Abstract
Pneumococcal surface protein C (PspC) is an important candidate for a cost-effective vaccine with broad coverage against pneumococcal diseases. Previous studies have shown that Streptococcus pneumoniae is able to bind to both human factor H (FH), an inhibitor of complement alternative pathway, and human secretory IgA (sIgA) via PspC. PspC was classified into 11 groups based on variations of the gene. In this work, we used three PspC fragments from different groups (PspC3, PspC5, and PspC8) to immunize mice for the production of antibodies. Immunization with PspC3 induced antibodies that recognized the majority of the clinical isolates as analyzed by Western blotting of whole-cell extracts and flow cytometry of intact bacteria, while anti-PspC5 antibodies showed cross-reactivity with the paralogue pneumococcal surface protein A (PspA), and anti-PspC8 antibodies reacted only with the PspC8-expressing strain. Most of the isolates tested showed strong binding to FH and weaker interaction with sIgA. Preincubation with anti-PspC3 and anti-PspC5 IgG led to some inhibition of binding of FH, and preincubation with anti-PspC3 partially inhibited sIgA binding in Western blotting. The analysis of intact bacteria through flow cytometry showed only a small decrease in FH binding after incubation of strain D39 with anti-PspC3 IgG, and one clinical isolate showed inhibition of sIgA binding by anti-PspC3 IgG. We conclude that although anti-PspC3 antibodies were able to recognize PspC variants from the majority of the strains tested, partial inhibition of FH and sIgA binding through anti-PspC3 antibodies in vitro could be observed for only a restricted number of isolates.
INTRODUCTION
Streptococcus pneumoniae is an important human pathogen, causing more than 800,000 deaths annually worldwide in children under the age of 5 (29). Currently available vaccines are based on the induction of antipolysaccharide antibodies, and the conjugate polysaccharide vaccines are very efficient in the prevention of invasive disease caused by serotypes present in the formulation. The widespread use of these vaccines has led to an increase in disease caused by nonvaccine serotypes through a phenomenon known as serotype replacement (1, 18, 44). Alternative strategies include the use of protein antigens such as pneumococcal surface protein C (PspC) as vaccines.
PspC has also been described as CbpA (choline-binding protein A) (43), SpsA (S. pneumoniae IgA-binding protein) (16), PbcA (C3-binding protein A) (5), and Hic (factor H-binding inhibitor of complement) (22). It is a multifunctional protein, capable of interacting with complement through binding to C3 (5) and human factor H (FH) (8, 22, 23), and acts as an adhesion molecule through interaction with the secretory component of human IgA (16) and the laminin receptor (33). Binding of pneumococci to the extracellular domain of polymeric Ig receptor (secretory component) via PspC was also shown to enhance bacterial adhesion and invasion of respiratory epithelial cells (12, 48). Binding to C3 and FH also seems to further increase adhesion to epithelial cells (15, 37, 45). PspC can interact concomitantly with FH and sIgA, since the domains responsible for the association with each of these components localize to different regions of the molecule (9), and binding to sIgA was shown to have an additive effect with FH on adherence to endothelial cells (37). Binding of pneumococcal isolates to C4b-binding protein (C4BP) was recently described and shown to be dependent on the expression of a specific PspC variant, PspC4 (PspC from group 4) (11).
PspC is composed of an N-terminal α-helical domain exposed at the surface of the bacteria, followed by a proline-rich region and a cell surface-anchoring motif (4). PspC molecules show variability between strains and were classified into 11 groups (21). PspCs from groups 1 to 6 have a repetitive choline-binding region at the C terminus, which anchors the protein to the bacterial surface through interaction with choline present in teichoic and lipoteichoic acids. PspCs from groups 7 to 11 have the LPXTG anchoring motif typical of Gram-positive bacteria and are also known as PspC-like proteins. PspC5 has a region with high similarity to the paralogue pneumococcal surface protein A (PspA) (4, 21).
FH binding for both PspC3 from D39 (8) and the PspC-like protein Hic from A66 (PspC11) (22, 23) has been described. FH binding was localized to a 12-amino-acid sequence at the N terminus of PspC from D39 (ALNIKLSAIKTK), and comparison with sequences from other strains has shown conservation of amino acids at positions 2 (leucine), 6 (leucine), 9 (isoleucine), and 10 (lysine). The 12-amino-acid sequence downstream of the FH-binding motif was also shown to be necessary for full FH binding capacity (26).
sIgA binding was mapped to the 6-amino-acid motif Y(H/R)RNYPT, which can be found in the direct repeat regions R1 and R2 of PspC. Amino acids YPT of the identified hexapeptide were further shown to be critical for binding to sIgA (17). The YRNYPT hexapeptide, related to binding to sIgA, was found only in PspC sequences from groups 1 to 7, not in the PspC-like proteins from groups 8 to 11 (21). Although PspC7 is also considered a PspC-like protein, it seems to be more closely related to the beta antigen of Streptococcus agalactiae than to other PspC variants (21).
PspC was shown to have an important role in colonization with pneumococci, since pspC-deficient strains were shown to be attenuated in carriage models using D39 in rats (43) and in CBA/N (2) and CD1 mice (31). On the other hand, the analysis of the role of PspC in pneumonia and bacteremia has provided conflicting data. A serotype 19 pspC-deficient strain was shown to have reduced virulence (2), while a D39 pspC mutant strain showed no significant attenuation in lung infection models in CBA/N mice (31). A D39 pspC-deficient strain was shown to behave like wild-type pneumococci in a bacteremia model of intravenous infection (2) and intraperitoneal challenge (31) of BALB/c mice, whereas the analysis of survival after an intravenous challenge with D39 and A66 pspC-deficient strains has shown a significant increase in survival of MF1 outbred mice (20). The conflicting data obtained by the different groups may be due to some differences in the challenge models. Furthermore, the role of PspC as a systemic and pulmonary virulence determinant in mice was shown to be strain dependent, with the deletion of pspC in strains of serotypes 2, 3, and 19F having no impact in a lethal pneumonia model in outbred MF1 mice, whereas reduced virulence was observed for a serotype 4 strain (24).
Intranasal immunization with PspC induced protection against colonization after an intranasal challenge (2), while parenteral immunization with different adjuvants induced protection after intraperitoneal challenge (32). The mixture of PspA and PspC was shown to induce higher protection levels than single antigens after an intraperitoneal challenge (30). Furthermore, cross-reactivity of anti-PspC antibodies with PspA has been described (4), and it was suggested to be related to the presence of domains with high similarity in both proteins. PspA and PspC were also shown to act in synergy to protect pneumococci from complement-dependent clearance during invasive infection, affecting immune adherence of erythrocytes and transfer of pneumococci to macrophages (25).
PspA is also a variable antigen, being divided into family 1 (clades 1 and 2), family 2 (clades 3, 4, and 5), and family 3 (clade 6). This variability found in PspA and PspC creates an additional problem with their use as vaccine antigens. We previously selected PspA molecules that induce antibodies that show broad reactivity with isolates expressing PspAs from different clades (7) and that induce protection against strains expressing PspAs from both family 1 and family 2 in an intranasal lethal challenge model in mice (28). In this work, we wished to analyze the reactivity of antibodies raised against PspC from different groups with clinical isolates of pneumococci in order to select a molecule with potential broad coverage. The inhibition of FH and sIgA binding through these antibodies was also analyzed.
MATERIALS AND METHODS
Bacterial strains.
Pneumococcal strains were isolated at the Clinical Laboratory Service of the University Hospital of the University of São Paulo (São Paulo, Brazil). Strains were serotyped using a previously described multiplex PCR method (10, 34), and the Quellung reaction was used when necessary (35). Thirteen strains were chosen based on the formulation of the 13-valent pneumococcal conjugate vaccine (Table 1). Strain St491/00 was kindly provided by Maria Cristina C. Brandileone (Instituto Adolfo Lutz, São Paulo, Brazil), TIGR4 was generously provided by Jeffrey N. Weiser (University of Philadelphia School of Medicine), and D39 was a gift from David E. Briles (University of Alabama at Birmingham). The D39 pspA mutant (D39-ΔpspA) was generated in our laboratory by insertion-deletion, using an erythromycin resistance cassette. The whole coding sequence of the gene was removed, and mutants were selected for erythromycin (1 μg/ml) resistance (42). Strains were grown in sheep blood agar and in Todd-Hewitt medium containing 0.5% yeast extract (THY). Frozen stocks were maintained at −80°C in THY containing 20% glycerol.
Table 1.
Pneumococcal isolates
| Isolate | Serotypea | Sourceb | Penicillin resistancec | PspA clade | PspC group | pspC GenBank accession no. |
|---|---|---|---|---|---|---|
| HU26/07 | 1 (HU1) | Blood | S | PspA1 | PspC6 | GU138629 |
| HU329/06 | 3 (HU3) | Blood | S | PspA2 | PspC8 | GU138630 |
| HU160/07 | 4 (HU4) | Blood | S | PspA4 | PspC3 | GU138631 |
| HU496/06 | 5 (HU5) | Blood | S | PspA1 | PspC3 | GU138632 |
| HU614/05 | 6A* (HU6A) | Blood | I | PspA2 | PspC3 | GU138633 |
| HU780/05 | 6B* (HU6B) | Blood | S | PspA3 | PspC4, PspC10 | GU138634, GU138635 |
| HU336/07 | 7F* (HU7F) | Blood | S | PspA3 | PspC6 | GU138636 |
| HU473/06 | 9V* (HU9V) | Blood | I | PspA3 | PspC3 | GU138637 |
| HU625/05 | 14 (HU14) | Blood | R | PspA1 | PspC3 | GU138638 |
| HU13/07 | 18C* (HU18C) | Blood | S | PspA4 | PspC6 | GU138639 |
| HU414/07 | 19A (HU19A) | CSF | I | PspA1 | PspC5 | GU138640 |
| HU470/06 | 19F (HU19F) | Blood | S | PspA4 | PspC9 | GU138641 |
| HU368/06 | 23F (HU23F) | CSF | I | PspA2 | PspC3 | GU138642 |
| St491/00 | 6B | PspA1 | PspC3 | EF424119 | ||
| TIGR4 | 4 | PspA3 | PspC3 | |||
| D39 | 2 | PspA2 | PspC3 |
Designations in parentheses are the identifications of the strains in the figures. *, serotyping was done by the Quellung reaction.
CSF, cerebrospinal fluid.
S, susceptible; I, intermediate; R, resistant.
Classification of PspC and PspA.
The complete pspC locus of all strains from the University Hospital was amplified by PCR from the genomic DNA of S. pneumoniae using primers that anneal in the conserved flanking regions: IF43 (5′ AATGAGAAACGAATCCTTAGCAATG 3′) and IF30 (5′ AAGATGAAGATCGCCTACGAACAC 3′) (21). The fragments were cloned into pGEM-T-Easy (Promega), and the complete genes were sequenced using an ABI Prism 3100 genetic analyzer (Applied Biosystems). The pspA gene was amplified using primers LSM12 (5′ CCGGATCCAGCGTCGCTATCTTAGGGGCTGGTT 3′) and SKH2 (5′ CCACATACCGTTTTCTTGTTTCCAGCC 3′) (19), and the clade-defining region was sequenced using primer SKH2.
Expression and purification of recombinant proteins.
N-terminal fragments of PspC3 (mature N-terminal region plus the proline-rich region from strain St491/00) (13), PspC5 (mature N-terminal region without the proline-rich region from strain HU414/07) and PspC8 (mature N-terminal region plus the proline-rich region from strain HU329/06) were expressed in Escherichia coli. The recombinant proteins were expressed in E. coli BL21 SI with an N-terminal 6× histidine tag using the pAE vector (38). Proteins were expressed in soluble form and purified by Ni+2 affinity chromatography as described before (13).
Immunization of mice.
Animal experimental protocols were approved by the Ethical Committee for Animal Research of Instituto Butantan (São Paulo, Brazil; CEUAIB 457/08). Animals were obtained from the Medical School of the University of São Paulo (São Paulo, Brazil). For the production of anti-PspC antisera, female BALB/c mice were immunized via the subcutaneous route with 5 μg of recombinant protein using aluminum hydroxide as the adjuvant (50 μg Al3+). Immunizations were performed once a week for three consecutive weeks, and animals were bled 2 weeks after the last immunization. Anti-PspA4 antibodies were previously obtained in our laboratory (28).
Purification of IgG.
Anti-PspC3 and anti-PspC5 IgG were purified through protein G affinity chromatography using HiTrap protein G-Sepharose columns (GE Healthcare). IgG against E. coli intimin (14), a protein not related to pneumococci, was also purified and used as a negative control.
Western blotting.
Western blotting experiments were performed based on a previously described method (26, 27). Pneumococcal strains were grown in THY to an optical density at 600 nm (OD600) of 0.6, and the cellular precipitates were lysed in a solution containing 0.01% sodium dodecyl sulfate, 0.1% sodium deoxycholate, and 0.15 M sodium citrate (40). Protein extracts (15 μg for antibody binding and FH and sIgA binding assays and 2.5 μg for inhibition of FH and sIgA binding assays) were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane. PspC was detected with the different anti-PspC antisera, followed by incubation with anti-mouse IgG conjugated to horseradish peroxidase (HRP) (Sigma-Aldrich). Detection was performed using an enhanced chemiluminescence (ECL) kit (GE Healthcare). For analysis of FH binding, membranes were incubated with 0.04 μg/ml human FH (C5813; Sigma-Aldrich), followed by incubation with goat anti-human FH (341276; Calbiochem) and detection with anti-goat IgG conjugated to HRP (Sigma-Aldrich) (27). For analysis of sIgA binding, the membrane was incubated with 0.4 μg/ml human sIgA (I2636; Sigma-Aldrich), followed by incubation with goat anti-human sIgA (S1640; Sigma-Aldrich) and detection with anti-goat IgG conjugated to HRP (27). The capacity of anti-PspC antibodies to block binding of human FH and human sIgA was evaluated through incubation with purified IgG against PspC3, PspC5, or intimin prior to incubation with FH or sIgA.
Flow cytometry analysis.
Binding of anti-PspC antibodies to intact bacteria was analyzed by flow cytometry as previously described (39). S. pneumoniae strains were grown in THY to a concentration of ∼108 CFU/ml (OD600, 0.4 to 0.5) and harvested by centrifugation. The pellets were incubated in the presence of heat-inactivated sera, followed by incubation with goat anti-mouse IgG Fc conjugated with fluorescein isothiocyanate (FITC) (MP Biomedicals). The analysis of FH and sIgA binding was performed based on previously described methods (9, 47). For the analysis of FH binding, bacteria were incubated with 1 μg/ml human FH, followed by incubation with goat anti-human FH and anti-goat IgG conjugated with FITC (Sigma-Aldrich). For the analysis of sIgA binding, bacteria were incubated with 30 μg/ml human sIgA, followed by incubation with goat anti-human sIgA and anti-goat IgG conjugated with FITC. The capacity of anti-PspC antibodies to block binding of human FH and human sIgA was evaluated through incubation with 1 μg IgG against PspC3, PspC5, and intimin prior to incubation with FH or sIgA. All samples were fixed in 2% formaldehyde in phosphate-buffered saline (PBS) and analyzed using FACSCalibur (BD Biosciences). A total of 10,000 gated events were acquired and analyzed in fluorescence intensity histograms.
RESULTS
Sequence analysis of PspC.
The PspC group was determined for each pneumococcal isolate, and among the 13 strains from the different serotypes analyzed, diversity similar to that found by Iannelli and collaborators (21) was observed, with six strains with PspC3, three strains with PspC6, and one strain each with PspC5, PspC8, and PspC9. One duplication with PspC4 and PspC10 was also found (Table 1). The alignment of the PspC sequences (see Fig. S1 in the supplemental material) showed the conservation of amino acids at positions 6 (leucine) and 9 (isoleucine) within the described motif for FH binding (26) in all strains. We also analyzed the PspC sequences for the presence of the hexapeptide Y(H/R)RNYPT, to which binding to sIgA was mapped (17). We found the hexapeptide in all sequences except those of PspC8, PspC9, and PspC10, which is in accordance with the analysis performed by Iannelli and collaborators showing that PspC-like proteins from groups 8 to 11 lack this motif (21). The majority of the strains have two copies of the hexapeptide motif in repeat regions R1 and R2, while strains HU1 (PspC6) and HU5 (PspC3) lack the first copy and strain HU6B (PspC4) lacks the second one. It is interesting that PspC4 was also shown to bind to C4BP (11). We detected a further polymorphism in the first amino acid of the hexapeptide with phenylalanine (F) and leucine (L), besides the described tyrosine (Y), histidine (H), and arginine (R). The critical amino acids YPT were present in all mapped motifs.
Reactivity of anti-PspC3, anti-PspC5, and anti-PspC8 sera with whole-cell extracts.
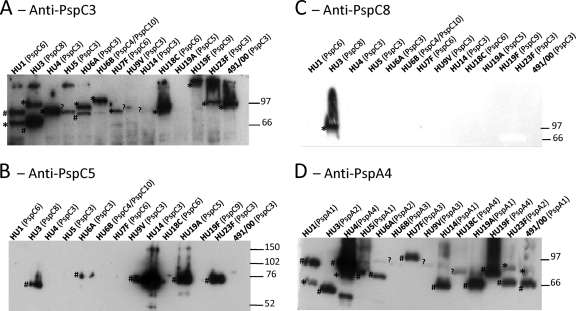
Whole-cell extracts of the isolates were tested for reactivity with polyclonal antibodies raised against PspC3, PspC5, and PspC8 in Western blotting. As seen in Fig. 1A, anti-PspC3 serum reacted with the majority of the isolates, showing broad reactivity. Lack of reactivity could be due to the absence of cross-reactivity or low expression of PspC. Furthermore, some strains showed more than one band, which could be due to cross-reaction with PspA. In fact, comparison with anti-PspA4 (PspA from clade 4) serum confirmed that some bands probably correspond to PspA (Fig. 1D). We could not determine which band corresponds to PspC and PspA by using the predicted molecular mass, since anomalous migration of both full-length PspA and PspC in SDS-PAGE has been described (26, 46, 48). Anti-PspC5 serum showed reactivity with about half of the isolates (Fig. 1B), but comparison with results for anti-PspA4 serum showed that the bands probably correspond to PspA (Fig. 1D). PspC5 has a region that has high similarity to PspA2 (PspA from clade 2), and only strains expressing PspA from family 1 (PspA1 and PspA2) showed reactivity with anti-PspC5. Anti-PspC8 serum showed reaction restricted to the PspC8-expressing strain (Fig. 1C).
Fig 1.
Western blotting analysis of reactivity of anti-PspC3, anti-PspC5, anti-PspC8, and anti-PspA4 antibodies with different pneumococcal strains. Polyclonal sera from mice immunized with recombinant PspC3 (A), PspC5 (B), PspC8 (C), and PspA4 (D) were tested for reactivity with cell lysates from 13 strains expressing different PspCs and PspAs. Identification of serotype and PspC group (A to C) or PspA clade (D) is shown on top of each blot. St491/00 is a serotype 6B strain from which pspC3 was cloned. The migration of molecular mass standards (in kDa) is indicated on the right. #, PspA; *, PspC; ?, bands not known to be either PspA or PspC.
Binding of FH and sIgA to whole-cell extracts.
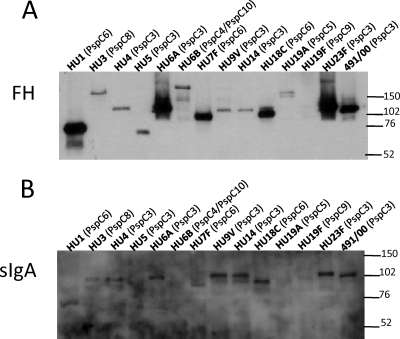
Binding of FH to the pneumococcal whole-cell extracts was analyzed by Western blotting. Figure 2A shows that FH binds to all strains, with bands of different intensities being detected. These differences were probably related to the higher affinity of the PspC variants for FH and not to the level of expression of PspC, since some of the strains that showed high-intensity bands with anti-PspC3 serum, such as HU4 (Fig. 1A), yielded low-intensity bands after interaction with FH. Binding of sIgA to the pneumococcal whole-cell extracts was also analyzed by Western blotting, and reactions were observed with the majority of the isolates. Overall, faint bands were observed for PspC4/PspC10-, PspC5-, PspC8-, and PspC9-expressing strains (Fig. 2B). PspC8, PspC9, and PspC10 lack the hexapeptide Y(H/R)RNYPT, to which binding to sIgA was mapped (21).
Fig 2.
Western blotting analysis of binding of FH and sIgA to whole-cell extracts. Binding of FH to cell extracts from different pneumococcal strains was analyzed after incubation with human FH and detection with anti-FH antibodies (A). Binding of sIgA to cell extracts from different pneumococcal strains was analyzed after incubation with human sIgA and detection with anti-sIgA antibodies (B). The serotype and PspC group are shown on top of each blot. St491/00 is a serotype 6B strain from which pspC3 was cloned. The migration of molecular mass standards (in kDa) is indicated on the right.
Inhibition of FH and sIgA binding to whole-cell extracts by anti-PspC IgG.
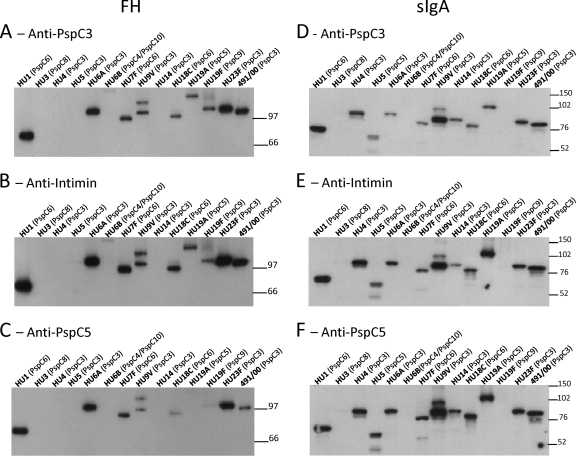
In order to evaluate whether the more broadly reactive anti-PspC3 and anti-PspC5 antibodies could inhibit interaction with FH and sIgA, we purified IgG from anti-PspC3 and anti-PspC5 sera to reduce contamination with mouse FH and sIgA from the sera. IgG against E. coli intimin was used as a negative control, and smaller amounts of cellular extracts were loaded in the gels (2.5 μg/lane, compared with 15 μg in Fig. 2A) in order to better visualize inhibition. Both anti-PspC3 and anti-PspC5 IgG could reduce interaction of FH with the whole-cell extracts to some extent (Fig. 3A and C). Interaction of FH with the strain expressing PspC5 (HU19A) was almost completely abolished by the homologous anti-PspC5 IgG (Fig. 3C), compared with anti-intimin IgG (Fig. 3B). When interaction with sIgA was analyzed, anti-PspC3 IgG seemed to inhibit the interaction more efficiently than anti-PspC5 IgG (Fig. 3D and F), compared with anti-intimin IgG (Fig. 3E). The densitometric analysis of the bands confirmed lower binding of FH in the blots incubated with anti-PspC3 and anti-PspC5 IgG (see Fig. S2A in the supplemental material) and lower binding of sIgA in blots incubated with anti-PspC3 IgG (see Fig. S2B).
Fig 3.
Western blotting analysis of inhibition of FH and sIgA binding to whole-cell extracts through anti-PspC IgG. The inhibition of FH binding to pneumococcal strains (A to C) was analyzed after incubation with anti-PspC3 IgG (A), anti-intimin IgG (B), and anti-PspC5 IgG (C). Membranes were the incubated with human FH, and detection was performed with anti-FH antibodies. The inhibition of sIgA binding to pneumococcal strains (D to F) was analyzed after incubation with anti-PspC3 IgG (D), anti-intimin IgG (E), and anti-PspC5 IgG (F). Membranes were incubated with human sIgA, and detection was performed using anti-sIgA antibodies. Anti-intimin IgG was used as a negative control. The serotype and PspC group are shown on top of each blot. St491/00 is a serotype 6B strain from which pspC3 was cloned. The migration of molecular mass standards (in kDa) is indicated on the right.
Binding of IgG, FH, and sIgA to intact bacteria.
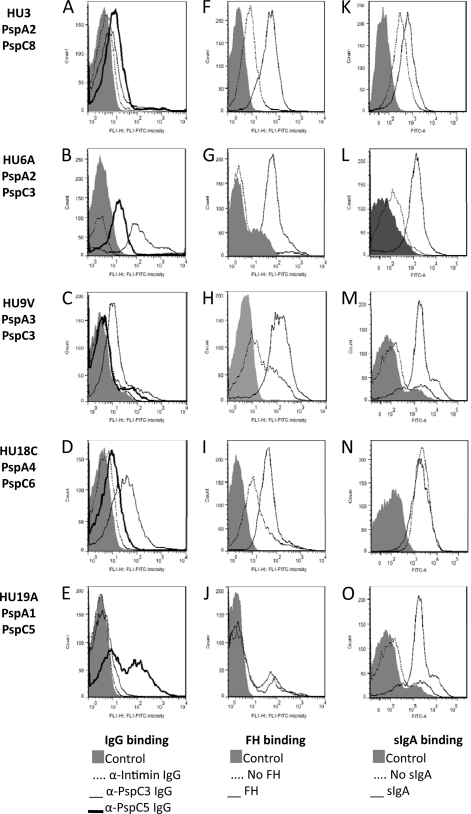
The binding of anti-PspC3 and anti-PspC5 IgG to intact pneumococci was analyzed by flow cytometry. Stronger binding of anti-PspC3 IgG was observed for PspC3- and PspC6-expressing strains (Fig. 4B, C, and D). Anti-PspC5 IgG reacted more with the PspC8- and PspC5-expressing strains (Fig. 4A and E). Once again, reactivity with the PspC8-expressing strain could be due to reactivity with PspA. Efficient binding of FH was observed for all strains analyzed, except for the PspC5-expressing strain (Fig. 4F to J). Binding of sIgA was also observed for two PspC3- and one PspC5-expressing strains. A high background in the control sample of the PspC6- and the PspC8-expressing strains makes it difficult to confirm binding (Fig. 4K to O). Since the PspC8-expressing strain lacks the hexapeptide sIgA binding motif, we consider the results to be negative for sIgA binding.
Fig 4.
Flow cytometry analysis of binding of IgG, FH and sIgA to clinical isolates. Binding of IgG (A to E), FH (F to J), and sIgA (K to O) to intact bacteria was analyzed by flow cytometry. Pneumococcal isolates HU3 (A, F, and K), HU6A (B, G, and L), HU9V (C, H, and M), HU18C (D, I, and N), and HU19A (E, J, and O) were used. Bacteria incubated only with the detection antibody conjugated with FITC were used as a control for each strain. Results for samples without incubation with FH and sIgA are also shown.
Inhibition of FH and sIgA binding to intact bacteria by anti-PspC IgG.
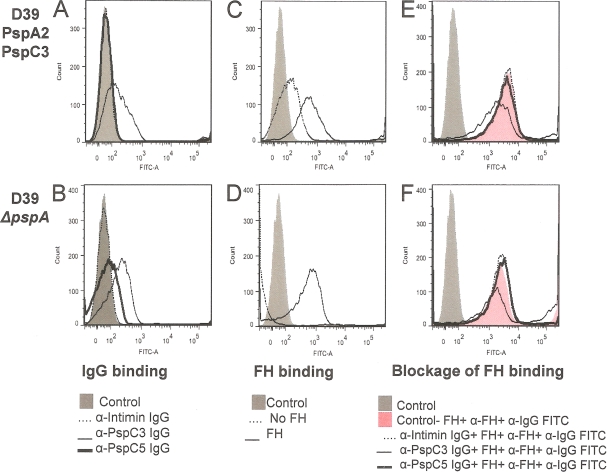
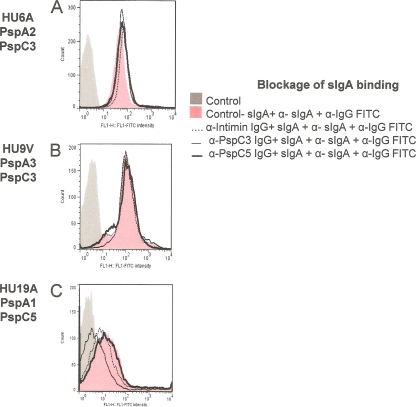
We next aimed to analyze the inhibition of binding of FH and sIgA to intact bacteria by anti-PspC3 and anti-PspC5 IgG. We did not observe inhibition of binding of FH for any of the clinical isolates (data not shown). We observed only a small decrease in FH binding after incubation of anti-PspC3 IgG with D39 and D39-ΔpspA (Fig. 5). When we analyzed binding of sIgA in the clinical isolates, only the PspC5-expressing strain showed some inhibition with anti-PspC3 IgG (Fig. 6).
Fig 5.
Flow cytometry analysis of inhibition of FH binding to D39 through anti-PspC IgG. Binding of IgG (A and B), binding of FH (C and D), and blockage of binding of FH by anti-PspC IgG (E and F) were analyzed by flow cytometry. The pneumococcal strains D39 (A, C, and E) and D39-ΔpspA (B, D, and F) were employed in the assays. Bacteria incubated only with the detection antibody conjugated with FITC were used as a control for each strain. Samples without incubation with FH (C and D) and samples incubated with anti-intimin IgG (E and F) are also shown.
Fig 6.
Flow cytometry analysis of inhibition of sIgA binding to clinical isolates through anti-PspC IgG. Blockage of binding of sIgA through anti-PspC IgG was analyzed by flow cytometry. The clinical isolates HU6A (A), HU9V (B), and HU19A (C) were employed in the assays. Bacteria incubated only with the detection antibody conjugated with FITC were used as a control for each strain. Results for samples incubated with anti-intimin IgG are also shown.
DISCUSSION
Analysis of the complete genome of 240 pneumococcal strains recently revealed that besides the capsule synthesis locus, pspA, pspC, and psrP are also hot spots of recombination, and it was proposed that strains would be able to respond rapidly to the introduction of protein vaccines based on these antigens (6). In order to develop an efficient vaccine formulation, it is thus crucial to select PspA and PspC molecules that induce broadly reacting antibodies, which would reduce problems with recombination and selection of escape mutants. The high variability of PspA and PspC is still limited by their function as virulence factors, which could enable correctly selected molecules to induce broad protection. We previously selected two PspA molecules with broad coverage potential (7, 28). We now show that immunization with PspC3 induces antibodies that recognize the majority of the pneumococcal isolates as analyzed by Western blotting of whole-cell extracts and flow cytometry of intact bacteria. A certain degree of reactivity with PspA was also observed, which was particularly pronounced when antibodies to PspC5 were used. PspC8 showed no cross-reactivity, and antibodies raised against this variant recognized only the PspC8-expressing strain. PspC8 is also described as a PspC-like protein, and it was recently shown that immunization with the N-terminal fragment of another PspC-like molecule, PspC11 from the serotype 3 strain HB565, elicited antibodies that bound efficiently to HB565 but not to D39 or TIGR4, both of which express PspC3. Furthermore, C3 deposition onto HB565 was increased in the presence of postimmune sera, while no effect was observed for D39 and TIGR4 (41). In our work, anti-PspC3 showed reactivity with the PspC-like proteins PspC8 and PspC9.
We have analyzed the capacity of the strains expressing different PspCs to interact with FH and sIgA, and most of the clinical isolates in the panel tested showed strong binding to FH and weaker interaction with sIgA. No interaction with sIgA was observed for the PspC4/PspC10-, PspC5-, PspC8-, and PspC9-expressing strains. Lu and collaborators analyzed 11 different clinical isolates and showed that most of them were able to bind FH and sIgA in Western blotting experiments (27). In that work, a serotype 3 strain expressing PspC8 did not bind FH or sIgA, and a serotype 23F strain expressing PspC3 did not bind sIgA. We detected binding of FH to a serotype 3 strain expressing PspC8 by Western blotting and flow cytometry analysis, albeit at lower intensity than the other strains. A 23F strain also expressing PspC3 showed strong binding of FH and sIgA in Western blotting. Previous work also analyzed 89 pneumococcal strains for FH binding through flow cytometry, but the PspC group of each strain was not described. The purpose of that work was to show that isolates from different pathological sources (systemic, mucosal, or carriage) could bind FH, and it was found that carriage isolates recruited significantly more FH than either systemic or mucosal isolates (36). More recently, 9 strains from different serotypes were analyzed for FH binding through flow cytometry, including four strains with PspC3, one strain with PspC6, one strain with PspC11, one strain with PspC6 and PspC9, and two strains with PspC of an unknown group. It was shown that the degree of FH binding varied between strains and was dependent on PspC, but this interaction was affected by capsular serotype (47).
Our final aim was to analyze whether the induced anti-PspC antibodies could block the FH and sIgA-binding functions of PspC, which would be desirable in a vaccine formulation. The analysis of preincubation of membranes containing whole-cell extracts of pneumococci showed that purified anti-PspC3 and anti-PspC5 IgG caused some reduction in the interaction with FH, while anti-PspC3 IgG led to reduced binding of sIgA. Almost complete blockage was observed only for one strain for FH. The analysis of this inhibition in intact bacteria by flow cytometry was not observed for the majority of the isolates. We could observe some degree of inhibition of FH binding to D39 through anti-PspC3 IgG and some inhibition of sIgA binding to a PspC5-expressing strain also through anti-PspC3 IgG. Complex interactions of PspC not only with capsule but also with other virulence factors in the pneumococcal surface may have influenced our results. These data are in accordance with results showing that pspC is a systemic and pulmonary virulence determinant for S. pneumoniae, but its effects are influenced by the pneumococcal strain (24).
In conclusion, our results show that PspC3 has potential to be used as a broad-coverage vaccine antigen, being able to induce antibodies that recognize different PspC groups. Some degree of inhibition was observed for FH and sIgA binding though anti-PspC3 IgG in vitro, but this inhibition was strain dependent. It will now be important to test the potential of PspC3 as a broad-coverage vaccine antigen in vivo. It is important to keep in mind that interaction of PspC is specific to human FH (27) and human sIgA (17) and that the several murine models of challenge with pneumococci would thus address only the capacity of anti-PspC molecules to opsonize the bacteria and to lead to subsequent phagocytosis but would not take into account blockage of PspC function. Recently, a human FH transgenic mouse model was used to evaluate the immunogenicity of the meningococcal factor H-binding protein (fHbp), and it was shown that a mutant protein that was not able to bind FH elicited antibodies with higher bactericidal activity than the native protein, with the induction of antibody responses being directed more at epitopes that would be obscured by FH bound to the wild-type protein (3). The use of such transgenic mouse models could thus be important to further evaluate pneumococcal vaccine antigens that show specific interactions with human proteins.
Supplementary Material
ACKNOWLEDGMENTS
We thank Fabiana Cristina Pimenta for advice and critical reading of the manuscript.
This work was supported by FAPESP, Fundação Butantan, and CNPq (Brazil).
Footnotes
Published ahead of print 15 February 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. Ardanuy C, et al. 2009. Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997–2007. Clin. Infect. Dis. 48: 57–64 [DOI] [PubMed] [Google Scholar]
- 2. Balachandran P, Brooks-Walter A, Virolainen-Julkunen A, Hollingshead SK, Briles DE. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70: 2526–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beernink PT, et al. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186: 3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brooks-Walter A, Briles DE, Hollingshead SK. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67: 6533–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng Q, Finkel D, Hostetter MK. 2000. Novel purification scheme and functions for a C3-binding protein from Streptococcus pneumoniae. Biochemistry 39: 5450–5457 [DOI] [PubMed] [Google Scholar]
- 6. Croucher NJ, et al. 2011. Rapid pneumococcal evolution in response to clinical interventions. Science 331: 430–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darrieux M, et al. 2008. Recognition of pneumococcal isolates by antisera raised against PspA fragments from different clades. J. Med. Microbiol. 57: 273–278 [DOI] [PubMed] [Google Scholar]
- 8. Dave S, Brooks-Walter A, Pangburn MK, McDaniel LS. 2001. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69: 3435–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dave S, Carmicle S, Hammerschmidt S, Pangburn MK, McDaniel LS. 2004. Dual roles of PspC, a surface protein of Streptococcus pneumoniae, in binding human secretory IgA and factor H. J. Immunol. 173: 471–477 [DOI] [PubMed] [Google Scholar]
- 10. Dias CA, Teixeira LM, da Gloria Carvalho G, Beall B. 2007. Sequential multiplex PCR for determining capsular serotypes of pneumococci recovered from Brazilian children. J. Med. Microbiol. 56: 1185–1188 [DOI] [PubMed] [Google Scholar]
- 11. Dieudonne-Vatran A, et al. 2009. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor C4b-binding protein in a PspC allele-dependent fashion. J. Immunol. 182: 7865–7877 [DOI] [PubMed] [Google Scholar]
- 12. Elm C, Rohde M, Vaerman JP, Chhatwal GS, Hammerschmidt S. 2004. Characterization of the interaction of the pneumococcal surface protein SpsA with the human polymeric immunoglobulin receptor (hpIgR). Indian J. Med. Res. 119(Suppl.): 61–65 [PubMed] [Google Scholar]
- 13. Ferreira DM, et al. 2009. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 16: 636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreira PC, et al. 2008. Immunization of mice with Lactobacillus casei expressing intimin fragments produces antibodies able to inhibit the adhesion of enteropathogenic Escherichia coli to cultivated epithelial cells. FEMS Immunol. Med. Microbiol. 54: 245–254 [DOI] [PubMed] [Google Scholar]
- 15. Hammerschmidt S, et al. 2007. The host immune regulator factor H interacts via two contact sites with the PspC protein of Streptococcus pneumoniae and mediates adhesion to host epithelial cells. J. Immunol. 178: 5848–5858 [DOI] [PubMed] [Google Scholar]
- 16. Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. 1997. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 25: 1113–1124 [DOI] [PubMed] [Google Scholar]
- 17. Hammerschmidt S, Tillig MP, Wolff S, Vaerman JP, Chhatwal GS. 2000. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol. Microbiol. 36: 726–736 [DOI] [PubMed] [Google Scholar]
- 18. Hicks LA, et al. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J. Infect. Dis. 196: 1346–1354 [DOI] [PubMed] [Google Scholar]
- 19. Hollingshead SK, Becker R, Briles DE. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68: 5889–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iannelli F, Chiavolini D, Ricci S, Oggioni MR, Pozzi G. 2004. Pneumococcal surface protein C contributes to sepsis caused by Streptococcus pneumoniae in mice. Infect. Immun. 72: 3077–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iannelli F, Oggioni MR, Pozzi G. 2002. Allelic variation in the highly polymorphic locus pspC of Streptococcus pneumoniae. Gene 284: 63–71 [DOI] [PubMed] [Google Scholar]
- 22. Janulczyk R, Iannelli F, Sjoholm AG, Pozzi G, Bjorck L. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275: 37257–37263 [DOI] [PubMed] [Google Scholar]
- 23. Jarva H, et al. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8–11 of factor H. J. Immunol. 168: 1886–1894 [DOI] [PubMed] [Google Scholar]
- 24. Kerr AR, et al. 2006. The contribution of PspC to pneumococcal virulence varies between strains and is accomplished by both complement evasion and complement-independent mechanisms. Infect. Immun. 74: 5319–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Glover DT, Szalai AJ, Hollingshead SK, Briles DE. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 75: 5877–5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu L, Ma Y, Zhang JR. 2006. Streptococcus pneumoniae recruits complement factor H through the amino terminus of CbpA. J. Biol. Chem. 281: 15464–15474 [DOI] [PubMed] [Google Scholar]
- 27. Lu L, et al. 2008. Species-specific interaction of Streptococcus pneumoniae with human complement factor H. J. Immunol. 181: 7138–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moreno AT, et al. 2010. Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin. Vaccine Immunol. 17: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Brien KL, et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374: 893–902 [DOI] [PubMed] [Google Scholar]
- 30. Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75: 350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogunniyi AD, et al. 2007. Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect. Immun. 75: 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogunniyi AD, Woodrow MC, Poolman JT, Paton JC. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69: 5997–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orihuela CJ, et al. 2009. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Invest. 119: 1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pai R, Gertz RE, Beall B. 2006. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 44: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pimenta FC, et al. 2011. Serotype and genotype distributions of pneumococcal carriage isolates recovered from Brazilian children attending day-care centers. J. Med. Microbiol. 60: 1455–1459 [DOI] [PubMed] [Google Scholar]
- 36. Quin LR, Onwubiko C, Carmicle S, McDaniel LS. 2006. Interaction of clinical isolates of Streptococcus pneumoniae with human complement factor H. FEMS Microbiol. Lett. 264: 98–103 [DOI] [PubMed] [Google Scholar]
- 37. Quin LR, et al. 2007. Factor H binding to PspC of Streptococcus pneumoniae increases adherence to human cell lines in vitro and enhances invasion of mouse lungs in vivo. Infect. Immun. 75: 4082–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramos CR, Abreu PA, Nascimento AL, Ho PL. 2004. A high-copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz J. Med. Biol. Res. 37: 1103–1109 [DOI] [PubMed] [Google Scholar]
- 39. Ren B, Szalai AJ, Hollingshead SK, Briles DE. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72: 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71: 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ricci S, et al. 2011. The factor H-binding fragment of PspC as a vaccine antigen for the induction of protective humoral immunity against experimental pneumococcal sepsis. Vaccine 29: 8241–8249 [DOI] [PubMed] [Google Scholar]
- 42. Roche AM, King SJ, Weiser JN. 2007. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect. Immun. 75: 2469–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenow C, et al. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25: 819–829 [DOI] [PubMed] [Google Scholar]
- 44. Singleton RJ, et al. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297: 1784–1792 [DOI] [PubMed] [Google Scholar]
- 45. Smith BL, Hostetter MK. 2000. C3 as substrate for adhesion of Streptococcus pneumoniae. J. Infect. Dis. 182: 497–508 [DOI] [PubMed] [Google Scholar]
- 46. Yother J, Handsome GL, Briles DE. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174: 610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yuste J, et al. 2010. The effects of PspC on complement-mediated immunity to Streptococcus pneumoniae vary with strain background and capsular serotype. Infect. Immun. 78: 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang JR, et al. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102: 827–837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.