A functional link is identified between Cdo and Stim1 that leads to NFATc3 activation. Stim1 is required for muscle differentiation via activation of the calcineurin/NFAT pathway. The netrin-2–mediated activation of NFATc3 is coincident with an interaction between Cdo and Stim1 via ERK-mediated phosphorylation of Stim1 at Ser-575.
Abstract
The promyogenic cell surface molecule Cdo is required for activation of extracellular signal-regulated kinase (ERK) and nuclear factor of activated T cells c3 (NFATc3) induced by netrin-2 in myogenic differentiation. However, the molecular mechanism leading to NFATc3 activation is unknown. Stromal interaction molecule 1 (Stim1), an internal calcium sensor of the endoplasmic reticulum store, promotes myogenesis via activation of NFATc3. In this study we investigated the functional interaction between Cdo and Stim1 in myogenic differentiation. Overexpression and depletion of Stim1 enhanced or decreased myotube formation, respectively. Of interest, Stim1 protein levels were decreased in Cdo-deficient perinatal hindlimb muscles or primary myoblasts; this correlates with defective NFATc3 activation in Cdo−/− myoblasts upon differentiation. Forced activation of NFATc3 by overexpression of calcineurin restored differentiation of Cdo-depleted C2C12 myoblasts. Furthermore, Cdo and Stim1 formed a complex in 293T cells or in differentiating C2C12 myoblasts. The netrin-2–mediated NFATc3 activation was coincident with robust interactions between Cdo and Stim1 in myoblasts and the ERK-mediated Stim1 phosphorylation at serine 575. The serine 575 phosphorylation was enhanced in C2C12 cells upon differentiation, and the alanine substitution of serine 575 failed to restore differentiation of Stim1-depleted myoblasts. Taken together, the results indicate that cell adhesion signaling triggered by netrin-2/Cdo induces Stim1 phosphorylation at serine 575 by ERK, which promotes myoblast differentiation.
INTRODUCTION
Skeletal myoblast differentiation is a well-coordinated process involving cell cycle withdrawal, expression of muscle-specific genes, and morphological alterations of myoblasts into multinucleated myotubes by fusion (Molkentin and Olson, 1996). This process is regulated by several families of transcription factors, including MyoD family factors MEF2 and nuclear factor of activated T cells c3 (NFATc3; Bergstrom et al., 2002; Pownall et al., 2002; Kang et al., 2004; Sartorelli and Caretti, 2005; Tapscott, 2005). The activities of these transcription factors are regulated tightly by extracellular cues and signaling pathways to ensure the efficient differentiation and to maintain the differentiated state of cells. Cell–cell contact between muscle precursors promotes myoblast differentiation, and Cdo appears to be a critical component that integrates cell contact–mediated signals from cell surface into the myogenic regulatory network (Krauss et al., 2005; Lu and Krauss, 2010).
Cdo is a member of the immunoglobulin/fibronectin superfamily of proteins that forms a complex with other cell adhesion molecules, such as cadherins and related proteins, biregional cell adhesion molecule–related/down-regulated by oncogenes (Cdon) binding protein (Boc), and neogenin and stimulates cell adhesion–mediated signaling pathways to promote myoblast differentiation (Kang et al., 1998, 2002, 2003, 2004). The complex emanates multiple signals, including activation of p38 mitogen-activated protein kinase (MAPK), Akt, focal adhesion kinase (FAK), and extracellular signal-regulated kinase (ERK), during myoblast differentiation (Takaesu et al., 2006; Kang et al., 2008; Bae et al., 2009a, 2009b, 2010). Cdo is expressed at high levels in the myogenic compartment during embryogenesis. Consistent with this expression pattern, Cdo-deficient mice display a delay in muscle development, leading to the formation of smaller muscle, and Cdo−/− myoblasts exhibit defects in myotube formation (Cole et al., 2004). Recently we showed that Cdo functions as a multifunctional coreceptor for two extracellular cues—sonic hedgehog and netrin-2 (Zhang et al., 2006; Bae et al., 2009b).
Netrins are implicated in multiple biological processes, including cell adhesion, axon guidance, and differentiation (Graef et al., 2003; Yebra et al., 2003; Cirulli and Yebra, 2007; Round and Stein, 2007). Our recent studies show that netrin-3 is mainly expressed in C2C12 myoblasts, and the soluble chicken netrin-2—most closely related to mammalian netrin-3—enhances myotube formation of C2C12 cells (Kang et al., 2004) via activation of several downstream signaling molecules, such as FAK and ERK, in a neogenin/Cdo–dependent manner (Bae et al., 2009b). In addition, Cdo is also required for NFATc3 activation induced by netrin-2 in myoblast differentiation (Kang et al., 2004). However, the molecular mechanism of how Cdo induces NFATc3 activation is not clear. In axon growth or guidance, netrin signaling induces activation of multiple downstream signaling regulators, including FAK and ERK (Forcet et al., 2002; Round and Stein, 2007), calcineurin/NFAT (Graef et al., 2003), and Ca2+ influx through canonical transient receptor potential (TRPC) Ca2+ channels, leading to axon turning (Hong et al., 2000; Wang and Poo, 2005).
Calcium signaling has been proposed to play an essential role in myogenic differentiation and muscle development (Shin and Muallem, 2008). During myogenic differentiation, Ca2+ activates several myogenic transcription factors, including NFATc3, via activation of Ca2+/calmodulin–dependent protein phosphatase, calcineurin (Friday et al., 2000). Calcineurin dephosphorylates members of NFAT, allowing NFAT to translocate to the nucleus, where it activates transcription of target genes. Forced calcineurin activity promotes myoblast differentiation via induction of NFATc3 nuclear translocation. Store-dependent Ca2+ entry (SOCE) appears to play important roles in muscle development and myoblast differentiation (Stiber et al., 2008). Stromal interaction molecule 1 (Stim1) has recently been identified as an endoplasmic reticulum (ER) calcium sensor (Liou et al., 2005; Roos et al., 2005). Stim1 is highly expressed in skeletal muscle, and Stim1-dependent SOCE has been implicated in muscle development and contractile activities (Shin and Muallem, 2008; Stiber et al., 2008; Darbellay et al., 2009). Consistently, Stim1-deficient mice are smaller and exhibit perinatal lethality due to skeletal myopathy. In addition, it has also been shown that SOCE conferred by Stim1 and Orai1 plays an essential role in the early process of myogenic differentiation via activation of calcineurin and NFAT (Stiber et al., 2005, 2008; Darbellay et al., 2009). Stim1 was originally identified as a phosphoprotein with multiple phosphorylation sites (Manji et al., 2000). Recent studies show that activities of Stim1 may be modulated by a differential phosphorylation of serine residues. The phosphorylation of serines 486 and 668 of Stim1 is upregulated in mitotically arrested cells, and these sites are targets for Cdk1, leading to suppression of SOCE during mitosis (Smyth et al. 2009). In addition, serines 519 and 575 are identified as phosphorylated from the phosphopeptide analysis of Stim1 in resting or store-depleted HEK293 cells by thapsigargin or 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment, and TPA modulates SOCE via Stim1 phosphorylation at target sites, including serine 575, by ERK1/2 activation (Pozo-Guisado et al., 2010).
In this study, we attempted to characterize the functional link between Cdo and Stim1 in NFATc3 activation and myoblast differentiation. In agreement with previous studies, Stim1 knockdown or overexpression decreased or enhanced myotube formation, respectively. Stim1 was decreased in perinatal Cdo−/− hindlimb muscles, and the differentiation-specific up-regulation of Stim1 was impaired in Cdo−/− primary myoblasts, which correlated well with defects in NFATc3 activation and myoblast differentiation. Activation of NFATc3 by expression of an active form of calcineurin restored differentiation of Cdo-depleted myoblasts. Cdo formed a complex with Stim1 in differentiating C2C12 myoblasts, and netrin-2 induced NFATc3 activation that coincided with a robust interaction between Cdo and Stim1 proteins in C2C12 cells, most likely via ERK-mediated phosphorylation of Stim1 at serine 575. The alanine substitution mutant of serine 575 lost the promyogenic activity of Stim1. Taking these results together, we propose that cell adhesion signaling triggered by netrin/Cdo induces Stim1 phosphorylation at serine 575 by ERK1/2, which promotes myoblast differentiation.
RESULTS
Stim1 is required for myotube formation, and its expression is impaired in Cdo−/− muscles and myoblasts during differentiation
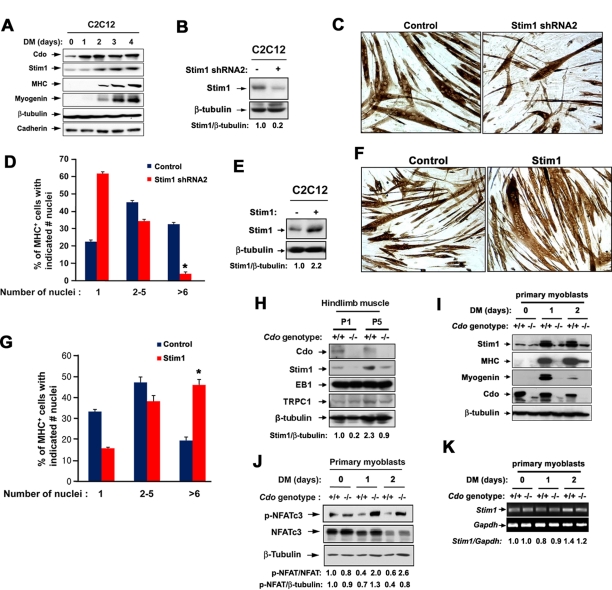
To investigate the functional link between Cdo and Stim1 in myoblast differentiation, we analyzed the role of Stim1 in C2C12 myoblast differentiation. C2C12 cells close to confluency (D0) were induced to differentiate by switching to differentiation medium (DM) for a total of 4 d. Lysates were analyzed for expression of Stim1, Cdo, myosin heavy chain (MHC), myogenin, cadherin, and β-tubulin as a loading control. Whereas Cdo levels were increased prior to initiation of MHC and myogenin expression, Stim1 expression coincided with the induction of the expression of muscle-specific markers (Figure 1A). To analyze the role of Stim1 in myoblast differentiation, we stably transfected C2C12 cells with the control or two different Stim1 short hairpin RNA (shRNA) expression vectors, and we analyzed cell lysates by immunoblotting for the degree of Stim1 depletion. Expression of either of two Stim1 shRNA constructs (designated as shStim1-1 and shStim1-2) decreased Stim1 protein levels to 18 and 7%, respectively, compared with control cells (Supplemental Figure S1A). Because shStim1-2 expression generally gave a greater knockdown effect, we used this construct for further study (Figure 2B). Control and Stim1-depleted cells were induced to differentiate for 3 d, followed by immunostaining with an antibody to MHC. In agreement with previous studies, Stim1 knockdown by the stable transfection of Stim1 shRNAs in C2C12 cells formed smaller myotubes with fewer nuclei compared with the control cells (Figure 1, C and D, and Supplemental Figure S1B). In contrast, overexpression of Stim1 in C2C12 cells enhanced myotube formation, with 2.5-fold more of larger myotubes containing more than six nuclei compared with the control transfected cells (Figure 1, E–G).
FIGURE 1:
Stim1 promotes myotube formation, and its expression is impaired in Cdo−/− developing muscles and differentiating myoblasts. (A) Lysates of C2C12 cells cultured at near confluence in growth medium (D0) or in differentiation medium (DM) for indicated times were immunoblotted with indicated antibodies. (B) C2C12 cells were stably transfected with the control or Stim1 shRNA expression vector, and cell lysates were Western blotted with the indicated antibodies. Depleted protein and β-tubulin loading control signals were quantified by densitometry; ratio is reported under each lane in arbitrary units, with control transfectants set to 1. (C) Control and Stim1 shRNA2-expressing cells were induced to differentiate for 3 d, followed by immunostaining with an antibody to MHC to analyze myotube formation. (D) Quantification of myotube formation shown in C. Values represent means ± SEM from three independent experiments with triple determinations (n = 3). *p < 0.01. (E) C2C12 myoblasts were stably transfected with pcDNA or Stim1 expression vectors and analyzed by immunoblotting. Overexpressed protein and β-tubulin loading control signals were quantified by densitometry; ratio is reported under each lane in arbitrary units, with control transfectants set to 1. (F) Cells shown in E were cultured in DM for 48 h and immunostained with MHC antibodies. (G) Quantification of myotube formation in F. Values represent means ± SEM from three independent experiments with triple determinations (n = 3). *p < 0.005. (H) Extracts of hindlimb muscles from postnatal day 1 (P1) and P5 mice were immunoblotted with indicated antibodies. Stim1 and β-tubulin loading control signals were quantified by densitometry; ratio is reported under each lane at the bottom in arbitrary units, with extracts from wild-type hindlimb muscles at P1 set to 1. (I) Lysates of Cdo+/+ and Cdo−/− primary myoblasts at D0 or cultured under differentiation conditions for 1 or 2 d were immunoblotted with indicated antibodies. (J) Lysates of Cdo+/+ and Cdo−/− primary myoblasts cultured under similar conditions were immunoblotted with antibodies to phosphorylated NFATc3 (an inactive form, p-NFATc3), NFATc3, and β-tubulin as a loading control. p-NFATc3 and NFATc3 signals, as well as p-NFATc3 and β-tubulin loading control signals, were quantified by densitometry; ratio is reported arbitrary units, with signals from Cdo+/+ myoblasts at D0 set to 1. (K) RNAs isolated from Cdo+/+ and Cdo−/− myoblasts at various differentiation time points were analyzed by RT-PCR. Stim1 and control glyceraldehyde-3-phosphate dehydrogenase signals were quantified by densitometry, and signals from Cdo+/+ myoblasts at D0 set to 1.
FIGURE 2:
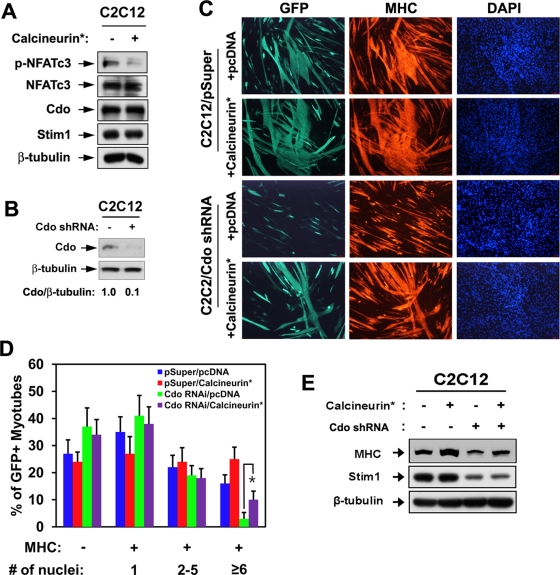
Forced expression of calcineurin restores differentiation of Cdo-depleted C2C12 cells. (A) Lysates of C2C12 cells transfected with a constitutively active form of Calcineurin (Calcineurin*) or control (−) expression vector were subjected to immunoblotting. (B) Lysates of C2C12 cells stably transfected with pSuper or Cdo shRNA were subjected to immunoblotting with antibodies to Cdo and β-tubulin. Cdo and the loading control β-tubulin signals were quantified by densitometry. (C) C2C12/pSuper and C2C12/Cdo shRNA cells were transiently transfected with pcDNA or calcineurin* expression vectors plus a GFP expression vector to mark transfectants. Cells at DM3 were immunostained for MHC (red) and visualized for GFP expression, followed by DAPI staining (blue). (D) Quantification of myotube formation of three independent experiments shown in C. Cultures were scored as MHC negative or MHC positive, with MHC-positive cells further scored as having a single nucleus, two to five nuclei, or six or more nuclei. Values represent means ± SEM from three independent experiments with triple determinations (n = 3). *p < 0.005. (E) Lysates of C2C12/pSuper and C2C12/Cdo shRNA transfected with pcDNA or calcineurin* expression vectors at DM2 were subjected to immunoblotting with antibodies to MHC, Stim1, and β-tubulin.
We next examined the expression pattern of Stim1 in Cdo-deficient muscles and primary myoblasts. Hindlimb muscles were isolated from wild-type and Cdo−/− mice at postnatal days 1 and 5 and analyzed for expression of Stim1 and two other Stim1-interacting proteins—EB1 and TRPC1. Stim1 levels were reduced in hindlimb muscles of Cdo−/− pups at postnatal days 1 and 5 compared with that of the wild-type muscles, whereas TRPC1 and EB1 proteins were only slightly affected or unchanged in Cdo−/− hindlimb muscles (Figure 1H). Primary myoblasts isolated from Cdo+/+ and Cdo−/− hindlimb muscles at high density were induced to differentiate for 2 d by the removal of basic fibroblast growth factor from the culture medium, and the lysates were analyzed by immunoblotting. After 1 d in DM (DM1), Cdo+/+ myoblasts showed a robust induction of muscle-specific markers MHC and myogenin, whereas Cdo−/− myoblasts exhibited defects in expression of these genes. The level of Stim1 was unaltered in high-density Cdo−/− myoblast cultures in the growth medium (DM0) compared with the protein in wild-type cells. At DM1, however, wild-type myoblasts displayed a drastic increase in Stim1 expression, whereas Cdo−/− myoblasts failed to do so (Figure 1I). We then analyzed activation of NFATc3 by using an antibody specific to the inactive, phosphorylated NFATc3 (p-NFATc3) in these cells. The level of p-NFATc3 was drastically reduced on differentiation in Cdo+/+ myoblasts, whereas total NFATc3 levels only decreased at DM2. In contrast, p-NFATc3 levels increased in Cdo−/− myoblasts upon differentiation, whereas NFATc3 levels decreased at DM2, similar to Cdo+/+ myoblasts. In agreement with the published data (Kang et al., 2004), these results further suggest that Cdo−/− myoblasts failed to activate NFATc3 upon induction of differentiation (Figure 1J). To address whether the Stim1 induction in differentiating myoblasts is caused by the increased gene transcription, we performed reverse transcription (RT)-PCR analysis with RNAs isolated from Cdo+/+ and Cdo−/− myoblasts. As shown in Figure 1K. the level of Stim1 RNA did not change significantly during myoblast differentiation. These data revealed that Stim1 expression levels during myoblast differentiation were primarily regulated at protein levels rather than transcriptionally (Figure 1K). Taken together, these data suggest that Stim1 is required for myoblast differentiation, and the reduction in Stim1 protein levels correlates with defects in differentiation and NFATc3 activation of Cdo−/− myoblasts.
Forced expression of calcineurin restores myogenic differentiation of Cdo-depleted myoblasts
We next analyzed whether reactivation of NFATc3 can rescue the defective differentiation of Cdo-depleted myoblasts. C2C12 cells were stably transfected with the control or an expression vector for a constitutively active form of calcineurin (calcineurin*) and analyzed for NFATc3 activation. As shown in Figure 2A, forced expression of calcineurin* decreased the level of p-NFATc3 without alteration in protein levels of total NFATc3, Cdo, and Stim1. We then asked whether defects in myotube formation observed in Cdo-depleted C2C12 cells can be rescued by expression of calcineurin*. C2C12 cells were stably transfected with pSuper and Cdo shRNA expression vectors. The level of Cdo in C2C12/Cdo shRNA was reduced to 10% of the control level (Figure 2B). C2C12/pSuper and C2C12/Cdo shRNA cells were then transiently transfected with pcDNA or calcineurin* plus green fluorescent protein (GFP) vectors to mark the transfectants and induced to differentiate for 3 d, followed by immunostaining with anti-MHC antibodies and 4′,6-diamidino-2-phenylindole (DAPI) staining. Expression of calcineurin* in C2C12/pSuper cells enhanced myotube formation, with more of the larger myotubes containing more than six nuclei compared with the control transfected cells. Whereas C2C12/Cdo shRNA cells transfected with the control pcDNA exhibited impaired myotube formation, the expression of calcineurin* in these cells restored myotube formation almost to the level of the control cells (Figure 2, C and D). Western blot analysis of these cells at DM2 revealed that the level of MHC in Cdo-depleted cells is comparable to that of control cells by calcineurin* expression (Figure 2E). Similar to Cdo−/− myoblasts, Stim1 levels were also significantly decreased in Cdo-depleted cells, and the forced expression of calcineurin did not alter the level of Stim1 in both control and Cdo-depleted cells. These data demonstrate that reactivation of NFATc3 restores differentiation of Cdo-depleted myoblasts.
Cdo and Stim1 form a complex in differentiating C2C12 cells, and netrin-2 enhances this complex formation
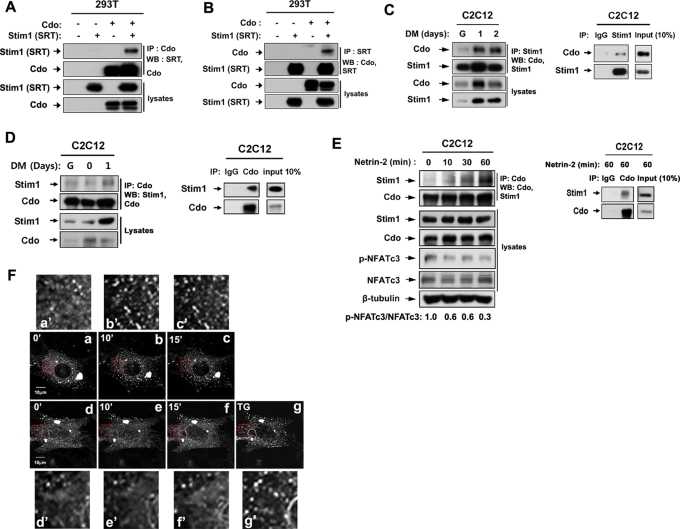
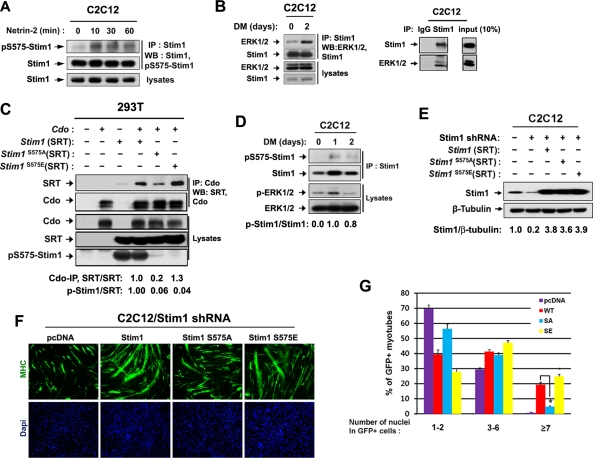
On the basis of the correlation that we observed, we next analyzed a potential involvement of Stim1 in Cdo-mediated NFATc3 activation. We first analyzed a potential interaction between Cdo and Stim1. To do so, expression vectors for SRT-tagged Stim1 and/or Cdo were transiently transfected into 293T cells, and the lysates were subjected to immunoprecipitation with antibodies for Cdo or SRT, followed by immunoblotting with the indicated antibodies. As shown in Figure 3, A and B, Cdo and Stim1 interacted in 293T cells. To analyze whether Cdo and Stim1 can interact endogenously in myoblasts, lysates of C2C12 cells from G, DM1, and DM2 were subjected to immunoprecipitation with anti-Stim1 antibodies and immunoblotted with antibodies to Cdo or Stim1. As control for Stim1 antibody, C2C12 lysates at DM1 were also immunoprecipitated with control rabbit immunoglobulin G (IgG) or Stim1 antibodies (Figure 3C). As shown in Figure 3C, endogenous Cdo and Stim1 proteins were coprecipitated with anti-Stim1 antibodies in differentiating C2C12 myoblasts, and this complex formation was increased upon differentiation, correlating well with the increase in Stim1 levels. This was also confirmed with a reverse immunoprecipitation with Cdo antibodies (Figure 3D). To analyze further this interaction, 293T cells were cotransfected with Stim1 and a glutathione S-transferase (GST)–tagged expression vector containing only the intracellular region of Cdo, followed by glutathione-bead pulldown and immunoblotting. As shown in Supplemental Figure S2A, the Cdo intracellular region failed to interact with Stim1, suggesting that Cdo interacts with Stim1 indirectly. These data suggested that other cytoplasmic interacting proteins of Cdo, such as JLP, Abl, or APPL1, may not be mediators of this interaction. We next asked whether components of the Cdo multiprotein complex, such as Boc or neogenin, interact with Stim1 in C2C12 myoblasts. Lysates of C2C12 cells at DM1 were subjected to immunoprecipitation with the control rabbit IgG or Stim1 antibodies, followed by immunoblotting with Boc or neogenin antibodies. Stim1 was coimmunoprecipitated with neogenin but not with Boc (Supplemental Figure S2, B and C). These data suggest that the interaction between Cdo and Stim1 and neogenin is included in this complex.
FIGURE 3:
Cdo and Stim1 form complexes in 293T cells and differentiating C2C12 cells, and netrin-2 enhances this interaction. (A) 293T cells were transfected with SRT-tagged Stim1, Cdo, or control (−) expression vectors, and 48 h later, lysates were immunoprecipitated with Cdo antibodies and immunoblotted with antibodies for SRT or Cdo. (B) Lysates of 293T cells transfected with indicated vectors were subjected to immunoprecipitation with an antibody to a SRT tag and immunoblotting with antibodies for SRT or Cdo. (C) Lysates of C2C12 cell cultures from G, DM1, or DM2 were immunoprecipitated with Stim1 antibodies and immunoblotted with antibodies for Cdo or Stim1 (left). Lysates of C2C12 cells at DM1 were immunoprecipitated with control rabbit IgG or Stim1 antibodies and immunoblotted with Cdo and Stim1 (right). (D) Lysates of C2C12 cell cultures from G, DM0, or DM1 were immunoprecipitated with Cdo antibodies and immunoblotted with antibodies for Cdo or Stim1 (left). As control, lysates of C2C12 cells at DM1 were immunoprecipitated with control rabbit IgG or Cdo antibodies and immunoblotted with Cdo and Stim1 (right). (E) Confluent C2C12 myoblasts were incubated for 8 h in DM and treated with netrin-2 for indicated times. Lysates were subjected to immunoprecipitation with Cdo antibodies and immunoblotting with indicated antibodies. p-NFATc3 and NFATc3 signals were quantified by densitometry. The signal ratio of C2C12 cells without netrin-2 was set to 1 (left). As control, lysates of C2C12 cells treated with netrin-2 for 60 min were immunoprecipitated with control rabbit IgG or Cdo antibodies and immunoblotted with Cdo and Stim1 (right). (F) Control (top; the red boxed areas in a–c are shown in a′–c′) and Cdo-depleted (bottom; the red boxed areas in d–e are shown in d′–e′) C2C12 cells transfected with Stim1-RFP were treated with netrin-2 for the indicated times, and live confocal images were taken. The TG (1 μM) treatment of the Cdo-depleted cell serves as the positive control.
We previously showed that netrin-2 activates NFATc3 in a Cdo-dependent manner (Bae et al., 2009b). We then examined whether netrin-2 enhances the interaction between Cdo and Stim1. Confluent C2C12 cells were incubated in DM for 8 h and treated with 100 ng/ml netrin-2 for the indicated times. Lysates were immunoprecipitated with anti-Cdo antibody and analyzed by immunoblotting with indicated antibodies. In addition, lysates of C2C12 treated with netrin-2 for 60 min were also immunoprecipitated with the control rabbit IgG or Cdo antibodies and immunoblotted with Cdo and Stim1 antibodies. The level of Stim1 found in the precipitates gradually increased upon netrin-2 treatment, whereas the level of Stim1 and Cdo stayed constant in the lysates. This increase in the amount of interacting Cdo and Stim1 coincided with the decrease of p-NFATc3 levels (Figure 3E), suggesting that netrin-2 may activate NFATc3, most likely via induction of the interaction between Cdo and Stim1. On ER calcium depletion, Stim1 is activated and relocalizes close to the plasma membrane into punctate structures in the ER and activates SOCE channels such as Orai1 (Putney, 2005; Baba et al., 2006; Wu et al., 2006). To further investigate the role of Stim1 in netrin-2-mediated NFATc3 activation, we examined whether Cdo is involved in Stim1 activation by analyzing the redistribution of Stim1 from a diffuse pattern to a punctate structure. To do so, C2C12/pSuper and C2C12/Cdo shRNA cells were transiently transfected with a Stim1-RFP expression vector and treated with netrin-2 for 10 and 15 min, and the localization of Stim1-RFP was analyzed by confocal microscopy. As previously reported, Stim1-RFP was organized in the diffuse structures in the control C2C12/pSuper and C2C12/Cdo shRNA cells (Figure 3F, a and d). Treatment of netrin-2 induced rearrangement of Stim1-RFP from a diffuse pattern to puncta structures (Figure 3F, a–c). In contrast, netrin-2 failed to induce rearrangement of Stim1-RFP in C2C12/Cdo shRNA cells (Figure 3F, d–f). However, treatment with thapsigargin (TG; 1 μM) induced redistribution of Stim1-RFP in the same cells (Figure 3F, g). Taken together, these results suggest that Cdo is required for netrin-2–mediated Stim1 redistribution but not for TG-induced Stim1 activation.
ERK2 activated by netrin-2 phosphorylates Stim1 on serine 575 and is required for NFATc3 activation
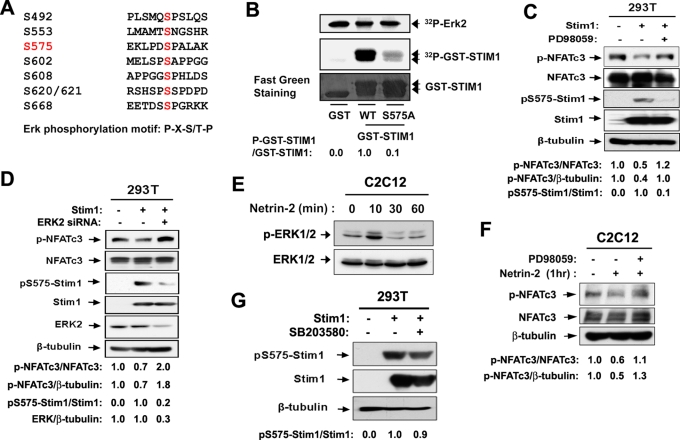
The increased interaction between Cdo and Stim1 by netrin-2 treatment for a short period without altered expression levels of these proteins suggests that posttranslational modifications may be involved in the regulation of this association. In our previous study, Cdo-deficient myoblasts showed defects in activation of ERK and NFATc3 in response to netrin-2 treatment (Bae et al., 2009b). This raised the interesting question of whether netrin-2–mediated ERK1/2 activation is involved in regulation of Cdo and Stim1 complex formation. Among the identified phosphorylation sites (Smyth et al., 2009; Pozo-Guisado et al., 2010) the peptide sequence around serine 575 contains an optimal ERK phosphorylation site with a consensus amino acid sequence (Pro-Xaa-Ser/Thr-Pro) that has been identified by a systematic analysis for the substrate specificity using synthetic peptides (Figure 4A; Gonzalez et al., 1991). To test whether serine 575 of Stim1 is phosphorylated by ERK, we generated constructs containing either the wild-type motif or a mutation of serine 575 to alanine residue (S575A) of GST-Stim1 harboring amino acids 542–607. These constructs were used in an in vitro kinase assay using the purified active form of ERK2 and GST-Stim1 proteins as substrates. As shown in Figure 4B, ERK2 phosphorylated robustly the wild-type GST-Stim1, whereas the Stim1S575A mutant displayed a blunted phosphorylation by ERK2, suggesting that serine 575 may be a potential phosphorylation site for ERK2.
FIGURE 4:
Netrin-2 induces phosphorylation of Stim1 at serine 575 via ERK activation. (A) The consensus ERK phosphorylation motif and a potential phosphorylation site at serine 575. (B) In vitro phosphorylation of the putative ERK phosphorylation site of Stim1 by ERK. The wild-type GST-Stim1 encompassing amino acids 542–607 or the alanine mutant form of GST-Stim1 at serine 575 (S575A) was used as substrate, and phosphorylation was measured by 32P incorporation. GST serves as a negative control. The substrate levels were determined using fast green staining. p-GST-STIM1 and GST-STIM1 signals were quantified by densitometry. The signal ratio of GST-STIM1WT was set to 1. (C) 293T cells transfected with Stim1 were treated with PD98059 for 1 h and analyzed by immunoblotting. Signals of p-NFATc3, NFATc3, p575-Stim1, Stim1, and β-tubulin were quantified by densitometry, and the ratio from control transfectants was set to 1. (D) Lysates of 293T cells cotransfected with control or Stim1 expression vector plus a scrambled siRNA or ERK2 siRNA were subjected to immunoblotting with antibodies to p-NFATc3, NFATc3, pS575-Stim1, Stim1, ERK2, and β-tubulin as a loading control. Signals of p-NFATc3, NFATc3, ERK2, and β-tubulin were quantified by densitometry; ratios are reported under each lane at the bottom in arbitrary units, with control transfectants set to 1. (E) Lysates from C2C12 cells treated with netrin-2 for indicated times were immunoblotted with antibodies to p-ERK1/2 or ERK1/2. (F) C2C12 cells were incubated for 8 h in DM and treated with netrin-2 for 1 h. For the treatment with PD98059, cells were pretreated with PD98059 for 1 h, prior to the netrin treatment. Lysates were immunoblotted with indicated antibodies. Signals of p-NFATc3, NFATc3, and β-tubulin were quantified by densitometry, and the ratio from the control transfectant was set to 1. (G) 293T cells transfected with Stim1 were treated with SB203580 for 1 h and analyzed by immunoblotting with antibodies to pS575-Stim1, Stim1, and β-tubulin as a loading control. Signals of pS575-Stim1 and Stim1 were quantified by densitometry, and the ratio from the control transfectant was set to 1.
To further assess Stim1 phosphorylation by ERK2, we transiently transfected 293T cells with Stim1 or the control expression vector and treated cells with the ERK inhibitor PD98059 for 1 h. Overexpression of Stim1 decreased p-NFATc3 levels (i.e., activation of NFATc3) to 50% of the control level, and the treatment with PD98059 blocked this NFATc3 activation by Stim1 (Figure 4C). To analyze Stim1 phosphorylation at serine 575, we raised a peptide antibody specifically recognizing a phosphorylated form of Stim1 at serine 575 (pS575-Stim1). This, preabsorbed with nonphosphopeptide and affinity purified against phosphopeptide pS575-Stim1 antibody, appears to be specific to p575-Stim1 since it did not recognize when serine 575 is mutated to alanine or glutamic acid (Figure 5C). As shown in Figure 4C, the ectopically expressed Stim1 was readily recognized by this antibody in the control-treated cells, whereas the PD98059 treatment dramatically decreased the level of pS575-Stim1, without alterations in total transfected Stim1 protein levels. To further confirm the role of ERK2 in Stim1 phosphorylation, we screened five different ERK2-specific siRNAs obtained from GenePharma. One siRNA that we used in the further study resulted in a strong knockdown effect, whereas others were weak or inconsistent in their knockdown effect. 293T cells were cotransfected with Stim1 expression vector plus either a control scrambled or ERK2 siRNA, and 48 h later, cells were lysed, followed by immunoblotting with ERK2 antibodies. Expression of ERK2 siRNA resulted in decreased ERK levels to 30% of the control siRNA. In agreement with data shown in Figure 4C, ERK2-knockdown cells exhibited a significant reduction in pS575-Stim1 levels and an increase in p-NFATc3 levels (Figure 4D). These data suggest that Stim1-dependent NFATc3 activation requires ERK activities, and ERK regulates this process most likely via phosphorylation of Stim1 at serine 575.
FIGURE 5:
Phosphorylation of Stim1 at serine 575 is required for the full promyogenic activity of Stim1. (A) Lysates of C2C12 cells treated with netrin-2 for indicated times were immunoprecipitated with a Stim1 antibody followed by immunoblotting with antibodies to pS575-Stim1 or Stim1. (B) Lysates of C2C12 cells at DM0 and DM2 are subjected to immunoprecipitation with Stim1 antibodies and immunoblotted with antibodies to ERK1/2 and Stim1 (left). As control, lysates of C2C12 cells at DM2 are immunoprecipitated with the control rabbit IgG or Stim1 antibodies, followed by immunoblotting (right). (C) 293T cells were transiently transfected with indicated expression vectors, followed by immunoprecipitation and immunoblotting. (D) Lysates from C2C12 cells at DM0, DM1, or DM2 were analyzed for the phosphorylation status of Stim1 at serine 575 and ERK1/2 by immunoblotting. Signals of p-Stim1 and Stim1 were quantified by densitometry, and the ratio from C2C12 cells at D0 was set to 1. (E) C2C12/pSuper and C2C12/Stim1 shRNA cells were transiently cotransfected with indicated expression vectors, and 48 h later, cells were analyzed by immunoblotting with antibodies to Stim1 and β-tubulin as a loading control. Stim1 and β-tubulin signals were quantified by densitometry; ratios are reported under each lane in arbitrary units, with control transfectants set to 1. (F) C2C12/Stim1 shRNA cells in E were induced to differentiate for 3 d and immunostained with GFP antibodies, followed by DAPI staining. (G) Quantification of myotube formation of three independent experiments shown in F. Cultures were scored as GFP-positive cells having one or two nuclei, three to six nuclei, or seven or more nuclei. Values represent means ± SEM from three independent experiments with triple determinations (n = 3). *p < 0.01.
In agreement with our previous studies, the phosphorylated form of ERK1/2 (the active ERK1/2) was significantly increased by netrin-2 treatment for 10 min in C2C12 cells and returned to the basal level thereafter (Figure 4E). To examine whether the netrin-2–mediated NFATc3 activation requires the activation of ERK1/2, C2C12 cells were treated with netrin-2 with or without the pretreatment with PD98059 for 1 h. As shown in Figure 4F, the pretreatment with PD98059 abrogated the activation of NFATc3 induced by netrin-2 treatment, suggesting that ERK1/2 activities are required for NFATc3 activation initiated by netrin-2. We next analyzed the effect of p38MAPK inhibition on Stim1 phosphorylation at serine 575. To do so, we transfected 293T cells with the control or Stim1 expression vectors and treated cells with a p38MAPK inhibitor, SB203580, for 1 h. As shown in Figure 4G, the level of pS575-Stim1 did not change significantly in cells treated with SB203580 compared with dimethyl sulfoxide vehicle–treated cells. Taken together, these data suggest that ERK phosphorylates Stim1, most likely at serine 575, which is required for NFATc3 activation induced by netrin-2.
Phosphorylation of Stim1 on serine 575 is enhanced by netrin-2 treatment or in differentiating myoblasts
Next we examined whether the netrin-2 treatment induces phosphorylation of Stim1 at serine 575. C2C12 cells were treated with netrin-2 for various times and immunoprecipitated with an antibody to Stim1, followed by immunoblotting with antibodies recognizing pS575-Stim1 or Stim1. The level of pS575-Stim1 was enhanced substantially after the netrin-2 treatment for 10 min and decreased slightly after 60 min (Figure 5A). We further asked whether endogenous Stim1 and ERK interacted in myoblasts. Lysates of C2C12 cells at D0 or DM2 were subjected to immunoprecipitate with anti-Stim1 antibodies and immunoblotted with antibodies against Stim1 or ERK. As a control, lysates of C2C12 at DM2 were immunoprecipitated with the control rabbit IgG or Stim1 antibodies and immunoblotted (Figure 5B). As shown in Figure 5B, ERK coprecipitated efficiently with anti-Stim1 antibodies in differentiating myoblasts at DM2. These data suggest that Stim1 can interact with ERK in differentiating myoblasts.
To explore whether Stim1 phosphorylation at serine 575 regulates complex formation between Cdo and Stim1, we generated expression vectors of SRT-tagged Stim1 containing a mutation of serine 575 to either alanine (S575A) or glutamic acid (S575E). These constructs were cotransfected with Cdo into 293T cells, and the lysates were subjected to coimmunoprecipitation analysis with anti-Cdo antibodies and immunoblotted with antibodies to SRT or Cdo. The expression level of three SRT-tagged Stim1 constructs was relatively constant among different transfectants. The wild type and Stim1S575E mutant coprecipitated efficiently with Cdo, whereas the S575A mutation showed a significant decrease in interaction with Cdo. The pS575-Stim1 antibody appears to be specific since both forms of the Stim1 point mutants (S575A or S575E) were not detected by pS575-Stim1 antibodies (Figure 5C). Lysates of C2C12 cells from DM0, DM1, and DM2 were analyzed for pS575-Stim1 levels and ERK1/2 activation. pERK1/2 levels were enhanced in cells at DM1, and these cells showed a concomitant increase in pS575-Stim1 levels (Figure 5D). Taken together, Stim1 phosphorylation on serine 575 is enhanced in myoblasts treated with netrin-2 or upon differentiation.
Mutation of serine 575 to alanine inhibits promyogenic function of Stim1
To test whether Stim1 phosphorylation on serine 575 is required for the promyogenic function of Stim1, we analyzed the differentiation-inducing abilities of Stim1 mutants. The Stim1 RNA interference–resistant human Stim1 expression vectors for wild type or mutants (Stim1S575A, Stim1S575E) or the control pcDNA were transiently cotransfected with a GFP expression vector to mark transfectants into C2C12/Stim1 shRNA cells. Cells were induced to differentiate for 3 d, followed by immunostaining with anti-GFP antibodies and DAPI staining. First we analyzed the expression levels of these Stim1 constructs in transfected cells. At 24 h after transient transfection, cells were harvested and analyzed for the expression of Stim1. As shown in Figure 5E, Stim1 was reduced to 20% of the control level in C2C12/Stim1 shRNA cells. The Stim1 levels were greatly enhanced by overexpression of Stim1 constructs; however, the expression level of Stim1 mutants was not significantly different from that for the wild-type Stim1 (Figure 5E). Expression of the wild-type Stim1 and Stim1S575E induced larger myotube formation with more nuclei compared with the control transfectants (Figure 5E). The control MHC-positive transfectants were scored as containing one to two nuclei (∼70%), three to six nuclei (∼29%), or seven or more nuclei (0%). Overexpression of the wild-type Stim1 or Stim1S575E increased the percentage of MHC-positive cells with one to two nuclei (∼39 or ∼27%, respectively), three to six (∼42 or ∼48%, respectively), or seven or more (∼19 or ∼25%, respectively). In contrast, cells expressing Stim1S575A were not greatly improved in myotube formation compared with the control transfectants (Figure 5G). Similar to the control transfectants, the percentage of MHC-positive Stim1S575A transfectants were scored as containing one to two nuclei (∼56%), three to six nuclei (∼38%), or seven or more nuclei (∼6%) (Figure 5G). Taken together, the expression of the wild-type Stim1 and Stim1S575Emutant induced efficient myotube formation, whereas Stim1S575A mutant did so very inefficiently in Stim1-knockdown C2C12 cells. Taken together, these data suggest that netrin-2 induces phosphorylation of Stim1 at serine 575 by ERK1/2, leading to complex formation of Stim1 with Cdo, and this serine 575 phosphorylation appears to be important for the promyogenic function of Stim1.
DISCUSSION
Stim1 is highly expressed in skeletal muscle and is required for activation of the calcineurin/NFAT signaling pathway, which plays essential roles in myoblast differentiation (Stiber et al., 2008; Johnstone et al., 2010). The identity of the upstream signaling to activate Stim1 during myoblast differentiation is not well understood. In axon growth and guidance, netrin signaling has been shown to induce activation of multiple downstream signaling regulators, including FAK and ERK (Forcet et al., 2002; Round and Stein, 2007), calcineurin/NFAT (Graef et al., 2003), and Ca2+ influx through TRPC Ca2+ channels, leading to axon turning (Hong et al., 2000; Wang and Poo, 2005). We previously showed that netrin induces NFATc3 activation required for myoblast differentiation, and Cdo−/− myoblasts show multiple defects in signaling pathways triggered by netrin-2, including activation of NFATc3 and ERK (Kang et al., 2004; Bae et al., 2009b). This correlation led us to postulate a functional link between Stim1 and Cdo in netrin signaling during myoblast differentiation. In this study, we demonstrated a functional link between a promyogenic cell surface molecule—Cdo—and Stim1 that led to NFATc3 activation and myoblast differentiation. Stim1 protein was strongly increased during myoblast differentiation, and the level of Stim1 protein was significantly decreased in Cdo-deficient muscles or myoblasts without a significant alteration of Stim1 transcripts. This decrease of Stim1 in Cdo-deficient myoblasts correlated well with the reduced NFATc3 activation observed in these cells. Among known Cdo interacting proteins, Gas1 also showed a specific decrease in Cdo-deficient myoblasts during differentiation, whereas neogenin, cadherin, and JLP did not change their expression (Kang et al., 2004; Takaesu et al., 2006; Bae et al., 2011; Leem et al., 2011). Similar to Stim1, Gas1 expression is also enhanced upon myoblast differentiation. Given that Cdo deficiency caused defective myoblast differentiation, the decrease of Stim1 might be partly due to differentiation defects of these cells. However, it appeared that Stim1 induction did not solely reflect the degree of differentiation, since the forced expression of a constitutively activated form of calcineurin restored differentiation of Cdo-depleted cells almost to the level of the control cells without altering Stim1 protein levels. In addition, Stim1 reduction caused by Cdo deficiency was not the result of a general defect in expression of SOCE components, since the expressions of EB1 and TRPC1 were not significantly affected in Cdo-deficient muscles. Additional directed investigation is required to understand the molecular mechanism by which Stim1 protein levels are regulated in myoblast differentiation.
Stim1 was originally identified as a phosphoprotein; however, the physiological role of phosphorylation in modulation of Stim1 activity is just beginning to be understood. Recently two protein kinases have been identified as phosphorylating Stim1, thereby regulating Stim1 activities. Cdk1 phosphorylates Stim1 at serines 486 and 668 and inhibits Stim1 activity, leading to suppression of SOCE during mitosis (Smyth et al., 2009). These authors also identified multiple potential serine phosphorylation sites, including serine 575, which appears not to be involved in Stim1 regulation during mitosis. More recently, ERK1/2 has been proposed to phosphorylate Stim1 and regulate Stim1 binding to Orai1, leading to activation of SOCE in TPA- or thapsigargin-treated HEK293 cells (Pozo-Guisado et al., 2010). These authors found that two serine residues, 519 and 575, are phosphorylated in these cells and a triple-alanine-substitution mutation, including serine 575 and two other potential ERK1/2 target sites (serines 608 and 621), failed to activate SOCE by altering Stim1 binding to Orai1. However, they did not analyze whether all three serine residues are truly phosphorylated in vivo and the role of the single serine 575 phosphorylation in TPA-triggered SOCE. It is conceivable that these serine residues are differentially phosphorylated in response to different extracellular stimuli, leading to modulation of Stim1 activity. We report here that netrin-2–activated ERK phosphorylated Stim1 at serine 575 resulted in enhancement of its interaction with Cdo and was required for NFATc3 activation in myoblasts. Our study provides further evidence that Stim1 phosphorylation is one way to positively or negatively regulate Stim1 activity. ERK1/2 has been shown to play both positive and negative roles in C2C12 myoblast differentiation (Wu et al., 2000; Li and Johnson, 2006; Cho et al., 2007; Yokoyama et al., 2007). The negative role of ERK for myoblast differentiation may be linked to its function as the major mediator of growth factor–mediated proliferative signals. Insulin-like growth factor 1 (IGF-1), a promyogenic signal, activates the calcineurin/NFAT signaling pathway in myogenic differentiation (Delling et al., 2000), and IGF's promyogenic function is partly mediated via ERK2 activation (Li and Johnson, 2006). It will be interesting to determine whether ERK activated by IGF-1 phosphorylates Stim1 in a similar manner in netrin-2 signaling.
We previously reported that netrin-3 and neogenin promoted myotube formation by C2C12 myoblasts (Kang et al., 2004). Neogenin forms cis complexes with Cdo, and both neogenin−/− and Cdo−/− myoblasts show multiple defects in signaling pathways triggered by netrin-2, including activation of NFATc3, FAK, or ERK (Bae et al., 2009b). Because we failed to observe an obvious binding of netrins to Cdo, we proposed that Cdo functions as a coreceptor for neogenin in the netrin-mediated signaling pathway. On the basis of the present data, we propose a model in which the promyogenic extracellular protein netrin binds to its receptor neogenin present in a complex with the coreceptor Cdo, which allows ERK activation. In turn the activated ERK phosphorylates Stim1 at serine 575, and this phosphorylation enhances complex formation of Stim1 with the plasma membrane–resident Cdo and neogenin, resulting in activation of Stim1 and calcinuerin/NFATc3 pathway during myoblast differentiation. Because Cdo is mainly a membrane-resident protein and the redistribution of Stim1 induced by netrin requires Cdo, it is tempting to speculate that Stim1 binding to Cdo complexes may position Stim1 in the vicinity of plasma membrane where SOCE channels are localized. Whether Cdo/neogenin protein complexes modulate Stim1 binding to Orai1 or TRPC channels, resulting in activation of SOCE during myoblast differentiation, will be the focus of future studies. Our study suggests that promyogenic signals such as netrin-2 induce phosphorylation of Stim1 at serine 575 by ERK1/2, leading to complex formation of Stim1 with Cdo, and this is required for NFATc3 activation and myoblast differentiation.
MATERIALS AND METHODS
Cell culture and transfection
293T cells and C2C12 myoblasts were cultured as previously described (Bae et al., 2010). Primary myoblasts were obtained from the hindlimbs of wild-type and Cdo−/− mice as previously described (Cole et al., 2004). To construct SRT-tagged Stim1 or Stim1 mutants (Stim1S575A, Stim1S575E), the full-length Stim1 cDNA was cloned into pcDNA3-SRT (Lee et al., 2001). For small interfering RNA studies, the following sequences were inserted into pSuper: Stim1#1, 5-GATCCCGCTGCTGGTTTGCCTATATTTCAAGAGAATATAGGCAAACCAGCAGCTTTTTTGGAAA-3, and Stim1#2, 5-GATCCCGCTGCTGCTGTCACATCTTTTCAAGAGAAAGATGTGACAGCAGCAGCTTTTTTGGAAA-3. To knock down Cdo, we used previously validated Cdo shRNA construct #1 (Zhang et al., 2006; Kang et al., 2008; Bae et al., 2009a; Leem et al., 2011). Five ERK2 siRNAs obtained from GenePharma (Shanghai, China) were screened for their effectiveness by transfection into 293T cells, and one siRNA (5′-GCUCCAGAAAUUAUGUUGATT-3′) with the strongest knockdown effect was used for the cotransfection study.
To generate C2C12 cell lines that stably overexpress Stim1, or shRNAs against Cdo or Stim1, cells were transfected with the indicated expression vectors and FuGENE6 (Promega, Madison, WI), and cultures were selected in the puromycin-containing medium. Drug-resistant cells were pooled and analyzed immediately. The transfection experiments were repeated at least three times, and freshly generated stable cell lines were used for each transfection-based study. The activated calcineurin (calcineurin*) expression plasmid was generously provided by G. K. Pavlath (Friday et al., 2000). For ERK and p38MAPK inhibitor studies, 293T cells were transfected with the control or Stim1 expression vectors, and 24 h later, cells were treated with PD98059 or SB203580 with a final concentration of 1 μM in fresh culture medium for 1 h. Cell lysates were harvested and analyzed by immunoblotting.
Western blot analysis and immunoprecipitation
Western blot analysis and immunoprecipitation were carried out as previously described (Bae et al., 2010). Primary antibodies used were as follows: Cdo (R&D Systems, Minneapolis, MN, and Zymed, San Francisco, CA), Stim1 (ProSci, Poway, CA, and Sigma-Aldrich, St. Louis, MO), Cadherin (Sigma-Aldrich), β-tubulin (Zymed), MHC (MF-20; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), myogenin (Santa Cruz Biotechnology, Santa Cruz, CA), EB1 (Santa Cruz Biotechnology), TRPC1 (Santa Cruz Biotechnology), ERK1/2, pERK1/2 (Sigma-Aldrich), p-NFATc3, NFATc3 (Sigma-Aldrich), and SRT (Lee et al., 2001). For the generation of phospho-specific pS575Stim1 antibody, the phospho-peptide (VEKLPDp-SPALAKK, corresponding to residues 571–583 of Stim1, where p-S denotes a phospho-serine) was synthesized, and pS575Stim1 antiserum was raised against this phospho-peptide (GenScript, Piscataway, NJ). This phospho-specific antibody was preabsorbed with unphosphorylated peptide, followed by affinity purification against the phospho-peptide.
Immunocytochemistry and confocal microscopy
Immunocytochemistry was carried out as previously described (Bae et al., 2010). For the MHC immunostaining, cells were differentiated for 2–3 d, fixed, and immunostained with anti-MHC antibodies (Developmental Studies Hybridoma Bank) or anti-GFP antibodies (Zymed), followed by incubation with Alexa Fluor 488–conjugated secondary antibody (Molecular Probes, Invitrogen, Carlsbad, CA) or Alexa Fluor 568–conjugated secondary (Molecular Probes). Images were captured and processed with the Nikon ECLIPS TE-2000U and NIS-Elements F software (Nikon, Melville, NY). Quantitative differentiation assays were performed for at least three independent experiments. Statistical analysis of myotube formation was done with a Mann–Whitney U test.
For the analysis of the Stim1 localization after netrin-2 treatment, stably transfected C2C12 cells with the control pSuper or pSuper/Cdo shRNA were grown on coverslips and transiently transfected with Stim1-RFP vectors. At 24 h later, coverslips were placed into a flowthrough chamber and treated with netrin-2 (100 ng/ml) or thapsigargin (1 μM) for the indicated times. Confocal imaging was performed using a Zeiss LSM 510 (Carl Zeiss, Jena, Germany), and a Zeiss C-Apochromat 63× (numerical aperture, 1.20) water immersion objective was used. All confocal images were collected with the pinhole set at 1 Airy unit. The 543-nm line of a HeNe laser was used to excite Stim1–red fluorescent protein, and emission was collected at between 565 and 615 nm. Cells were randomly selected and used for imaging and analysis, and imaging experiments were repeated at least five times.
In vitro kinase assay
An Stim1 fragment (amino acid 542–607) containing a putative ERK phosphorylation site (serine 575) was amplified with the oligonucleotides 5-TAGGATCCCGTGCTGCAGACGAGGCTCTCAAT-3 and 5-GCCACCAGGTGGGGCTGAGGG-3 and subcloned into pGEX-4T-1 vector (Amersham-Pharmacia Biotech, GE Healthcare Bio-Sciences, Piscataway, NJ). Recombinant GST-Stim1 wild-type or S575A mutant proteins were purified by using glutathione S–Sepharose (Amersham-Pharmacia Biotech). In vitro kinase assays were performed in 30 μl of 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.5), 10 mM MgCl2, 1 mM dithiothreitol, 0.05 mM sodium orthovanadate, and 10 μCi of [γ-32P]ATP with active ERK2 (Millipore, Billerica, MA) and GST, GST-Stim1 (amino acids 542–607) wild-type, or S575A mutant proteins at 30°C for 45 min. After gel electrophoresis, proteins were transferred to nitrocellulose. Radiolabeled proteins were visualized and quantified on BAS (Fujifilm, Tokyo, Japan).
Statistical analysis
Statistical analysis for the quantification of the myotube formation was expressed as mean ± SEM from three independent experiments with triplicate determinations. Error bars represent SEM. For comparison between multiple groups, statistical significance was tested by a Mann–Whitney U test using SPSS, version 12.0 (IBM, Armonk, NY).
Supplementary Material
Acknowledgments
We thank Ruth Simon for critical reading of the manuscript. This work was supported by the National Research Foundation of Korea, which is funded by the Korean Government, Ministry of Education, Science and Technology (2011-0017315), to J.S.K.
Abbreviations used:
- DM
differentiation medium
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- MAPK
mitogen-activated protein kinase
- MHC
myosin heavy chain
- Stim1
stromal interaction molecule 1
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-07-0634) on February 1, 2012.
REFERENCES
- Baba Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Domene S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS. Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet. 2011;89:231–240. doi: 10.1016/j.ajhg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Kim BG, Lee HJ, Oh JE, Lee SJ, Zhang W, Krauss RS, Kang JS. Cdo binds Abl to promote p38alpha/beta mitogen-activated protein kinase activity and myogenic differentiation. Mol Cell Biol. 2009a;29:4130–4143. doi: 10.1128/MCB.00199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Lee JR, Kim BG, Han JW, Leem YE, Lee HJ, Ho SM, Hahn MJ, Kang JS. Cdo interacts with APPL1 and activates Akt in myoblast differentiation. Mol Biol Cell. 2010;21:2399–2411. doi: 10.1091/mbc.E09-12-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Yang YJ, Jiang G, Hong M, Lee HJ, Tessier-Lavigne M, Kang JS, Krauss RS. Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro. Mol Biol Cell. 2009b;20:4920–4931. doi: 10.1091/mbc.E09-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom DA, Penn BH, Strand A, Perry RL, Rudnicki MA, Tapscott SJ. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol Cell. 2002;9:587–600. doi: 10.1016/s1097-2765(02)00481-1. [DOI] [PubMed] [Google Scholar]
- Cho YY, et al. RSK2 mediates muscle cell differentiation through regulation of NFAT3. J Biol Chem. 2007;282:8380–8392. doi: 10.1074/jbc.M611322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- Cole F, Zhang W, Geyra A, Kang JS, Krauss RS. Positive feedback regulation of myogenic differentiation by CDO. Dev Cell. 2004;7:843–854. doi: 10.1016/j.devcel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Darbellay B, Arnaudeau S, König S, Jousset H, Bader C, Demaurex N, Bernheim L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009;284:5370–5380. doi: 10.1074/jbc.M806726200. [DOI] [PubMed] [Google Scholar]
- Delling U, Tureckova J, Lim HW, De Windt LJ, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy chain expression. Mol Cell Biol. 2000;20:6600–6611. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcet C, Stein E, Pays L, Corset V, Llambi F, Tessier-Lavigne M, Mehlen P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- Friday BB, Horsley V, Pavlath GK. Calcineurin activity required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149:657–665. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FA, Raden DL, Davis RJ. Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem. 1991;266:22159–22163. [PubMed] [Google Scholar]
- Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- Johnstone LS, Graham SJ, Dziadek MA. STIM proteins: integrators of signalling pathways in development, differentiation and disease. J Cell Mol Med. 2010;14:1890–1903. doi: 10.1111/j.1582-4934.2010.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Bae GU, Yi MJ, Yang YJ, Oh JE, Takaesu G, Zhou YT, Low BC, Krauss RS. A Cdo-Bnip-2-Cdc42 signaling pathway regulates p38alpha/beta MAPK activity and myogenic differentiation. J Cell Biol. 2008;182:497–507. doi: 10.1083/jcb.200801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Feinleib JL, Knox S, Ketteringham MA, Krauss RS. Pro-myogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc Natl Acad Sci USA. 2003;100:3989–3994. doi: 10.1073/pnas.0736565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Mulieri PJ, Hu Y, Taliana L, Krauss RS. BOC: an immunoglobulin superfamily member that associates with CDO to positively regulate myogenic differentiation. EMBO J. 2002;21:114–124. doi: 10.1093/emboj/21.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Mulieri PJ, Miller C, Sassoon DA, Krauss RS. CDO, a Robo-related cell surface protein that mediates myogenic differentiation. J Cell Biol. 1998;143:403–413. doi: 10.1083/jcb.143.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Yi MJ, Zhang W, Feinleib JL, Cole F, Krauss RS. Netrins and neogenin promote myotube formation. J Cell Biol. 2004;167:493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss RS, Cole F, Gaio U, Takaesu G, Zhang W, Kang JS. Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J Cell Sci. 2005;118:2355–2362. doi: 10.1242/jcs.02397. [DOI] [PubMed] [Google Scholar]
- Lee JR, Chang YY, Hahn MJ. Development of a new epitope tag recognized by a monoclonal antibody to Rickettsia typi. Biotechniques. 2001;31:541–545. doi: 10.2144/01313st08. [DOI] [PubMed] [Google Scholar]
- Leem YE, Han JW, Lee HJ, Ha HL, Kwon YL, Ho SM, Kim BG, Tran P, Bae GU, Kang JS. Gas1 cooperates with Cdo and promotes myogenic differentiation via activation of p38MAPK. Cell Signal. 2011;23:2021–2029. doi: 10.1016/j.cellsig.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Li J, Johnson SE. ERK2 is required for efficient terminal differentiation of skeletal myoblasts. Biochem Biophys Res Commun. 2006;345:1425–1433. doi: 10.1016/j.bbrc.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM1 is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Krauss RS. N-cadherin ligation, but not Sonic hedgehog binding, initiates Cdo-dependent p38alpha/beta MAPK signaling in skeletal myoblasts. Proc Natl Acad Sci USA. 2010;107:4212–4217. doi: 10.1073/pnas.0908883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Gustafsson MK, Emerson CP., Jr Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- Pozo-Guisado E, Campbell DG, Deak M, Alvarez-Barrientos A, Morrice NA, Alvarez IS, Alessi DR, Martín-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J Cell Sci. 2010;123:3084–3093. doi: 10.1242/jcs.067215. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J, Stein E. Netrin signaling leading to directed growth cone steering. Curr Opin Neurobiol. 2007;17:15–21. doi: 10.1016/j.conb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Caretti G. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr Opin Genet Dev. 2005;15:528–535. doi: 10.1016/j.gde.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Muallem S. Skeletal muscle dressed in SOCs. Nat Cell Biol. 2008;10:639–641. doi: 10.1038/ncb0608-639. [DOI] [PubMed] [Google Scholar]
- Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiber J, et al. STIM1 signaling controls store operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiber JA, Tabatabaei N, Hawkins AF, Hawke T, Worley PF, Williams RS, Rosenberg P. Homer modulates NFAT-dependent signaling during myoblast differentiation. Dev Biol. 2005;287:213–224. doi: 10.1016/j.ydbio.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Kang JS, Bae GU, Yi MJ, Lee CM, Reddy EP, Krauss RS. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol. 2006;175:383–388. doi: 10.1083/jcb.200608031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yebra M, et al. Recognition of the neural chemoattractant netrin-1 by integrins a6b4 and a3b1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Takano K, Yoshida A, Katada F, Sun P, Takenawa T, Andoh T, Endo T. DA-Raf1, a competent intrinsic dominant-negative antagonist of the Ras-ERK pathway, is required for myogenic differentiation. J Cell Biol. 2007;177:781–793. doi: 10.1083/jcb.200703195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the sonic hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell. 2006;10:657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.