrenergic system are involved in the enhancement of memories that are emotionally arousing. They also support the notion that the amygdala interacts with other brain regions during the consolidation period to strengthen emotionally arousing memories. Alterations to these systems may result in pathological memory for traumatic events.
A role for the vagus nerve in translating peripheral release of stress hormones to memory and brain plasticity is strongly supported by the animal research. Interestingly, poor vagal tone is a common predictor of anxiety disorders in humans (Friedman, 2007) including PTSD (Sack et al., 2004; Sahar et al., 2001). This observation may be relevant to recent research examining memory extinction. Extinction of conditioned fear is not forgetting but, rather, replacing the associations of cues that once marked danger with more neutral associations. In a controlled laboratory setting, identical twins – one with PTSD and the other without – were trained on a fear conditioning task where a colored light signaled a mild electrical shock. Fear conditioning was measured by galvanic skin response following exposure to the conditioned stimulus (the colored light). Extinction trials were administered the following day. During extinction trials, the colored light was presented in the absence of the electrical shock. Results suggested that the twins with PTSD are impaired in their ability to extinguish conditioned fear (Milad et al., 2008). An example of natural extinction would be seen in the responses of a soldier returning home from combat. The sound of a helicopter may provoke a strong fear response at first but, over time, the veteran might acquire new associations with the sound of the helicopter and no longer express fear in response to the sound. Alternatively, if the fear response did not resolve over time and instead continued to worsen, the veteran would likely be diagnosed as having PTSD. Approximately 20–30% of people exposed to a trauma develop PTSD (Resnick et al., 1993). Therefore, PTSD is not always the predicted consequence of traumatic experiences. Results of the twin study suggest that an environmental factor contributes to the ability to extinguish conditioned fear and the development of PTSD. An attractive hypothesis based on these findings is that the normal vagal response to fear-conditioned cue-induced epinephrine release facilitates the consolidation of new memories and thus enables the extinction of conditioned fear. Therefore, poor vagal tone could contribute to the impairment observed in extinction in PTSD patients because reduced vagal responsiveness would mark impaired memory consolidation and extinction of old memories requires consolidation of new memories.
6.2 Treating memory and anxiety disorders
Research examining the influence of stress on memory has also provided potential tools to be used in the treatment of memory and anxiety disorders. Therapies founded on this research may intervene at several points in the circuitry described above. One site for potential intervention is the vagus nerve. An attractive hypothesis based on the work described here is that cognitive behavior therapy (CBT), a primary treatment approach in PTSD, may be paired with vagus nerve stimulation (VNS) to enhance the effects of the therapy. Extinction in rats is an established model for a form of CBT called exposure therapy in humans (Anderson and Insel, 2006; Milad et al., 2006). Previous findings indicate that posttraining infusions of norepinephrine directly into the BLA enhance the effects of extinction (Berlau and McGaugh, 2006) and VNS increases norepinephrine levels in the BLA (Hassert et al., 2004). Taken together, these findings suggest that VNS should enhance both extinction and exposure therapy. VNS has been used safely to treat intractable epilepsy since 1994 and was given approval by the Food and Drug Administration in the United States in 1997 (George and Aston-Jones, 2010). Stimulation similar to that given in epilepsy treatment enhances memory consolidation in humans (Clark et al., 1999) and VNS repeatedly paired with exposure to a tone produces dramatic auditory cortex plasticity (Engineer et al., 2011). Information gained from VNS research may be used to conceive of new approaches to enhance the efficacy of exposure therapy. Walker and colleagues (Walker et al., 2002) found that systemic or intra-amygdala infusions of the partial NMDA agonist d-cycloserine enhanced extinction of fear conditioning in rats and this research led directly to clinical trials demonstrating that d-cycloserine enhances the effect of exposure therapy in patients with acrophobia (Ressler et al., 2004). Such adjunct therapy has the potential to benefit patients suffering from several disorders, such as phobia, obsessive compulsive disorder, PTSD, and may even facilitate the prevention of substance abuse in addicts.
Many studies have attempted to block the effects of epinephrine by administering the beta-adrenergic receptor antagonist propranolol. Based on the observations in animals that elevated epinephrine levels following an aversive event modulate the storage of memory for the event, Pitman and colleagues administered propranolol to patients as soon after the trauma as possible (Pitman et al., 2002). This was a departure from the more common approach of chronic administration of anxiolytic drugs, including propranolol, to patients already diagnosed with PTSD. Pitman reasoned that this post-trauma approach was a relatively safe, prophylactic measure to prevent the development of PTSD. Results of the early pilot study were encouraging, but findings of subsequent studies are inconsistent. In one study, rates of PTSD were lower in patients given propranolol 2–20 hours after the trauma (Vaiva et al., 2003). Another study measured no effect of propranolol on incidence of PTSD, however propranolol was administered within 48 hours after the trauma (Stein et al., 2007). According to results of animal research, posttraining drug administration is generally effective only up to 4 hours after training, and is most effective immediately after training (McGaugh and Roozendaal, 2009). After that timeframe, the window of opportunity to influence the consolidation of the memory may be passed. Interestingly, a recent examination of PTSD diagnosis in military personnel suffering from traumatic brain injury determined that administration of morphine shortly after trauma significantly reduced the incidence of PTSD (Holbrook et al., 2010). This finding is noteworthy in the context of this review because the opioid morphine reduces norepinephrine levels in the amygdala and impairs the consolidation of long-term memory when administered immediately after training (Introini et al., 1985; Quirarte et al., 1998). While the obvious conclusion is that morphine reduces the pain and therefore lessens the degree of trauma, an alternative explanation is that morphine administration impairs the consolidation of the memory through its effects on amygdala norepinephrine.
Findings of animal research reviewed here indicate that glucocorticoids, released by the adrenal cortex, play a role in the consolidation of emotionally arousing memories. However, de Quervain and colleagues (1998; 2000) found that elevated glucocorticoid levels at the time of retrieval impaired memory performance in rats and humans. According to extensive research carried out by Yehuda and others, cortisol levels are reduced in PTSD patients and individuals who have lower cortisol responses to trauma are more likely to develop PTSD (Yehuda, 2002). This finding may seem to contradict the results of animal studies showing glucocorticoid-induced enhancement of memory consolidation, but PTSD is not necessarily a consequence of enhanced consolidation of a traumatic memory. In some cases, PTSD patients have very poor memories of the events to which they attribute their PTSD. Rather, PTSD is characterized by intrusive recollections, flashbacks and nightmares of highly disturbing events, suggesting that excessive memory retrieval may be a culprit in the memory disorder. Consistent with this theory, Schelling and colleagues reported a beneficial effect of chronic cortisol administration in intensive care patients. The patients given doses of hydrocortisone that were comparable to levels produced by stress were less likely to show symptoms of PTSD following septic shock and related treatment (2001). Cortisol administration offers an additional benefit of enhancing the consolidation of extinction memory (Cai et al., 2006), potentially providing further protection from the symptoms of PTSD. Based on these apparent benefits of cortisol, Aerni and colleagues carried out a pilot study to investigate the effect of chronic administration of low-dose cortisol on PTSD symptoms and reported that a significant reduction in PTSD symptoms was observed in cortisol-treated patients (Aerni et al., 2004). For review of glucocorticoids, memory and PTSD, see (Schelling et al., 2004) and (de Quervain, 2008).
7. CONCLUSIONS
The amygdala is well-positioned to translate sympathetic arousal into synaptic plasticity that is distributed throughout the brain. A physiological mechanism to promote brain plasticity and rapid consolidation of memory for events that have a bearing on survival and well-being can have both adaptive and maladaptive implications. Here, we propose that the sympathetic “fight or flight” response goes beyond the peripheral nervous system by implementing epinephrine stimulation of the vagus nerve, which bridges the peripheral and central nervous systems by stimulating the nucleus of the solitary tract in the brainstem. As a result of adrenergic actions on the vagus nerve, norepinephrine is released from neurons of the locus coeruleus that are activated by afferents of the nucleus of the solitary tract (Figure 1). Levels of the neuromodulator norepinephrine increase in the BLA following stress hormone administration, stimulation of the vagus nerve or nucleus of the solitary tract, or training on an aversive task (Table 2). Norepinephrine administration promotes plasticity in cortical and hippocampal synapses, especially when paired with emotional arousal or amygdala stimulation. In consideration of evidence indicating amygdala interactions with limbic and temporal lobe structures in human and non-human animals, emotional arousal-induced norepinephrine actions promote amygdala modulation of synapses in target areas that are engaged in memory processing. Target regions respond to sensory and cognitive input while they are simultaneously infused with the neuromodulators norepinephrine and acetylcholine, and tuned by BLA influences (Figure 2). Although this cannot be a complete picture, the model is presumed to be the beginning of an assembly of critical mechanisms, processes and substrates necessary for the enhancement of emotionally arousing memory.
Table 2.
Treatment effects on memory and amygdala norepinephrine levels
| Treatmenta | Memory effect | Amygdala norepinephrine | Reference |
|---|---|---|---|
| Footshock (FS) | Varies with FS intensity | Varies with FS intensity | Quirarte et al. 1998 |
| Vagus Stimulation | Enhances | Increases | Hassert et al., 2004 |
| IA training | Varies with FS intensity | Increasesb | McIntyre et al. 2002 |
| Epinephrine | Enhancesc | Increases | Williams et al. 1998 |
| Corticosterone | Enhancesc | Increases | McReynolds et al., 2010 |
| Muscimol | Impairs | Decreases | Hatfield et al. 1999 |
| Picrotoxin | Enhances | Increases | Hatfield et al. 1999 |
| β-endorphin | Impairs | Decreases | Quirarte et al. 1998 |
| Naloxone | Enhances | Increases | Quirarte et al. 1998 |
All treatments given immediately posttraining.
Training-induced increase in norepinephrine correlates with retention performance tested 24 hours later.
Epinephrine and corticosterone produce inverted U dose-response effects on memory retention. Effects presented here are based on administration of memory-enhancing doses.
Highlights
Stress hormones contribute to fight-or-flight response and enhance memory for important events
The vagus nerve bridges the peripheral stress response with memory processes in the brain
The amygdala influences synaptic strength in other areas of the brain that are involved in memory
Results reveal potential underlying causes of and therapies for stress-related memory disorders
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Chambers AS, Greischar L, Monticelli RM. Orienting, emotion, and memory: phasic and tonic variation in heart rate predicts memory for emotional pictures in men. Neurobiol Learn Mem. 2008;90:644–650. doi: 10.1016/j.nlm.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Allchin RE, Batten TF, McWilliam PN, Vaughan PF. Electrical stimulation of the vagus increases extracellular glutamate recovered from the nucleus tractus solitarii of the cat by in vivo microdialysis. Exp Physiol. 1994;79:265–268. doi: 10.1113/expphysiol.1994.sp003761. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Yamaguchi Y, Grabski W, Lacka D. Emotional memories are not all created equal: evidence for selective memory enhancement. Learn Mem. 2006;13:711–718. doi: 10.1101/lm.388906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KC, Insel TR. The promise of extinction research for the prevention and treatment of anxiety disorders. Biol Psychiatry. 2006;60:319–321. doi: 10.1016/j.biopsych.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Arai T, Watanabe T, Nagaro T, Matsuo S. Blood-brain barrier impairment after cardiac resuscitation. Crit Care Med. 1981;9:444–448. doi: 10.1097/00003246-198106000-00002. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci U S A. 2009;106:14599–14604. doi: 10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado JA, Almaguer-Melian W, Kostenko S, Frey S, Frey JU. Behavioral reinforcement of long-term potentiation in rat dentate gyrus in vivo is protein synthesis-dependent. Neurosci Lett. 2003;351:56–58. doi: 10.1016/s0304-3940(03)00943-1. [DOI] [PubMed] [Google Scholar]
- Bergado JA, Lucas M, Richter-Levin G. Emotional tagging-A simple hypothesis in a complex reality. Prog Neurobiol. 2011;94:64–76. doi: 10.1016/j.pneurobio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci. 2003;18:2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Bevilaqua L, Ardenghi P, Schröder N, Bromberg E, Quevedo J, Schmitz PK, Bianchin M, Walz R, Schaeffer E, Medina JH, Izquierdo I. Agents that affect cAMP levels or protein kinase A activity modulate memory consolidation when injected into rat hippocampus but not amygdala. Braz J Med Biol Res. 1997;30:967–970. doi: 10.1590/s0100-879x1997000800009. [DOI] [PubMed] [Google Scholar]
- Borrell J, De Kloet ER, Versteeg DH, Bohus B. Inhibitory avoidance deficit following short-term adrenalectomy in the rat: the role of adrenal catecholamines. Behav Neural Biol. 1983;39:241–258. doi: 10.1016/s0163-1047(83)90910-x. [DOI] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biol Psychiatry. 2010;68:1023–1030. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci U S A. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cai WH, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. J Neurosci. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178:1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol Learn Mem. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- Coupland RE, Parker TL, Kesse WK, Mohamed AA. The innervation of the adrenal gland. III. Vagal innervation. J Anat. 1989;163:173–181. [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Dahlgren N, Rosén I, Sakabe T, Siesjö BK. Cerebral functional, metabolic and circulatory effects of intravenous infusion of adrenaline in the rat. Brain Res. 1980;184:143–152. doi: 10.1016/0006-8993(80)90593-4. [DOI] [PubMed] [Google Scholar]
- de Paula PM, Tolstykh G, Mifflin S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2259–2265. doi: 10.1152/ajpregu.00760.2006. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Prog Brain Res. 2008;167:239–247. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Descartes R. The Passions of the Soul. In: Cottingham J, Stoothoff R, Murdoch D, editors. The Philosphical Writings of Descartes. Cambridge University Press; Cambridge, England: 1647. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318:890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Kuo MC, Tomaszek S. Stabilization of thalamo-cortical long-term potentiation by the amygdala: cholinergic and transcription-dependent mechanisms. Eur J Neurosci. 2004;20:557–565. doi: 10.1111/j.1460-9568.2004.03515.x. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: an amygdaloid activating system. Exp Brain Res. 1996;108:285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Elwood SW, Ferguson HB, Thakar J. Catecholamine response of children in a naturally occurring stressor situation. J Human Stress. 1986;12:154–161. doi: 10.1080/0097840X.1986.9936782. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol Learn Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) Neuropsychopharmacology. 2010;35:301–316. doi: 10.1038/npp.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Fertomani G, Zaimovic A, Caccavari R, Reali N, Maestri D, Avanzini P, Monica C, Delsignore R, Brambilla F. Neuroendocrine responses to emotional arousal in normal women. Neuropsychobiology. 1996;33:173–181. doi: 10.1159/000119273. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Posttraining brain norepinephrine concentrations: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav Biol. 1978;23:509–520. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- Granata AR, Reis DJ. Release of [3H]L-glutamine acid (L-Glu) and [3H]D-aspartic acid (D-Asp) in the area of nucleus tractus solitarius in vivo produced by stimulation of the vagus nerve. Brain Res. 1983;259:77–93. doi: 10.1016/0006-8993(83)91068-5. [DOI] [PubMed] [Google Scholar]
- Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett. 2005;379:174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Hauer D, Weis F, Papassotiropoulos A, Schmoeckel M, Beiras-Fernandez A, Lieke J, Kaufmann I, Kirchhoff F, Vogeser M, Roozendaal B, et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit Care Med. 2011;39:643–650. doi: 10.1097/CCM.0b013e318206bae6. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G, Labar KS. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front Hum Neurosci. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer F, Reisberg D. Vivid memories of emotional events: the accuracy of remembered minutiae. Mem Cognit. 1990;18:496–506. doi: 10.3758/bf03198482. [DOI] [PubMed] [Google Scholar]
- Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- Holdefer RN, Jensen RA. The effects of peripheral D-amphetamine, 4-OH amphetamine, and epinephrine on maintained discharge in the locus coeruleus with reference to the modulation of learning and memory by these substances. Brain Res. 1987;417:108–117. doi: 10.1016/0006-8993(87)90184-3. [DOI] [PubMed] [Google Scholar]
- Holloway CM, McIntyre CK. Post-training disruption of Arc protein expression in the anterior cingulate cortex impairs long-term memory for inhibitory avoidance training. Neurobiol Learn Mem. 2011;95:425–432. doi: 10.1016/j.nlm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, Vogeley K, Maier W, Dolan RJ. Noradrenergic modulation of emotion-induced forgetting and remembering. J Neurosci. 2005;25:6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Nakanishi K, Saito H, Abe K. Amygdala beta-noradrenergic influence on hippocampal long-term potentiation in vivo. Neuroreport. 1997;8:3143–3146. doi: 10.1097/00001756-199709290-00027. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci Res. 1995;22:203–207. doi: 10.1016/0168-0102(95)00894-7. [DOI] [PubMed] [Google Scholar]
- Introini IB, McGaugh JL, Baratti CM. Pharmacological evidence of a central effect of naltrexone, morphine, and beta-endorphin and a peripheral effect of met- and leu-enkephalin on retention of an inhibitory response in mice. Behav Neural Biol. 1985;44:434–446. doi: 10.1016/s0163-1047(85)90832-5. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I, Saghafi D, Novack GD, McGaugh JL. Memory-enhancing effects of post-training dipivefrin and epinephrine: involvement of peripheral and central adrenergic receptors. Brain Res. 1992;572:81–86. doi: 10.1016/0006-8993(92)90454-h. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schaeffer E, Schmitz PK, Medina JH. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur J Neurosci. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo JA, Insua JA, Biscardi AM, Izquierdo IA. Some observations on the responses to the afferent stimulation of the vagus. Med Exp Int J Exp Med. 1959;1:325–332. doi: 10.1159/000134813. [DOI] [PubMed] [Google Scholar]
- Izquierdo JA, Insua JA, Biscardi AM, Izquierdo IA. Effects on reserpine and diphenydramine on the responses to stimulation of the afferent vagus nerve. Med Exp Int J Exp Med. 1960;2:8–14. doi: 10.1159/000134835. [DOI] [PubMed] [Google Scholar]
- Jodo E, Aston-Jones G. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res. 1997;768:327–332. doi: 10.1016/s0006-8993(97)00703-8. [DOI] [PubMed] [Google Scholar]
- Jurado-Berbel P, Costa-Miserachs D, Torras-Garcia M, Coll-Andreu M, Portell-Cortés I. Standard object recognition memory and “what” and “where” components: Improvement by post-training epinephrine in highly habituated rats. Behav Brain Res. 2010;207:44–50. doi: 10.1016/j.bbr.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Heggelund P. Single cell responses in cat visual cortex to visual stimulation during iontophoresis of noradrenaline. Exp Brain Res. 1982;45:317–327. doi: 10.1007/BF01208591. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci U S A. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot EC, Chattillion EA, Williams CL. Functional interactions between the nucleus tractus solitarius (NTS) and nucleus accumbens shell in modulating memory for arousing experiences. Neurobiol Learn Mem. 2008;89:47–60. doi: 10.1016/j.nlm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot EC, Williams CL. Interactions between brainstem noradrenergic neurons and the nucleus accumbens shell in modulating memory for emotionally arousing events. Learn Mem. 2011;18:405–413. doi: 10.1101/lm.2108911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS. The biogenic amines in the central nervous system: their possile roles in arousal, emotion and learning. In: Schmidt FO, editor. The Neurosciences: second study program. Rockefeller Press; New York: 1970. pp. 324–335. [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- King SO, Williams CL. Novelty-induced arousal enhances memory for cued classical fear conditioning: interactions between peripheral adrenergic and brainstem glutamatergic systems. Learn Mem. 2009;16:625–634. doi: 10.1101/lm.1513109. [DOI] [PubMed] [Google Scholar]
- Kollmann J. Ueber den Verlauf des Lungenmagennerven in der Bauchh6hle. Zeitschrift fur wissenschaftliche Zoologie. 1860;10:413–448. [Google Scholar]
- Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci. 2006;120:217–223. doi: 10.1037/0735-7044.120.1.217. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Nawar EM, McGaugh JL. Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent dopamine receptor activation in both brain regions. Learn Mem. 2005;12:296–301. doi: 10.1101/lm.93205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Watkins D, Jarrott B. Visualization of beta-adrenoceptor binding sites on human inferior vagal ganglia and their axonal transport along the rat vagus nerve. J Hypertens. 1995;13:631–635. doi: 10.1097/00004872-199506000-00009. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Waterhouse BD. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. J Comp Neurol. 2005;481:179–193. doi: 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- Lesting J, Geiger M, Narayanan RT, Pape HC, Seidenbecher T. Impaired extinction of fear and maintained amygdala-hippocampal theta synchrony in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:337–346. doi: 10.1111/j.1528-1167.2010.02758.x. [DOI] [PubMed] [Google Scholar]
- Liang KC, Chen LL, Huang TE. The role of amygdala norepinephrine in memory formation: involvement in the memory enhancing effect of peripheral epinephrine. Chin J Physiol. 1995;38:81–91. [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Liang KC, McGaugh JL. Lesions of the stria terminalis attenuate the amnestic effect of amygdaloid stimulation on avoidance responses. Brain Res. 1983;274:309–318. doi: 10.1016/0006-8993(83)90709-6. [DOI] [PubMed] [Google Scholar]
- Liang KC, McGaugh JL, Yao HY. Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Loizou LA. Projections of the nucleus locus coeruleus in the albino rat. Brain Res. 1969;15:563–566. doi: 10.1016/0006-8993(69)90185-1. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Mabry TR, Gold PE, McCarty R. Age-related changes in plasma catecholamine and glucose responses of F-344 rats to a single footshock as used in inhibitory avoidance training. Neurobiol Learn Mem. 1995;64:146–155. doi: 10.1006/nlme.1995.1054. [DOI] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol Learn Mem. 2007;87:295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ, Calabrese P, Würker M, Durwen HF, Kessler J, Babinsky R, Brechtelsbauer D, Heuser L, Gehlen W. The amygdala's contribution to memory--a study on two patients with Urbach-Wiethe disease. Neuroreport. 1994;5:1349–1352. [PubMed] [Google Scholar]
- McCarty R, Gold PE. Plasma catecholamines: effects of footshock level and hormonal modulators of memory storage. Horm Behav. 1981;15:168–182. doi: 10.1016/0018-506x(81)90026-x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991a;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991b;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Yim TT, Lehmann H, Sparks FT, Zelinski EL, Sutherland RJ, Hong NS. Expression of a conditioned place preference or spatial navigation task following muscimol-induced inactivations of the amygdala or dorsal hippocampus: A double dissociation in the retrograde direction. Brain Res Bull. 2010;83:29–37. doi: 10.1016/j.brainresbull.2010.06.001. [DOI] [PubMed] [Google Scholar]
- McGaugh J. Memory and Emotion: The Making of Lasting Memories. Weidenfeld & Nicolson; London: 2003. [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci U S A. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem. 2010;93:312–321. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: involvement of the basolateral amygdala. Learn Mem. 2004;11:312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski JF. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J Neurosci. 2009;29:898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem. 2008;89:269–284. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Glutamatergic transmission in the nucleus of the solitary tract modulates memory through influences on amygdala noradrenergic systems. Behav Neurosci. 2002;116:13–21. doi: 10.1037//0735-7044.116.1.13. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Epinephrine administration increases neural impulses propagated along the vagus nerve: Role of peripheral beta-adrenergic receptors. Neurobiol Learn Mem. 2006;85:116–124. doi: 10.1016/j.nlm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Moor T, Mundorff L, Bohringer A, Philippsen C, Langewitz W, Reino ST, Schachinger H. Evidence that baroreflex feedback influences long-term incidental visual memory in men. Neurobiol Learn Mem. 2005;84:168–174. doi: 10.1016/j.nlm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS. fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2010;48:3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cogn Neurosci. 2009;21:1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo- hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Jensen RA. Beta-adrenergic receptor antagonist antihypertensive medications impair arousal-induced modulation of working memory in elderly humans. Behav Neural Biol. 1994;62:190–200. doi: 10.1016/s0163-1047(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Yee D, Erickson KI. Memory enhancement by a semantically unrelated emotional arousal source induced after learning. Neurobiol Learn Mem. 2005;84:49–56. doi: 10.1016/j.nlm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Niijima A. Electrophysiological study on the vagal innervation of the adrenal gland in the rat. J Auton Nerv Syst. 1992;41:87–92. doi: 10.1016/0165-1838(92)90130-9. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci U S A. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Gabriele A. Peripheral anxiogenic drug injections differentially affect cognitive and habit memory: role of basolateral amygdala. Neuroscience. 2009;164:457–462. doi: 10.1016/j.neuroscience.2009.07.054. [DOI] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–880. doi: 10.1002/hipo.20120. [DOI] [PubMed] [Google Scholar]
- Parent MB, Avila E, McGaugh JL. Footshock facilitates the expression of aversively motivated memory in rats given post-training amygdala basolateral complex lesions. Brain Res. 1995;676:235–244. doi: 10.1016/0006-8993(95)00095-8. [DOI] [PubMed] [Google Scholar]
- Parent MB, West M, McGaugh JL. Memory of rats with amygdala lesions induced 30 days after footshock-motivated escape training reflects degree of original training. Behav Neurosci. 1994;108:1080–1087. doi: 10.1037//0735-7044.108.6.1080. [DOI] [PubMed] [Google Scholar]
- Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Paré D, Dong J, Gaudreau H. Amygdalo-entorhinal relations and their reflection in the hippocampal formation: generation of sharp sleep potentials. J Neurosci. 1995;15:2482–2503. doi: 10.1523/JNEUROSCI.15-03-02482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JG, Likhtik E, Filali M, Paré D. Lasting increases in basolateral amygdala activity after emotional arousal: implications for facilitated consolidation of emotional memories. Learn Mem. 2005;12:96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta R, Lossinsky AS, Wiśniewski HM, Mossakowski MJ. Early blood-brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res. 1994;633:41–52. doi: 10.1016/0006-8993(94)91520-2. [DOI] [PubMed] [Google Scholar]
- Power AE, Thal LJ, McGaugh JL. Lesions of the nucleus basalis magnocellularis induced by 192 IgG-saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala. Proc Natl Acad Sci U S A. 2002;99:2315–2319. doi: 10.1073/pnas.022627799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Sant'Anna MK, Madruga M, Lovato I, de-Paris F, Kapczinski F, Izquierdo I, Cahill L. Differential effects of emotional arousal in short- and long-term memory in healthy adults. Neurobiol Learn Mem. 2003;79:132–135. doi: 10.1016/s1074-7427(02)00034-5. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Racotta IS, Richard D, Piñón-López MJ, Naimi N. Effect of adrenaline on food consumption and C-fos expression in the central nervous system. Gac Med Mex. 1995;131:409–416. [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci U S A. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Revelle W, Loftus DA. The implications of arousal effects for the study of affect and memory. In: Christianson S, editor. The Handbook of Emotion and Memory. Lawrance Erlbaum; Hillsdale: 1992. pp. 113–150. [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. Emotional tagging of memory formation--in the search for neural mechanisms. Brain Res Brain Res Rev. 2003;43:247–256. doi: 10.1016/j.brainresrev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. Eur J Neurosci. 2002;15:905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009a;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Res. 1996;709:243–250. doi: 10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009b;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci U S A. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav Neurosci. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: heart rate dynamics and individual differences in arousal regulation. Biol Psychiatry. 2004;55:284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- Sahar T, Shalev AY, Porges SW. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biol Psychiatry. 2001;49:637–643. doi: 10.1016/s0006-3223(00)01045-3. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Noradrenergic modulation of selective attention: its role in memory retrieval. Ann N Y Acad Sci. 1985;444:178–193. doi: 10.1111/j.1749-6632.1985.tb37588.x. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, De Quervain DJ. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann N Y Acad Sci. 2004;1032:158–166. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- Schreurs J, Seelig T, Schulman H. Beta 2-adrenergic receptors on peripheral nerves. J Neurochem. 1986;46:294–296. doi: 10.1111/j.1471-4159.1986.tb12961.x. [DOI] [PubMed] [Google Scholar]
- Segal M, Bloom FE. The action of norepinephrine in the rat hippocampus. III. Hippocampal cellular responses to locus coeruleus stimulation in the awake rat. Brain Res. 1976a;107:499–511. doi: 10.1016/0006-8993(76)90140-2. [DOI] [PubMed] [Google Scholar]
- Segal M, Bloom FE. The action of norepinephrine in the rat hippocampus. IV. The effects of locus coeruleus stimulation on evoked hippocampal unit activity. Brain Res. 1976b;107:513–525. doi: 10.1016/0006-8993(76)90141-4. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci U S A. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. Eur J Neurosci. 2000;12:367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S, Razik F, Anderson AK. Emotion enhanced retention of cognitive skill learning. Emotion. 2011;11:12–19. doi: 10.1037/a0020288. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20:923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Sumal KK, Blessing WW, Joh TH, Reis DJ, Pickel VM. Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Thorén P. Brain noradrenergic neurons in the locus coeruleus: inhibition by blood volume load through vagal afferents. Brain Res. 1979;172:174–178. doi: 10.1016/0006-8993(79)90908-9. [DOI] [PubMed] [Google Scholar]
- Teitelbaum HA. The nature of thoracic and abdominal distribution of the vagus. Anatomical Record. 1933;55:297–317. [Google Scholar]
- Uzakov S, Frey JU, Korz V. Reinforcement of rat hippocampal LTP by holeboard training. Learn Mem. 2005;12:165–171. doi: 10.1101/lm.89305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH. The role of the noradrenergic system in emotional memory. Acta Psychol (Amst) 2008;127:532–541. doi: 10.1016/j.actpsy.2007.10.004. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Everaerd W, Cahill L, McGaugh JL, Gooren LJ. Memory for emotional events: differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology (Berl) 1998;138:305–310. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JP, Rombouts SA. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993a;264:R41–50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in the nucleus tractus solitarius mediate the responses to the stimulation of cardio-pulmonary vagal afferent C fiber endings. Brain Res. 1993b;618:23–31. doi: 10.1016/0006-8993(93)90424-l. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Vincent HH, Boomsma F, Man in 't Veld AJ, Schalekamp MA. Stress levels of adrenaline amplify the blood pressure response to sympathetic stimulation. J Hypertens. 1986;4:255–260. doi: 10.1097/00004872-198604000-00018. [DOI] [PubMed] [Google Scholar]
- Vincent JH, Boomsma F, Man in 't Veld AJ, Schalekamp MA. Beta-receptor stimulation by adrenaline elevates plasma noradrenaline and enhances the pressor responses to cold exposure and isometric exercise. J Hypertens Suppl. 1983;1:74–76. [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EL. Action decrement and its relation to learning. Psychological Review. 1958;65:129–142. [Google Scholar]
- Walling SG, Harley CW. Locus ceruleus activation initiates delayed synaptic potentiation of perforant path input to the dentate gyrus in awake rats: a novel beta-adrenergic- and protein synthesis-dependent mammalian plasticity mechanism. J Neurosci. 2004;24:598–604. doi: 10.1523/JNEUROSCI.4426-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: receptive fields plasticity, model, and mechanisms. Audiol Neurootol. 1998;3:145–167. doi: 10.1159/000013787. [DOI] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci. 1993;107:955–962. [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC. The effects of noradrenergic activation of the nucleus tractus solitarius on memory and in potentiating norepinephrine release in the amygdala. Behav Neurosci. 2000;114:1131–1144. doi: 10.1037//0735-7044.114.6.1131. [DOI] [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behav Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- Witvliet CV, Vrana SR. Psychophysiological responses as indices of affective dimensions. Psychophysiology. 1995;32:436–443. doi: 10.1111/j.1469-8986.1995.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Moises HC, Waterhouse BD, Hoffer BJ, Freedman R. Modulatory actions of norepinephrine in the central nervous system. Fed Proc. 1979;38:2109–2116. [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:341–368. vii. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Sacco T, Strata P, Sacchetti B. Basolateral Amygdala Inactivation Impairs Learning-Induced Long-Term Potentiation in the Cerebellar Cortex. Plos One. 2011;6 doi: 10.1371/journal.pone.0016673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Clark B, Warnecke A, Smith L, Tabar J, Talbot JN. Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiol Behav. 2011;103:467–476. doi: 10.1016/j.physbeh.2011.01.016. [DOI] [PubMed] [Google Scholar]