Mesenchymal stem cells inhibit experimental autoimmune uveitis, and their immunomodulatory function is due at least in part to the induction of antigen-specific Treg in a paracrine fashion by the secretion of TGFβ.
Abstract
Purpose.
Mesenchymal stem/progenitor cells (MSCs) have regenerative and immunomodulatory properties, exerted by cell-cell contact and in a paracrine fashion. Part of their immunosuppressive activity has been ascribed to their ability to promote the induction of CD4+CD25+FoxP3+ T lymphocytes with regulatory functions (Treg). Here the authors studied the effect of MSCs on the induction of Treg and on the development of autoimmunity, and they examined the possibility that MSC-mediated Treg induction could be attributed to the secretion of soluble factors.
Methods.
The authors induced experimental autoimmune uveitis (EAU) in mice by immunization with the 1–20 peptide of the intraphotoreceptor binding protein. At the same time, some of the animals were treated intraperitoneally with syngeneic MSCs. The authors checked T-cell responses and in vitro Treg conversion by cell proliferation and blocking assays, in cell-cell contact and transwell settings. TGFβ and TGFβ receptor gene expression analyses were performed by real-time PCR.
Results.
The authors found that a single intraperitoneal injection of MSCs was able to significantly attenuate EAU and that a significantly higher percentage of adaptive Treg was present in MSC-treated mice than in MSC-untreated animals. In vitro blocking of antigen presentation by major histocompatibility complex class II precluded priming and clonal expansion of antigen-specific Treg, whereas blockade of TGFβ impaired the expression of FoxP3, preventing the conversion of CD4+ T cells into functionally active Treg.
Conclusions.
The authors demonstrated that MSCs can inhibit EAU and that their immunomodulatory function is due at least in part to the induction of antigen-specific Treg in a paracrine fashion by secreting TGFβ.
Bone marrow–derived mesenchymal stem/progenitor cells (MSCs) represent nonhematopoietic stem cells that make up a small proportion of the stromal cell population in the bone marrow. MSCs can generate bone, cartilage, and adipose tissues and can support the formation of blood and fibrous connective tissues.
Clinical applications of MSCs are based on the plasticity of their differentiation potential combined with their role in modulating the immune response.1 In both cases, MSCs exert their action locally in a cell-cell contact fashion or by secreting potent combinations of trophic factors to evoke responses from a wide range of cells.2 The paracrine effects of MSCs are transient, but they nevertheless endow the MSCs with regenerative2 and immunomodulatory3 functions. Although compelling evidence for the existence of MSC paracrine actions exists, much less is known about their nature, the stimuli for their release, and their mechanisms of action.4
The clinical use of MSCs requires high cell doses, usually up to millions of cells per kilogram of patient body weight, administered in multiple or single injections,5 currently obtained with labor-intensive methods and high reagent costs. Moreover, the use of xenogeneic serum-containing media represents a risk of contamination and raises safety concerns. Lately, the use of bioreactor systems in combination with novel xeno-free medium formulations has proved to be a viable alternative to reproducibly achieve safe and reliable MSC doses relevant for cell therapy.6 The possibility that the clinical efficacy of MSCs might be due to their paracrine action on other effector cells might activate routes of cell administration other than the ones currently used, eventually requiring fewer MSCs and thus decreasing transplantation risks, safety concerns, and production costs.
MSCs can modulate the immune response by direct interaction with cells, contributing to the activation and regulation of the innate and adaptive immune response or by the expression of cytokines participating in the immunomodulatory molecular pathways.7 It has been demonstrated that human and murine MSCs can induce the generation of CD4+CD25+ T lymphocytes with regulatory functions in both in vitro and in vivo settings.3,8–11 MSCs recruit, regulate, and maintain Treg phenotype and function over time12 because of enhanced MSC/T lymphocyte contact,13 but other mechanisms cannot be excluded.
In the present study, we focused our attention on the effect of MSCs on the induction of Treg, possibly not mediated by cell contact. We used a well-established mouse model of autoimmune uveitis known as experimental autoimmune uveitis (EAU).14 This EAU model is particularly well suited to examining the role and mechanism of MSCs in autoimmune disease. It represents autoimmune uveitis in humans, which is a group of diseases affecting the eye and estimated to be responsible for 10% of the cases of severe visual handicap that show associations with major histocompatibility complex (MHC) class I or class II molecules,15 and MHC-restricted presentation of uveitogenic antigens has been demonstrated to be a key event in the pathogenesis of uveitis in humans16 and in animal models.17 As in clinical uveitis, susceptibility to EAU is genetically controlled, and the responsiveness to EAU induction is determined by the effect of genes belonging to the MHC, H2 in mouse, and other non-MHC genes.15 MHC gene control is determined largely by epitope recognition, and in mouse some H2 haplotypes, if present on the appropriate background, are conducive to disease induction. Highly to moderately susceptible H2 haplotypes are H2r (carried by the B10.RIII mouse strain), H2k (carried by the B10.BR mouse strain), and H2b (carried by the C57BL/6 mouse strain), in that order.18 The uveitogenic peptides presented by the MHC class II molecules coded by the alleles of these haplotypes are residues 161–180 of a major retinal antigen, the interphotoreceptor binding protein (IRBP) for H2r,19 residues 201–216 of IRBP for H2k,20 and residues 1–20 of IRBP for H2b.21
Strain dependence is also observed in the differentiation potential and immunomodulation function of MSCs. In fact, it has been described that MSCs from mice with disparate genetic backgrounds show variable morphologies and phenotypes.22,23 In our hands, among other different strains, MSCs isolated from C57Bl/6 mice showed the best differentiation and immunomodulatory potential (Tasso R et al., unpublished data, 2008).
Because it was critical for this study to use a model in which the bias caused by possible crossover and antigen spreading was minimal and the immunomodulatory function of MSC was optimal, we chose C57Bl/6 strain as EAU-suseptible strain and MSC donor.
A role for Treg in controlling the pathogenesis of uveitis in humans24 and animal models has been demonstrated.25,26 It has also been shown that in murine EAU the frequency of Treg in the draining lymph nodes was increased on day 7, reached its peak on day 14, and maintained a high level up to day 42, corresponding approximately to the induction, full-blown, and resolution phases of clinical disease, respectively.27 Moreover, CD4+CD25+ Treg cells obtained from mice on days 14 and 28 after immunization (full-blown disease) showed a stronger inhibitory effect on lymphocyte proliferation and cytokine production.26
Methods
Mice
Six-week-old C57Bl/6 (H2b) mice were purchased from Charles River Laboratories (Calco, LC, Italy). Mice were bred and maintained at the institution's animal facility. Animal care and use were in compliance with the laws of the Italian Ministry of Health and the guidelines of the European Community.
MSC Isolation, Expansion, and Characterization
MSCs were isolated from C57Bl/6 mice. Bone marrow cells were flushed out from femurs and tibiae with cold PBS and were collected. Cells were cultured at a concentration of 10 × 106 nucleated cells/10-cm Petri dish in Coon's modified Ham's F12 medium (Biochrom AG, Berlin, Germany) supplemented with 10% fetal calf serum (Gibco, S. Giuliano Milanese, Milan, Italy), 1% glutamine, and 1% penicillin-streptomycin (standard medium). No cytokines were added at any stage. Cultures (passage [P] 0 stage) were incubated at 37°C in a 5% CO2 atmosphere. After 7 days, nonadherent cells were removed. When they reached 80% of confluence in the dish, approximately 15 days after plating, adherent cells were trypsinized (0.05% trypsin/EDTA at 37°C for 10 minutes) and expanded (P1 stage).
As quality controls, the capacity of isolated cells to form colonies (CFU-f) and their ability to undergo differentiation into chondrocytes, osteocytes, and adipocytes were evaluated. To evaluate the clonogenic capacity of cultured MSCs, after 14 days in culture and before colony coalescence, cells in the low-density plates were washed with PBS, fixed with 3.7% paraformaldehyde, washed, and stained with methylene blue for 2 hours to identify all colonies. To evaluate the in vitro differentiation ability of cultured MSCs, cells were trypsinized, harvested, and plated at 5 × 104 cells per well in 24-well plates in standard medium. Medium was replaced 24 hours after plating. Osteogenic differentiation was induced in MSC cultures by adding ascorbic acid (50 μg/mL), sodium b-glycerophosphate (10 mM), and dexamethasone (10−8 M) in standard medium. After 2 weeks, plates were washed with PBS, fixed with 3.7% paraformaldehyde, and stained with 1% alizarin red. Adipogenic differentiation was induced in another aliquot of cells of the initial culture by the addition of dexamethasone (10−7 M) and insulin (6 ng/mL), in F12 medium supplemented with 1% fetal calf serum, 1% glutamine, and 1% penicillin-streptomycin to the cell culture. After 3 weeks, plates were washed with PBS, fixed with 3.7% paraformaldehyde, and stained with Sudan black. Chondrogenic differentiation was in vitro investigated performing the 3D chondrogenic pellet culture. Briefly, confluent cells at P0 were detached, and 7.5 × 105 cells were pelleted at 500g for 6 minutes in 15 mL polypropylene conical tubes and cultured for 2 weeks. The basal chondrogenic medium was Coon's modified Ham's F12 supplemented with 6.25 μg/mL bovine insulin, 6.25 μg/mL human apo-transferrin, 5.35 μg/mL bovine serum albumin, 1.25 μg/mL linoleic acid, and 1 mM sodium pyruvate (all from Sigma, Milan, Italy). To induce chondrogenic differentiation, 10 ng/mL rhTGFβ1 (PeproTech, Rocky Hill, NJ), 10−7 M dexamethasone (Sigma), and 50 μg/mL ascorbic acid (Sigma) were added. Cultures were incubated at 37°C in an atmosphere containing 5% CO2, and the medium was changed every other day. To monitor chondrogenesis, pellet cultures were harvested at 2 weeks, fixed with 4% paraformaldehyde in PBS for 10 to 15 minutes, and routinely embedded in paraffin. The 4-μm-thick paraffin sections were stained with toluidine blue and viewed in transmitted light microscopy.
We checked the immunophenotype of MSCs by flow cytometry using monoclonal antibodies to H2-Kb, H2-IAb, CD11b, CD13, CD14, CD44, CD45.2, CD34, CD28, CD68, CD80, CD86 (clone AF6–88.5, AF6–120.1, M1/70, R3–242, rmC5–3, IM7, 104, RAM34, 37.51,1G10, and GL1, respectively) (BD PharMingen, Milan, Italy), CD68 (clone FA-11) (AbD Serotec, Milan, Italy), CD146 (clone P1H12) (Santa Cruz Biotechnology, Heidelberg, Germany), and PD1 (clone J43) (eBioscience, San Diego, CA).
EAU Induction and Scoring
EAU was induced by active immunization with the IRBP peptide 1–20, GPTHLFQPSLVLDMAKVLLD, synthesized as previously described.21 The peptide (500 μg) was emulsified in complete Freund's adjuvant (Sigma), 1:1 vol/vol, and 200 μL of the emulsion was injected in both thighs and in the base of the tail of each mouse. As an adjuvant, 0.3 μg Bordetella pertussis toxin (Sigma) was injected intraperitoneally in each mouse. Fourteen mice were also intraperitoneally injected with a 200 μL suspension of 5 × 106 MSCs in PBS.
Mice were euthanized at the end of the experiment, 21 days after immunization. Spleens were collected for further study. Eyes were enucleated and eyeballs were prefixed for 1 hour in 4% glutaraldehyde in PBS. The prefixing solution was then aspirated and replaced with 10% formaldehyde in PBS. Fixed eyeballs were paraffin-embedded using standard histologic techniques. Serial 4-μm sections were cut and stained with hematoxylin and eosin to examine morphologic features and to assess the histologic disease score. Eyes were examined, and a score was assigned ranging from 0 to 4, depending on the extent of inflammation and tissue damage. Briefly, eyes were assigned a score ranging from 0 to 4, depending on the extent of inflammation and tissue damage. The minimal criterion to score an eye as positive by histopathology was inflammatory cell infiltration of the ciliary body, choroid, vitreous, or retina (EAU grade 0.5–1). Progressively higher grades were assigned for the presence of discrete lesions in the tissue, such as vasculitis, granuloma formation, retinal folding or detachment, and photoreceptor damage. Specifically, eyes assigned with a disease score of 2 showed a disorganized retinal architecture, inflammatory cell infiltration, and a damaged photoreceptor layer; a disease score of 3 implied extensive destruction of the photoreceptor cell layer; finally, a maximal disease score of 4 reflected extensive retinal damage with complete destruction of the photoreceptor cell layers, including photoreceptor cells.
Delayed-Type Hypersensitivity Assay
The delayed-type hypersensitivity (DTH) response of immunized animals was tested by treating mice with a suspension of 10 μg immunizing peptide in a volume of 10 μL administered intradermally into the pinna of one ear. The other ear was injected with 0.1 N acetic acid diluted in PBS. Ear swelling was measured 48 hours later with a spring-loaded micrometer. DTH is expressed as the difference in mm (Δmm) between the peptide- and the PBS-injected ears.
Lymphocyte Isolation and Characterization
Splenocytes were obtained by mincing the spleens, collecting them in RPMI 1640 medium (Sigma) supplemented with 2-ME, glutamine, nonessential amino acids, sodium pyruvate, antibiotics (Sigma), and 1% normal mouse serum, and pooling them within experimental groups. T lymphocytes were separated using an isolation kit (Pan T Cell Isolation Kit; Miltenyi Biotec, Bergisch Gladbach, Germany) by depletion of non-T cells. Then we isolated Treg using another isolation kit (CD4+ CD25+ Regulatory T Cell Isolation Kit; Miltenyi Biotec).
Cells were counted and examined by flow cytometry to evaluate the purity of the cell suspension using monoclonal antibodies to CD4, CD25 (clones GK1.5 and PC61, respectively) (BD PharMingen, Milan, Italy), and FoxP3 (clone NRRF-30; eBioscience). The purity of the isolated population was approximately 85%.
In Vitro Treg Induction
Splenocytes from naive mice were plated at a density of 1 × 106 cells in the upper insert of a six-well transwell system (Corning B.V., Schiphol-Rijk, Netherlands). The lower part of the system was filled with 1 × 106 MSCs cultured in the presence or absence of 0.4 μM PHA or IRBP peptide 1–20. In some wells, 0.1 μg/mL antibody α-H2-IAb (BD PharMingen) or α-TGFβ (R&D Systems, Madrid, Spain) was also added. No additional cytokines were present in the medium.
The cultures were maintained for 10 days at 37°C, 5% CO2. After 10 days, at the end of the experiment, cells cultured in the upper inserts were harvested, counted, examined by flow cytometry, and used in proliferation assays.
Proliferation and Blocking Assay
We tested the proliferation of splenocytes derived from immunized mice to stimulation with 0.2 μM of the mitogenic stimulus PHA (Sigma) or with 25 μg/mL IRBP 1–20 peptide in the presence or absence of γ-irradiated syngeneic Treg isolated from the splenocytes of immunized mice, treated or untreated with MSCs.
We plated 5 × 105 responder cells per well in round-bottomed 96-well plates (Corning BW Lifescience, Pero, Milan, Italy) in proliferation assay. Cultures were incubated for 72 hours and were pulsed with [3H] thymidine (1.0 μCi/well) (Amersham Biosciences, Milan, Italy) for at least 16 hours. Cells were harvested, and cell proliferation was evaluated by counting thymidine uptake, and the averaged proliferation rate was measured as counts per minute (cpm).
In another experimental setting, we tested the proliferation of splenocytes derived from immunized mice to stimulation with 25 μg/mL IRBP peptide 1–20, in the presence or absence of 5 × 105 γ-irradiated Treg in vitro converted as previously described.
TGFβ and TGFβ Receptor Gene Expression Analysis
We isolated total RNA (RNeasy Mini kit; Qiagen, Milan, Italy). RNA was treated with DNase (RNase-free DNase set; Qiagen) to avoid contamination of genomic DNA, and cDNA was synthesized (Superscript II First Strand Synthesis System; Invitrogen, San Giuliano Milanese, Milan, Italy).
Real-time PCR was performed using specific primers and probes, as follows: TGFβ—forward primer (5′-3′) CCTGCAAGACCATCGACATG, reverse primer (5′-3′) ACAGGATCTGGCCACGGAT, probe (5′-3′) ACAGGATCTGGCCACGGAT; TGFβ receptor 2—forward primer (5′-3′) GAGACTTTGACCGAGTGCTGG, reverse primer (5′-3′) CTGAAGCGCTCTGCCACAC, probe (5′-3′) CCGAAGCCCGTCTCACAGCACAGT. We measured gene expression level as the ratio between expression values and internal GAPDH (Rodent GAPDH Control Reagents; Applied Biosystem, Monza, Italy). Probes for real-time PCR were 6-FAM-labeled. The probe for the reporter gene was VIC-labeled.
Statistical Analysis
Disease score was assessed for each treated animal as previously described. The results of four independent immunization experiments were compiled, and the statistical significance of observed differences among experimental groups was calculated with the nonparametric Mann-Whitney U test.
Means and standard deviations were calculated from at least three independent experiments to assess DTH response and frequency of CD4+CD25+CD27+ T lymphocytes assessed by flow cytometric analysis of pooled spleen cell populations, proliferation assays, and gene expression analysis. A two-tailed t-test was used to evaluate the statistical significance of the observed differences. Data are presented as compiled results.
Results
Infusion of MSCs Protects from EAU
EAU was induced in 30 C57Bl/6 mice by active immunization with IRBP peptide 1–20.21 Fourteen of these mice simultaneously received intraperitoneal injection of syngeneic MSCs as immunosuppression therapy. Only MSC cultures that had clonogenic potential and were able to differentiate into osteoblasts, chondroblasts, and adipoblasts were used (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-8211/-/DCSupplemental).
In previous experiments performed to set up the uveitogenic protocol, and in accordance with the described model,21,27 we observed the highest averaged disease score in mice immunized with IRBP 1–20 at day 21 after immunization and an almost complete resolution of histologic signs of disease after 28 to 30 days following immunization (data not shown). Disease scores were assigned by histopathology, as previously described. Based on these preliminary results, we euthanized the MSC-treated mice and the controls at day 21 after immunization and treatment.
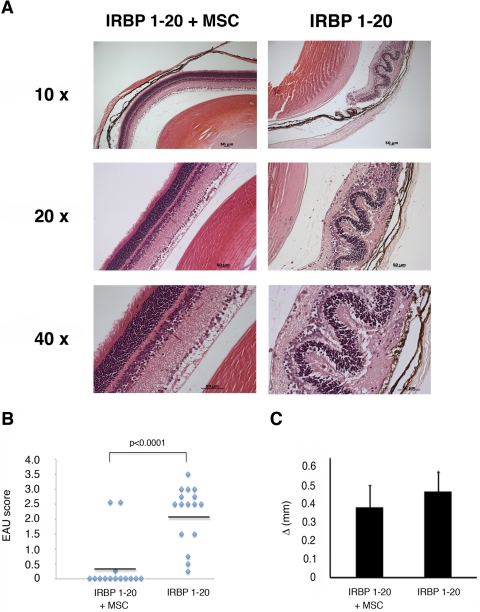
A single injection of MSCs, administered at the time of immunization when the induction of the pathogenic mechanisms began, significantly reduced the incidence and severity of EAU. Only 2 of 14 EAU-immunized mice treated with MSCs developed EAU, and one developed a trace of disease, whereas most remained disease free. In contrast, all 16 control animals developed the disease with an average score of 2.188 ± 0.981 SD (P < 0.0001) (Figs. 1A, 1B).
Figure 1.
MSCs prevented the activation of EAU pathogenic mechanisms and retinal damage. (A) Representative histologic features of eyes from immunized mice treated or untreated with MSCs. Left: eye of an immunized and MSC-treated mouse with a disease score of 0 because the retinal layers remain ordered and well preserved (top left, magnification 10×; middle left, magnification 20×; bottom left, magnification 40×). Right: eye of an immunized, but not MSC-treated, mouse with a disease score of 2, in which the retinal architecture is disorganized, inflammatory cell infiltration is present, and the photoreceptor layer is damaged (top right, magnification 10×; middle right, magnification 20×; bottom right, magnification 40×). Data are representative of three independent experiments, in which EAU was induced in a total of 30 mice and 14 of these mice simultaneously received intraperitoneal injections of syngeneic MSCs. (B) Disease scores in individual C57Bl/6 mice after immunization with IRBP 1–20 with or without MSCs. Bars indicate mean values. (C) DTH responses in MSC-treated or untreated IRBP-20–immunized mice. Shown is the difference in ear thickness between ears injected with peptide and with 0.1 N acetic acid in PBS, 48 hours after challenge. Bars represent mean ± SD values.
Age-specific DTH responses were measured. No statistically significant differences between groups were observed, although the responses appeared to be less vigorous in MSC-treated mice (Fig. 1C), suggesting that the presence of MSCs did not interfere with the mechanisms of T-cell priming and activation of the immunologic response to the antigen.
MSCs Induce Conversion of CD4+ to Antigen-Specific CD4+CD25+FoxP3+ Treg
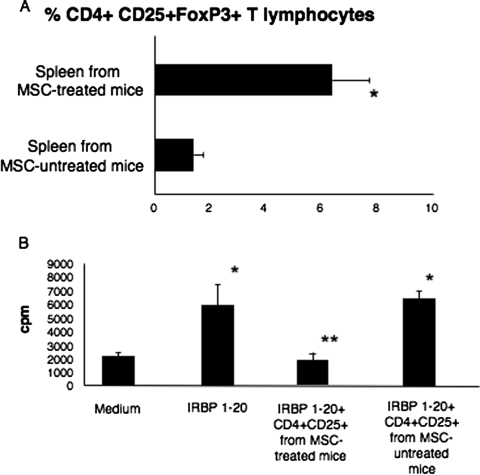
We compared the frequency of CD4+CD25+FoxP3+ T lymphocytes within the bulk of the spleen cell population isolated from uveitic mice with the frequency of these cells in the spleens of MSC-treated mice, and we found that the spleens of mice immunized and treated with a single intraperitoneal injection of MSCs contained an average of 6.4% CD4+CD25+FoxP3+ T lymphocytes, whereas this percentage appeared significantly decreased in the spleens of uveitic, MSC-untreated mice (1.4%; P < 0.01) (Fig. 2A). In addition, only CD4+CD25+ T cells isolated from MSC-treated mice behaved as antigen-specific functional Treg, able to significantly suppress the proliferation of primed T lymphocytes from uveitic mice to the IRBP 1–20 peptide, in contrast to CD4+CD25+ T cells isolated from control mice, which did not show antigen-specific suppressive function under the same conditions (P < 0.001) (Fig. 2B).
Figure 2.
In vivo conversion of CD4+ T lymphocytes into functionally active antigen-specific Treg. (A) Percentage of CD4+CD25+FoxP3+ T lymphocytes out of the total splenocytes isolated from the spleens of MSC-treated or untreated immunized mice. Shown are the compiled results of three independent experiments. *P < 0.001. (B) The histogram shows the compiled results of three proliferation assays in which responder peptide-specific T lymphocytes were in vitro challenged with IRBP 1–20 peptide in the presence or absence of Treg cells isolated from MSC-treated or untreated immunized mice. *P < 0.001 comparing the proliferation of peptide-challenged responder cells with the baseline (medium). **P < 0.001 comparing the proliferation of peptide-challenged responder cells with the proliferation of primed lymphocytes in the presence of Treg derived from MSC-treated animals (IRBP1–20 + Treg from MSC-treated mice).
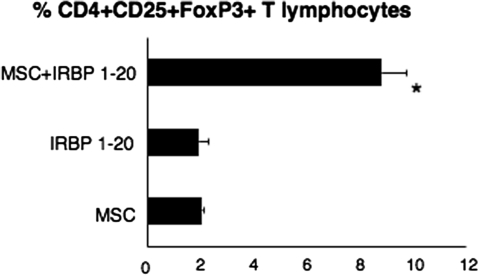
Importantly, we were able to replicate Treg induction from spleens of uveitic mice by MSCs in vitro using a transwell system. We set up experiments in which we seeded splenocytes isolated from naive mice on the top compartment of a transwell plate, and in the bottom we put MSCs alone, IRBP 1–20 peptide, or both MSCs and IRBP 1–20 peptide (Fig. 3). In the presence of MSCs and peptide, we could retrieve an average of 8.8% CD4+CD25+FoxP3+ lymphocytes, whereas only approximately 2% of converted cells was recovered in all the other conditions, representing a more than fourfold reduction (P < 0.0002) (Fig. 3).
Figure 3.
In vitro conversion of CD4+ T lymphocytes into functionally active antigen-specific Treg. Efficiency of CD4+CD25+FoxP3+ conversion in transwell cultures. Shown are the results of three independent experiments in which splenocytes from naive mice were seeded on the top of the transwell and MSCs were plated on the bottom part of the transwell with IRBP 1–20 (MSC+ IRBP 1–20) or without IRBP 1–20 (MSC) peptide added to the medium. The IRBP 1–20 bar refers to transwells in which splenocytes were seeded on the top part of the transwell, the IRBP peptide was added to the culture medium, and the bottom part of the transwell remained empty. *P < 0.0002 comparing the percentage of Treg generated in the presence of MSCs in vitro stimulated with the peptide and the other conditions.
MSCs Produce TGFβ, Which Is Required for FoxP3 Expression and Treg Induction
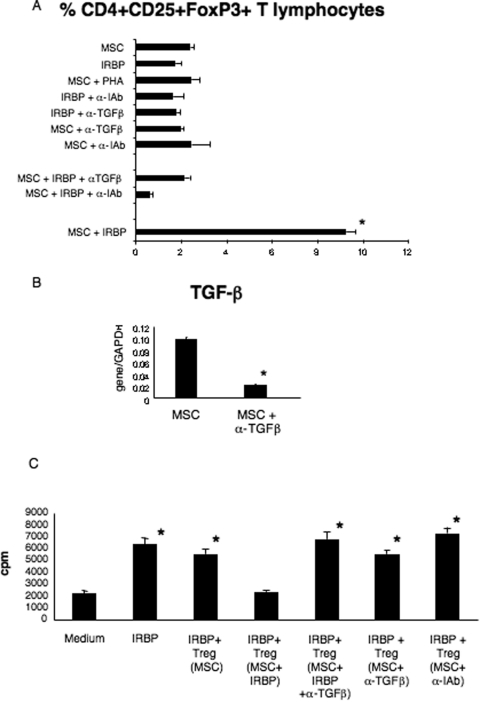
It is known that TGFβ is necessary for the production of functionally active Tregs28 and that antigen-specific Tregs are generated after antigen presentation in the context of MHC molecules.29 We therefore performed transwell experiments set up as previously described, including wells in which ant–MHC class II blocking antibodies (α-IAb) or anti–TGFβ blocking antibodies (α-TGFβ) were added (Fig. 4A). We found that either MHC class II blockade or TGFβ neutralization prevented the peptide-driven conversion of purified conventional T cells to Treg. Namely, in the presence of MSCs, peptide, and α-IAb antibodies, we were able to recover only approximately 0.65% CD4+CD25+FoxP3+ T lymphocytes (Fig. 4A), a value not only significantly lower than the frequency of Tregs induced by MSCs plus peptide (P < 0.0001) but significantly lower than the background in wells in which only MSCs were present (P < 0.0001). Similarly, only approximately 2% CD4+CD25+FoxP3+ T lymphocytes were recovered from wells in which MSCs, peptide, and α-TGFβ were present (Fig. 4A), a value significantly lower than in the presence of MSCs and IRBP 1–20 (approximately 9%) (P < 0.0001) (Fig. 4A). Thus, both antigen presentation and TGFβ secretion are required for the conversion of antigen-specific T cells to Tregs in these cultures. The conversion observed under other experimental conditions, considered as controls, did not significantly differ from baseline (Fig. 4A).
Figure 4.
Effect of α-MHC class II or α-TGFβ antibodies on in vitro conversion of CD4+ T lymphocytes into functionally active antigen-specific Treg. (A) Percentage of CD4+CD25+FoxP3+ T lymphocytes isolated from the top portion of transwell experiments in which splenocytes from naive mice were seeded. *P < 0.001 comparing the percentage of Treg generated in the presence of MSCs and peptide (MSC+IRBP) with all the other conditions. **P < 0.001 comparing wells in which Treg were generated in the presence of MSC, IRBP peptide, and α-IAb blocking antibodies (MSC+IRBP+α-IAb) with all the other conditions. (B) TGFβ expression by MSCs isolated from transwell plates in the absence (MSC) or presence of α-TGFβ blocking antibodies (MSC+α-TGFβ). Shown are the compiled results of three independent experiments. *P < 0.001 comparing the two different conditions. (C) Proliferation of T lymphocytes isolated from uveitic mice to the immunizing antigen in the presence or absence of CD4+CD25+FoxP3+ T lymphocytes (Treg) isolated from the transwell experiments. Shown are compiled results of three independent experiments. *P < 0.001 comparing the proliferation observed in any experimental condition with the baseline value (medium). Bars indicate mean + SD.
We analyzed the expression of TGFβ by MSCs seeded in the bottom part of the transwell in the presence or absence of α-TGFβ. Results showed that the expression of TGFβ mRNA in MSCs was significantly decreased in the presence of the blocking antibodies (P < 0.0001) (Fig. 4B). We also noted a concomitant decrease of TGFβ receptor, although this decrease did not achieve statistical significance (data not shown). These results suggested that the TGFβ required for stimulating the expression of FoxP3 by CD4+CD25+ T cells was produced mostly by the MSCs in the coculture.
We next tested whether CD4+CD25+ T lymphocytes, generated in vitro in the presence of MSCs and peptide, were functionally active Treg (Fig. 4C). CD4+CD25+ T lymphocytes purified from the transwell cultures were able to suppress the proliferation of primed CD4+ cells, isolated from the spleens of uveitic mice, to the immunizing antigen (P < 0.0001) (Fig. 4C), demonstrating that they were bona fide functional Treg. In contrast, the presence of either one of the blocking antibodies resulted in spleen cell populations lacking functional Treg that were unable to suppress the proliferation of responder cells to the immunizing antigen (Fig. 4C).
Discussion
Cell therapy with MSCs for autoimmune diseases is a fast-growing field; however, a broader application of stem cell therapies requires better understanding of how the use of adult stem cells can modify the autoimmune process and interfere with hyperactivation of the host immune responses. MSCs can modulate the immune response by direct interaction with cells contributing to the activation and regulation of the innate and adaptive immune response, or by the expression of cytokines participating in immunomodulatory molecular pathways.30,31
A recently published work32 demonstrated the efficacy of MSCs as immunosuppressant therapy of EAU, modulating the immune response to the immunizing antigen, including the upregulation of Treg cells. Here, we essentially observed, and confirmed, the effect of MSCs on EAU induction and progression and on the immunologic mechanisms underlying the pathogenesis of the disease. Despite these similarities, some notable differences arose. First, we used a mouse model of EAU. Murine EAU appears to be less acute than rat EAU, with a later onset and longer duration. In addition, pathologic changes in murine EAU primarily involve the posterior segment with little anterior segment involvement, whereas rat EAU usually manifests inflammation in both anterior and posterior segments.27,33 Given that mouse EAU has some elements of chronicity, we opted for this model to contribute to the ongoing debate on the efficacy of MSC treatment of chronic or relapsing immune-mediated disease and on the optimal protocol of administration, although further studies using different models of spontaneous or chronic EAU would be appropriate.34 Second, our main goal was to explore the mechanisms of the paracrine effects of MSCs on the downmodulation of the immune response in autoimmunity, specifically in EAU. To this end, it has to be used a model in which cell contact between MSCs and immune effector cells would be delayed. We previously demonstrated that MSCs injected intraperitoneally or seeded onto a bioscaffold remained in the site of seeding for a few days before migrating into the bloodstream2,3,8; using the intraperitoneal route of administration, we prevented rapid contact between MSCs and circulating lymphocytes or lymph node cells, thus optimizing the achievement of our goal. Moreover, given that intraperitoneal or intravenous injection had a similar outcome in terms of blocking the pathogenesis of disease, innovative routes of cell administration that are possibly less invasive and involve fewer risks can be explored.
Interestingly, the inhibition of disease occurrence and histologic damage in MSC-treated animals was not paralleled by a concomitant decrease in the DTH response. In fact, DTH was positive in both MSC-treated and untreated mice, and the observed differences were not statistically significant. Given that a positive DTH response indicates that no major breakdown in the immunologic machinery has occurred and that the antigen-specific T-cell priming and the expansion of memory cells were preserved, we assumed that other mechanisms blocking the extent of the immune response and the occurrence of the histologic damage must have been activated. We reached a similar conclusion studying the therapeutic effect of MSCs on a mouse model of rheumatoid arthritis, suggesting that the inhibitory function of MSCs could be exerted by the action of antigen-specific Treg.3 Here we took a step further, investigating how MSCs could convert CD4+ T lymphocytes into antigen-specific CD4+CD25+FoxP3+ Treg and whether this function might involve MSC-mediated paracrine mechanisms. We found indeed that the presence of MSCs does not prevent antigen presentation by professional and nonprofessional antigen-presenting cells, including MSCs themselves, and that they contribute to Treg clonal expansion in a paracrine fashion by secreting TGFβ.
We have already demonstrated that Treg induced in vivo by MSCs are antigen specific because they are able to suppress proliferation to the immunizing antigen but not to an irrelevant antigen (e.g., ovalbumin).3 We know that antigen-specific Treg are generated after antigen presentation in the context of MHC molecules.29 Although MSCs are considered nonprofessional APCs because they constitutively express MHC class I molecules, they can express MHC class II molecules under appropriate conditions.35,36 In the in vitro experimental conditions used in this work, antigen presentation to CD4+ T lymphocytes occurred primarily by APCs in the bulk of the splenocyte cell population, but the contribution of MSCs themselves cannot be excluded in vivo. We have previously shown that CD4+CD25− T lymphocytes isolated from naive mice could be converted to tumor-specific Treg by MSCs in a transwell system,8 suggesting the possibility that soluble MHC/peptide complexes excreted from MSCs could engage TCR to the induction of antigen-specific Treg. In fact, a role for antigen presentation by soluble nonclassical MHC class I molecules in the MSC-mediated expansion of Treg clones suppressing T-cell proliferation to allogeneic stimuli has been demonstrated,10 and soluble MHC class II molecules loaded with antigenic peptides can be found in mouse sera that are able to bind TCR and activate CD4+ T-cell responses.37
Soluble factors are essential for MSCs to regulate the immune response. In our hands, TGFβ represented the primary player among other cytokines involved in the immunomodulatory cascade (e.g., IL-10).3,38 It is known that TGFβ is necessary for the production of functionally active Treg39 and that TGFβ-transduced MSCs are highly effective in ameliorating experimental autoimmune arthritis modulating the Treg/Th17 balance.40 We found that MSCs produced the necessary amount of TGFβ required for stimulating the expression of FoxP3 by CD4+CD25+ T cells without the need for external manipulation.
Taken together, these data indicated that MSCs work as catalyzers of antigen-specific Treg conversion. It is, in fact, conceivable that Treg cells generated in vivo in the presence of MSCs would persist and expand in the event that a relapse of the autoimmune disease occurred to achieve prompt control of the disease. It has been demonstrated in mice that the peripheral Treg pool is not only highly stable but largely self-sustained, with a relatively minor contribution by newly generated thymic emigrants.41 The success of cell therapy using FoxP3+ Treg is likely to be heavily dependent on how Treg cells are prepared as a pure population and how they can be maintained as a functionally stable lineage after they are expanded in an antigen-specific manner.42 If a single injection of exogenous, short-lived MSCs could act as catalysts in expanding long-lasting antigen-specific Treg cells, it would have important implications for Treg cell therapy. Furthermore, the administration of cells that are unable to engraft, either intravenously or by the use of an opportune device, would require fewer external manipulations,43 decreasing the possibility of adverse events, thus representing a safer approach to cell therapy for uveitis and other immune-mediated diseases.
Supplementary Material
Footnotes
Supported by grants from the Italian Ministry of University and Research. RRC is supported by National Eye Institute/National Institutes of Health intramural funding.
Disclosure: R. Tasso, None; C. Ilengo, None; R. Quarto, None; R. Cancedda, None; R.R. Caspi, None; G. Pennesi, None
References
- 1. Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tasso R, Augello A, Boccardo S, et al. Recruitment of a host's osteoprogenitor cells using exogenous mesenchymal stem cells seeded on porous ceramic. Tissue Eng Part A. 2009;15:2203–2212 [DOI] [PubMed] [Google Scholar]
- 3. Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186 [DOI] [PubMed] [Google Scholar]
- 4. Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441 [DOI] [PubMed] [Google Scholar]
- 6. Santos FD, Andrade PZ, Abecasis MM, et al. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods. 2011;17:1201–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736 [DOI] [PubMed] [Google Scholar]
- 8. Tasso R, Augello A, Carida M, et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30:150–157 [DOI] [PubMed] [Google Scholar]
- 9. Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525 [PubMed] [Google Scholar]
- 10. Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222 [DOI] [PubMed] [Google Scholar]
- 11. Svobodova E, Krulova M, Zajicova A, et al. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-Cell or proinflammatory helper T-cell 17 population. Stem Cells Dev. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318 [DOI] [PubMed] [Google Scholar]
- 13. Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312 [DOI] [PubMed] [Google Scholar]
- 14. Caspi RR, Silver PB, Luger D, et al. Mouse models of experimental autoimmune uveitis. Ophthalmic Res. 2008;40:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pennesi G, Caspi RR. Genetic control of susceptibility in clinical and experimental uveitis. Int Rev Immunol. 2002;21:67–88 [DOI] [PubMed] [Google Scholar]
- 16. Pennesi G, Mattapallil MJ, Sun SH, et al. A humanized model of experimental autoimmune uveitis in HLA class II transgenic mice. J Clin Invest. 2003;111:1171–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caspi RR, Grubbs BG, Chan CC, Chader GJ, Wiggert B. Genetic control of susceptibility to experimental autoimmune uveoretinitis in the mouse model: concomitant regulation by MHC and non-MHC genes. J Immunol. 1992;148:2384–2389 [PubMed] [Google Scholar]
- 18. Cortes LM, Mattapallil MJ, Silver PB, et al. Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice. Invest Ophthalmol Vis Sci. 2008;49:1946–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silver PB, Rizzo LV, Chan CC, Donoso LA, Wiggert B, Caspi RR. Identification of a major pathogenic epitope in the human IRBP molecule recognized by mice of the H-2r haplotype. Invest Ophthalmol Vis Sci. 1995;36:946–954 [PubMed] [Google Scholar]
- 20. Namba K, Ogasawara K, Kitaichi N, et al. Identification of a peptide inducing experimental autoimmune uveoretinitis (EAU) in H-2Ak-carrying mice. Clin Exp Immunol. 1998;111:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Avichezer D, Chan CC, Silver PB, Wiggert B, Caspi RR. Residues 1–20 of IRBP and whole IRBP elicit different uveitogenic and immunological responses in interferon gamma deficient mice. Exp Eye Res. 2000;71:111–118 [DOI] [PubMed] [Google Scholar]
- 22. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668 [DOI] [PubMed] [Google Scholar]
- 23. Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406 [DOI] [PubMed] [Google Scholar]
- 24. Sugita S, Yamada Y, Kaneko S, Horie S, Mochizuki M. Induction of regulatory T cells by infliximab in Behçet's disease. Invest Ophthalmol Vis Sci. 2011;52:476–484 [DOI] [PubMed] [Google Scholar]
- 25. Grajewski RS, Silver PB, Agarwal RK, et al. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J Exp Med. 2006;203:851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun M, Yang P, Du L, Zhou H, Ren X, Kijlstra A. Contribution of CD4+CD25+ T cells to the regression phase of experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2010;51:383–389 [DOI] [PubMed] [Google Scholar]
- 27. Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease: experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495 [PubMed] [Google Scholar]
- 28. Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770 [DOI] [PubMed] [Google Scholar]
- 29. Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tasso R, Pennesi G. When stem cells meet immunoregulation. Int Immunopharmacol. 2009;9:596–598 [DOI] [PubMed] [Google Scholar]
- 31. Macdonald GI, Augello A, De Bari C. Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:2547–2557 [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Ren X, Li G, et al. Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Invest Ophthalmol Vis Sci. 2011;52:3143–3152 [DOI] [PubMed] [Google Scholar]
- 33. van Veen T, Elofsson R, Hartwig HG, et al. Retinal S-antigen: immunocytochemical and immunochemical studies on distribution in animal photoreceptors and pineal organs. Exp Biol. 1986;45:15–25 [PubMed] [Google Scholar]
- 34. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romieu-Mourez R, Francois M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179:1549–1558 [DOI] [PubMed] [Google Scholar]
- 36. Francois M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–2638 [DOI] [PubMed] [Google Scholar]
- 37. Marios-Frankiskos S, Panagiota M, Katerina B, Athanassakis I. Serum-derived MHC class II molecules: potent regulators of the cellular and humoral immune response. Immunobiology. 2010;215:194–205 [DOI] [PubMed] [Google Scholar]
- 38. Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490 [DOI] [PubMed] [Google Scholar]
- 39. Wan YY, Flavell RA. Regulatory T cells, transforming growth factor-beta, and immune suppression. Proc Am Thorac Soc. 2007;4:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park MJ, Park HS, Cho ML, et al. Transforming growth factor beta-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum. 2011;63:1668–1680 [DOI] [PubMed] [Google Scholar]
- 41. Rubtsov YP, Niec RE, Josefowicz S, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500 [DOI] [PubMed] [Google Scholar]
- 43. Pennesi G, Scaglione S, Giannoni P, Quarto R. Regulatory influence of scaffolds on cell behavior: how cells decode biomaterials. Curr Pharm Biotechnol. 2011;12:151–159 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.