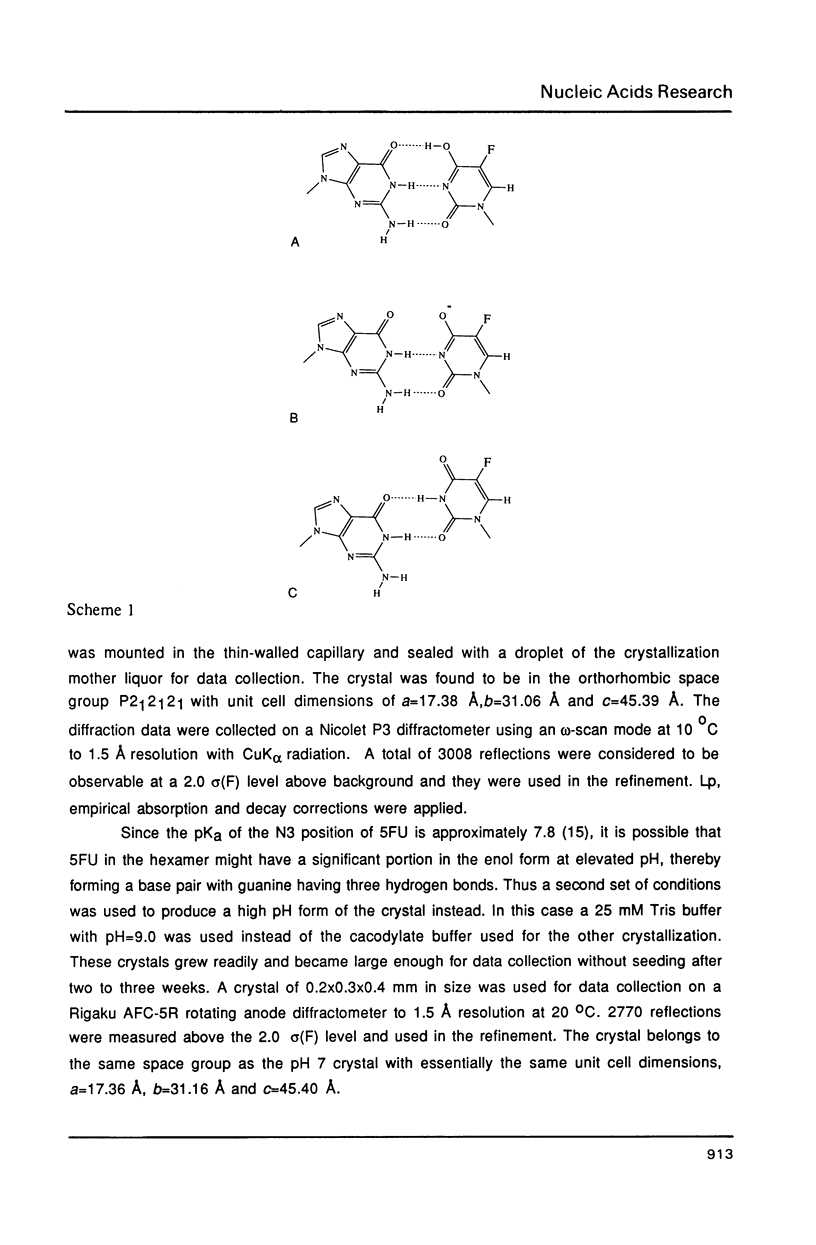
Abstract
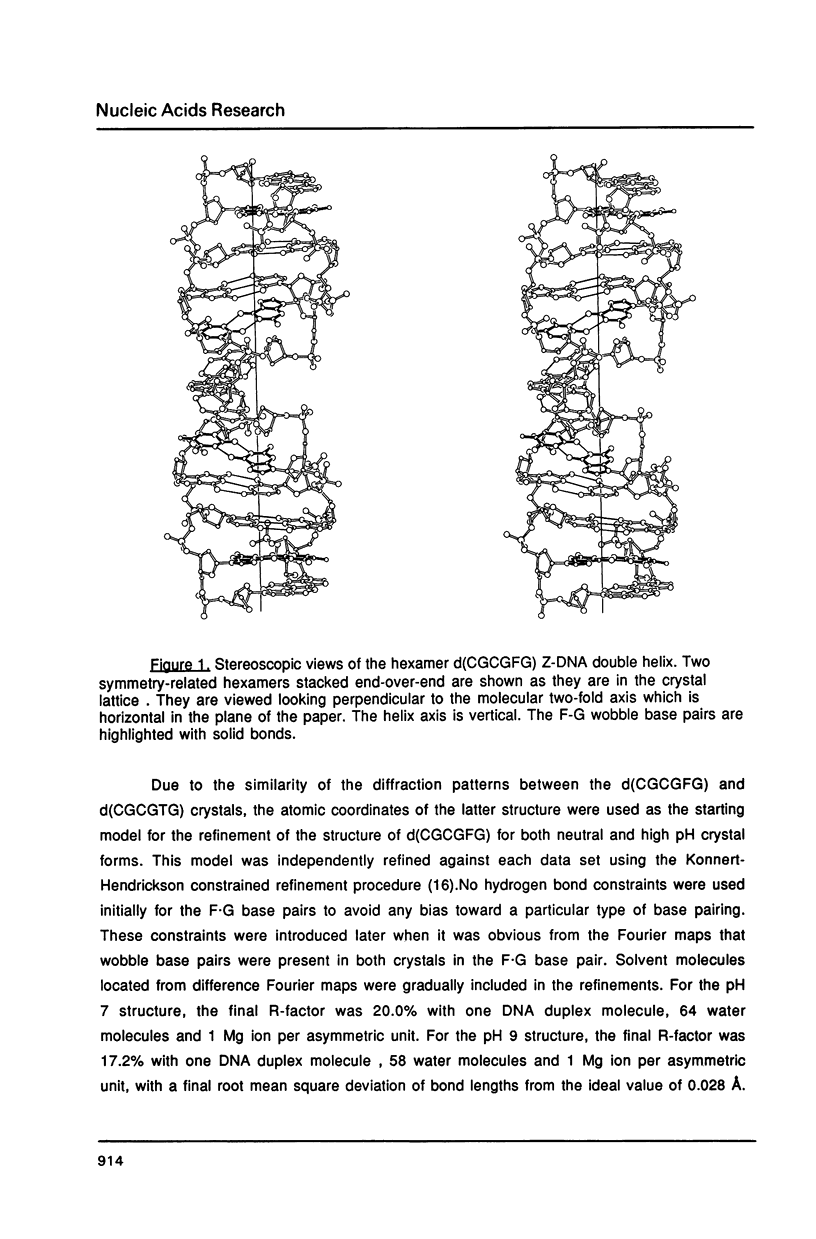
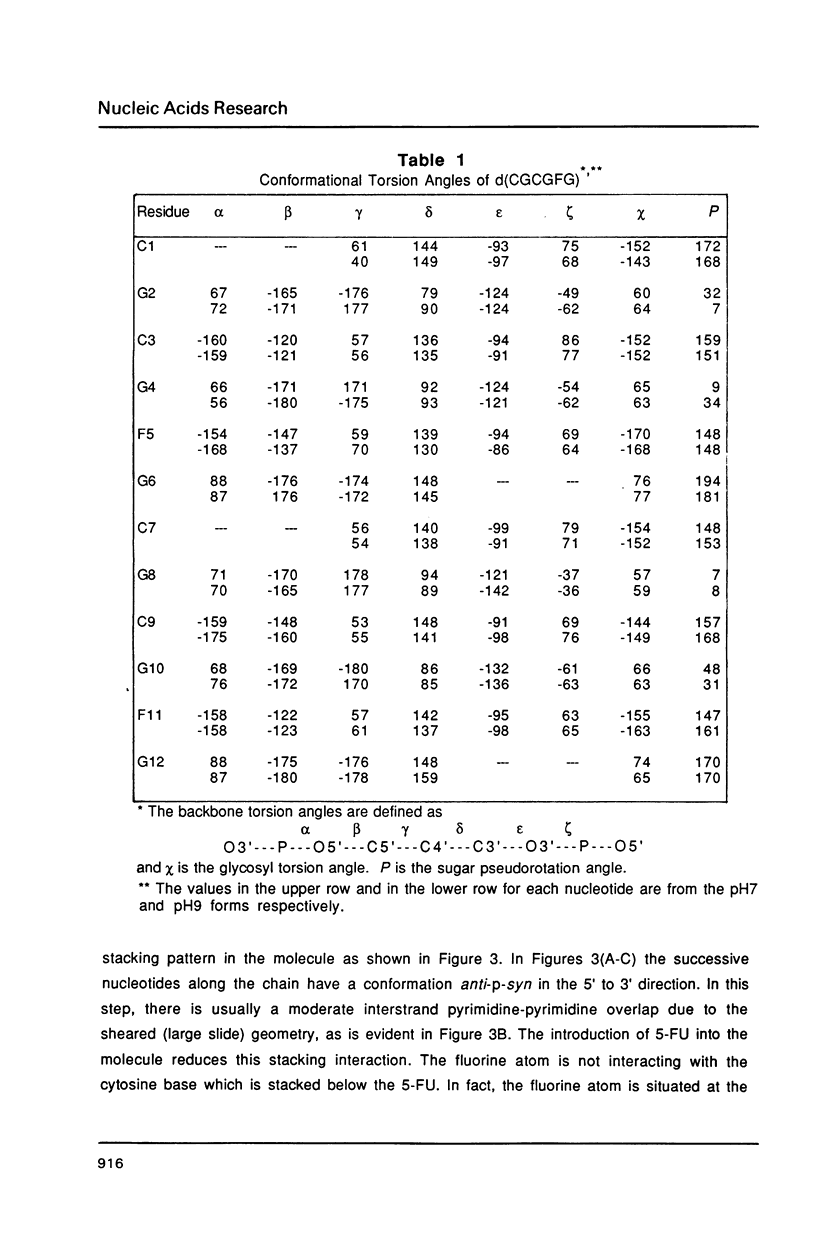
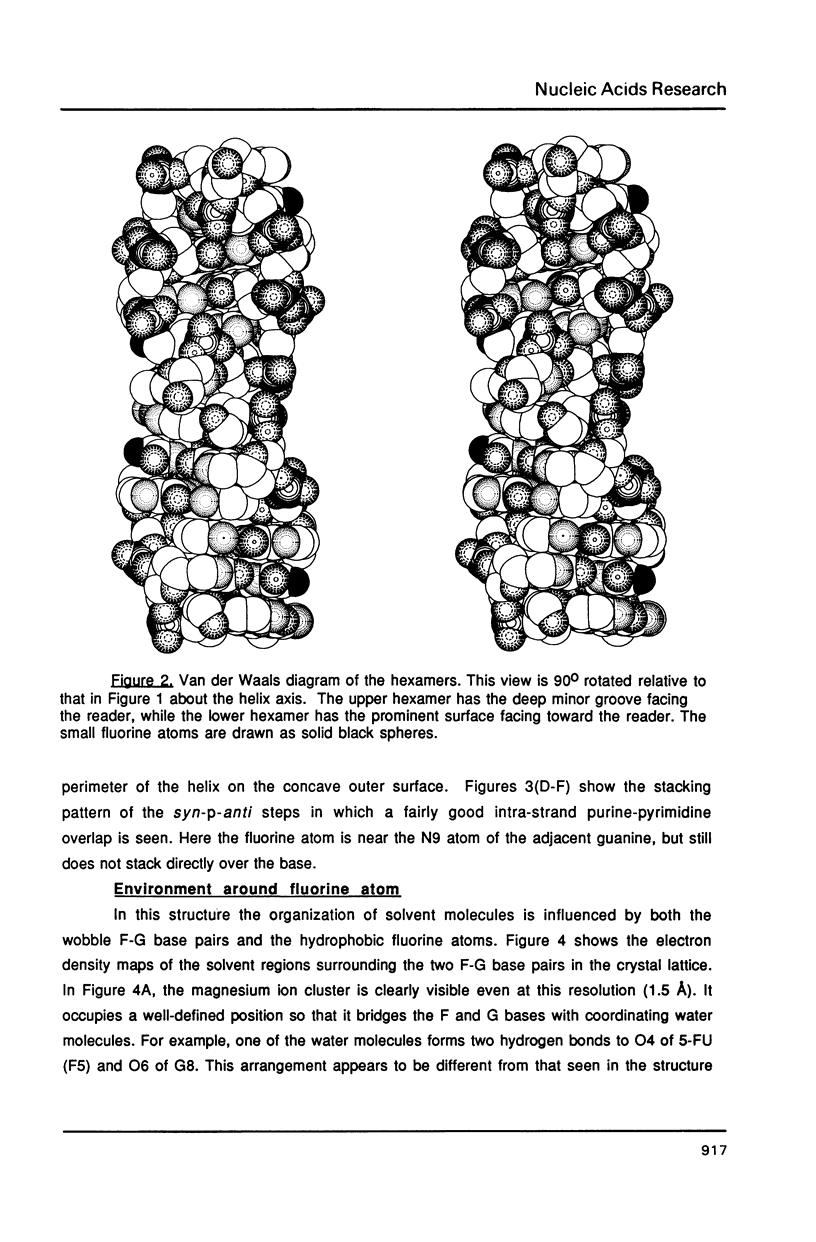
The chemotherapeutic agent 5-fluorouracil is a DNA base analogue which is known to incorporate into DNA in vivo. We have solved the structure of the oligonucleotide d(CGCGFG), where F is 5-fluorouracil (5FU). The DNA hexamer crystallizes in the Z-DNA conformation at two pH values with the 5FU forming a wobble base pair with guanine in both crystal forms. No evidence of the enol or ionized form of 5FU is found under either condition. The crystals diffracted X-rays to a resolution of 1.5 A and their structures have been refined to R-factors of 20.0% and 17.2%, respectively, for the pH = 7.0 and pH = 9.0 forms. By comparing this structure to that of d(CGCGCG) and d(CGCGTG), we were able to demonstrate that the backbone conformation of d(CGCGFG) is similar to that of the archetypal Z-DNA. The two F-G wobble base pairs in the duplex are structurally similar to the T-G base pairs both with respect to the DNA helix itself and its interactions with solvent molecules. In both cases water molecules associated with the wobble base pairs bridge between the bases and stabilize the structure. The fluorine in the 5FU base is hydrophobic and is not hydrogen bonded to any solvent molecules.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T., Kneale G., Hunter W. N., Kennard O. Structural characterisation of the bromouracil.guanine base pair mismatch in a Z-DNA fragment. Nucleic Acids Res. 1986 Feb 25;14(4):1801–1809. doi: 10.1093/nar/14.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg C. E., Thomas J. M., Sundaralingam M., Rao S. T. Stereochemistry of nucleic acids and their constituents. X. Solid-state base-stacking patterns in nucleic acid constituents and polynucleotides. Biopolymers. 1971;10(1):175–219. doi: 10.1002/bip.360100113. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Nakayama K. Effects of 5-fluoro-2'-deoxyuridine on DNA metabolism in HeLa cells. Mol Pharmacol. 1983 Jan;23(1):171–174. [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Quigley G. J., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. Methylation of the EcoRI recognition site does not alter DNA conformation: the crystal structure of d(CGCGAm6ATTCGCG) at 2.0-A resolution. J Biol Chem. 1988 Nov 25;263(33):17872–17879. doi: 10.2210/pdb4dnb/pdb. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Saal D., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. The crystal structure of d(GGm5CCGGCC): the effect of methylation on A-DNA structure and stability. Biopolymers. 1987;26 (Suppl):S145–S160. doi: 10.1002/bip.360260014. [DOI] [PubMed] [Google Scholar]
- HARTMAN K. A., Jr, RICH A. THE TAUTOMERIC FORM OF HELICAL POLYRIBOCYTIDYLIC ACID. J Am Chem Soc. 1965 May 5;87:2033–2039. doi: 10.1021/ja01087a031. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Vo C. D., Le D. B., Gryan G. P., Ercolani L., Wang A. H. 5-Fluorodeoxyuridine as an alternative to the synthesis of mixed hybridization probes for the detection of specific gene sequences. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1735–1739. doi: 10.1073/pnas.85.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. S., Frederick C. A., Quigley G. J., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. G.T wobble base-pairing in Z-DNA at 1.0 A atomic resolution: the crystal structure of d(CGCGTG). EMBO J. 1985 Dec 16;4(13A):3617–3623. doi: 10.1002/j.1460-2075.1985.tb04125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T., Watanabe T., Kufe D. W. Effects of 5-fluorouracil on globin mRNA synthesis in murine erythroleukemia cells. Biochemistry. 1986 May 6;25(9):2703–2707. doi: 10.1021/bi00357a063. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Chandrasegaran S., Pulford S. M., Miller P. S. Detection of a guanine X adenine base pair in a decadeoxyribonucleotide by proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4263–4265. doi: 10.1073/pnas.80.14.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard O. Structural studies of DNA fragments: the G.T wobble base pair in A, B and Z DNA; the G.A base pair in B-DNA. J Biomol Struct Dyn. 1985 Oct;3(2):205–226. doi: 10.1080/07391102.1985.10508412. [DOI] [PubMed] [Google Scholar]
- Kneale G., Brown T., Kennard O., Rabinovich D. G . T base-pairs in a DNA helix: the crystal structure of d(G-G-G-G-T-C-C-C). J Mol Biol. 1985 Dec 20;186(4):805–814. doi: 10.1016/0022-2836(85)90398-5. [DOI] [PubMed] [Google Scholar]
- Kremer A. B., Mikita T., Beardsley G. P. Chemical consequences of incorporation of 5-fluorouracil into DNA as studied by NMR. Biochemistry. 1987 Jan 27;26(2):391–397. doi: 10.1021/bi00376a009. [DOI] [PubMed] [Google Scholar]
- Kufe D. W., Major P. P., Egan E. M., Loh E. 5-Fluoro-2'-deoxyuridine incorporation in L1210 DNA. J Biol Chem. 1981 Sep 10;256(17):8885–8888. [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Marky L. A., Rice J. A., Broka C., Dallas J., Itakura K., Breslauer K. J. Structure, dynamics, and energetics of deoxyguanosine . thymidine wobble base pair formation in the self-complementary d(CGTGAATTCGCG) duplex in solution. Biochemistry. 1982 Feb 2;21(3):437–444. doi: 10.1021/bi00532a003. [DOI] [PubMed] [Google Scholar]
- Privé G. G., Heinemann U., Chandrasegaran S., Kan L. S., Kopka M. L., Dickerson R. E. Helix geometry, hydration, and G.A mismatch in a B-DNA decamer. Science. 1987 Oct 23;238(4826):498–504. doi: 10.1126/science.3310237. [DOI] [PubMed] [Google Scholar]
- Teng M. K., Usman N., Frederick C. A., Wang A. H. The molecular structure of the complex of Hoechst 33258 and the DNA dodecamer d(CGCGAATTCGCG). Nucleic Acids Res. 1988 Mar 25;16(6):2671–2690. doi: 10.1093/nar/16.6.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibanyenda N., De Bruin S. H., Haasnoot C. A., van der Marel G. A., van Boom J. H., Hilbers C. W. The effect of single base-pair mismatches on the duplex stability of d(T-A-T-T-A-A-T-A-T-C-A-A-G-T-T-G) . d(C-A-A-C-T-T-G-A-T-A-T-T-A-A-T-A). Eur J Biochem. 1984 Feb 15;139(1):19–27. doi: 10.1111/j.1432-1033.1984.tb07970.x. [DOI] [PubMed] [Google Scholar]


