Abstract
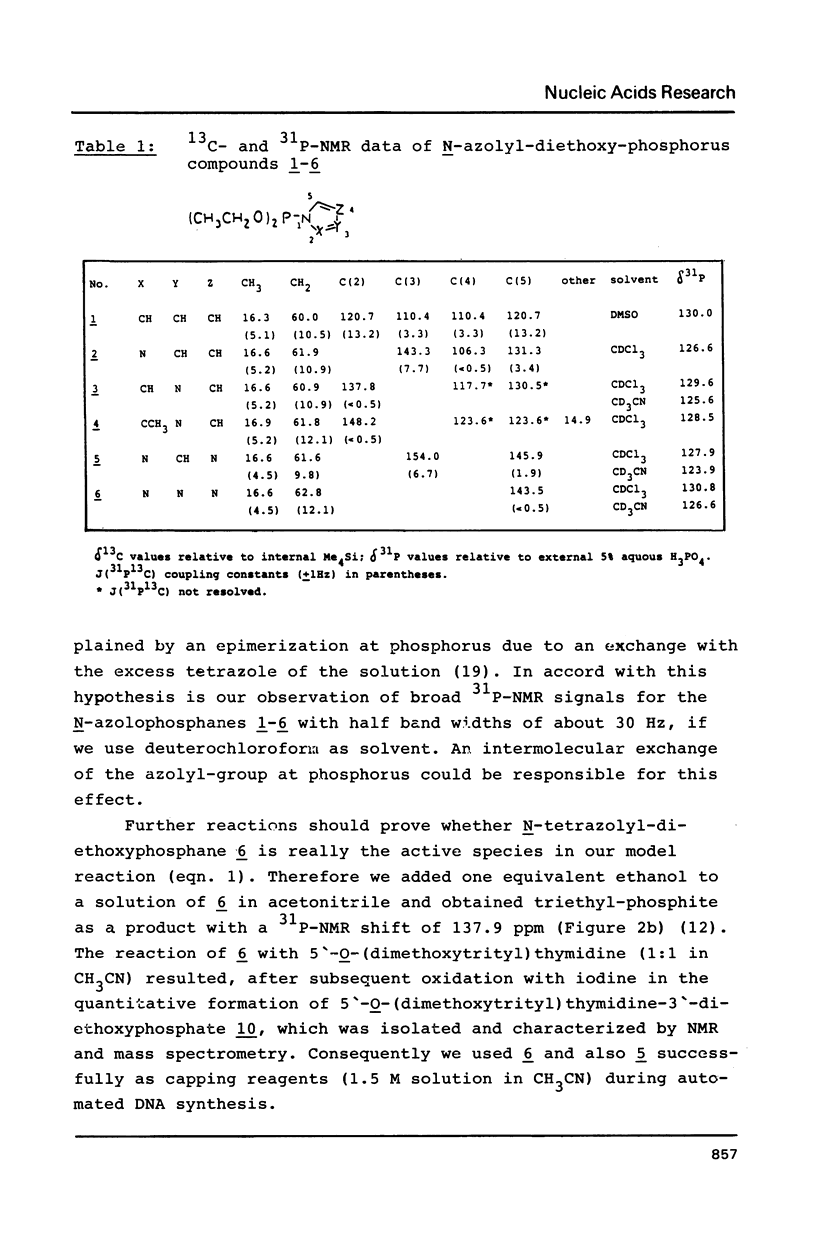
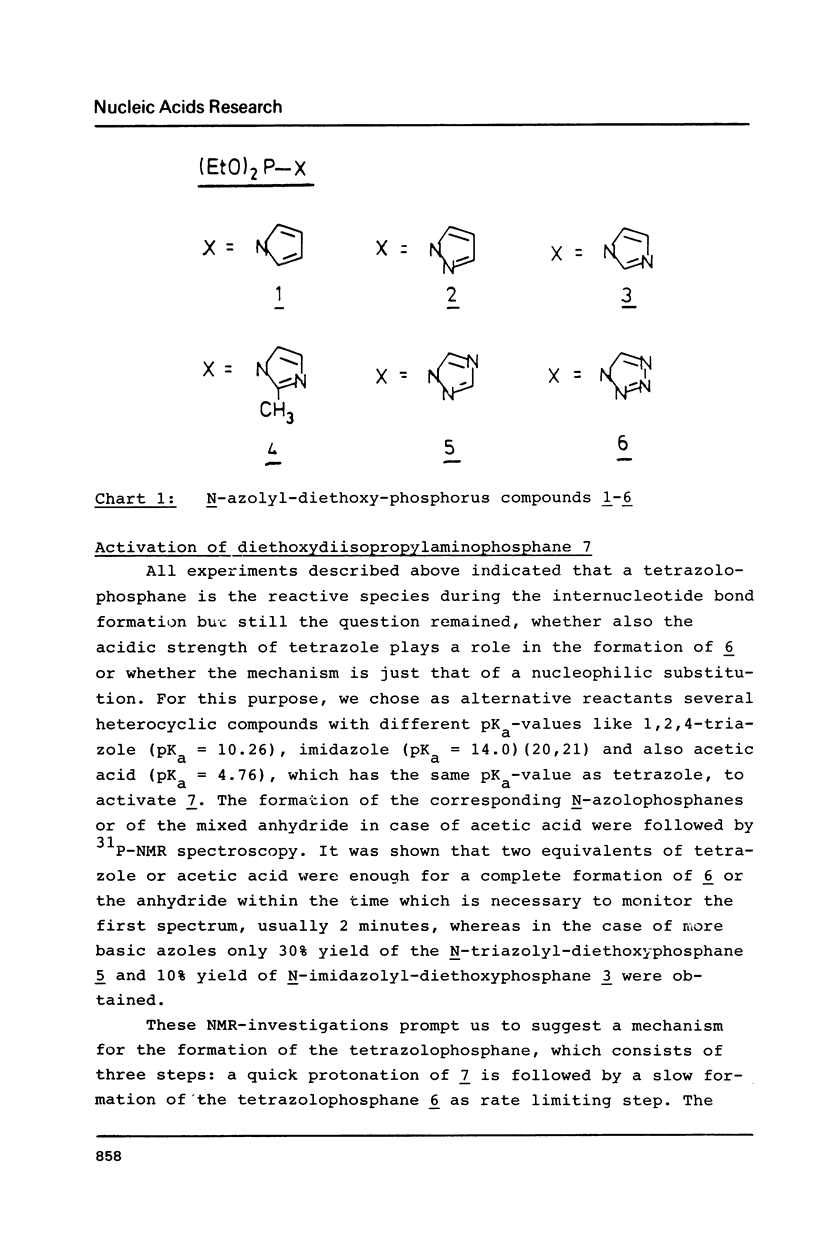
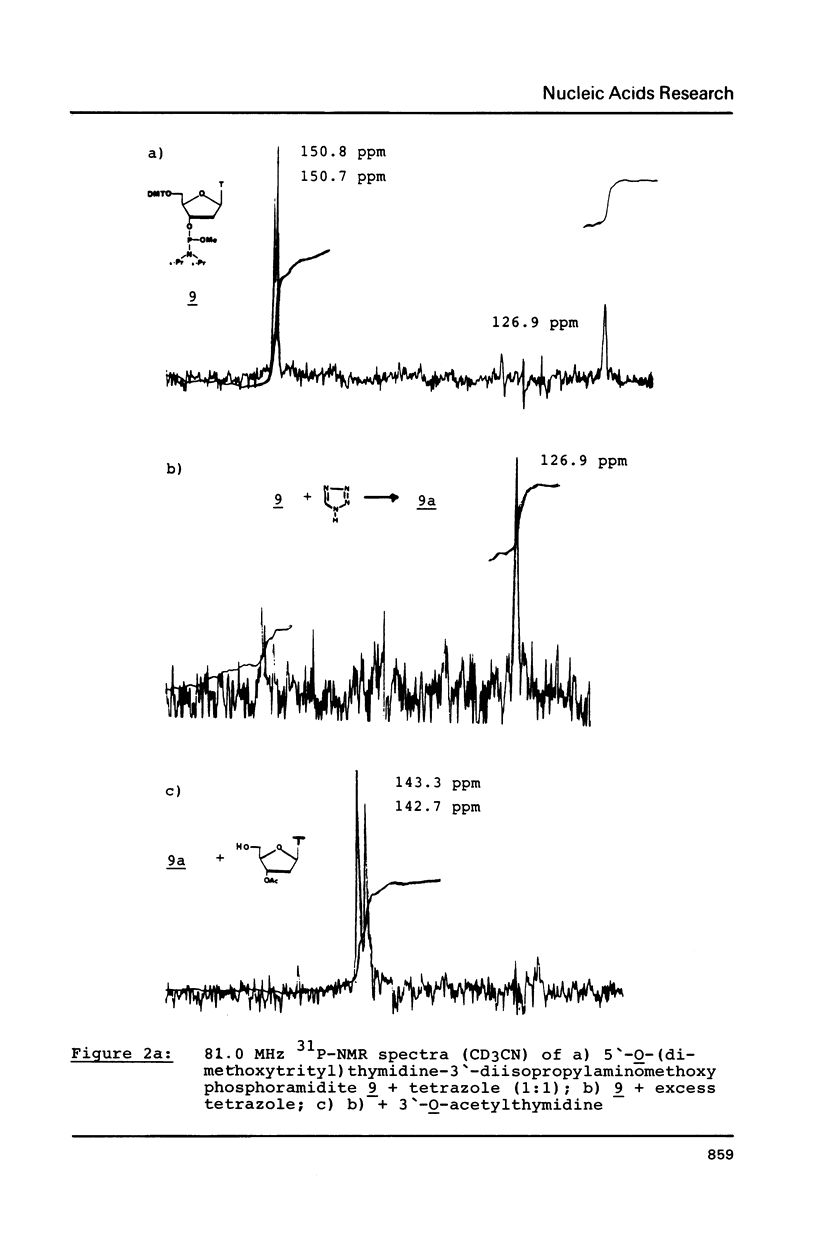
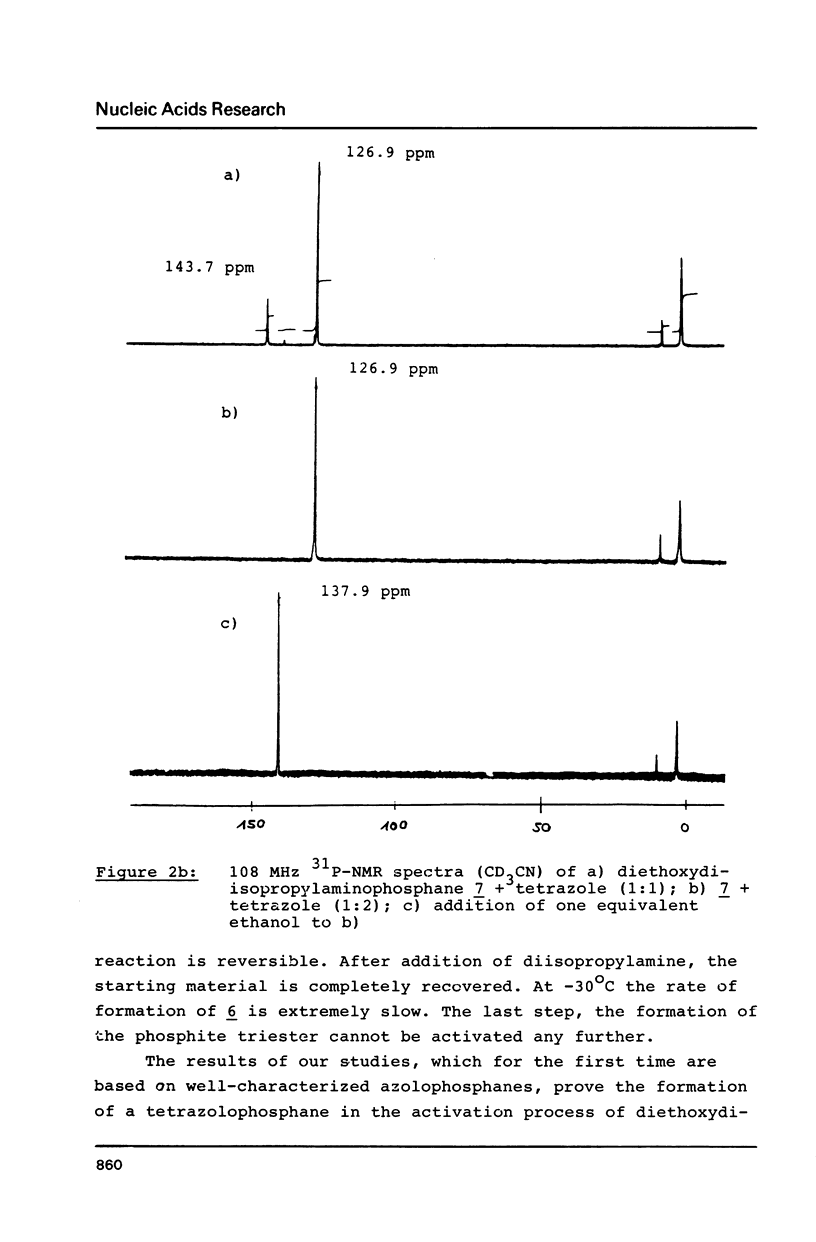
The mechanism of the tetrazole-activated coupling step in the synthesis of oligonucleotides via phosphoramidites is studied with the help of model reactions: Treatment of diethoxydiisopropylaminophosphane with two equivalents of tetrazole resulted in a diethoxy-tetrazolophosphane, whose (31P)-NMR shift of 126 ppm is identical with the signal observed during internucleotide bond formation. A series of different related diethoxy-phosphorous-acid derivatives were also synthesized; their (31P)-NMR signals between 123.9 and 130.8 ppm are additional evidence for the intermediacy of a tetrazolide species. Further NMR investigations with more basic azoles showed that tetrazole is also active as a proton donor.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahl B. H., Nielsen J., Dahl O. Mechanistic studies on the phosphoramidite coupling reaction in oligonucleotide synthesis. I. Evidence for nucleophilic catalysis by tetrazole and rate variations with the phosphorus substituents. Nucleic Acids Res. 1987 Feb 25;15(4):1729–1743. doi: 10.1093/nar/15.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Damha M. J., Ogilvie K. K. Modification of guanine bases by nucleoside phosphoramidite reagents during the solid phase synthesis of oligonucleotides. Nucleic Acids Res. 1985 Sep 25;13(18):6447–6465. doi: 10.1093/nar/13.18.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]


