Abstract
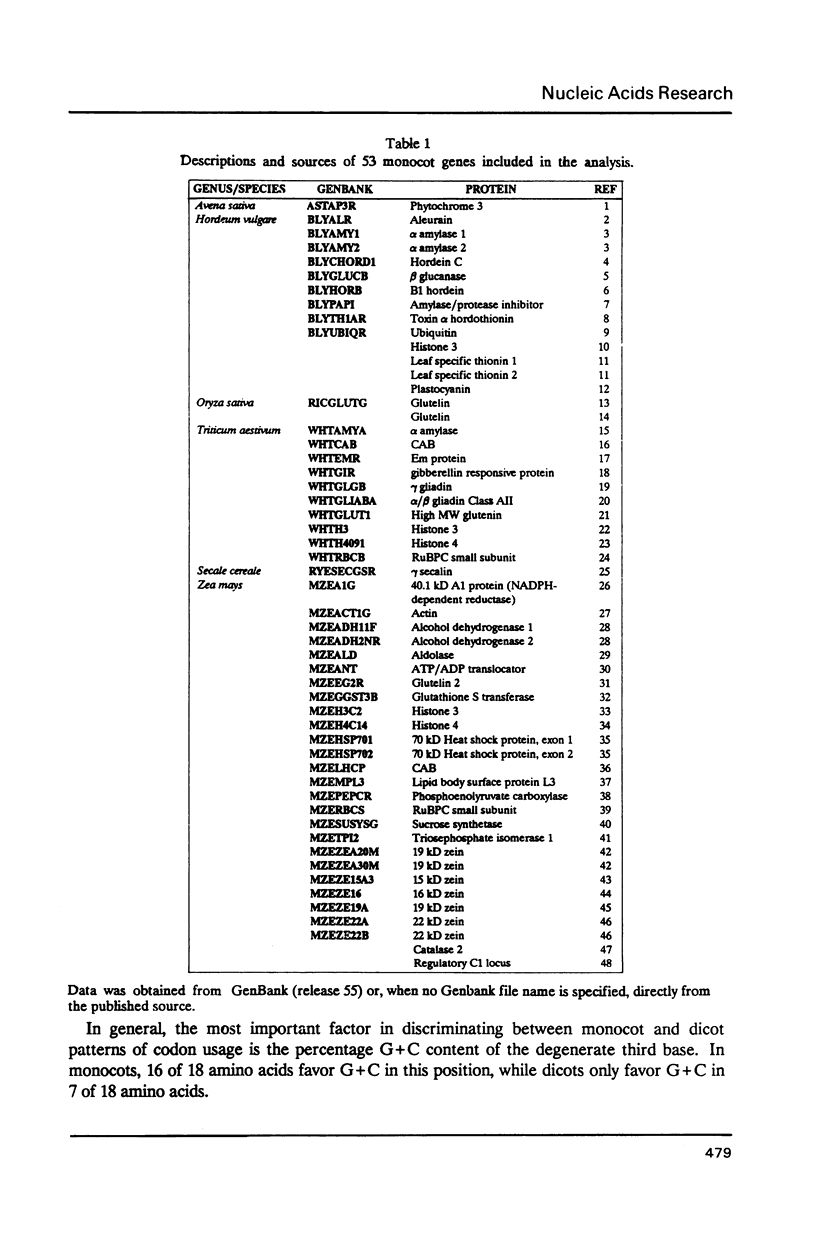
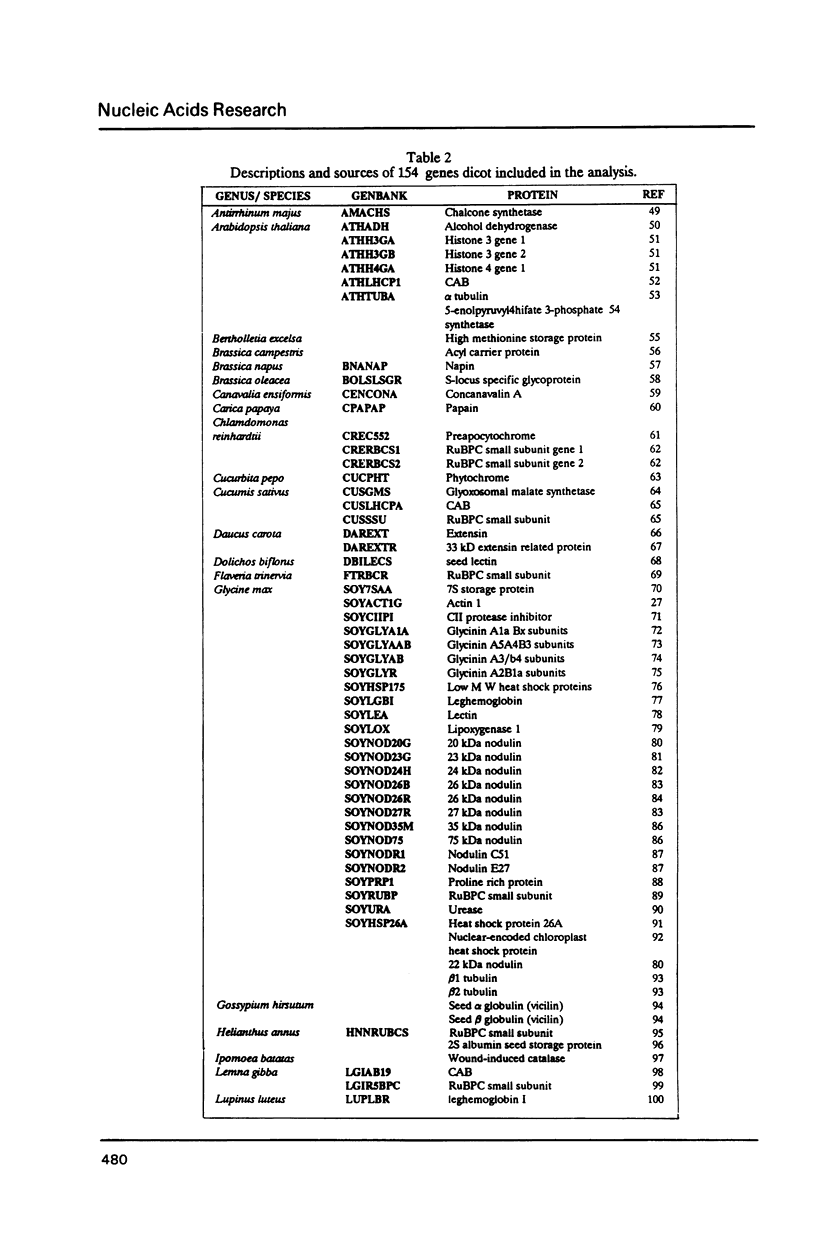
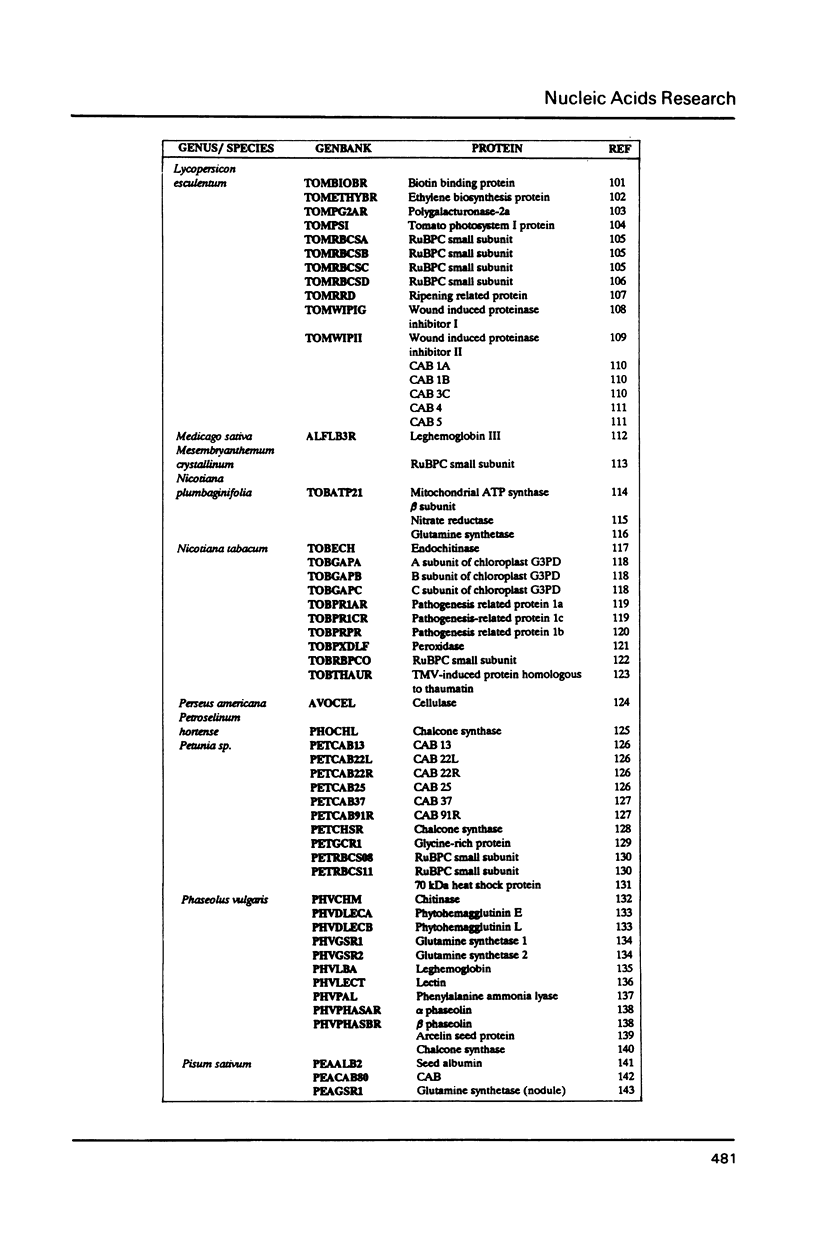
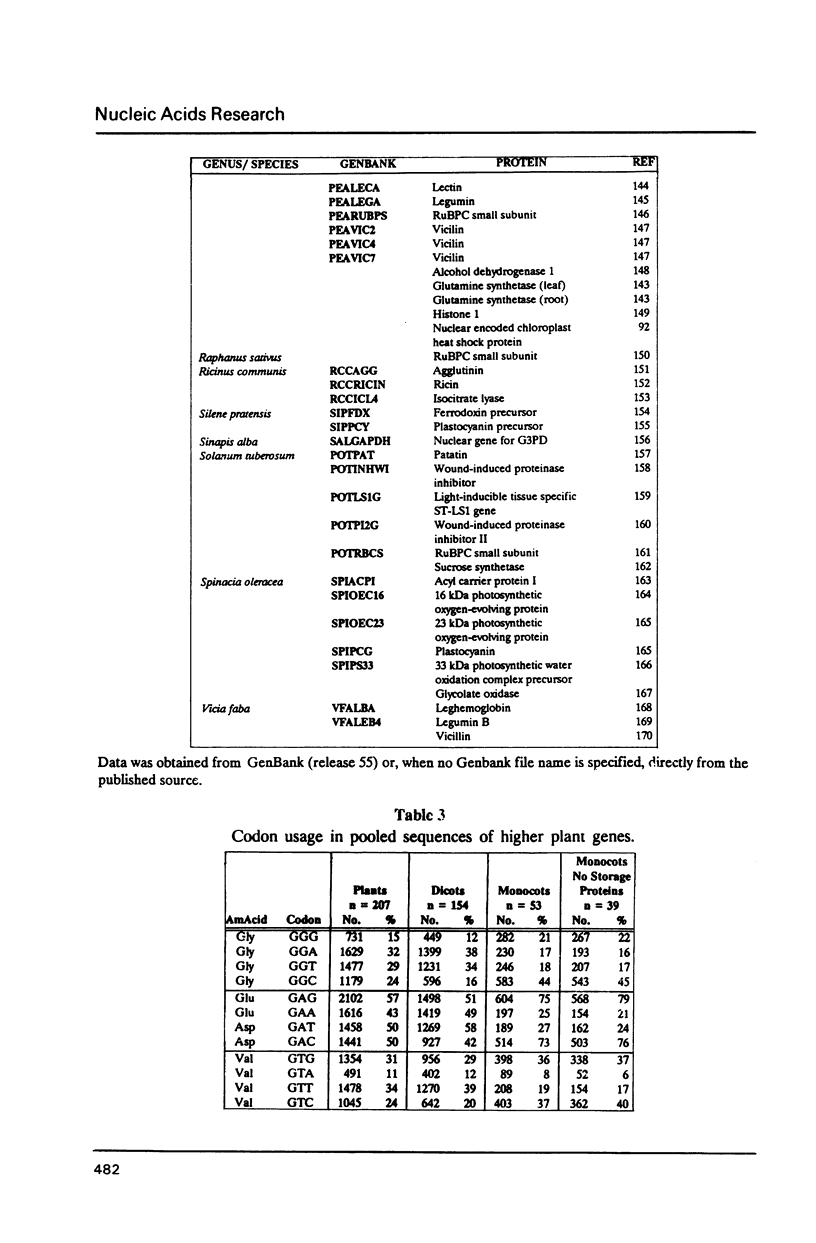
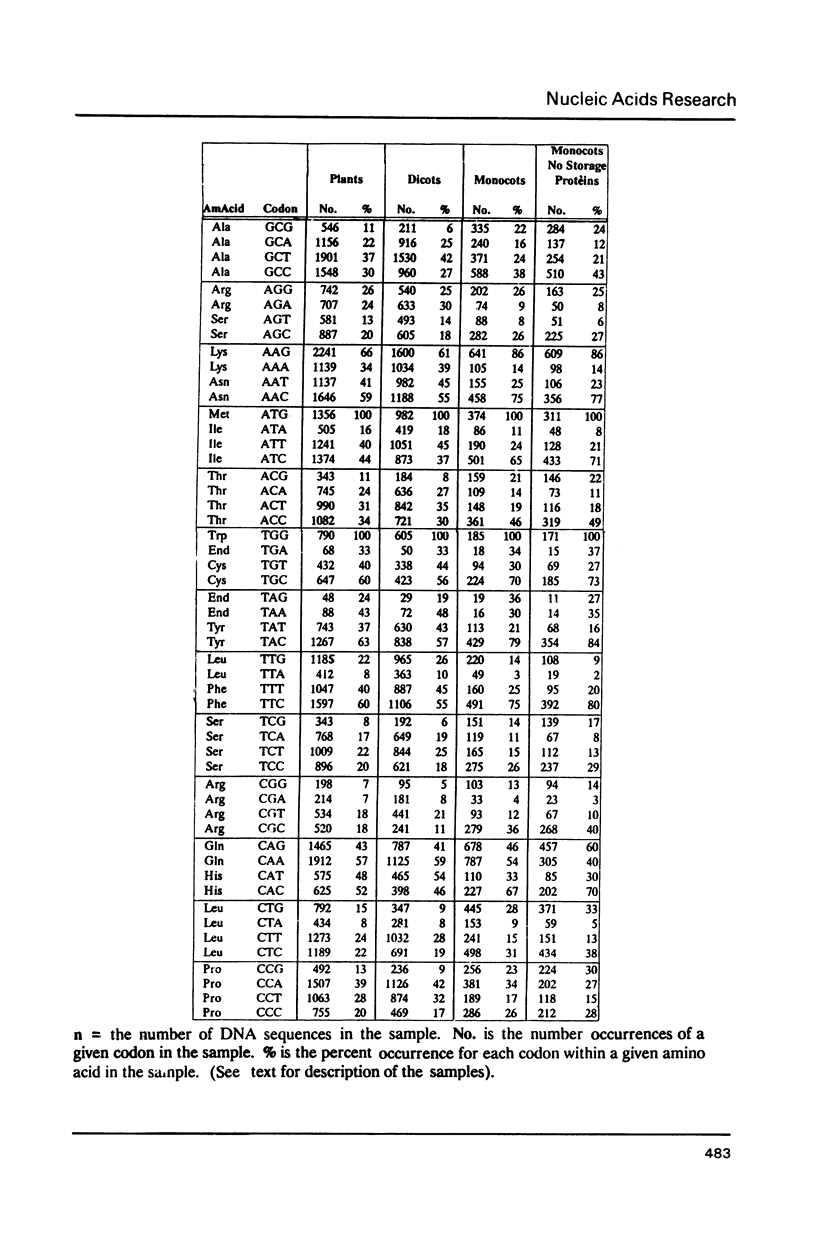
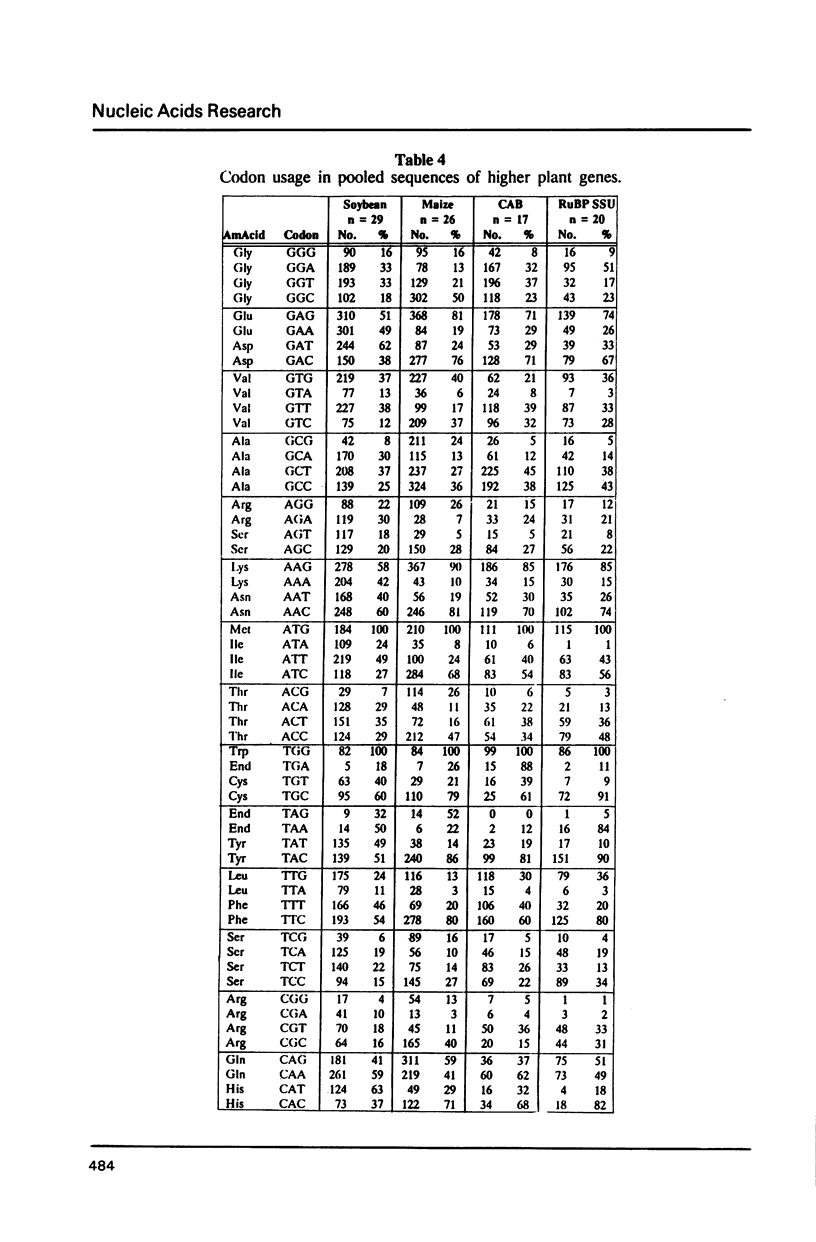
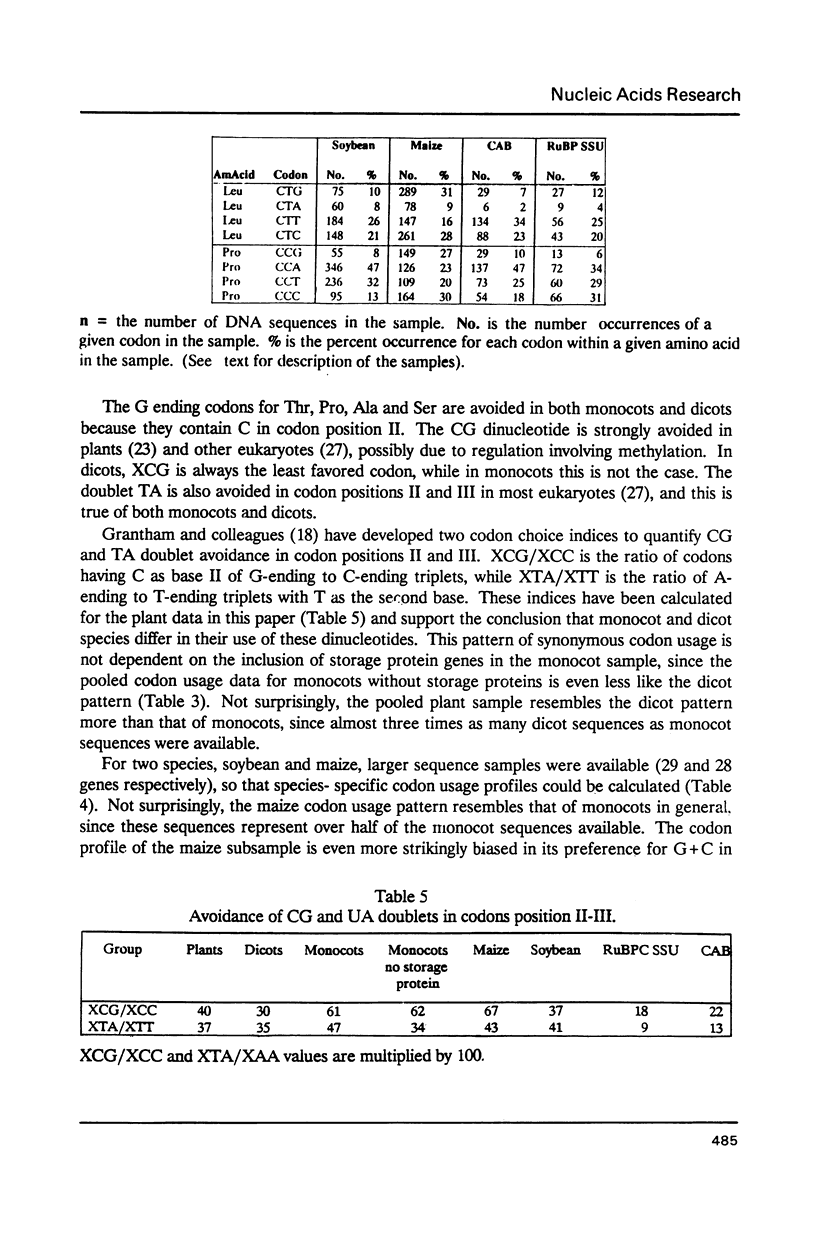
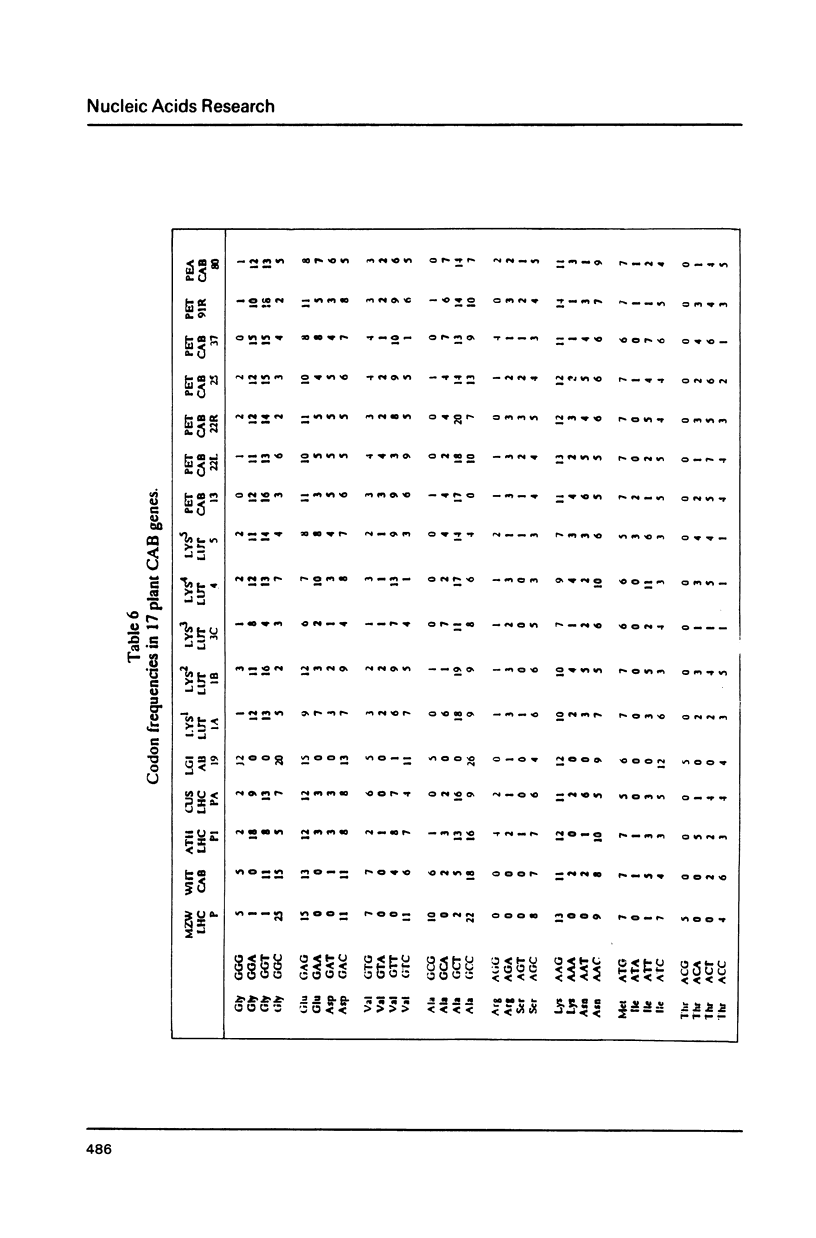
We have examined codon bias in 207 plant gene sequences collected from Genbank and the literature. When this sample was further divided into 53 monocot and 154 dicot genes, the pattern of relative use of synonymous codons was shown to differ between these taxonomic groups, primarily in the use of G + C in the degenerate third base. Maize and soybean codon bias were examined separately and followed the monocot and dicot codon usage patterns respectively. Codon preference in ribulose 1,5 bisphosphate and chlorophyll a/b binding protein, two of the most abundant proteins in leaves was investigated. These highly expressed are more restricted in their codon usage than plant genes in general.
Full text
PDF

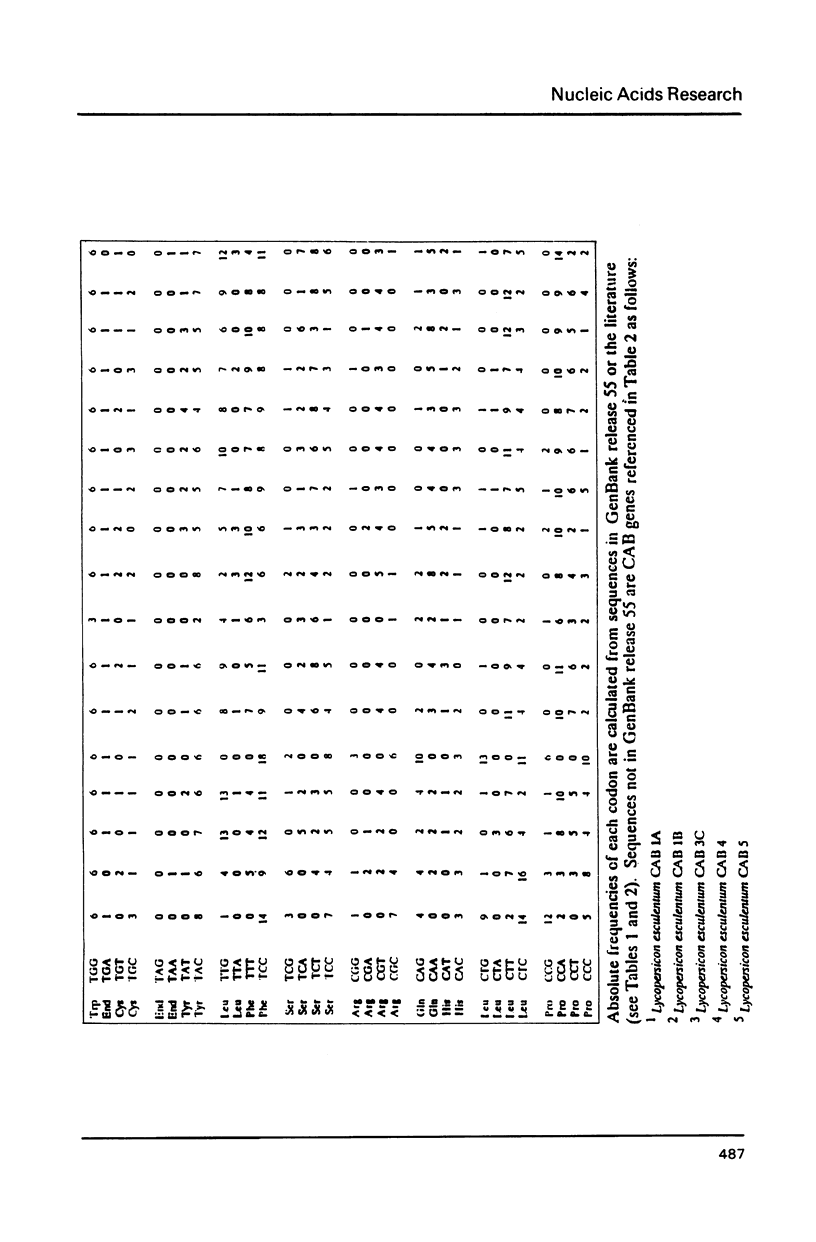
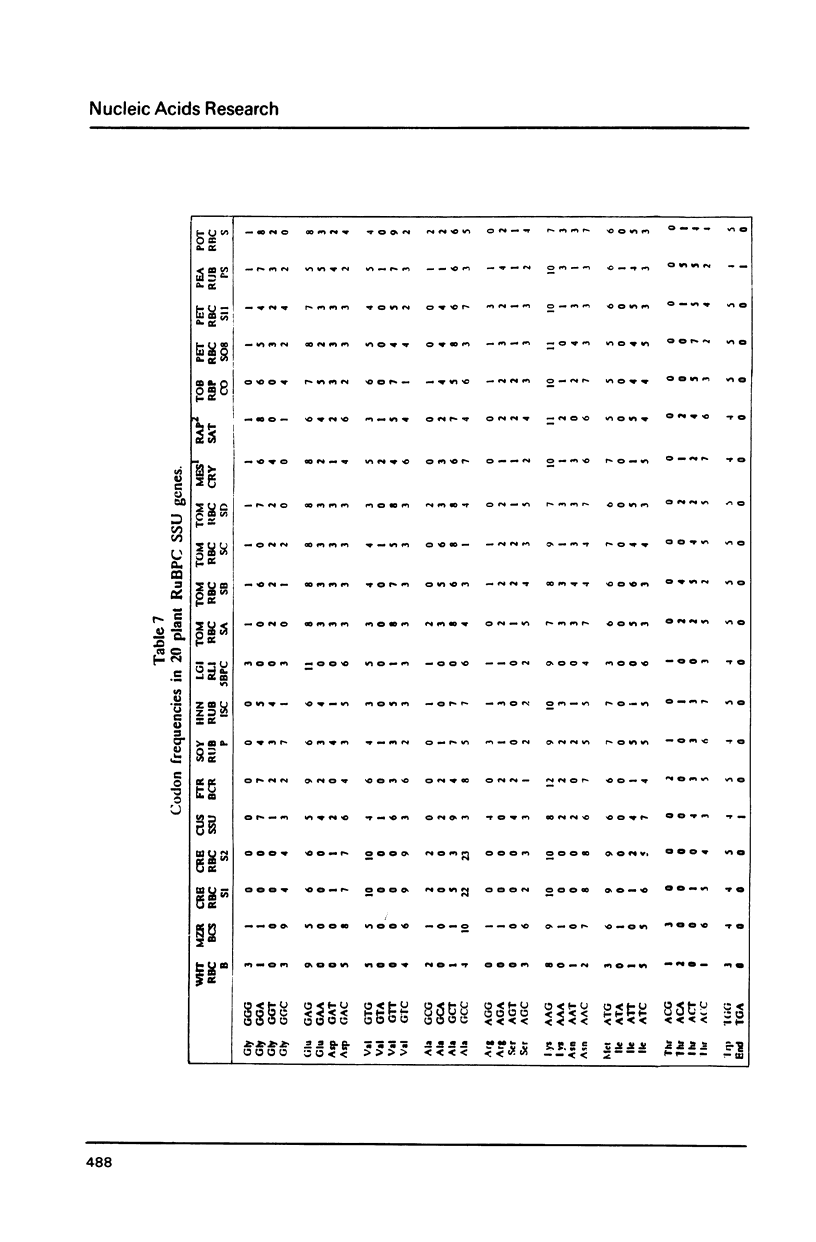
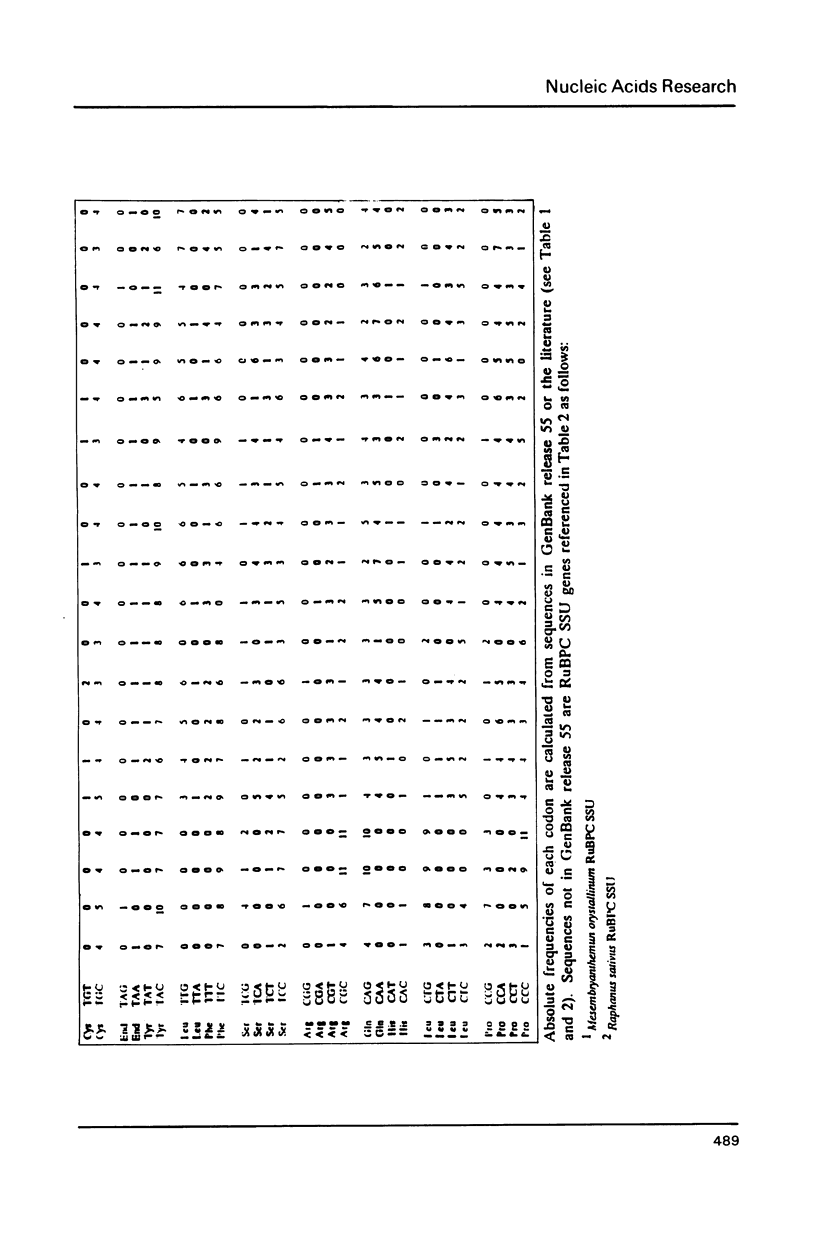









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Babcock M., Leung F., Sun S. M. Sequence of a ribulose 1,5-bisphosphate carboxylase/oxygenase cDNA from the C4 dicot Flaveria trinervia. Nucleic Acids Res. 1987 Feb 25;15(4):1875–1875. doi: 10.1093/nar/15.4.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D., Cohen E. A., Vonder Haar R. A., Adams C. A., Ma D. P., Nessler C. L., Thomas T. L. Sequence and expression of a gene encoding an albumin storage protein in sunflower. Mol Gen Genet. 1987 Dec;210(2):211–218. doi: 10.1007/BF00325686. [DOI] [PubMed] [Google Scholar]
- Aota S., Gojobori T., Ishibashi F., Maruyama T., Ikemura T. Codon usage tabulated from the GenBank Genetic Sequence Data. Nucleic Acids Res. 1988;16 (Suppl):r315–r402. doi: 10.1093/nar/16.suppl.r315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A., Leaver C. J. Isolation and sequence analysis of a cDNA encoding the ATP/ADP translocator of Zea mays L. Nucleic Acids Res. 1985 Aug 26;13(16):5857–5867. doi: 10.1093/nar/13.16.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassüner R., Hai N. V., Jung R., Saalbach G., Müntz K. The primary structure of the predominating vicilin storage protein subunit from field bean seeds (Vicia faba L. var. minor cv. Fribo). Nucleic Acids Res. 1987 Nov 25;15(22):9609–9609. doi: 10.1093/nar/15.22.9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D C, Huttly A K, Martienssen R A, Barker R F, Jarvis M G. A novel wheat alpha-amylase gene (alpha-Amy3). Mol Gen Genet. 1987 Aug;209(1):33–40. doi: 10.1007/BF00329833. [DOI] [PubMed] [Google Scholar]
- Baulcombe D. C., Barker R. F., Jarvis M. G. A gibberellin responsive wheat gene has homology to yeast carboxypeptidase Y. J Biol Chem. 1987 Oct 5;262(28):13726–13735. [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bernardi G., Bernardi G. Codon usage and genome composition. J Mol Evol. 1985;22(4):363–365. doi: 10.1007/BF02115693. [DOI] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Bethards L. A., Skadsen R. W., Scandalios J. G. Isolation and characterization of a cDNA clone for the Cat2 gene in maize and its homology with other catalases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6830–6834. doi: 10.1073/pnas.84.19.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson N., Verma D. P. Soybean leghemoglobin gene family: normal, pseudo, and truncated genes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4055–4059. doi: 10.1073/pnas.79.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumlein H., Wobus U., Pustell J., Kafatos F. C. The legumin gene family: structure of a B type gene of Vicia faba and a possible legumin gene specific regulatory element. Nucleic Acids Res. 1986 Mar 25;14(6):2707–2720. doi: 10.1093/nar/14.6.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R, Huttner E, Vincentz M, Rouzé P, Galangau F, Vaucheret H, Chérel I, Meyer C, Kronenberger J, Caboche M. Cloning of DNA fragments complementary to tobacco nitrate reductase mRNA and encoding epitopes common to the nitrate reductases from higher plants. Mol Gen Genet. 1987 Oct;209(3):552–562. doi: 10.1007/BF00331162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R. Structure and expression of a pea nuclear gene encoding a chlorophyll a/b-binding polypeptide. Proc Natl Acad Sci U S A. 1984 May;81(10):2960–2964. doi: 10.1073/pnas.81.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. W., Coghlan V. M., Dihel L. C. Cloning and sequencing of papain-encoding cDNA. Gene. 1986;48(2-3):219–227. doi: 10.1016/0378-1119(86)90080-6. [DOI] [PubMed] [Google Scholar]
- Coraggio I., Compagno C., Martegani E., Ranzi B. M., Sala E., Alberghina L., Viotti A. Transcription and expression of zein sequences in yeast under natural plant or yeast promoters. EMBO J. 1986 Mar;5(3):459–465. doi: 10.1002/j.1460-2075.1986.tb04234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Hooft van Huijsduijnen R. A., Bol J. F. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. 1986 May 29-Jun 4Nature. 321(6069):531–532. doi: 10.1038/321531a0. [DOI] [PubMed] [Google Scholar]
- Cornelissen B. J., Hooft van Huijsduijnen R. A., Van Loon L. C., Bol J. F. Molecular characterization of messenger RNAs for 'pathogenesis related' proteins la, lb and lc, induced by TMV infection of tobacco. EMBO J. 1986 Jan;5(1):37–40. doi: 10.1002/j.1460-2075.1986.tb04174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecka E., Nagao R. T., Key J. L., Gurley W. B. Characterization of Gmhsp26-A, a stress gene encoding a divergent heat shock protein of soybean: heavy-metal-induced inhibition of intron processing. Mol Cell Biol. 1988 Mar;8(3):1113–1122. doi: 10.1128/mcb.8.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRocher E. J., Ramage R. T., Michalowski C. B., Bohnert H. J. Nucleotide sequence of a cDNA encoding rbcS from the desert plant Mesembryanthemum crystallinum. Nucleic Acids Res. 1987 Aug 11;15(15):6301–6301. doi: 10.1093/nar/15.15.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Sachs M. M., Gerlach W. L., Finnegan E. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase 2 (Adh2) gene of maize. Nucleic Acids Res. 1985 Feb 11;13(3):727–743. doi: 10.1093/nar/13.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. L., Rödin J., Lenman M., Glimelius K., Josefsson L. G., Rask L. Structure of the rapeseed 1.7 S storage protein, napin, and its precursor. J Biol Chem. 1986 Nov 5;261(31):14576–14581. [PubMed] [Google Scholar]
- Fincher G. B., Lock P. A., Morgan M. M., Lingelbach K., Wettenhall R. E., Mercer J. F., Brandt A., Thomsen K. K. Primary structure of the (1-->3,1-->4)-beta-D-glucan 4-glucohydrolase from barley aleurone. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2081–2085. doi: 10.1073/pnas.83.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Heyworth A., Pywell J., Kreis M. Nucleotide sequence of a B1 hordein gene and the identification of possible upstream regulatory elements in endosperm storage protein genes from barley, wheat and maize. Nucleic Acids Res. 1985 Oct 25;13(20):7327–7339. doi: 10.1093/nar/13.20.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Kreis M., Williamson M. S., Fry R. P., Pywell J., Shewry P. R., Bunce N., Miflin B. J. Short tandem repeats shared by B- and C-hordein cDNAs suggest a common evolutionary origin for two groups of cereal storage protein genes. EMBO J. 1985 Jan;4(1):9–15. doi: 10.1002/j.1460-2075.1985.tb02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M. G., Morrison N. A., Verma D. P. Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 1987 Jan 26;15(2):813–824. doi: 10.1093/nar/15.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H. J., Nap J. P., Gloudemans T., Stiekema W., Van Dam H., Govers F., Louwerse J., Van Kammen A., Bisseling T. Characterization of cDNA for nodulin-75 of soybean: A gene product involved in early stages of root nodule development. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4495–4499. doi: 10.1073/pnas.84.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa C., Momma T., Hirano H., Harada K., Udaka K. Glycinin A3B4 mRNA. Cloning and sequencing of double-stranded cDNA complementary to a soybean storage protein. J Biol Chem. 1985 May 25;260(10):6234–6239. [PubMed] [Google Scholar]
- Fukazawa C., Udaka K., Murayama A., Higuchi W., Totsuka A. Expression of soybean glycinin subunit precursor cDNAs in Escherichia coli. FEBS Lett. 1987 Nov 16;224(1):125–127. doi: 10.1016/0014-5793(87)80434-9. [DOI] [PubMed] [Google Scholar]
- Gantt J. S., Key J. L. Molecular cloning of a pea H1 histone cDNA. Eur J Biochem. 1987 Jul 1;166(1):119–125. doi: 10.1111/j.1432-1033.1987.tb13490.x. [DOI] [PubMed] [Google Scholar]
- Gatehouse J. A., Bown D., Evans I. M., Gatehouse L. N., Jobes D., Preston P., Croy R. R. Sequence of the seed lectin gene from pea (Pisum sativum L.). Nucleic Acids Res. 1987 Sep 25;15(18):7642–7642. doi: 10.1093/nar/15.18.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby A. A., van der Vies S. M., Rothstein S. J. Co-expression of both the maize large and wheat small subunit genes of ribulose-bisphosphate carboxylase in Escherichia coli. Eur J Biochem. 1987 Oct 1;168(1):227–231. doi: 10.1111/j.1432-1033.1987.tb13409.x. [DOI] [PubMed] [Google Scholar]
- Gausing K., Barkardottir R. Structure and expression of ubiquitin genes in higher plants. Eur J Biochem. 1986 Jul 1;158(1):57–62. doi: 10.1111/j.1432-1033.1986.tb09720.x. [DOI] [PubMed] [Google Scholar]
- Gebhardt C., Oliver J. E., Forde B. G., Saarelainen R., Miflin B. J. Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J. 1986 Jul;5(7):1429–1435. doi: 10.1002/j.1460-2075.1986.tb04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D. E., Messing J., Rubenstein I. Sequence analysis and comparison of cDNAs of the zein multigene family . EMBO J. 1982;1(11):1329–1335. doi: 10.1002/j.1460-2075.1982.tb01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L. H., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of pre-inhibitor II. J Biol Chem. 1985 Jun 10;260(11):6561–6564. [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M. Codon frequencies in 119 individual genes confirm consistent choices of degenerate bases according to genome type. Nucleic Acids Res. 1980 May 10;8(9):1893–1912. doi: 10.1093/nar/8.9.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Mercier R., Pavé A. Codon catalog usage and the genome hypothesis. Nucleic Acids Res. 1980 Jan 11;8(1):r49–r62. doi: 10.1093/nar/8.1.197-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson D., Tucker G. A., Keen J., Ray J., Bird C. R., Schuch W. Sequencing and identification of a cDNA clone for tomato polygalacturonase. Nucleic Acids Res. 1986 Nov 11;14(21):8595–8603. doi: 10.1093/nar/14.21.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidet F., Fourcroy P. Nucleotide sequence of a radish ribulose 1,5 bisphosphate carboxylase small subunit (rbcS) cDNA. Nucleic Acids Res. 1988 Mar 25;16(5):2336–2336. doi: 10.1093/nar/16.5.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. W., Foard D. E., Larkins B. A. Molecular cloning and analysis of a gene coding for the Bowman-Birk protease inhibitor in soybean. J Biol Chem. 1984 Aug 10;259(15):9883–9890. [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr Codon usage in muscle genes and liver genes. J Mol Evol. 1983;19(3-4):214–218. doi: 10.1007/BF02099968. [DOI] [PubMed] [Google Scholar]
- Hershey H. P., Barker R. F., Idler K. B., Lissemore J. L., Quail P. H. Analysis of cloned cDNA and genomic sequences for phytochrome: complete amino acid sequences for two gene products expressed in etiolated Avena. Nucleic Acids Res. 1985 Dec 9;13(23):8543–8559. doi: 10.1093/nar/13.23.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi W., Fukazawa C. A rice glutelin and a soybean glycinin have evolved from a common ancestral gene. Gene. 1987;55(2-3):245–253. doi: 10.1016/0378-1119(87)90284-8. [DOI] [PubMed] [Google Scholar]
- Hoekema A., Kastelein R. A., Vasser M., de Boer H. A. Codon replacement in the PGK1 gene of Saccharomyces cerevisiae: experimental approach to study the role of biased codon usage in gene expression. Mol Cell Biol. 1987 Aug;7(8):2914–2924. doi: 10.1128/mcb.7.8.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M., Donaldson D. D. Characterization of two Phaseolus vulgaris phytohemagglutinin genes closely linked on the chromosome. EMBO J. 1985 Apr;4(4):883–889. doi: 10.1002/j.1460-2075.1985.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M. Structure of a chromosomal Phaseolus vulgaris lectin gene and its transcript. J Mol Appl Genet. 1984;2(5):447–453. [PubMed] [Google Scholar]
- Hoffman N. E., Pichersky E., Cashmore A. R. A tomato cDNA encoding a biotin-binding protein. Nucleic Acids Res. 1987 May 11;15(9):3928–3928. doi: 10.1093/nar/15.9.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Pichersky E., Malik V. S., Castresana C., Ko K., Darr S. C., Cashmore A. R. A cDNA clone encoding a photosystem I protein with homology to photosystem II chlorophyll a/b-binding polypeptides. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8844–8848. doi: 10.1073/pnas.84.24.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth M. J., Bird C. R., Ray J., Schuch W., Grierson D. Structure and expression of an ethylene-related mRNA from tomato. Nucleic Acids Res. 1987 Jan 26;15(2):731–739. doi: 10.1093/nar/15.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. C., Nagao R. T., Key J. L. Characterization and sequence analysis of a developmentally regulated putative cell wall protein gene isolated from soybean. J Biol Chem. 1987 Jun 15;262(17):8367–8376. [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985 Jan;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Izui K., Ishijima S., Yamaguchi Y., Katagiri F., Murata T., Shigesada K., Sugiyama T., Katsuki H. Cloning and sequence analysis of cDNA encoding active phosphoenolpyruvate carboxylase of the C4-pathway from maize. Nucleic Acids Res. 1986 Feb 25;14(4):1615–1628. doi: 10.1093/nar/14.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs F. A., Zhang M., Fortin M. G., Verma D. P. Several nodulins of soybean share structural domains but differ in their subcellular locations. Nucleic Acids Res. 1987 Feb 11;15(3):1271–1280. doi: 10.1093/nar/15.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Kohorn B. D., Thornber J. P., Tobin E. M. A chlorophyll a/b-protein encoded by a gene containing an intron with characteristics of a transposable element. J Mol Appl Genet. 1985;3(1):45–61. [PubMed] [Google Scholar]
- Katinakis P., Verma D. P. Nodulin-24 gene of soybean codes for a peptide of the peribacteroid membrane and was generated by tandem duplication of a sequence resembling an insertion element. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4157–4161. doi: 10.1073/pnas.82.12.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Tolan D. R. The complete amino Acid sequence for the anaerobically induced aldolase from maize derived from cDNA clones. Plant Physiol. 1986 Dec;82(4):1076–1080. doi: 10.1104/pp.82.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G. B., Végh Z., Vincze E. Nucleotide sequence of a cDNA clone encoding leghemoglobin III (LbIII) from Medicago sativa. Nucleic Acids Res. 1987 Apr 24;15(8):3620–3620. doi: 10.1093/nar/15.8.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H. J., Muskopf Y. M., Gasser C. S. Cloning of an Arabidopsis thaliana gene encoding 5-enolpyruvylshikimate-3-phosphate synthase: sequence analysis and manipulation to obtain glyphosate-tolerant plants. Mol Gen Genet. 1987 Dec;210(3):437–442. doi: 10.1007/BF00327194. [DOI] [PubMed] [Google Scholar]
- Koes R. E., Spelt C. E., Reif H. J., van den Elzen P. J., Veltkamp E., Mol J. N. Floral tissue of Petunia hybrida (V30) expresses only one member of the chalcone synthase multigene family. Nucleic Acids Res. 1986 Jul 11;14(13):5229–5239. doi: 10.1093/nar/14.13.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A. Nucleotide sequence of lupin leghemoglobin I cDNA. Nucleic Acids Res. 1987 Aug 25;15(16):6742–6742. doi: 10.1093/nar/15.16.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis M., Forde B. G., Rahman S., Miflin B. J., Shewry P. R. Molecular evolution of the seed storage proteins of barley, rye and wheat. J Mol Biol. 1985 Jun 5;183(3):499–502. doi: 10.1016/0022-2836(85)90017-8. [DOI] [PubMed] [Google Scholar]
- Krueger R. W., Holland M. A., Chisholm D., Polacco J. C. Recovery of a soybean urease genomic clone by sequential library screening with two synthetic oligodeoxynucleotides. Gene. 1987;54(1):41–50. doi: 10.1016/0378-1119(87)90345-3. [DOI] [PubMed] [Google Scholar]
- Lagrimini L. M., Burkhart W., Moyer M., Rothstein S. Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: Molecular analysis and tissue-specific expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7542–7546. doi: 10.1073/pnas.84.21.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G. K., Morelli G., Chua N. H. Structure and developmental regulation of a wheat gene encoding the major chlorophyll a/b-binding polypeptide. Mol Cell Biol. 1985 Jun;5(6):1370–1378. doi: 10.1128/mcb.5.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M., Waksman G., Freyssinet G. Nucleotide sequence of a gene encoding corn ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit (rbcs). Nucleic Acids Res. 1987 May 26;15(10):4360–4360. doi: 10.1093/nar/15.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Brown W. E., Graham J. S., Pearce G., Fox E. A., Dreher T. W., Ahern K. G., Pearson G. D., Ryan C. A. Molecular characterization and phylogenetic studies of a wound-inducible proteinase inhibitor I gene in Lycopersicon species. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7277–7281. doi: 10.1073/pnas.83.19.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Verma D. P. Structure and chromosomal arrangement of leghemoglobin genes in kidney bean suggest divergence in soybean leghemoglobin gene loci following tetraploidization. EMBO J. 1984 Dec 1;3(12):2745–2752. doi: 10.1002/j.1460-2075.1984.tb02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutwiler L. S., Meyerowitz E. M., Tobin E. M. Structure and expression of three light-harvesting chlorophyll a/b-binding protein genes in Arabidopsis thaliana. Nucleic Acids Res. 1986 May 27;14(10):4051–4064. doi: 10.1093/nar/14.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litts J. C., Colwell G. W., Chakerian R. L., Quatrano R. S. The nucleotide sequence of a cDNA clone encoding the wheat Em protein. Nucleic Acids Res. 1987 Apr 24;15(8):3607–3618. doi: 10.1093/nar/15.8.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn D. J., Finnegan E. J., Ellis J. G., Dennis E. S., Peacock W. J. Structure and expression of an alcohol dehydrogenase 1 gene from Pisum sativum (cv. "Greenfeast"). J Mol Biol. 1987 May 5;195(1):115–123. doi: 10.1016/0022-2836(87)90331-7. [DOI] [PubMed] [Google Scholar]
- Ludwig S. R., Oppenheimer D. G., Silflow C. D., Snustad D. P. Characterization of the alpha-tubulin gene family of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5833–5837. doi: 10.1073/pnas.84.16.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G. W., Croy R. R., Shirsat A. H., Boulter D. The complete nucleotide sequence of a legumin gene from pea (Pisum sativum L.). Nucleic Acids Res. 1984 Jun 11;12(11):4493–4506. doi: 10.1093/nar/12.11.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G. W., Delauney A. J., Gatehouse J. A., Gilroy J., Croy R. R., Boulter D. The vicilin gene family of pea (Pisum sativum L.): a complete cDNA coding sequence for preprovicilin. Nucleic Acids Res. 1983 Apr 25;11(8):2367–2380. doi: 10.1093/nar/11.8.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni M., Gilbert W. The triosephosphate isomerase gene from maize: introns antedate the plant-animal divergence. Cell. 1986 Jul 4;46(1):133–141. doi: 10.1016/0092-8674(86)90867-6. [DOI] [PubMed] [Google Scholar]
- Marks M. D., Larkins B. A. Analysis of sequence microheterogeneity among zein messenger RNAs. J Biol Chem. 1982 Sep 10;257(17):9976–9983. [PubMed] [Google Scholar]
- Marks M. D., Lindell J. S., Larkins B. A. Nucleotide sequence analysis of zein mRNAs from maize endosperm. J Biol Chem. 1985 Dec 25;260(30):16451–16459. [PubMed] [Google Scholar]
- Martin W., Cerff R. Prokaryotic features of a nucleus-encoded enzyme. cDNA sequences for chloroplast and cytosolic glyceraldehyde-3-phosphate dehydrogenases from mustard (Sinapis alba). Eur J Biochem. 1986 Sep 1;159(2):323–331. doi: 10.1111/j.1432-1033.1986.tb09871.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Kano-Murakami Y., Tanaka Y., Ozeki Y., Yamamoto N. Nucleotide sequence of cDNA encoding the small subunit of ribulose-1,5-bisphosphate carboxylase from maize. J Biochem. 1987 Oct;102(4):673–676. doi: 10.1093/oxfordjournals.jbchem.a122103. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Kano-Murakami Y., Yamamoto N. Nucleotide sequence of cDNA encoding the light-harvesting chlorophyll a/b binding protein from maize. Nucleic Acids Res. 1987 Aug 11;15(15):6302–6302. doi: 10.1093/nar/15.15.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D. J., Cantwell B. A., Devine K. M., Forage A. J., Laoide B. M., O'Kane C., Ollington J. F., Sharp P. M. Genetic engineering of extracellular enzyme systems of Bacilli. Ann N Y Acad Sci. 1986;469:1–17. doi: 10.1111/j.1749-6632.1986.tb26480.x. [DOI] [PubMed] [Google Scholar]
- McKnight T. D., Alexander D. C., Babcock M. S., Simpson R. B. Nucleotide sequence and molecular evolution of two tomato genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. Gene. 1986;48(1):23–32. doi: 10.1016/0378-1119(86)90348-3. [DOI] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. The Cu(II)-repressible plastidic cytochrome c. Cloning and sequence of a complementary DNA for the pre-apoprotein. J Biol Chem. 1987 Jul 5;262(19):9062–9067. [PubMed] [Google Scholar]
- Mignery G. A., Pikaard C. S., Hannapel D. J., Park W. D. Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res. 1984 Nov 12;12(21):7987–8000. doi: 10.1093/nar/12.21.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momma T., Negoro T., Hirano H., Matsumoto A., Udaka K., Fukazawa C. Glycinin A5A4B3 mRNA: cDNA cloning and nucleotide sequencing of a splitting storage protein subunit of soybean. Eur J Biochem. 1985 Jun 18;149(3):491–496. doi: 10.1111/j.1432-1033.1985.tb08951.x. [DOI] [PubMed] [Google Scholar]
- Moore R. E., Davies M. S., O'Connell K. M., Harding E. I., Wiegand R. C., Tiemeier D. C. Cloning and expression of a cDNA encoding a maize glutathione-S-transferase in E. coli. Nucleic Acids Res. 1986 Sep 25;14(18):7227–7235. doi: 10.1093/nar/14.18.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R. T., Czarnecka E., Gurley W. B., Schöffl F., Key J. L. Genes for low-molecular-weight heat shock proteins of soybeans: sequence analysis of a multigene family. Mol Cell Biol. 1985 Dec;5(12):3417–3428. doi: 10.1128/mcb.5.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro T., Momma T., Fukazawa C. A cDNA clone encoding a glycinin A1a subunit precursor of soybean. Nucleic Acids Res. 1985 Sep 25;13(18):6719–6731. doi: 10.1093/nar/13.18.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J. D., Litts J. C., Anderson O. D., Greene F. C., Stiles J. I. Expression of a wheat alpha-gliadin gene in Saccharomyces cerevisiae. Gene. 1987;55(2-3):303–317. doi: 10.1016/0378-1119(87)90290-3. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N. Markedly unbiased codon usage in Bacillus subtilis. Gene. 1985;40(1):145–150. doi: 10.1016/0378-1119(85)90035-6. [DOI] [PubMed] [Google Scholar]
- Okita T. W., Cheesbrough V., Reeves C. D. Evolution and heterogeneity of the alpha-/beta-type and gamma-type gliadin DNA sequences. J Biol Chem. 1985 Jul 5;260(13):8203–8213. [PubMed] [Google Scholar]
- Osborni T. C., Alexander D. C., Sun S. S., Cardona C., Bliss F. A. Insecticidal activity and lectin homology of arcelin seed protein. Science. 1988 Apr 8;240(4849):207–210. doi: 10.1126/science.240.4849.207. [DOI] [PubMed] [Google Scholar]
- Paz-Ares J., Ghosal D., Wienand U., Peterson P. A., Saedler H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987 Dec 1;6(12):3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K., Devereux J., Wilson D. R., Sheldon E., Larkins B. A. Cloning and sequence analysis reveal structural variation among related zein genes in maize. Cell. 1982 Jul;29(3):1015–1026. doi: 10.1016/0092-8674(82)90465-2. [DOI] [PubMed] [Google Scholar]
- Pfitzinger H., Guillemaut P., Weil J. H., Pillay D. T. Adjustment of the tRNA population to the codon usage in chloroplasts. Nucleic Acids Res. 1987 Feb 25;15(4):1377–1386. doi: 10.1093/nar/15.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzner U. M., Goodman H. M. Isolation and characterization of cDNA clones encoding pathogenesis-related proteins from tobacco mosaic virus infected tobacco plants. Nucleic Acids Res. 1987 Jun 11;15(11):4449–4465. doi: 10.1093/nar/15.11.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps G., Chaubet N., Chaboute M. E., Ehling M., Gigot C. Genomic organization and nucleotide sequences of two corn histone H4 genes. Gene. 1986;42(2):225–229. doi: 10.1016/0378-1119(86)90301-x. [DOI] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Breidenbach R. B., Kausch A. P., Cashmore A. R. Molecular characterization and genetic mapping of two clusters of genes encoding chlorophyll a/b-binding proteins in Lycopersicon esculentum (tomato). Gene. 1985;40(2-3):247–258. doi: 10.1016/0378-1119(85)90047-2. [DOI] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Cashmore A. R. Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3880–3884. doi: 10.1073/pnas.83.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponz F., Paz-Ares J., Hernández-Lucas C., García-Olmedo F., Carbonero P. Cloning and nucleotide sequence of a cDNA encoding the precursor of the barley toxin alpha-hordothionin. Eur J Biochem. 1986 Apr 1;156(1):131–135. doi: 10.1111/j.1432-1033.1986.tb09557.x. [DOI] [PubMed] [Google Scholar]
- Prat S., Cortadas J., Puigdomènech P., Palau J. Nucleic acid (cDNA) and amino acid sequences of the maize endosperm protein glutelin-2. Nucleic Acids Res. 1985 Mar 11;13(5):1493–1504. doi: 10.1093/nar/13.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat S., Pérez-Grau L., Puigdomènech P. Multiple variability in the sequence of a family of maize endosperm proteins. Gene. 1987;52(1):41–49. doi: 10.1016/0378-1119(87)90393-3. [DOI] [PubMed] [Google Scholar]
- Rafalski J. A. Structure of wheat gamma-gliadin genes. Gene. 1986;43(3):221–229. doi: 10.1016/0378-1119(86)90210-6. [DOI] [PubMed] [Google Scholar]
- Ray J., Bird C., Maunders M., Grierson D., Schuch W. Sequence of pTOM5, a ripening related cDNA from tomato. Nucleic Acids Res. 1987 Dec 23;15(24):10587–10587. doi: 10.1093/nar/15.24.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold U., Kröger M., Kreuzaler F., Hahlbrock K. Coding and 3' non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J. 1983;2(10):1801–1805. doi: 10.1002/j.1460-2075.1983.tb01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C., Dean D., Heck G. R. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6512–6516. doi: 10.1073/pnas.82.19.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E., DeJesus C. E., Moylan S. L., Ridge N. P., Scherer D. E., Knauf V. C. The nucleotide sequence of a cDNA clone encoding acyl carrier protein (ACP) from Brassica campestris seeds. Nucleic Acids Res. 1987 Sep 11;15(17):7197–7197. doi: 10.1093/nar/15.17.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother C., Jansen T., Tyagi A., Tittgen J., Herrmann R. G. Plastocyanin is encoded by an uninterrupted nuclear gene in spinach. Curr Genet. 1986;11(3):171–176. doi: 10.1007/BF00420603. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Lahners K. N., Lazarus C. M., Baulcombe D. C., Gatenby A. A. Synthesis and secretion of wheat alpha-amylase in Saccharomyces cerevisiae. Gene. 1987;55(2-3):353–356. doi: 10.1016/0378-1119(87)90296-4. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Ryder T. B., Hedrick S. A., Bell J. N., Liang X. W., Clouse S. D., Lamb C. J. Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet. 1987 Dec;210(2):219–233. doi: 10.1007/BF00325687. [DOI] [PubMed] [Google Scholar]
- Sakajo S., Nakamura K., Asahi T. Molecular cloning and nucleotide sequence of full-length cDNA for sweet potato catalase mRNA. Eur J Biochem. 1987 Jun 1;165(2):437–442. doi: 10.1111/j.1432-1033.1987.tb11457.x. [DOI] [PubMed] [Google Scholar]
- Salanoubat M., Belliard G. Molecular cloning and sequencing of sucrose synthase cDNA from potato (Solanum tuberosum L.): preliminary characterization of sucrose synthase mRNA distribution. Gene. 1987;60(1):47–56. doi: 10.1016/0378-1119(87)90212-5. [DOI] [PubMed] [Google Scholar]
- Sandal N. N., Bojsen K., Marcker K. A. A small family of nodule specific genes from soybean. Nucleic Acids Res. 1987 Feb 25;15(4):1507–1519. doi: 10.1093/nar/15.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell D. J., Etzler M. E. Primary structure of the Dolichos biflorus seed lectin. J Biol Chem. 1987 May 25;262(15):7220–7225. [PubMed] [Google Scholar]
- Schuler M. A., Ladin B. F., Pollaco J. C., Freyer G., Beachy R. N. Structural sequences are conserved in the genes coding for the alpha, alpha' and beta-subunits of the soybean 7S seed storage protein. Nucleic Acids Res. 1982 Dec 20;10(24):8245–8261. doi: 10.1093/nar/10.24.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Shepherd N., Tacke E., Gierl A., Rohde W., Leclercq L., Mattes M., Berndtgen R., Peterson P. A., Saedler H. Influence of transposable elements on the structure and function of the A1 gene of Zea mays. EMBO J. 1987 Feb;6(2):287–294. doi: 10.1002/j.1460-2075.1987.tb04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Genes encoding actin in higher plants: intron positions are highly conserved but the coding sequences are not. J Mol Appl Genet. 1983;2(1):111–126. [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J Mol Evol. 1986;24(1-2):28–38. doi: 10.1007/BF02099948. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Tuohy T. M., Mosurski K. R. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986 Jul 11;14(13):5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R. A., Lissemore J. L., Quail P. H. Nucleotide and amino acid sequence of a Cucurbita phytochrome cDNA clone: identification of conserved features by comparison with Avena phytochrome. Gene. 1986;47(2-3):287–295. doi: 10.1016/0378-1119(86)90072-7. [DOI] [PubMed] [Google Scholar]
- Shibata D., Steczko J., Dixon J. E., Hermodson M., Yazdanparast R., Axelrod B. Primary structure of soybean lipoxygenase-1. J Biol Chem. 1987 Jul 25;262(21):10080–10085. [PubMed] [Google Scholar]
- Shih M. C., Lazar G., Goodman H. M. Evidence in favor of the symbiotic origin of chloroplasts: primary structure and evolution of tobacco glyceraldehyde-3-phosphate dehydrogenases. Cell. 1986 Oct 10;47(1):73–80. doi: 10.1016/0092-8674(86)90367-3. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Drong R. F., Klassy R. C., Hoffman L. M. Nucleotide sequences from phaseolin cDNA clones: the major storage proteins from Phaseolus vulgaris are encoded by two unique gene families. Nucleic Acids Res. 1985 Sep 25;13(18):6483–6498. doi: 10.1093/nar/13.18.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Bedbrook J., Speirs J. Characterisation of three cDNA clones encoding different mRNAs for the precursor to the small subunit of wheat ribulosebisphosphate carboxylase. Nucleic Acids Res. 1983 Dec 20;11(24):8719–8734. doi: 10.1093/nar/11.24.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Leaver C. J. Glyoxysomal Malate Synthase of Cucumber: Molecular Cloning of a cDNA and Regulation of Enzyme Synthesis during Germination. Plant Physiol. 1986 Jul;81(3):762–767. doi: 10.1104/pp.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiekema W. J., Wimpee C. F., Tobin E. M. Nucleotide sequence encoding the precursor of the small subunit of ribulose 1,5-bisphosphate carboxylase from Lemna gibba L.G-3. Nucleic Acids Res. 1983 Nov 25;11(22):8051–8061. doi: 10.1093/nar/11.22.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Rafalski A., Peterson D., Söll D. A wheat HMW glutenin subunit gene reveals a highly repeated structure. Nucleic Acids Res. 1985 Dec 20;13(24):8729–8737. doi: 10.1093/nar/13.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T., Iwabuchi M. Molecular cloning and nucleotide sequence of a variant wheat histone H4 gene. Gene. 1984 Nov;31(1-3):285–289. doi: 10.1016/0378-1119(84)90223-3. [DOI] [PubMed] [Google Scholar]
- Tingey S. V., Coruzzi G. M. Glutamine Synthetase of Nicotiana plumbaginifolia: Cloning and in Vivo Expression. Plant Physiol. 1987 Jun;84(2):366–373. doi: 10.1104/pp.84.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Walker E. L., Coruzzi G. M. Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J. 1987 Jan;6(1):1–9. doi: 10.1002/j.1460-2075.1987.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., Clark W. G., Tabor G. J., Hironaka C. M., Fraley R. T., Shah D. M. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986 Apr 25;14(8):3325–3342. doi: 10.1093/nar/14.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V. B., Huang A. H. The major protein from lipid bodies of maize. Characterization and structure based on cDNA cloning. J Biol Chem. 1987 Aug 15;262(23):11275–11279. [PubMed] [Google Scholar]
- Vierling E., Nagao R. T., DeRocher A. E., Harris L. M. A heat shock protein localized to chloroplasts is a member of a eukaryotic superfamily of heat shock proteins. EMBO J. 1988 Mar;7(3):575–581. doi: 10.1002/j.1460-2075.1988.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti A., Balducci C., Weil J. H. Adaptation of the tRNA population of maize endosperm for zein synthesis. Biochim Biophys Acta. 1978 Jan 26;517(1):125–132. doi: 10.1016/0005-2787(78)90040-0. [DOI] [PubMed] [Google Scholar]
- Vodkin L. O., Rhodes P. R., Goldberg R. B. cA lectin gene insertion has the structural features of a transposable element. Cell. 1983 Oct;34(3):1023–1031. doi: 10.1016/0092-8674(83)90560-3. [DOI] [PubMed] [Google Scholar]
- Volokita M., Somerville C. R. The primary structure of spinach glycolate oxidase deduced from the DNA sequence of a cDNA clone. J Biol Chem. 1987 Nov 25;262(33):15825–15828. [PubMed] [Google Scholar]
- Waksman G., Freyssinet G. Nucleotide sequence of a cDNA encoding the ribulose-1,5-bisphosphate carboxylase/oxygenase from sunflower (Helianthus annuus). Nucleic Acids Res. 1987 Feb 11;15(3):1328–1328. doi: 10.1093/nar/15.3.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werr W., Frommer W. B., Maas C., Starlinger P. Structure of the sucrose synthase gene on chromosome 9 of Zea mays L. EMBO J. 1985 Jun;4(6):1373–1380. doi: 10.1002/j.1460-2075.1985.tb03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Allenza P., Stachel S. E., McBride K. E., Nester E. W. Characterization of the virE operon of the Agrobacterium Ti plasmid pTiA6. Nucleic Acids Res. 1987 Jan 26;15(2):825–837. doi: 10.1093/nar/15.2.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F. P., Fritz C. C., Willmitzer L., Schell J., Schreier P. H. rbcS genes in Solanum tuberosum: conservation of transit peptide and exon shuffling during evolution. Proc Natl Acad Sci U S A. 1988 Feb;85(3):846–850. doi: 10.1073/pnas.85.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vies S. M., Bradley D., Gatenby A. A. Assembly of cyanobacterial and higher plant ribulose bisphosphate carboxylase subunits into functional homologous and heterologous enzyme molecules in Escherichia coli. EMBO J. 1986 Oct;5(10):2439–2444. doi: 10.1002/j.1460-2075.1986.tb04519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


