Abstract
Airway hyperresponsiveness (AHR), goblet cell metaplasia, and mucus overproduction are important features of bronchial asthma. To elucidate the molecular mechanisms behind these pulmonary pathologies, we examined for genes preferentially expressed in the lungs of a murine model of allergic asthma by using suppression subtractive hybridization (SSH). We identified a gene called gob-5 that had a selective expression pattern in the airway epithelium with AHR. Here, we show that gob-5, a member of the calcium-activated chloride channel family, is a key molecule in the induction of murine asthma. Intratracheal administration of adenovirus-expressing antisense gob-5 RNA into AHR-model mice efficiently suppressed the asthma phenotype, including AHR and mucus overproduction. In contrast, overexpression of gob-5 in airway epithelia by using an adenoviral vector exacerbated the asthma phenotype. Introduction of either gob-5 or hCLCA1, the human counterpart of gob-5, into the human mucoepidermoid cell line NCI-H292 induced mucus production as well as MUC5AC expression. Our results indicated that gob-5 may play a critical role in murine asthma, and its human counterpart hCLCA1 is therefore a potential target for asthma therapy.
Bronchial asthma is a serious chronic illness characterized by one or more of a number of symptoms, including episodic shortness of breath, wheezing, coughing, and chest tightness (1). These nonspecific symptoms are associated with reversible airway obstruction and airway hyperresponsiveness (AHR), which is believed to result from chronic inflammation of the bronchial mucosa. This inflammation involves leukocyte infiltration of the bronchial tissues, excessive mucus production, epithelial damage, basement membrane thickening, and smooth muscle hypertrophy (2, 3). Although many studies have focused on cytokine production, secretion of chemical mediators, and eosinophil infiltration, little is known of the changes in gene expression within asthmatic airways.
To help elucidate the molecular mechanisms of AHR, we decided to analyze the differential gene expression in the airways during the process of epithelial inflammation. To this end, we used a suppression subtractive hybridization (SSH) technique that has been shown to be highly effective in the identification of disease-related, developmental stage-specific, tissue-specific, and other differentially expressed genes (4). By comparing gene expression in ovalbumin (OVA)-sensitized mice after OVA-challenge with that of normal mice, the gob-5 gene was identified as being specifically induced in the murine lung with bronchial hyperreactivity. Gob-5 was recently cloned from a mouse intestinal cDNA library, and was found to be a member of the calcium-dependent chloride channel family (5). In this study, we demonstrated that in vivo adenoviral gene transfer with an antisense gob-5 construct suppressed the pathology of murine allergic asthma through the prevention of AHR and mucus overproduction. In contrast, overexpression of gob-5 in airway epithelia, by using adenoviral vector with sense gob-5 construct, induced mucus overproduction and AHR.
Materials and Methods
Murine AHR Models.
Male BALB/c mice were immunized by i.p. injection of 20 μg of OVA (grade V; Sigma) with 2 mg of aluminum hydroxide (Alum). One week after the first immunization, mice were further sensitized by an i.p. injection of 10 μg of OVA with 1 mg of Alum. Two weeks after the first immunization, mice were challenged for 25 min with an aerosol of 5% (wt/vol) OVA in 0.5× PBS each day for 7 consecutive days. Sham-challenged mice inhaled an aerosol of 0.5× PBS alone by using the same protocol. Airway responsiveness was measured as previously described (6, 7) with minor modifications. Twenty-four hours after the final inhalation, airway pressure was measured with a pressure transducer via the port of a tracheal cannula. After establishing a stable baseline airway pressure (6–7 cm H2O), acetylcholine chloride (ACh; Tokyo Kasei, Tokyo, Japan) was injected i.v. (62.5–1000 μg/kg), and changes in airway pressure were recorded. Airway responsiveness was defined as a change in the peak airway pressure from baseline. For measurement of infiltrating cells, bronchoalveolar lavage (BAL) was performed as described (8). Cytospin preparations of BAL cells were stained with Diff-Quik (Baxter Healthcare, Miami, FL), and cell identification, based on morphology and staining characteristics, was performed on at least 400 cells. All values were represented as mean ± SEM. Statistical analyses were carried out by using sas software (SAS Institute Inc., Cary, NC).
Subtractive Hybridization and Differential Screening.
Total RNA from lung tissues was obtained by using ISOGEN (Wako Pure Chemicals, Osaka) according to the manufacturer's instructions. Poly(A)+ RNA was isolated by using an mRNA purification kit (Amersham Pharmacia). Two-microgram aliquots of lung poly(A)+ RNA from normal and AHR-model mice were used to make driver and tester cDNA, respectively. SSH was performed by using the PCR-select cDNA Subtraction Kit (CLONTECH). Subtracted PCR products were ligated into the pT7 Blue-T vector (Novagen), and ligation mixtures were transformed into Escherichia coli DH-5α (Toyobo, Osaka). Differentially expressed products were selected by using the PCR-select Differential Screening Kit (CLONTECH), and the cloned products were sequenced by using an ABI PRISM 377 DNA Sequencer (Perkin-Elmer). Sequencing results were compared with GenBank database sequences by using the blast homology search program (National Institutes of Health, Bethesda, MD; ref. 9).
Full-Length Cloning of Mouse Gob-5 and Human CLCA1.
AHR-model mouse lung cDNA was reverse transcribed from poly(A)+ RNA by using a cDNA synthesis kit (Takara, Kyoto), and full-length gob-5 gene sequence were amplified by PCR by using specific primers (5′-GGGAAAGCTGCAGGATGGAATC-3′ and 5′-TATACCGCCCCCTACAGGAAGTCTAA-3′). A full-length hCLCA1 gene sequence was PCR amplified from a human small intestine cDNA library (CLONTECH) by using specific primers (5′-AATCACAGGGAGATGTACAGC-3′ and 5′-TTTTATAATCAAAAAAAGGATG-3′). PCR products were cloned into the pT7 Blue-T and pCRII-TOPO (Invitrogen) vectors, giving pT7-gob-5 and pCRII-hCLCA1 plasmids, respectively. For expression of gob-5 and hCLCA1, cloned gob-5 and hCLCA1 cDNA were subcloned into pcDNA3.1 (Invritogen), by using the HindIII and KpnI sites for gob-5 (pcDNA-gob-5), and the KpnI and NotI sites for hCLCA1 (pcDNA-hCLCA1).
Northern Analysis.
Tissue expression and distribution of gob-5 was determined by Northern blot analysis of poly(A)+ RNA extracted at the indicated time points from samples of brain, heart, liver, lung, kidney, thymus, spleen, stomach, small intestine, and large intestine of both normal and AHR-model mice. Aliquots of 0.5 μg poly(A)+ RNA were electrophoresed on 1.1% agarose gels containing 2.2 M formaldehyde, and blotted onto Hybond- N+ membranes (Amersham Pharmacia). Filters were hybridized to gob-5 cDNA, 32P-labeled by using the BcaBEST labeling kit (Takara), in Express Hyb hybridization solution (CLONTECH). GAPDH and β-actin probes (CLONTECH) were used as controls for RNA loading.
In Situ Hybridization.
Digoxigenin (DIG)-labeled 0.5-kb RNA probes were transcribed in vitro from PCR product amplified from gob-5 cDNA by using a DIG labeling kit (Roche Diagnostics). In situ hybridization was performed on paraformaldehyde-fixed murine lung sections by using the ISHR starting kit (Nippon Gene, Tokyo, Japan). Bound probes were detected by alkaline phosphatase-conjugated anti-DIG antibodies (Roche Diagnostics) and Nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche Diagnostics) was used as substrate. Hybridizations of several sections with gob-5 sense and antisense probes were used as negative and positive controls, respectively.
Construction of Recombinant Adenoviruses.
Gob-5 cDNA fragments were isolated from pT7-gob-5 after digestion with restriction enzymes HincII and SmaI, and ligated into Swa I-digested pAxCAwt (Takara). Orientation of gob-5 cDNA was confirmed by digestion with EcoRI. The resulting plasmids (pAxCAgob-5-S and pAxCAgob-5-AS) were transfected into HEK-293 cells (ATCC CRL-1573) following the protocol of the Adenovirus Expression Kit (Takara). Recombinant replication-deficient adenoviruses (Ad-gob-5-S and Ad-gob-5-AS) were rescued by homologous recombination. The presence of gob-5 cDNA was verified by analysis of viral genomic DNA fragments after XhoI digestion. Control adenovirus vector, pAxCAwt-derived Ad-wt, was constructed and characterized in the same manner. Viruses were expanded, purified, and plaque-titered in HEK-293 cells according to the manufacturer's protocols.
Administration of Recombinant Adenoviruses to Mouse Lung.
Ad-gob-5-S, Ad-gob-5-AS, and Ad-wt (5 × 108 pfu) in 40 μl distilled water were instilled intratracheally into the lungs of anesthetized BALB/c mice. Recombinant adenoviruses were injected 1 day before the start of OVA inhalation. Airway reactivity was measured 24 h after final OVA inhalation, given for 3 consecutive days in the case of Ad-gob-5-S, and 6 consecutive days in the case of Ad-gob-5-AS, as described above. Lungs were then removed, fixed by perfusion with paraformaldehyde, and periodic acid/Schiff reagent (PAS) stained.
Transient Transfection and PAS Staining.
Human pulmonary mucoepidermoid NCI-H292 cells (ATCC CRL-1848) were transfected with 3 μg pcDNA-gob-5, pcDNA-hCLCA1, or vector alone (pcDNA3.1) by using FuGENE6 (Roche Diagnostics) as a carrier. After 4 days culture, cells were fixed with formalin, and mucus glycoconjugates were visualized by PAS staining.
Determination of MUC5AC Gene Expression.
Total RNA was extracted from NCI-H292 cells by using ISOGEN following the manufacturer's protocols. Reverse transcription was performed on 0.5 μg total RNA by using an oligo(dT) primer, and cDNA corresponding to 50 ng total RNA was amplified by PCR by using specific primers. Primers used for gob-5 detection were 5′-GCCAAGGAGCCTCGCCTATTCTCAGG-3′ and 5′-GAAGCTCTCCCGTGGTCGTAG-3′. Primers used for hCLCA1 detection were 5′-TGATGCTACTAAGGATGAC-3′ and 5′-TTCCTCAGAGTTGGCTTCCT-3′. Primers used for MUC5AC detection were 5′-GTGGAACCACGATGACAGC-3′ and 5′-TCAGCACATAGCTGCAGTCG-3′ (10). Primers used for GAPDH internal control were 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′.
Results
Identification of Gob-5 as an AHR-Related Gene.
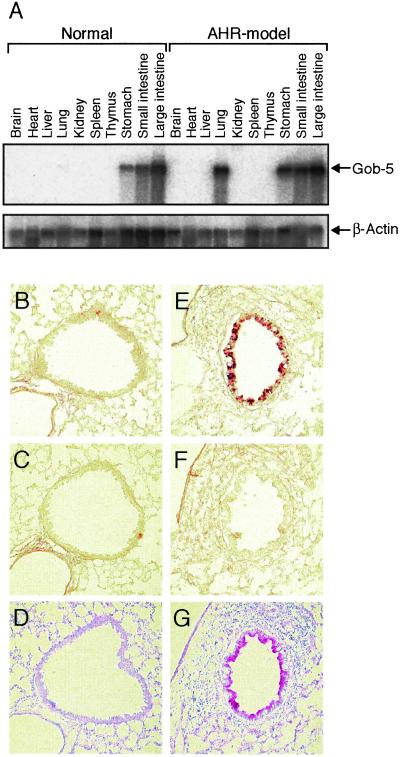
To search for genes preferentially expressed in lungs with bronchial hyperreactivity, we compared gene expression in the lungs from OVA-sensitized and -challenged mice (AHR-model mice) with that of normal mouse lungs. By using SSH, we isolated a pool of differentially expressed genes as subtracted PCR products. Of the 864 clones screened, 90 produced stronger signals with probes from AHR-model mice than probes from normal mice. One positive clone, designated 4-26, consisted of a 500-bp DNA fragment that matched to the partial sequence of a known gene, gob-5, in the GenBank database. Northern blot analysis of normal and AHR-model mice confirmed that clone 4-26 was strongly induced in the lung of AHR-model mice. It was reported that gob-5 was abundantly expressed in the small intestine, colon, stomach, and uterus, with only slight expression in tracheal tissue (5). We confirmed gob-5 expression in the stomach, small intestine, and large intestine by Northern blot analysis on both AHR-model and normal mice, with similar expression levels being observed in both digestive tracts (Fig. 1A). However, gob-5 mRNA was strongly expressed in lung tissues from AHR-model mice, whereas signal was scarcely detected in the normal lung. Thus, gob-5 was induced specifically in lung tissue associated with AHR.
Figure 1.
Selective expression of gob-5 mRNA in airway epithelia from AHR-model mice. (A) Tissue distribution of gob-5 was determined by Northern blot analysis in normal and AHR-model mice. (Upper) The results obtained by hybridization with a gob-5 cDNA probe. The murine tissues from which the poly(A)+ RNA was prepared are indicated at the top. The same filter was rehybridized with a β-actin probe to control for equal loading (Lower). (B, C, E, and F) Lung sections of normal (B and C) and AHR-model mice (E and F) were in situ hybridized with DIG-labeled, single-stranded antisense (B and E) and sense (C and F) RNA probes. (D and G) The same lung sections of normal (D) and AHR-model mice (G) were stained with PAS and counterstained with hematoxylin. Original magnification, ×200. Three different experiments showed similar results.
Next, in situ hybridization was carried out by using a DIG-labeled antisense RNA probe to identify cell-specific gob-5 expression. Strong signals were detected throughout the bronchial lining of lungs from AHR-model mice but not from normal mice (Fig. 1 B and E). The gob-5-expressing areas coincided completely with the surface areas positively stained by PAS reaction, which indicated that gob-5 expression was strictly localized to the airway epithelium, especially to mucus-producing cells (Fig. 1 E and G). Hybridization with the sense probe revealed no staining, confirming the specificity of the assay (Fig. 1 C and F). No signals were detected in infiltrating cells or in endothelial cells of AHR-model mice, indicating that these cells did not express gob-5 transcripts during airway inflammation. The combination of in situ hybridization and PAS staining analysis revealed that the location of gob-5 transcripts was the goblet cells, the function of which are to secrete mucins.
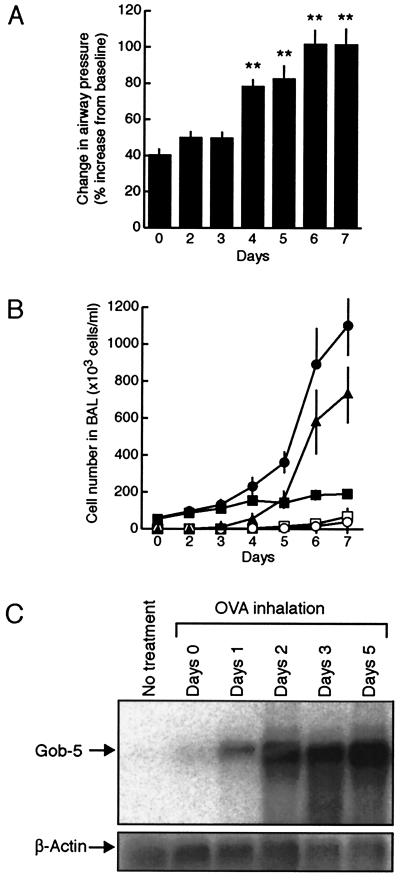
To elucidate the relationship between the development of AHR and gob-5 expression, we measured airway responsiveness and counted infiltrating cells by performing BAL every day during OVA inhalation (Fig. 2 A and B). Expression levels of gob-5 mRNA in the lung were also evaluated by Northern blot analysis (Fig. 2C). Whereas development of AHR and eosinophil infiltration was observed from day 4 after the start of OVA inhalation, gob-5 expression was markedly induced from day 2. Thus, gob-5 mRNA was induced before the onset of AHR and eosinophilic infiltration.
Figure 2.
Induction of gob-5 before the onset of AHR and eosinophilic inflammation. (A) Time-related changes in airway responsiveness after OVA inhalation were measured (n = 7–11). **, P < 0.01, compared with day 0 group. (B) Time-related changes in cell recruitment in BAL fluid after OVA inhalation. BAL was performed 24 h after inhalation of aerosolized OVA (n = 6–7). Total cells (●) were classified as macrophages (■), eosinophils (▴), neutrophils (○), or lymphocytes (□). (C) Time-dependent expression of gob-5 was determined by Northern blot analysis in lung tissue after OVA inhalation. Presented figures are representative of three separate experiments. Three different experiments showed similar results.
Silencing of Gob-5 Gene Expression Suppressed AHR and Mucus Overproduction.
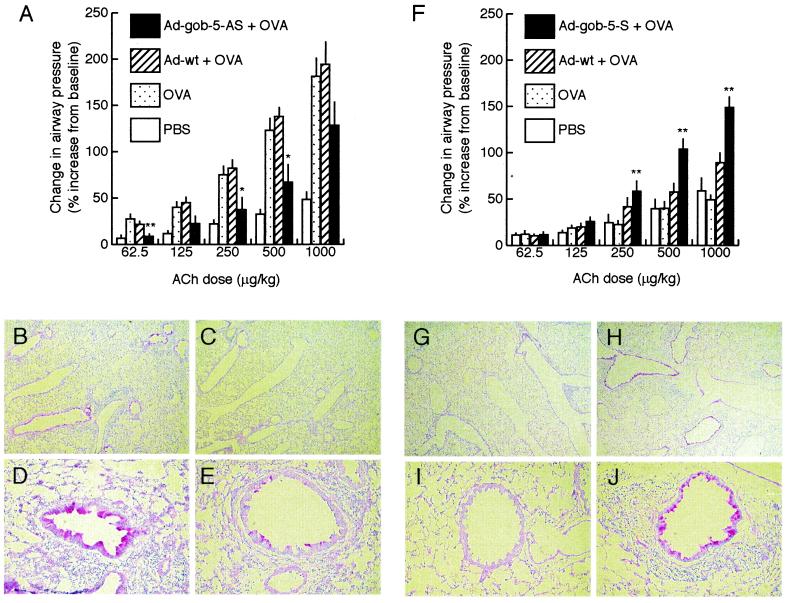
In vivo adenovirus-mediated gene delivery is an efficient strategy for the study of gene function in the lung (11–14). To assess the efficacy of intratracheal delivery of recombinant adenovirus, BALB/c mice were administered 1 × 108 plaque-forming units (pfu) of adenovirus containing the LacZ (β-galactosidase) gene. Lungs were harvested 24 h later, and sections were stained for β-galactosidase activity. Strong staining was observed in the airway epithelium and confirmed the efficacy of the recombinant adenovirus-mediated gene delivery system (data not shown). This system was then used to clarify the functional role of gob-5 in the development of AHR. Mice were administered either control adenovirus (Ad-wt) or adenovirus expressing antisense gob-5 RNA (Ad-gob-5-AS) intratracheally at a dose of 5 × 108 pfu 1 day before the start of OVA inhalation. OVA inhalation was then performed for 6 consecutive days, and airway responsiveness was measured 24 h after the final inhalation. The endogenous gob-5 expression was almost fully suppressed by this treatment (see Fig. 6, which is published as supplemental data on the PNAS web site, www.pnas.org). Intratracheal challenge with Ad-gob-5-AS significantly suppressed AHR, whereas no inhibitory effect on airway reactivity was observed after administration of Ad-wt (Fig. 3A). In pulmonary tissue sections from mice treated with control adenovirus, marked induction of extensive metaplasia in mucus-producing airway goblet cells was observed (Fig. 3 B and D). However, in pulmonary tissue sections from mice treated with Ad-gob-5-AS, the secretion of mucins from around the airway lumen was dramatically decreased (Fig. 3 C and E). PAS-stained area and the number of infiltrated eosinophils were also significantly decreased in lungs from Ad-gob-5-AS-treated mice compared with lungs from Ad-wt-treated mice (see supplemental Table 1 and supplemental Materials and Methods). These results indicate that the suppression of gob-5 induction by adenoviral antisense gene transfer ameliorated the murine asthma phenotype.
Figure 3.
Functional analysis of gob-5 in the development of AHR and mucus production by in vivo adenoviral gene transfer. (A) Reduction in airway responsiveness by the administration of adenovirus-expressing gob-5 antisense RNA in AHR-model mice (n = 5–10). Recombinant adenovirus-expressing gob-5 antisense RNA transcripts (Ad-gob-5-AS) and control adenovirus (Ad-wt) were intratracheally administered one day before the start of OVA inhalation. Airway responsiveness was measured 24 h after the final inhalation carried out for 6 consecutive days. *, P < 0.05; **, P < 0.01, compared with the OVA-inhaled group by Dunnett type test. (B–E) Suppression of airway mucus secretion by the administration of adenovirus-expressing gob-5 antisense RNA in AHR-model mice. Lung sections of Ad-wt-administered mice (B and D) and Ad-gob-5-AS-administered mice (C and E) were stained with PAS, counterstained with hematoxylin. Original magnifications, ×40 (B and C), ×200 (D and E). (F) Promotion of airway responsiveness by overexpressing gob-5 in OVA-inhaled mice (n = 6–10). Recombinant adenovirus expressing gob-5 RNA transcripts (Ad-gob-5-S) and control adenovirus (Ad-wt) were intratracheally administered 1 day before the start of OVA inhalation. Airway reactivity was measured 24 h after the final inhalation carried out for 3 consecutive days. **, P < 0.01, compared with OVA-inhaled group by Dunnett type test. (G–J) Induction of airway mucus secretion by overexpressing gob-5. Lung sections of Ad-wt-administered mice (G and I) and Ad-gob-5-S-administered mice (H and J) were stained with PAS, counterstained with hematoxylin. Original magnifications, ×40 (G and H), ×200 (I and J).
Overexpression of Gob-5 in Airway Epithelia Induced Mucus Production and Airway Hyperresponsiveness.
We next introduced the gob-5 gene into airway epithelia of OVA-sensitized mice by using an adenoviral vector to study whether gob-5 overexpression could promote the development of AHR and mucus production. Mice were administered adenovirus containing the gob-5 gene (Ad-gob-5-S) intratracheally at a dose of 5 × 108 pfu 1 day before the start of OVA inhalation. To focus on the enhancing effects of gob-5, OVA inhalation was performed for 3 consecutive days, and airway responsiveness was measured 24 h after final inhalation, at which point airway inflammation had not yet occurred (Fig. 2 A and B). Mice treated with Ad-gob-5-S showed significantly increased airway responsiveness compared with mice treated with control Ad-wt (Fig. 3F). Histochemical analysis of epithelia from most airway lumens of Ad-gob-5-S-treated mice demonstrated positive staining for mucins by PAS (Fig. 3 H and J), whereas no staining was observed in pulmonary tissue sections from control mice (Fig. 3 G and I). PAS-stained area was significantly increased in lungs from Ad-gob-5-S-treated mice compared with lungs Ad-wt-treated mice. The number of eosinophils in peribronchial distribution was also increased in lungs from mice treated with Ad-gob-5-S (see supplemental Table 1). These results indicate that the overexpression of the gob-5 gene in airway epithelia exacerbated the murine asthma phenotype, including AHR, goblet cell metaplasia, mucus overproduction, and eosinophil infiltration. When Ad-gob-5-S was administered to mice without OVA inhalation, only slight, but significant, enhancement of airway responsiveness was observed. Prominent mucus production and goblet cell metaplasia were still observed in lungs treated with sense gob-5 construct alone (see supplemental Fig. 5 and supplemental Table 2).
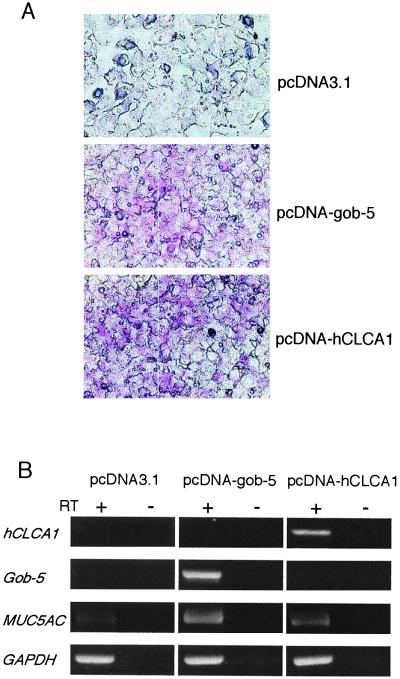
Mucus Production in Human Mucoepidermoid Cells Transfected with Gob-5 or hCLCA1.
Structurally, gob-5 belongs to a family of calcium-activated chloride channel (CaCCs) (15–19). The gob-5 protein shares 75% similarity with the human protein hCLCA1/hCaCC1, 55% with hCLCA4/hCaCC2, and 38% with hCLCA2/hCaCC3. Given the high structural homology and similarity of tissue distribution, it is likely that the human counterpart of gob-5 is hCLCA1 (16, 17). Therefore, we examined the relationship between mucus production and these molecules, hCLCA1 and gob-5. Expression vectors for hCLCA1 (pcDNA-hCLCA1) or gob-5 (pcDNA-gob-5) were introduced into the human pulmonary mucoepidermoid cell line NCI-H292. After transient transfection, cells were cultured for 4 days and assayed for mucus production by PAS staining. The number and intensity of PAS-stained cells were significantly increased in both hCLCA1-transfected and gob-5-transfected NCI-H292 cells, whereas no change was observed in mock-transfected cells (Fig. 4A). We also analyzed MUC5AC gene expression, a marker of goblet cell metaplasia (20). Although NCI-H292 cells displayed basal MUC5AC expression, introduction of hCLCA1 or gob-5 genes led to significant induction of MUC5AC expression (Fig. 4B). These results indicate that hCLCA1, the human counterpart of gob-5, may also play a direct role in mucus production, one of the main clinical features of human asthma.
Figure 4.
Mucus production in human mucoepidermoid cells transfected with gob-5 or hCLCA1. (A) PAS staining was performed on NCI-H292 cells after transfection with control vector (pcDNA3.1), gob-5 expression vector (pcDNA-gob-5), or hCLCA1 expression vector (pcDNA-hCLCA1). (B) Induction of MUC5AC gene expression by transfection with gob-5 or hCLCA1 genes in NCI-H292 cells. Total RNA was extracted from transfected cells 3 days after transfection, and reverse transcription (RT)-PCR amplification was performed for hCLCA1, gob-5, and MUC5AC gene detection. Amplification of the GAPDH gene was used as a control for RNA loading. cDNA reactions in which the RT was omitted (− lanes) were used as negative controls for each condition. These data are representative of three independent experiments.
Discussion
We have identified the gob-5 gene that was found to be markedly induced in the lung of AHR-model mice. Komiya et al. reported that gob-5 was abundantly expressed in the small intestine, colon, stomach, and uterus, with only slight expression in tracheal tissues (5). Our study confirmed expression in the small intestine, colon, and stomach of both normal and AHR-model mice, with similar expression levels. However, striking up-regulation of gob-5 expression was observed in lung from AHR-model mice compared with normal lung. The combination of in situ hybridization and PAS staining analysis revealed that gob-5 transcripts were localized to goblet cells, the function of which is to secrete mucins. The number of goblet cells in the airway epithelium is markedly increased in asthma and chronic bronchitis (21), and mucin overproduction is due to goblet cell metaplasia. As a result, the airway lumen becomes plugged with mucus, which leads to airway obstruction. We observed the induction of goblet cell metaplasia in the lung of AHR-model mice, and also observed that gob-5 expression coincided completely with the location of the goblet cells. Recently, it was reported that the mucin MUC5AC was a marker of goblet cell metaplasia in murine airways (20). In our experiments, MUC5AC mRNA was induced in a mucoepidermoid cell line by transient transfection of gob-5, suggesting that gob-5 may be an important gene in the regulation of MUC5AC expression.
To investigate the role of gob-5 in the development of murine bronchial hyperreactivity, we chose an in vivo transgene approach by using the recombinant adenovirus system. The airway epithelium has a very large absorption surface, to which adenovirus can be approached with relative specificity. It has also been reported that recombinant adenovirus can continue to overexpress transduced genes in the lung for more than a week after instillation (11–14). The administration of adenovirus-carrying antisense gob-5 sequence (Ad-gob-5-AS) dramatically suppressed PAS staining of bronchial epithelia, indicating a reduction in mucus production by the airway goblet cells. In contrast, the introduction of the gob-5 gene by adenovirus-carrying sense sequence into murine epithelia markedly enhanced mucus production around the airway lumen. These results clearly show that gob-5 is an essential molecule in the regulation of goblet cell mucus production. AHR and infiltrating cell numbers were also suppressed by Ad-gob-5-AS administration. Although it is generally accepted that AHR is caused by inflammation of the asthmatic airways (22), the mechanism by which asthmatic inflammation leads to AHR is poorly understood. It was demonstrated that IL-13 is a potent inducer of AHR (23), and that IL-13 caused goblet cell hyperplasia and mucus hyperproduction. We have not confirmed whether the mucus-producing activity of gob-5 was mediated by Th2 cytokines such as IL-13 or not. Further study focusing on the mechanism responsible for the induction of AHR and mucus production by gob-5 is warranted.
Gob-5 is a member of a family of calcium-activated chloride channel proteins (CaCCs) (15–19). In the mammalian intestine, fluid secretion is driven by the active export of chloride ions. The process of active chloride ion secretion has been associated with mucin release, and sustained active chloride ion secretion appears to involve vacuolated columnar cells rather than goblet cells (24). However, these studies examined cAMP-dependent chloride channel activity rather than calcium-activated chloride channel activity. Although it has not yet been confirmed whether gob-5 has calcium-activated chloride channel activity, if it does, gob-5 may enhance mucin secretion by mediating the active transport of chloride ions.
The gob-5 protein shares 75% similarity with the human protein hCLCA1/hCaCC1, 55% with hCLCA4/hCaCC2, and 38% with hCLCA2/hCaCC3 (16–18). Of these calcium-activated chloride channel family members, the transcription of hCLCA1/hCaCC1 is strikingly associated with the digestive tract, including the small intestine, colon, and appendix, and is exclusively expressed by intestinal basal crypt epithelia and goblet cells (16). hCaCC2 is widely distributed in a variety of tissues (17), particularly in the colon, and also in the trachea, whereas hCaCC3 is observed mainly in the trachea, and is faintly detectable in the uterus, prostate, testis, and kidney (17). Judging from both protein sequence similarity and tissue distribution, hCLCA1/hCaCC1 appears to be the most likely candidate of the human homolog of gob-5. Our results support this hypothesis as we observed that the transfection of the hCLCA1 gene into a mucoepidermoid cell line enhanced PAS staining and MUC5AC expression, indicating that hCLCA1 may play an important role in mucus production. It has since been confirmed that hCLCA1 has calcium-activated chloride channel activity (16), so that hCLCA1 is likely to enhance mucus secretion by mediating the active transport of chloride ions via a calcium-activated chloride channel. Further studies are required to determine whether the hCLCA1/hCaCC1 gene is induced in airway goblet cells of asthmatic patients, or if the functions of hCaCC2 or hCaCC3 are substituted for that of gob-5 in human AHR.
In conclusion, we have isolated an asthma-related gene gob-5 by SSH performed on lung cDNA from normal and AHR-model mice, and demonstrated that gob-5 may play critical roles in goblet cell metaplasia, mucus hypersecretion, and AHR. Goblet cell metaplasia and mucus hypersecretion are important features not only of acute asthma, but also of many chronic airway diseases including chronic bronchitis and cystic fibrosis (25, 26). The mechanisms involved in goblet cell metaplasia are poorly understood, and there are no effective therapies able to treat its symptoms. Our findings may provide new insights into the regulation of phenomena associated with the asthma disease process. Functional inhibition of the human calcium-activated chloride channel protein hCLCA1 may therefore provide a new therapeutic strategy for hypersecretory airway diseases.
Supplementary Material
Acknowledgments
We thank M. Hoshino, J. Tamaoki, K. Fujimoto, and M. Ichinose for scientific discussions. We also thank H. Nagaya, T. Matsumoto, M. Fujisawa, and T. Kurokawa for their advice and comments, and T. Nagi and K. Obi for excellent technical assistance.
Abbreviations
- AHR
airway hyperresponsiveness
- SSH
suppression subtractive hybridization
- OVA
ovalbumin
- BAL
bronchoalveolar lavage
- PAS
periodic acid/Schiff reagent
- DIG
digoxigenin
- CaCC
calcium-activated chloride channel
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.McFadden E R, Gilbert I A. N Engl J Med. 1992;327:1928–1937. doi: 10.1056/NEJM199212313272708. [DOI] [PubMed] [Google Scholar]
- 2.Boushey H A, Fahy J V. Environ Health Perspect. 1995;103, Suppl. 6:229–233. doi: 10.1289/ehp.95103s6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes P J. J Allergy Clin Immunol. 1989;83:1013–1026. doi: 10.1016/0091-6749(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 4.Diatchenko L, Lau Y-F C, Campbell A P, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov E D, Siebert P D. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komiya T, Tanigawa Y, Hirohashi S. Biochem Biophys Res Commun. 1999;255:347–351. doi: 10.1006/bbrc.1999.0168. [DOI] [PubMed] [Google Scholar]
- 6.Konzett H, Rössler R. Arch Exp Path Pharmakol. 1940;195:71–74. [Google Scholar]
- 7.Corry D B, Folkesson H G, Warnock M L, Erle D J, Matthay M A, Wiener-Kronish J P, Locksley R M. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur M, Herrmann K, Li X, Qin Y, Weinstock J, Elliott D, Monahan J, Padrid P. Am J Respir Crit Care Med. 1999;159:580–587. doi: 10.1164/ajrccm.159.2.9712018. [DOI] [PubMed] [Google Scholar]
- 9.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louahed J, Toda M, Jen J, Hamid Q, Renauld J-C, Levitt R C, Nicolaides N C. Am J Respir Cell Mol Biol. 2000;22:649–656. doi: 10.1165/ajrcmb.22.6.3927. [DOI] [PubMed] [Google Scholar]
- 11.Sime P J, Xing Z, Graham F L, Csaky K G, Gauldie J. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing Z, Braciak T, Ohkawara Y, Sallenave J-M, Foley R, Sime P J, Jordana M, Graham F L, Gauldie J. J Leukocyte Biol. 1996;59:481–488. doi: 10.1002/jlb.59.4.481. [DOI] [PubMed] [Google Scholar]
- 13.Xing Z, Ohkawara Y, Jordana M, Graham F L, Gauldie J. J Clin Invest. 1996;97:1102–1110. doi: 10.1172/JCI118503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standiford T J, Wilkowski J M, Sisson T H, Hattori N, Mehrad B, Bucknell K A, Moore T A. Human Gene Therapy. 1999;10:899–909. doi: 10.1089/10430349950018300. [DOI] [PubMed] [Google Scholar]
- 15.Fuller C M, Benos D J. News Physiol Sci. 2000;15:165–171. [PubMed] [Google Scholar]
- 16.Gruber A D, Elble R C, Ji H-L, Schreur K D, Fuller C M, Pauli B U. Genomics. 1998;54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- 17.Agnel M, Vermet T, Culouscou J-M. FEBS Lett. 1999;455:295–301. doi: 10.1016/s0014-5793(99)00891-1. [DOI] [PubMed] [Google Scholar]
- 18.Gruber A D, Schreur K D, Ji H-L, Fuller C M, Pauli B U. Am J Physiol. 1999;276:C1261–C1270. doi: 10.1152/ajpcell.1999.276.6.C1261. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi R, Elble R C, Gruber A D, Schreur K D, Ji H-L, Fuller C M, Pauli B U. J Biol Chem. 1998;273:32096–32101. doi: 10.1074/jbc.273.48.32096. [DOI] [PubMed] [Google Scholar]
- 20.Alimam M Z, Piazza F M, Selby D M, Letwin N, Huang L, Rose M C. Am J Respir Cell Mol Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 21.Blyth D I, Pendrick M S, Savage T J, Bright H, Beesley J E, Sanjar S. Am J Respir Cell Mol Biol. 1998;19:38–54. doi: 10.1165/ajrcmb.19.1.2930. [DOI] [PubMed] [Google Scholar]
- 22.Weinberger S. N Engl J Med. 1993;328:1389–1397. doi: 10.1056/NEJM199305133281906. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Homer R J, Wang Z, Chen Q, Geba G P, Wang J, Zhang Y, Elias J A. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halm D R, Halm S T, DiBona D R, Frizzell R A, Johnson R D. Am J Physiol. 1995;269:C929–C942. doi: 10.1152/ajpcell.1995.269.4.C929. [DOI] [PubMed] [Google Scholar]
- 25.Sinder G L, Faling L J, Rennard S I. Textbook of Respiratory Medicine. New York: Saunders; 1994. , Chap. 41. [Google Scholar]
- 26.Sinder G L, Faling L J, Rennard S I. Textbook of Respiratory Medicine. New York: Saunders; 1994. , Chap. 43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.