Abstract
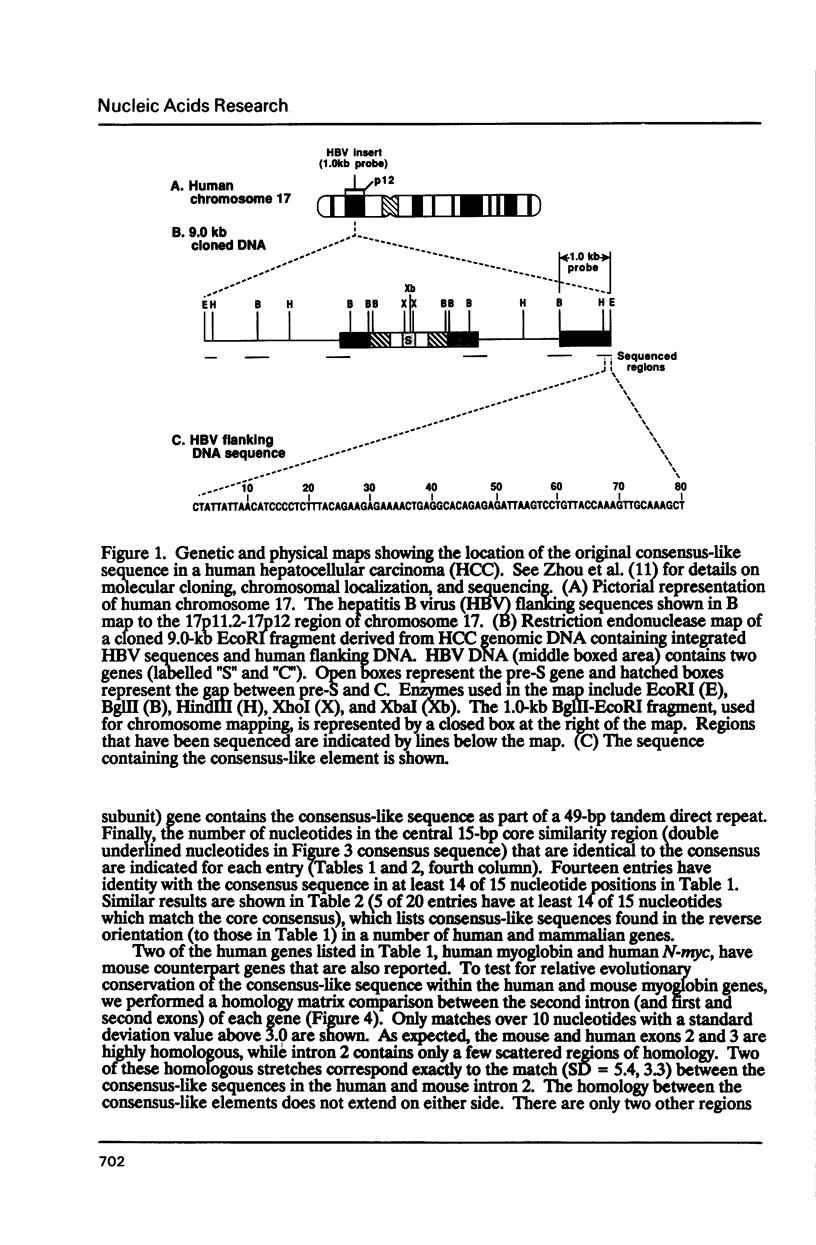
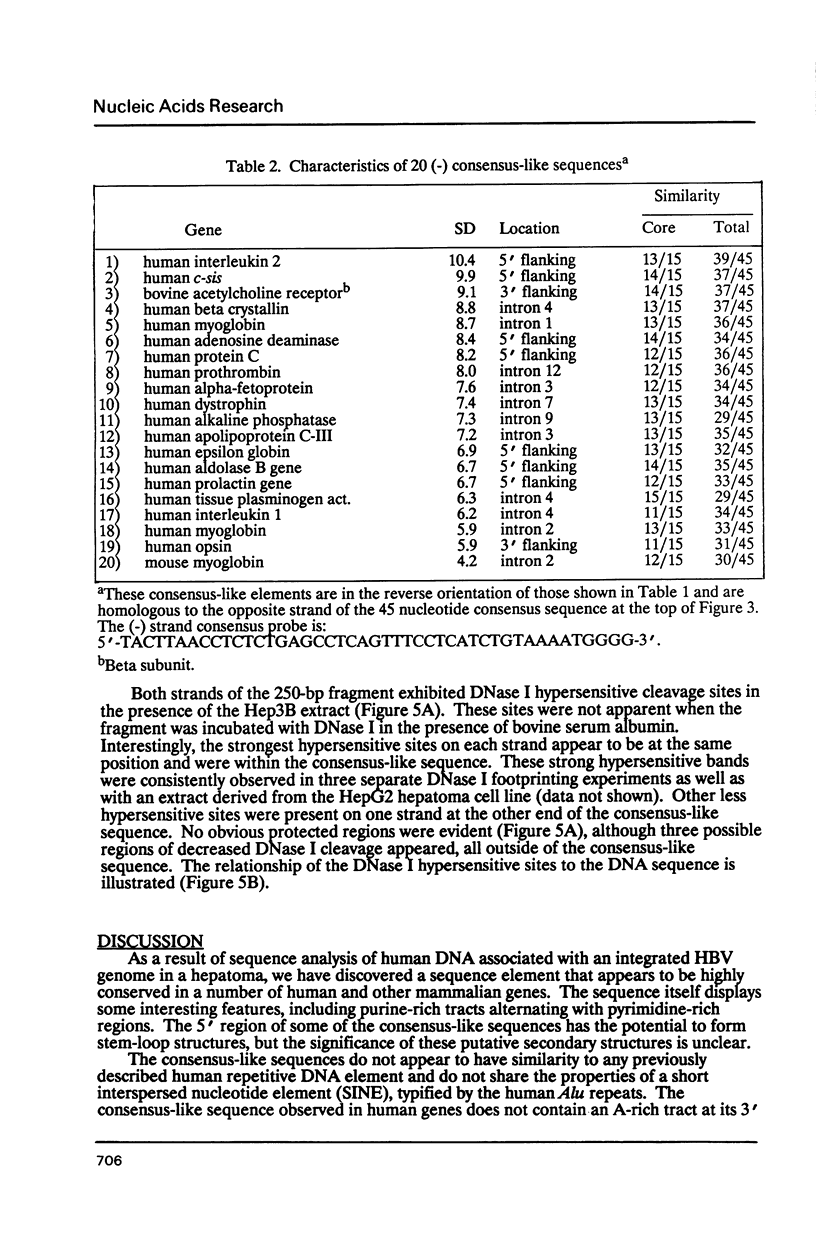
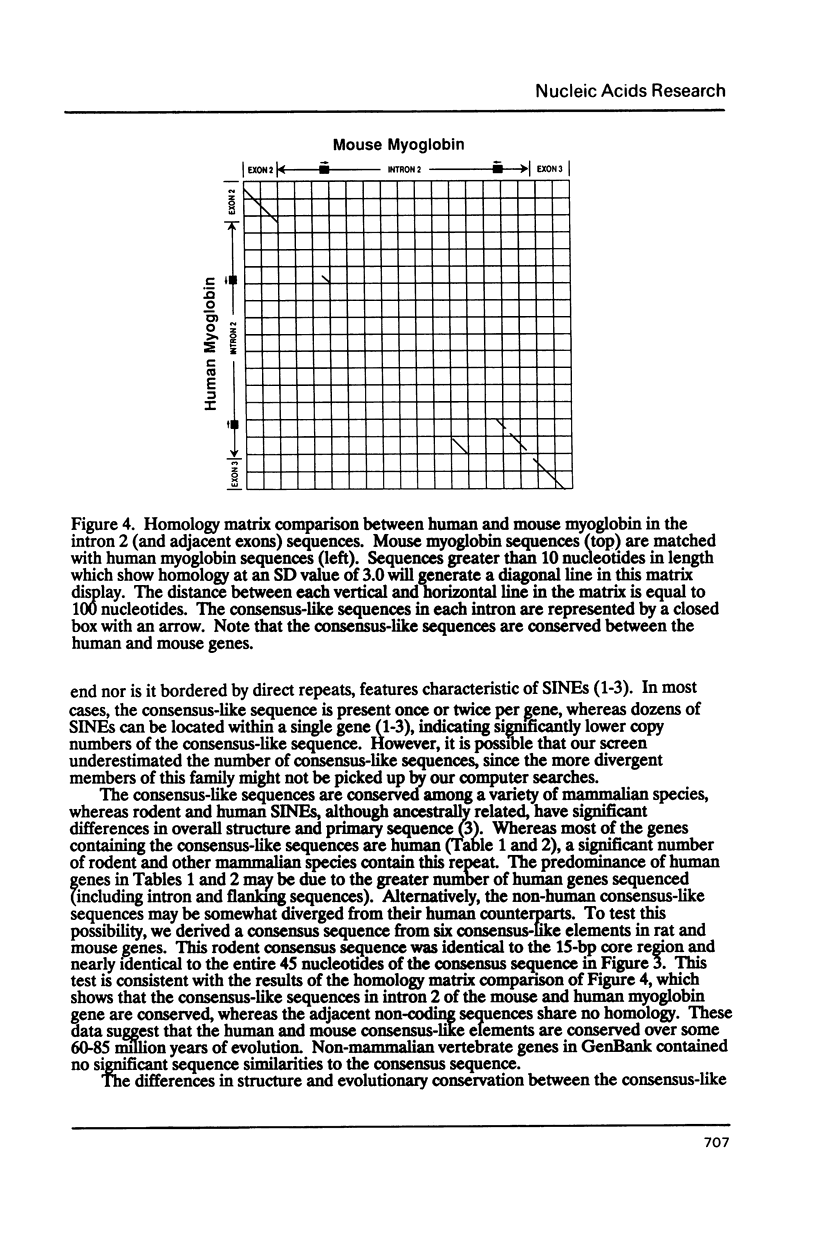
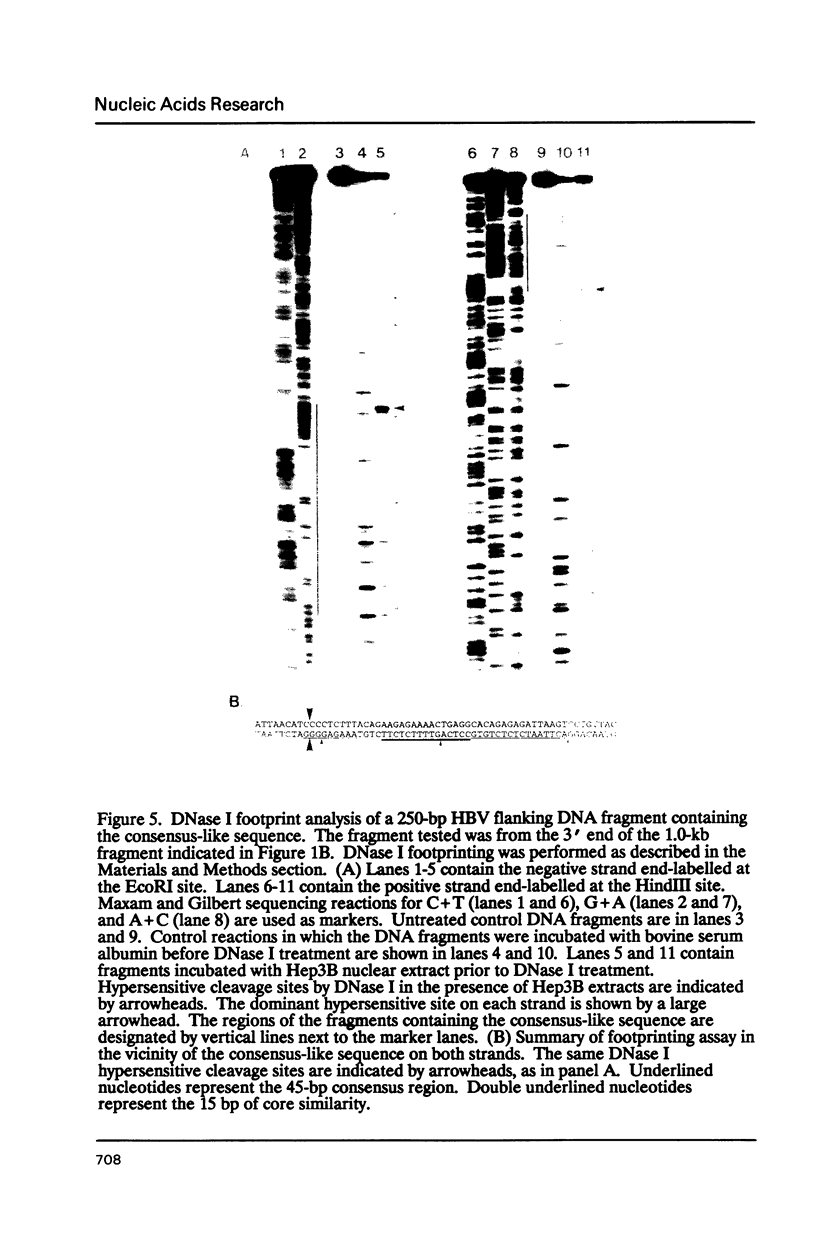
We have analyzed a sequence of approximately 70 base pairs (bp) that shows a high degree of similarity to sequences present in the non-coding regions of a number of human and other mammalian genes. The sequence was discovered in a fragment of human genomic DNA adjacent to an integrated hepatitis B virus genome in cells derived from human hepatocellular carcinoma tissue. When one of the viral flanking sequences was compared to nucleotide sequences in GenBank, more than thirty human genes were identified that contained a similar sequence in their non-coding regions. The sequence element was usually found once or twice in a gene, either in an intron or in the 5' or 3' flanking regions. It did not share any similarities with known short interspersed nucleotide elements (SINEs) or presently known gene regulatory elements. This element was highly conserved at the same position within the corresponding human and mouse genes for myoglobin and N-myc, indicating evolutionary conservation and possible functional importance. Preliminary DNase I footprinting data suggested that the element or its adjacent sequences may bind nuclear factors to generate specific DNase I hypersensitive sites. The size, structure, and evolutionary conservation of this sequence indicates that it is distinct from other types of short interspersed repetitive elements. It is possible that the element may have a cis-acting functional role in the genome.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Erickson B. W. Optimal sequence alignment using affine gap costs. Bull Math Biol. 1986;48(5-6):603–616. doi: 10.1007/BF02462326. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Morishima J. K., Devarayalu S., Gelinas R. E. Regulatory elements in the first intron contribute to transcriptional control of the human alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8869–8873. doi: 10.1073/pnas.84.24.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Coulombe B., Ponton A., Daigneault L., Williams B. R., Skup D. Presence of transcription regulatory elements within an intron of the virus-inducible murine TIMP gene. Mol Cell Biol. 1988 Aug;8(8):3227–3234. doi: 10.1128/mcb.8.8.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Lawrence C. B., Goldman D. A. Definition and identification of homology domains. Comput Appl Biosci. 1988 Mar;4(1):25–33. doi: 10.1093/bioinformatics/4.1.25. [DOI] [PubMed] [Google Scholar]
- Lawrence C. B., Goldman D. A., Hood R. T. Optimized homology searches of the gene and protein sequence data banks. Bull Math Biol. 1986;48(5-6):569–583. doi: 10.1007/BF02462324. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Krystal M., Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981 Nov 25;9(22):5931–5947. doi: 10.1093/nar/9.22.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel J. F., Norbury C. J., Tuite M. F., Dobson M. J., Mills J. S., Kingsman A. J., Kingsman S. M. Characterization of human chromosomal DNA sequences which replicate autonomously in Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Jan 25;12(2):1049–1068. doi: 10.1093/nar/12.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Reisman D., Greenberg M., Rotter V. Human p53 oncogene contains one promoter upstream of exon 1 and a second, stronger promoter within intron 1. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5146–5150. doi: 10.1073/pnas.85.14.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer J. D., Lerman M. I. Unusual class of Alu sequences containing a potential Z-DNA segment. Mol Cell Biol. 1983 May;3(5):960–964. doi: 10.1128/mcb.3.5.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Ben-Levy R. Multiple nuclear proteins in liver cells are bound to hepatitis B virus enhancer element and its upstream sequences. EMBO J. 1987 Jul;6(7):1913–1920. doi: 10.1002/j.1460-2075.1987.tb02451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Spence S. E., Young R. M., Garner K. J., Lingrel J. B. Localization and characterization of members of a family of repetitive sequences in the goat beta globin locus. Nucleic Acids Res. 1985 Mar 25;13(6):2171–2186. doi: 10.1093/nar/13.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Paulson K. E., Schmid C. W., Kadyk L., Leinwand L. Non-Alu family interspersed repeats in human DNA and their transcriptional activity. Nucleic Acids Res. 1984 Mar 26;12(6):2669–2690. doi: 10.1093/nar/12.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Tsukada T., Notake M., Nakanishi S., Numa S. Structural analysis of repetitive DNA sequences in the bovine corticotropin-beta-lipotropin precursor gene region. Nucleic Acids Res. 1982 Mar 11;10(5):1459–1469. doi: 10.1093/nar/10.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]



