Abstract
The effects of CO2 enrichment on growth and development of Impatiens hawkeri, an important greenhouse flower, were investigated for the purpose of providing scientific basis for CO2 enrichment to this species in greenhouse. The plants were grown in CO2-controlled growth chambers with 380 (the control) and 760 (CO2 enrichment) μmol·mol−1, respectively. The changes in morphology, physiology, biochemistry, and leaf ultrastructure of Impatiens were examined. Results showed that CO2 enrichment increased flower number and relative leaf area compared with the control. In addition, CO2 enrichment significantly enhanced photosynthetic rate, contents of soluble sugars and starch, activities of peroxidase (POD), superoxide dismutase (SOD), and ascorbate peroxidase (APX), but reduced chlorophyll content and malondialdehyde (MDA) content. Furthermore, significant changes in chloroplast ultrastructure were observed at CO2 enrichment: an increased number of starch grains with an expanded size, and an increased ratio of stroma thylakoid to grana thylakoid. These results suggest that CO2 enrichment had positive effects on Impatiens, that is, it can improve the visual value, promote growth and development, and enhance antioxidant capacity.
1. Introduction
Atmospheric concentrations of greenhouse gases such as CO2, N2O, and CH4 are increasing quickly since the beginning of the industrial revolution, which results in a rise in ground-level air temperatures [1–4]. These global climate changes will have produced profound effects on plant physiology and growth, structure and function of plant populations, and species distributions [2–4]. Among these factors, CO2 is one of the raw materials of photosynthesis and has great influences on plant growth and development. The current atmospheric CO2 concentration is about 380 μmol·mol−1, which is far below the optimum concentration of plant photosynthesis [5], especially for those plants such as ornamentals grown in greenhouse where ventilation is so limited to supplement the CO2 consumed by plant photosynthesis, thus seriously affecting the growth, development, yield, and visual value of greenhouse-grown ornamentals [6, 7].
Over the past three decades, a large number of studies have focused on the effects of CO2 enrichment (elevated CO2) on the growth and development of plants. Generally, plants grown at elevated CO2 relative to those grown at ambient CO2 often exhibit increased growth and photosynthesis, lower transpiration, inhibited respiration, improved water use efficiency, decreased mineral nutrient concentrations, increased plant hormones contents, reduced stomatal density and conductance, and so forth [8–11]. However, most of these studies are focused on trees, steppe plants, crop plants, and greenhouse-grown vegetables, but substantial knowledge about potential influences of CO2 enrichment on greenhouse-grown ornamentals is lacking [12]. Besides, available studies on greenhouse-grown ornamentals are usually focused on the morphology, photosynthesis, yield, and visual value of plants [7], few of them have investigated the impacts of CO2 enrichment on antioxidant enzyme system and leaf ultrastructure, which are very important for an integrative understanding of plant responses to elevated CO2.
Impatiens hawkeri or Impatiens New Guinea is a perennial species with rich colors and long flowering period, which has become a popular and important potted flowering plant throughout the world in recent years [13]. According to our knowledge, a study concentrating on the responses of Impatiens New Guinea to CO2 enrichment has not been reported until now. Therefore, to better understand the effects of CO2 enrichment on growth and development of Impatiens New Guinea, we investigated the influences of CO2 enrichment on its morphological characters, photosynthetic rate, chlorophyll content, nonstructural carbohydrates content, in particular antioxidant enzyme activity, and leaf ultrastructure. Although the results in the artificial greenhouse environment are possibly different from the results expected in the real world because of the nonlinear nature of the CO2 concentrations and the temporal and spatial variability of the CO2 concentration in the filed observations [1–3], our study will provide useful knowledge for supplementing CO2 during growth of ornamentals and even other horticultural crops in greenhouse.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Seedlings of Impatiens were bought from a local company (Changshu Agricultural Technology Development Co., Ltd.) and rooted in 200 cm3 plastic pots filled with medium consisting of a 2 : 1 [volume/volume (v/v)] mixture of peat and vermiculite. After that, plants were cultivated for ten weeks in CO2-controlled growth chambers which can automatically and accurately control environmental factors including temperature, light, relative humidity, photosynthetically active radiation (PAR), and CO2 concentration according to preset data. The plants were alternately watered to saturation with Murashige-Skoog (MS) solution or deionized water. During the first four weeks, plants were fertilized to saturation with 1/3 MS solution every two weeks and with 1/2 MS solution every two weeks in the following six weeks. Plants were grown under a 12 h photoperiod with 300 μmol·m−2 ·s−1 PAR and day : night temperatures of 25 : 18°C. The relative humidities during daytime and at time were maintained at 70–80% and over 90%, respectively. Control plants were grown in one CO2-controlled growth chamber with CO2 concentration of 380 ± 30 μmol·mol−1, while the treated plants were grown in another one with CO2 concentration of 760 ± 50 μmol·mol−1 during daytime and 380 ± 30 μmol·mol−1 at night. The purity of CO2 applied in this study was 99.999%. Except for CO2 concentration of CO2, other environmental conditions in two growth chambers were common. In order to keep the potential for interactive effects between the chambers and the developmental stage of the plants to a minimum, the CO2 concentrations of the two chambers were swapped, and the pots were moved between chambers and randomly rearranged weekly. This purpose is to average out any possible effects from the chambers and pot positions within the chambers.
2.2. Growth and Development Analysis
After ten weeks' cultivation under either 380 μmol·mol−1 or 760 μmol·mol−1 CO2 concentration, plant growth (three plants for each treatment) was assessed by regular (at 9:00 am every day) and nondestructive measurements of leaf length and leaf width (the third or fourth round of leaves), flower diameter, flower number, flower bud number, and lateral branch number for one month. Half of the product of leaf length multiplied by leaf width was regarded as the relative leaf area, and the mean of three times measurements of a flower's diameter from different angles was defined as the flower diameter. Flowers with fully unfolded corolla and buds with initial appearance of petals were included in the number of flower and flower bud, respectively. Leaf shape was quantified by means of bivariate allometric relationships between log-transferred leaf length and leaf width (n = 34), and the relationship between leaf length and leaf width determined the leaf shape of Impatiens New Guinea [12].
2.3. Determination of Photosynthetic Rate
Leaf Photosynthetic rate was measured with a Portable Photosynthesis system LI-6400 (LI-COR Inc., Lincoln, Neb, USA) after 45 days with three fully expanded leaves from each of five plants randomly selected from each treatment. The measurements for ambient CO2-grown plants were carried out at 1500 μmol·m−2 ·s−1 PAR, 2.0–2.5 KPa vapour pressure deficit (VPD), 23 ± 1°C and 380 μmol·mol−1 CO2, and for elevated CO2-grown plants at 1500 μmol·m−2 ·s−1 PAR, 2.0–2.5 KPa VPD, 23 ± 1°C and 760 μmol·mol−1 CO2.
2.4. Determination of Physiological and Biochemical Indexes
Leaves were obtained at the end of ten weeks' treatment from five plants of the control and elevated CO2-grown Impatiens New Guinea. Parts of the leaves were oven-dried at 60°C for the determination of starch and soluble sugars, and the other parts were stored in −80°C refrigerator following freezing in liquid nitrogen for the determination of contents of chlorophyll, MDA and activities of POD, SOD, APX. The content of starch was measured with the iodine colorimety method [14]. Chlorophyll, soluble sugars, MDA contents, and activities of POD, SOD were measured according to the methods of Wang [15]. The activity of APX was measured by method of Chen and Wang [16].
2.5. Leaf Ultrastructure Analysis
Areas beside the primary veins of fully expanded leaves were dissected into 1-2 mm2 squares and immediately fixed in 2.5% (v/v) glutaraldehyde (in 0.1 mol·L−1 phosphate buffer, pH 7.0) for 24 h. Then the samples were washed 5 times with the same buffer and postfixed in 1% osmium tetroxide for 3 h. After being washed with the same buffer for 3 times, leaf tissues were immediately passed through an ethanol dehydration series and then infiltrated and embedded in epoxy resin Epon-812. An ultramicrotome LKB-5 was used to cut sections. Thin sections were stained with uranyl acetate and lead citrate, and finally observations were carried out with a transmission electron microscope JEM-1200EX [9].
2.6. Statistical Analysis
The data are shown as mean ± standard deviation to indicate significant differences. Data were subjected to one-way analysis of variance and t-test using the SPSS software 16.0 (SPSS Inc, Chicago, Ill, USA). Morphological characteristics were determined based on 3 plants per sample, whereas physiological, chemical, and cellular characteristics were determined based on 5 plants per sample. All the analyses were repeated three times.
3. Results
3.1. Vegetative and Developmental Responses to Elevated CO2
The Impatiens New Guinea used in this study displayed altered external features when subjected to elevated CO2 (Figures 1, 2, 3 and Table 1). The most conspicuous change of Impatiens New Guinea was the increased flower number per individual plant (Figures 1, 2), which was significantly enhanced by 72.18% (P < 0.0001), leading to higher visual value. Elevated CO2 also increased flower bud number per individual plant by 14.97% (Figure 2, P = 0.003). However, elevated CO2 had no significant effects on flower diameter (Table 1, P = 0.131). Besides, lateral branch number per individual plant was significantly reduced by 29.39% (Figure 2, P < 0.0001), thus inducing a relatively well-proportioned plant shape compared with plants in ambient CO2 which had more little lateral branches to occupy more space but seldom flowered (Figure 1).
Figure 1.

Growth situation of Impatiens New Guinea grown under two CO2 concentrations: ambient CO2 (380 μmol·mol−1) and elevated CO2 (760 μmol·mol−1) for 10 weeks.
Figure 2.
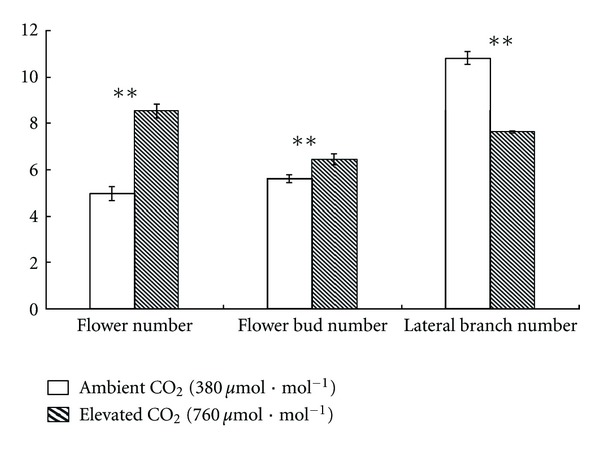
The numbers of flower, flower bud, and lateral branch of Impatiens New Guinea grown under two CO2 concentrations: ambient CO2 (380 μmol·mol−1) and elevated CO2 (760 μmol·mol−1). Asterisks show statistically significant differences (*P < 0.05; **P < 0.01; Student's t-test; n = 34 samples, with 3 plants per sample) and the bar is the standard deviation.
Figure 3.
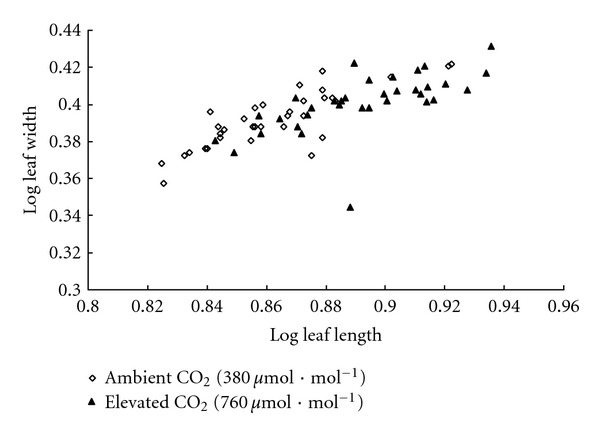
Allometric relationships between leaf length and leaf width for Impatiens New Guinea grown under two CO2 concentrations: ambient CO2 (380 μmol·mol−1) and elevated CO2 (760 μmol·mol−1). n = 34 samples, with 9 leaves from 3 plants per sample.
Table 1.
Leaf parameters and flower diameter of Impatiens New Guinea grown under two CO2 concentrations: ambient CO2 (380 μmol·mol−1) and elevated CO2 (760 μmol·mol−1).
| Ambient CO2 (380 μmol·mol−1) | Elevated CO2 (760 μmol·mol−1) | % increase | P value | |
|---|---|---|---|---|
| Log leaf length | 0.863 ± 0.004 | 0.893 ± 0.004 | 3.5 | <0.001 |
| Log leaf width | 0.392 ± 0.003 | 0.401 ± 0.003 | 2.3 | 0.009 |
| Relative leaf area (cm2) | 9.016 ± 0.137 | 9.864 ± 0.140 | 9.4 | <0.001 |
| Flower diameter (cm) | 6.615 ± 0.028 | 6.656 ± 0.023 | 0.6 | 0.131 |
Values given are mean ± standard deviation. Mean values (n = 34 samples, with 9 leaves or flowers from 3 plants per sample) were compared by Student's t-test.
Elevated CO2 also increased relative leaf area by 9.4% (Table 1, P < 0.001). The allometric relationships between leaf length and leaf width were analyzed to predict the changes of leaf shape (Figure 3). Log leaf length showed a 3.5% (Table 1, P < 0.0001) increase and log leaf width showed a 2.3% (Table 1, P = 0.009) increase, indicating that leaf length had a relatively bigger increase. Analyzing Table 1 and Figure 3 together, mean value of (log leaf length, log leaf width) of plants in ambient CO2 was (0.863, 0.392) and that of plants in elevated CO2 was (0.893, 0.401), showing that leaves in elevated CO2 appeared to be longer and wider than leaves in ambient CO2. Although the change of leaf shape seemed to be small, it was noticeable and measurable.
3.2. Responses of Photosynthetic Rate and Chlorophyll Content to Elevated CO2
Photosynthetic rate of Impatiens New Guinea grown in elevated CO2 was significantly accelerated compared with that of Impatiens New Guinea grown in ambient CO2 (Table 2), showing a 25.9% (P < 0.001) increase. However, total chlorophyll (chlorophyll a and chlorophyll b) content per unit leaf fresh weight of Impatiens New Guinea in elevated CO2 was significantly reduced (Table 2), showing an 18.2% (P < 0.001) decrease from that of Impatiens New Guinea in ambient CO2. Both chlorophyll a and chlorophyll b contributed to that response, which have a 26.2% and a 13.3% decrease, respectively, in spite of the indistinctive decrease of chlorophyll b (P = 0.15). The radio of chlorophyll a/b also dropped by 13.6% (Table 2, P = 0.004), indicating that the significant decrease of total chlorophyll mainly resulted from the decrease of chlorophyll a rather than chlorophyll b.
Table 2.
Chlorophyll content and photosynthetic rate of Impatiens New Guinea grown under two CO2 concentrations: ambient CO2 (380 μmol·mol−1) and elevated CO2 (760 μmol·mol−1).
| Ambient CO2 (380 μmol·mol−1) | Elevated CO2 (760 μmol·mol−1) | % increase | P value | |
|---|---|---|---|---|
| Chlorophyll a (μg·mg−1) | 1.03 ± 0.02 | 0.76 ± 0.01 | −26.2 | <0.001 |
| Chlorophyll b (μg·mg−1) | 0.45 ± 0.01 | 0.39 ± 0.01 | −13.3 | 0.15 |
| Total chlorophyll (μg·mg−1) | 1.81 ± 0.03 | 1.48 ± 0.02 | −18.2 | <0.001 |
| Chlorophyll a/b radio | 2.28 ± 0.02 | 1.97 ± 0.06 | −13.6 | 0.004 |
| Photosynthetic rate (μmol·m−2 ·s−1) | 11.09 ± 0.30 | 13.96 ± 0.30 | 25.9 | <0.001 |
Values given are mean ± standard deviation. Mean values (n = 3 samples, with 5 plants per sample) were compared by Student's t-test. Total chlorophyll: Chlorophyll a + Chlorophyll b. FW: fresh weight.
3.3. Responses of Nonstructural Carbohydrates to Elevated CO2
Main nonstructural carbohydrates found in leaves are total soluble sugars and starch. Figure 4 shows that elevation of CO2 had significant effect on nonstructural carbohydrates content per unit leaf dry weight of Impatiens New Guinea plants, which dramatically improved contents of soluble sugars and starch, showing a 77.81% (P < 0.001) and a 122.39% (P < 0.001) increase, respectively. Hence, the total nonstructural carbohydrates content of plants grown in elevated CO2 was doubled (a 103.5% increase, P < 0.001) compared with that of plants grown under ambient CO2.
Figure 4.
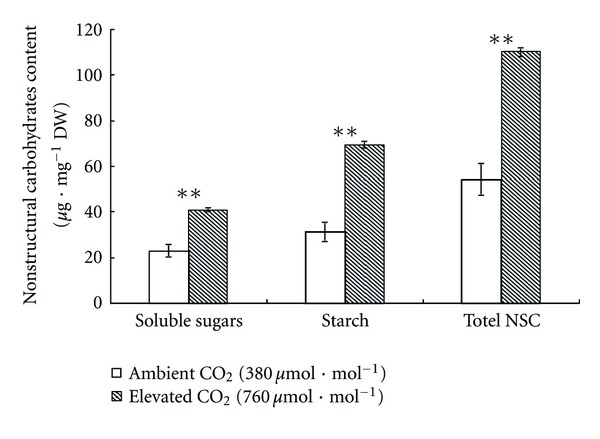
Nonstructural carbohydrates content of Impatiens New Guinea grown under two CO2 concentration: ambient CO2 (380 μmol·mol−1) and elevated CO2 (760 μmol·mol−1). Asterisks show statistically significant differences (*P < 0.05; **P < 0.01; Student's t-test; n = 3 samples, with 5 plants per sample) and the bar is the standard deviation. Total NSC= soluble sugars + starch.
3.4. Responses of Antioxidant Enzyme Activity and MDA Content to Elevated CO2
Elevated CO2 stimulated the activity of antioxidant enzymes, including peroxidase (POD), superoxide dismutase (SOD), and ascorbate peroxidase (APX) in these experiments of Impatiens New Guinea leaves (Table 3). The activity of POD, SOD, and APX increased by 119.78%, 11.01%, 73.26%, respectively, among which the increases of POD activity and APX activity reached significance level (P = 0.05), whereas the increase of SOD activity did not (P > 0.05). Besides, MDA content of Impatiens New Guinea leaves was remarkably reduced by 61.13% (P = 0.006) due to CO2 elevation (Table 3).
Table 3.
Antioxidant enzyme activity and MDA content of Impatiens New Guinea grown under two CO2 concentration: ambient CO2 (380 μmol·mol−1) and elevated CO2 (760 μmol·mol−1).
| Ambient CO2 (380 μmol·mol−1) | Elevated CO2 (760 μmol·mol−1) | % increase | P-value | |
|---|---|---|---|---|
| POD (U/(g∗min)) | 10.77 ± 2.83 | 23.67 ± 2.22 | 119.78 | 0.011 |
| SOD (U/g) | 527.75 ± 4.47 | 585.83 ± 39.51 | 11.01 | 0.141 |
| APX ((U/(g∗min)) | 20.64 ± 0.88 | 35.76 ± 4.41 | 73.26 | 0.039 |
| MDA (μmol/g) | 0.01114 ± 0.00142 | 0.00433 ± 0.00062 | −61.13 | 0.006 |
Values given are mean ± standard deviation. Mean values (n = 3 samples, with 5 plants per sample) were compared by Student's t-test.
3.5. Responses of Leaf Ultrastructure to Elevated CO2
Figures 5(a) and 5(b) show cross-sections through typical cells of Impatiens New Guinea leaves in ambient CO2 and elevated CO2, respectively. Chloroplasts were located peripherally and almost occupied half of the volume in the typical cell in elevated CO2 (Figure 5(b)). Starch grains were also observed, most of which are located at lateral sides of chloroplasts (Figures 5(a) and 5(b), arrows), leading to an ear-shaped protuberance arising from the surface. Figures 5(c) and 5(d) show that grana can be found throughout the chloroplast other than the volume occupied by starch grains. Figures 5(e) and 5(f) clearly show that grana were composed of numbers of grana thylakoids which were stacked orderly, making grana regular shape of cylinders. Moreover, stroma thylakoids were seen between grana (Figures 5(e) and 5(f), arrows), and plastoglobules were observed to spread in chloroplast around grana (Figure 5(f), arrows).
Figure 5.

Transmission electron micrographs showing leaf ultrastructure of Impatiens New Guinea grown under two CO2 concentrations: ambient CO2 (a, c, e, 380 μmol·mol−1) and elevated CO2 (b, d, f, 760 μmol·mol−1). s: starch; ch: chloroplast; g: grana; gt: grana thylakoid; st: stroma thylakoids; p: plastoglobuli. Bars: 5 μm (a and b); 2 μm (c and d); 0.5 μm (e and f).
As a consequence of elevated CO2, striking changes of the leaf ultrastructure were seen in the chloroplast. Firstly, chloroplasts showed an increased number of starch grains with expanded size (Figures 5(a), 5(b), 5(c), and 5(d)). Secondly, the number of grana in chloroplasts with elevated CO2 treatment seemed to be smaller than that of grana in ambient CO2 (Figures 5(c) and 5(d)), because of the greater number and size of starch grains. Furthermore, grana seemed to be reduced in size (Figures 5(e) and 5(f)), namely, the number of thylakoids making-up grana was reduced. However, elevated CO2 increased the number of stroma thylakoids between grana (Figures 5(e) and 5(f)), leading to an increased ratio of stroma thylakoid to grana thylakoid combining the opposite responses of stroma thylakoids and grana thylakoid numbers to CO2 elevation. Grana thylakoids in ambient CO2 were closely and orderly aligned (Figure 5(e)), whereas grana thylakoids in elevated CO2 were relatively loosely aligned (Figure 5(f)). This phenomenon was significantly observed in grana next to starch grains (Figures 5(c) and 5(d)). Finally, more plastoglobules seemed to be observed in chloroplasts with elevated CO2 treatment compared with those in ambient CO2 (Figures 5(e) and 5(f)).
4. Discussion
4.1. Morphological Characters
Flower number and flower bud number of Impatiens New Guinea were both significantly increased by elevated CO2. Similar results were found in other species such as Miniature rose, Alstroemeria, and Lilium [17–19]. Jablonski et al. [20] who used meta-analysis to integrate data from 159 enrichment papers providing information on 79 species found that growth at elevated CO2 resulted in the production of more (+19%) flowers. The significant stimulation of flower number and flower bud number of plants may primarily be a consequence of increased relative growth rate and accelerated developmental process which make plants reach the minimum size required for flowering earlier and have more resources available for reproduction, and finally increase flower number and flower bud number under elevated CO2 [9]. As flower number per individual plant is an important indicator for quality of ornamental plants, the remarkable enhancement of flower number and flower bud number by CO2 enrichment will be of great importance to improving the ornamental quality and market competitiveness of Impatiens New Guinea.
Besides, elevated CO2 also noteworthily increased relative leaf area of Impatiens New Guinea, that is, both leaf length and leaf width showed measurable increase, but leaf length had a relatively bigger increase. This is in accordance with many early reports [21, 22]. This increase of plant leaf area under elevated CO2 is probably an outcome of the well documented issue that elevated CO2 increased photosynthesis and carbohydrate production (discussed below). Ainsworth et al. [23] further demonstrated that at the transcript and metabolite level, CO2 enrichment stimulate the respiratory breakdown of carbohydrates, which provides increased energy and biochemical precursors for leaf expansion and growth, thus leading to increased leaf area of plants under elevated CO2. However, in a recent study, elevated CO2 had distinct effects on two Aechmea hybrids: A. fasciata “Maya” showed for both CO2 concentrations (380 μmol·mol−1 and 750 μmol·mol−1) an equal leaf area enhancement throughout the experimental period, whereas A. fasciata “Primera” showed a reduction of total leaf area by 41% [12]. A possible reason for this discrepancy is that the period of elevated CO2 treatment to this two Aechmea hybrids was too long (34 weeks), leading to the occurrence of CO2 acclimation, that is, the positive effects of long-term and high concentration CO2 treatments on plants will disappear gradually over time [6, 7].
4.2. Photosynthetic Rate, Chlorophyll Content, and Nonstructural Carbohydrates
In our study, photosynthetic rate of Impatiens New Guinea grown in elevated CO2 was significantly accelerated. This result was in accordance with many previous studies [8, 19, 21, 22], thus giving more evidence to the publicly accepted conclusion that CO2 enrichment is able to promote plant photosynthesis to some extent. However, in our study, total chlorophyll and chlorophyll per unit leaf fresh weight of Impatiens New Guinea in elevated CO2 was significantly reduced. We suppose that there is at least two reasons responsible for this result: firstly, the reduction of chlorophyll content may be caused by the dilution from excess accumulation of carbohydrates (discussed below); secondly, as Teng et al. [9] have proved of elevated CO2-induced reductions of mineral nutrient concentrations in plant leaves, including some of the basic components of chlorophyll such as N and Mg, so we hypothesize that the reductions of some necessary mineral nutrient may eventually affect the synthesis of chlorophyll.
A large number of studies have shown significant enhancement of carbohydrates (sugars and starches) in plant leaves by elevated CO2 [24–26]. It was reported that soluble sugars and starch contents increased by 50% and 160% on average, respectively [27]. We verified the previous studies with the result that contents of soluble sugars and starch of Impatiens New Guinea showed a 77.81% and a 122.39% increase, respectively, causing total nonstructural carbohydrates content doubled. We have documented above that elevated CO2 enhanced photosynthesis, which is in favor of the assimilation of nonstructural carbohydrates; in addition, limited sink capacity as well as some functional restriction such as limited phloem loading capacity and low efficiency of assimilate transport are likely to aggravate the accumulation of nonstructural carbohydrates in plant leaves under elevated CO2 [12].
However, with the increase of CO2 concentration or application time, plants tended to bring about the phenomenon of CO2 acclimation, which have been proved by many past studies to have a close relationship with these excess carbohydrates in plant leaves. Given the links between the phenomenon of CO2 acclimation and carbohydrates, it can be concluded as two aspects, one was that the excess accumulation of starch in plant leaves caused some physical damage to chloroplast ultrastructure (discussed below); the other was that soluble sugars suppressed photosynthesis by feedback inhibition [28].
4.3. Antioxidant Enzyme System
Antioxidant enzymes including peroxidase (POD), superoxide dismutase (SOD), and ascorbate peroxidase (APX) can neutralize free radicals, ridding the plant body of their harmful effects, therefore the activity of antioxidant enzymes is an important indicator of plant stress resistance [29]. Malonaldehyde (MDA) is a decomposition of peroxidized membrane lipid, so its content, which is closely related to the activity of antioxidant enzymes, can indicate the extent of membrane lipid peroxidation [30]. In our study, CO2 enrichment significantly improved activities of POD, SOD, and APX, but reduced MDA content, illustrating that antioxidant capacity of Impatiens New Guinea leaves was enhanced by elevated CO2. This phenomenon may be resulted from the significant stimulation of nonstructural carbohydrates in Impatiens New Guinea leaves (Figure 4), which can provide more energy substances for antioxidative metabolism, leading to improved activities of antioxidant enzymes and inhibited production of MDA, and eventually enhanced antioxidant capacity. Another possible interpretation for this is that elevated CO2 causes oxidative stress, thus signaling the need to increase the activity of antioxidant enzymes.
Because of the well-established link between antioxidant enzyme system and plant stress resistance, we suppose that stress resistance of Impatiens New Guinea would be strengthened. Several other plants have been reported with strengthened stress tolerance under elevated CO2 [31–35]. For instance, Sehmer et al. [31] found that under elevated CO2, the activities of SOD and APX of Norway spruce were enhanced when imposed to O3 stress to reduce the harm of O3 on its leaf tissue. Besides, as plant senescence is usually associated with the reduction of antioxidant enzymes activities [33], so we predict that elevated CO2 would delay the senescence of Impatiens New Guinea because of the significant enhancement of antioxidant activities. Rae et al. [34] proved at gene level that senescence was delayed under elevated CO2. In the investigation of Wang et al. [35] on effects of elevated CO2 on cut chrysanthemum, which were in great accordance with our findings, also found that elevated CO2 enhanced activities of POD and SOD, but reduced MDA content, therefore indirectly retarded cell degeneration and finally extended the life of cut flowers.
4.4. Leaf Ultrastructure
Many studies about effects of CO2 enrichment on plant leaf ultrastructure found that elevated CO2 increased the number and size of starch grains in chloroplast [6, 36, 37], which were consistent with our findings in biochemical assay of nonstructural carbohydrates (Figure 4) as well as leaf ultrastructure analysis (Figure 5) of Impatiens New Guinea. However, in an earlier study on young wheat leaves, more starch accumulation was observed in leaves in ambient CO2, whereas small starch grains were found to disperse throughout the stroma of chloroplasts in leaves grown under elevated CO2 [38]. A possible reason provided by the authors for this phenomenon was that during the early developmental stages of plants under elevated CO2, seedlings had a higher need for energy and carbon skeletons because of fast growth than those under ambient CO2, thus consuming more starch and leading to relatively less starch accumulated in young wheat leaves grown in elevated CO2.
In addition, elevated CO2 increased the ratio of stroma thylakoid to grana thylakoid, which agreed with many previous reports [9, 33, 39]. Griffin et al. [39] conjectured that these changes in the chloroplast structure maybe an approach to maintain leaf-level energy balance. They determined that, as elevated CO2 lead to a higher photosynthetic rate, increasing the demand for reductant to be used in carbon fixation and stroma thylakoids are enriched in photosystem I centers where NADPH is produced for reduction of CO2, so increased ratio of stroma thylakoid to grana thylakoid can ensure plant more efficiently fixation of CO2 into sugar products by increasing reductant production. Meanwhile, we found that grana thylakoids in ambient CO2 were closely and orderly aligned, whereas grana thylakoids in elevated CO2 were relatively loosely aligned, and this phenomenon was significantly observed in grana next to starch grains. There were several previous reports on elevated CO2-induced structural changes of thylakoids which were always accompanied with excess accumulation of starch grains [40, 41]. These findings indicated that structural changes of thylakoids may be caused by excess accumulation of starch grains which press against and separate grana thylakoids, thus changing the original close arrangement of grana thylakoids into loose. Taken together with elevated CO2-induced structural changes of thylakoids, these findings indicate that elevated CO2 damage the structure of leaf chloroplast to a certain extent, so we doubt whether the positive effects of elevated CO2 on growth and development of Impatiens New Guinea will continue or not with the increase of CO2 concentration or application time. Therefore, more work is needed to examine the detailed effects of higher concentration or long-term CO2 enrichment on growth and development of Impatiens New Guinea.
5. Conclusions
According to the above results, we are able to make a conclusion that CO2 enrichment has positive effects on Impatiens New Guinea, which can improve the visual value by increasing flower number and leaf area, promote growth by raising photosynthetic rate, nonstructural carbohydrates content, and ratio of stroma thylakoid to grana thylakoid, and enhance antioxidant capacity by improving activity of antioxidant enzymes. However, whether these positive effects of CO2 enrichment on Impatiens New Guinea will continue with the increase of CO2 concentration or application time still needs more investigations.
Acknowledgments
This work was supported by the National Science Fund of China (31171983, 30700081, 30870436), the Natural Science Fund of Jiangsu Province (BK2010447), the funding from the International Foundation for Science for Dr. Nian-Jun Teng (Reference no. C/4560-1), and the Student Research Training Project of Nanjing Agricultural University to Fan-Fan Zhang (0903A06).
References
- 1.Kondratyev KY, Varotsos C. Atmospheric greenhouse effect in the context of global climate change. Nuovo Cimento della Societa Italiana di Fisica C-Geophysics and Space Physics. 1995;18(2):123–151. [Google Scholar]
- 2.Varotsos C, Ondov J, Efstathiou M. Scaling properties of air pollution in Athens, Greece and Baltimore, Maryland. Atmospheric Environment. 2005;39(22):4041–4047. [Google Scholar]
- 3.Varotsos C, Assimakopoulos MN, Efstathiou M. Technical note: long-term memory effect in the atmospheric CO2 concentration at Mauna Loa. Atmospheric Chemistry and Physics. 2007;7(3):629–634. [Google Scholar]
- 4.Jin B, Wang L, Wang J, et al. The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana. BMC Plant Biology. 2011;11, article 35 doi: 10.1186/1471-2229-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng N, Jin B, Wang Q, et al. No detectable maternal effects of elevated CO2 on Arabidopsis thaliana over 15 generations. PLoS One. 2009;4(6) doi: 10.1371/journal.pone.0006035. Article ID e6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan HT, Liu XL, Zhang QX. Influences of CO2 enrichment on ornamental plant production. Journal of Beijing Forestry University. 2003;25(1):93–99. [Google Scholar]
- 7.Zhang FF, Teng NJ. A review on effects of CO2 enrichment on greenhouse flowers. Journal of Anhui Agricultural Sciences. 2010;38(31):17482–17495. [Google Scholar]
- 8.Hui JA, Li YH, Li Z, Ye QS. Effect of elevated CO2 concentration on photosynthesis, growth and development of Guzmania ‘Luna’. Acta Horticulturae Sinica. 2006;33(5):1027–1032. [Google Scholar]
- 9.Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana . New Phytologist. 2006;172(1):92–103. doi: 10.1111/j.1469-8137.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 10.Teng NJ, Chen T, Lin JX. A review on responses of plant sexul reproduction to elevated CO2 . Journal of Plant Ecology. 2006;30(6):1054–1063. [Google Scholar]
- 11.Pandey R, Chacko PM, Choudhary ML, Prasad KV, Pal M. Higher than optimum temperature under CO2 enrichment influences stomata anatomical characters in rose (Rosa hybrida) Scientia Horticulturae. 2007;113(1):74–81. [Google Scholar]
- 12.Croonenborghs S, Ceusters J, Londers E, De Proft MP. Effects of elevated CO2 on growth and morphological characteristics of ornamental bromeliads. Scientia Horticulturae. 2009;121(2):192–198. [Google Scholar]
- 13.Li RH, Fang Z. Effects of aluminium on growth and flower color of Impatiens hawkeri . Journal of Agricultural University of Hebei. 2006;29(5):32–36. [Google Scholar]
- 14.Xu CJ, Chen WJ, Chen KS, Zhang SL. A simple method for determining the content of starch—iodine colorimety. Biotechnology. 1998;8(2):41–43. [Google Scholar]
- 15.Wang XK. Principles and Techniques of Plant Physiological Biochemical Experiment. 2nd edition. Beijing, China: Higher Education Press; 2006. [Google Scholar]
- 16.Chen JX, Wang XF. Guidance for Plant Physiological Experiment. Guangzhou, China: South China University of Technology Press; 2006. [Google Scholar]
- 17.Mortensen LM, Moe R. Effects of temperature, carbon dioxide concentration, day length and photo flux density on growth, morphology and flowering of miniature roses. Acta Horticulturae. 1995;378:63–70. [Google Scholar]
- 18.Van Labeke MC, Dambre P. Effect of supplementary lighting and CO2 enrichment on yield and flower stem quality of Alstroemeria cultivars. Scientia Horticulturae. 1998;74(4):269–278. [Google Scholar]
- 19.Wei SL, Liu YH, Qu HY, Fu SL, Fu YL. Effects of high CO2 concentration on physiological and biochemical processes in Lily(Lilium Dauricum) Acta Phytoecologica Sinica. 2001;25(4):410–413. [Google Scholar]
- 20.Jablonski LM, Wang X, Curtis PS. Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytologist. 2002;156(1):9–26. [Google Scholar]
- 21.Wang JM, Li YH, Huang SQ, Liu LN, Ye QS. Effects of elevated CO2 concentration on Photosynthetic rate, growth and development in Anthurium andraeanum Lind. Leaves. Acta Horticulturae Sinica. 2005;32(2):335–338. [Google Scholar]
- 22.Li YH, Wu RH, Yang QS, Ye QS. Effects of short-term CO2 enrichment on photosynthetic rate and growth in Anthurium andraeanum L. Seedlings. Journal of Henan Agricultural University. 2006;40(6):607–610. [Google Scholar]
- 23.Ainsworth EA, Rogers A, Vodkin LO, Walter A, Schurr U. The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiology. 2006;142(1):135–147. doi: 10.1104/pp.106.086256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants face the future. Annual Review of Plant Biology. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 25.Uprety DC, Sunita K, Neeta D, Rajat M. Effect of elevated CO2 on the growth and yield of rice. Indian Journal of Plant Physiology. 2006;5:105–107. [Google Scholar]
- 26.Mao ZJ, Jia GM, Liu LX, Zhao M. Combined effects of elevated temperature, elevated CO2 and nitrogen supply on non-structural carbohydrate accumulation and allocation in Quercus mongolica seedlings. Chinese Journal of Plant Ecology. 2010;34(10):1174–1184. [Google Scholar]
- 27.Long SP, Drake BG. Crop Photosynthesis: Spatial and Temporal Determinants. 1992. Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations; pp. 69–95. [Google Scholar]
- 28.Sun J, Gibson KM, Kiirats O, Okita TW, Edwards GE. Interactions of nitrate and CO2 enrichment on growth, carbohydrates, and rubisco in arabidopsis starch mutants. Significance of starch and hexose. Plant Physiology. 2002;130(3):1573–1583. doi: 10.1104/pp.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Fang Z, Chen DF, Li WJ, Zhang SH. Comparison of physiological characteristics of different Impatiens hawkeri varieties under solution culture. Journal of Agricultural University of Hebei. 2003;5(26):134–136. [Google Scholar]
- 30.Zhang Y, Fang Z, Li YA, Li RH, Zhao HP. Effect of different aeration hours on the growth and development of New Guinea impatiens under solution culture. Journal of Agricultural University of Hebei. 2006;29(4):23–26. [Google Scholar]
- 31.Sehmer L, Fontaine V, Antoni F, Dizengremel P. Effects of ozone and elevated atmospheric carbon dioxide on carbohydrates metabolism of spruce needles. Catabolic and detoxification pathways. Physiologia Plantarum. 1998;102(4):605–611. [Google Scholar]
- 32.Velikova V, Tsonev T, Barta C, et al. BVOC emissions, photosynthetic characteristics and changes in chloroplast ultrastructure of Platanus orientalis L. exposed to elevated CO2 and high temperature. Environmental Pollution. 2009;157(10):2629–2637. doi: 10.1016/j.envpol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Wang H, Li H, et al. Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat (Triticum aestivum L.) Physiologia Plantarum. 2006;127(4):701–709. [Google Scholar]
- 34.Rae AM, Ferris R, Tallis MJ, Taylor G. Elucidating genomic regions determining enhanced leaf growth and delayed senescence in elevated CO2 . Plant, Cell and Environment. 2006;29(9):1730–1741. doi: 10.1111/j.1365-3040.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang YL, Huang ZZ, Sun R, Chen FD, Teng NJ. Effects of elevated CO2 on vase quality, physiological and structural characteristics of cut Chrysanthemum. Scientia Agricultura Sinica. 2010;43(21):4463–4472. [Google Scholar]
- 36.Zuo BY, Zhang Q, Jiang GZ, Bai KZ, Kuang TY. Effects of doubled-CO2 concentration on ultrastructure, supramolecular architecture and spectral characteristics of chloroplasts from wheat. Acta Botanica Sinica. 2002;44(8):908–912. [Google Scholar]
- 37.Wang X, Anderson OR, Griffin KL. Chloroplast numbers, mitochondrion numbers and carbon assimilation physiology of Nicotiana sylvestris as affected by CO2 concentration. Environmental and Experimental Botany. 2004;51(1):21–31. [Google Scholar]
- 38.Robertson EJ, Leech RM. Significant changes in cell and chloroplast develoment in young wheat leaves (Triticum aestivum cv Hereward) grown in elevated CO2 . Plant Physiology. 1995;107(1):63–71. doi: 10.1104/pp.107.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin KL, Anderson OR, Gastrich MD, et al. Plant growth in elevated CO2 alters mitochondrial number and chloroplast fine structure. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2473–2478. doi: 10.1073/pnas.041620898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritchard SG, Peterson CM, Prior SA, Rogers HH. Elevated atmospheric CO2 differentially affects needle chloroplast ultrastructure and phloem anatomy in Pinus palustris: interactions with soil resource availability. Plant, Cell and Environment. 1997;20(4):461–471. [Google Scholar]
- 41.Utriainen J, Janhunen S, Helmisaari HS, Holopainen T. Biomass allocation, needle structural characteristics and nutrient composition in scots pine seedlings exposed to elevated CO2 and O3 concentrations. Trees. 2000;14(8):475–484. [Google Scholar]


