Abstract
Since the 2006 discovery of the Acinetobacter baumannii strain AYE AbaR1 resistance island, similar elements have been reported in numerous members of this species. As AbaR1 is distantly related to Tn7, we have renamed it TnAbaR1. TnAbaR transposons are known to carry multiple antibiotic resistance- and efflux-associated genes, although none have been experimentally studied en bloc. We deleted the TnAbaR transposon in A. baumannii A424, which we have designated TnAbaR23, and characterized independent deletion mutants DCO163 and DCO174. The NotI pulsed-field gel electrophoresis (PFGE) profile of strain DCO174 was consistent with targeted deletion of TnAbaR23 alone, but strain DCO163 apparently harbored a second large genomic deletion. Nevertheless, “subtractive amplification” targeting 52 TnAbaR and/or resistance-associated loci yielded identical results for both mutants and highlighted genes lost relative to strain A424. PCR mapping and genome sequencing revealed the entire 48.3-kb sequence of TnAbaR23. Consistent with TnAbaR23 carrying two copies of sul1, both mutants exhibited markedly increased susceptibility to sulfamethoxazole. In contrast, loss of tetAR(A) resulted in only a minor and variable increase in tetracycline susceptibility. Despite not exhibiting a growth handicap, strain DCO163 was more susceptible than strain DCO174 to 9 of 10 antibiotics associated with mutant-to-mutant variation in susceptibility, suggesting impairment of an undefined resistance-associated function. Remarkably, despite all three strains sharing identical gyrA and parC sequences, the ciprofloxacin MIC of DCO174 was >8-fold that of DCO163 and A424, suggesting a possible paradoxical role for TnAbaR23 in promoting sensitivity to ciprofloxacin. This study highlights the importance of experimental scrutiny and challenges the assumption that resistance phenotypes can reliably be predicted from genotypes alone.
INTRODUCTION
The clinical significance of Acinetobacter baumannii has grown substantially over the last few decades largely due to the fact that this species possesses an extensive repertoire of innate antimicrobial resistance mechanisms and a remarkable capacity to acquire and express a diverse range of foreign resistance determinants. Whole-genome sequence analysis of A. baumannii is providing valuable insights into the genetic complexity and genomic agility of this pathogen and has also revealed extensive strain-to-strain variation in the repertoires of known and putative resistance-associated genes.
Over the last 4 years, it has been increasingly recognized that many A. baumannii strains possess highly clustered assemblages of resistance genes and associated mobile elements mapping to genomic segments that are closely related to AbaR1, an ∼86-kb element found in A. baumannii strain AYE which was predicted to encode resistance via 45 genes to several heavy metals and multiple classes of antimicrobial agents (14). This single, potentially mobile element, through diverse genes which have probably been acquired from several bacterial genera, is predicted to encode resistance to β-lactams, aminoglycosides, chloramphenicol, rifampin, tetracyclines, sulfonamides, and several heavy metals (2, 14). This resistance island type architecture also provides the perfect storehouse for class 1 integrons, insertion sequences, and transposons, thus aiding the transfer of drug resistance determinants (14).
Increasing numbers of AbaR1-like resistance islands have been identified in the last 2 years. These have been numbered consecutively and include at least 20 members identified in A. baumannii strains belonging to the European clone I lineage (1, 2, 18, 19, 24, 25). In addition, AbaR2 from A. baumannii ACICU, a likely remnant of an AbaR1-like element, was until recently the only such element found in a European clone II strain to have been characterized (17).
A feature common to all but one of the characterized AbaR1-like elements is that these elements disrupt the comM gene, which codes for a predicted competence-associated protein, in a site-specific manner. The comM gene also serves as an integration hot spot, though much less frequently, for several other evolutionarily distinct, horizontally acquired DNA sequences (2, 14, 29). PCR interrogation of the comM locus in 52 genotypically diverse multidrug-resistant A. baumannii clinical isolates suggested a comM-associated AbaR1-like carriage rate of ∼65% in this species (M. Kochar and K. Rajakumar, unpublished data). Critically, detailed bioinformatic analyses of the termini of AbaR1-like elements across multiple A. baumannii strains has provided the strongest evidence yet that these elements have arisen from an ancestral Tn7-like transposon. Accordingly, we have renamed AbaR1 TnAbaR1 and extended this nomenclature to the wider family of these elements (27). In all likelihood, the ancestral TnAbaR transposon diversified through acquisition, often in a nested fashion, of a large variety of transposons, integrons, and resistance gene cassettes, supplemented by deletions, duplications, rearrangements and/or the acquisition of point mutations. TnAbaR transposons carry genes predicted to code for resistance to a wide range of antibiotics and several efflux-associated systems. The latter include likely representatives of resistance-nodulation-division (RND), major facilitator superfamily (MFS), multidrug and toxic compound extrusion (MATE), and small multidrug resistance (SMR) transporter superfamilies, other examples of which have been shown to contribute to resistance in A. baumannii (11, 31, 33).
In this study, we have deleted en bloc the TnAbaR transposon in A. baumannii strain A424 (TnAbaR23) and performed resistance testing and genetic characterization of two independent TnAbaR-minus mutants and the wild-type parent to gain insight into the phenotypic contribution of this element. A. baumannii A424 was originally isolated from a case of invasive infection in Croatia. We propose that this large-scale genomic deletion approach could facilitate systematic analyses of resistance in Acinetobacter and provide phenotypic profiles for TnAbaR elements and other resistance-associated assemblages.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Details of bacterial strains and plasmids used in this study are listed in Table 1. A. baumannii strain A424, provided by Kevin Towner, Queen's Medical Centre, Nottingham, United Kingdom, was originally isolated from a patient in Croatia with an invasive infection. All strains were grown at 37°C at 200 rpm in Luria-Bertani (LB) medium, except for selection of A. baumannii merodiploids which was performed on Simmons citrate agar (Oxoid, Basingstoke, United Kingdom) (13). Gentamicin (20 μg/ml or 100 μg/ml) and chloramphenicol (20 μg/ml) were added as required.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Description, genotype, or relevant characteristicsa | Reference or source |
|---|---|---|
| Acinetobacter baumannii strains | ||
| AYE | Epidemic MDR type strain | 14 |
| AB0057 | MDR type strain | 2 |
| ATCC 19606 | Type strain | Salmonella Genetic Stock Centre |
| ACICU | Epidemic MDR type strain | 17 |
| A424 | Clinical isolate from Croatia | Kevin Towner |
| A424ΔTnAbaR23-163 | A424 derivative with TnAbaR23 deleted plus an additional genomic deletion and/or rearrangement | This study |
| A424ΔTnAbaR23-174 | A424 derivative with TnAbaR23 deleted | This study |
| Escherichia coli strains | ||
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ−thi-1 | Lab stock |
| CC118λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA thi-1 rpsE rpoB argE(Am) recA1; lysogenized with λpir phage | 16 |
| S17.1λpir | hsdR recA pro RP4-2 (Tc::Mu; Km::Tn7)(λpir) | 12 |
| Plasmids | ||
| pJTOOL-3 | Derivative of suicide vector pDS132; R6K ori mobRP4 sacB; Cmr | 32 |
| pJTAG | pJTOOL-3 derivative carrying the SOE-PCR product of TnAbaR1 UF–aacC1–TnAbaR1 DF; Cmr Gmr | This study |
Abbreviations: MDR, multidrug resistant; Cmr, chloramphenicol resistance; Gmr, gentamicin resistance.
DNA analysis and manipulation procedures.
Genomic and plasmid DNA purification, enzymatic manipulation of DNA, other routine recombinant DNA technology methods, and pulsed-field gel electrophoresis (PFGE) experiments were performed according to previously described protocols (15, 26). A. baumannii strain A424 was sequenced by 454 GS FLX pyrosequencing (Roche, Branford, CT) according to the standard protocol for whole-genome shotgun sequencing and assembled using the xBASE de novo assembly pipeline (9) and Newbler 2.5 (Roche). pJTAG was constructed by amplifying ∼750 to 800 bp of sequence in the regions immediately upstream and downstream of TnAbaR1 in strain AYE using the primer pairs AbaRUF/AbaRURGm (Gm stands for gentamicin) and AbaRDFGm/AbaRDR, respectively (see Fig. S1 in the supplemental material). These fragments were joined by splicing overlap extension (SOE) PCR to a Flippase recognition target (FRT)-flanked aacC1 cassette amplified with primers GmF (F stands for forward) and GmR (R stands for reverse) from pUC18T-mini-Tn7T-Gm (10). KOD Hot Start DNA polymerase (Novagen, Merck Biosciences, United Kingdom) was used according to the manufacturer's instructions for the SOE-PCR protocol. The resulting 2.7-kb SOE PCR product was restricted with NotI and cloned into the pDS132-derived suicide vector pJTOOL-3 using the Escherichia coli CC118λpir host (16, 32). The resultant plasmid, pJTAG, was transferred into E. coli S17.1λpir (12) and then conjugally mobilized into A. baumannii strain A424 (Fig. S1). Details of primers used in this study are shown in Table S1 in the supplemental material.
Construction of TnAbaR23-minus mutants by conjugative delivery of pJTAG.
Overnight cultures of the recipient A. baumannii A424 and the donor E. coli S17.1λpir/pJTAG were subcultured into antibiotic-free medium and grown to mid-log phase. The cells were pelleted, resuspended in 1 ml prewarmed LB medium, combined in a 1:1 ratio, and then centrifuged at low speed at room temperature. The supernatant was discarded, and the cells were resuspended in 50 μl of 10% glycerol and spotted onto the center of a nonselective LB agar plate. This mating plate was left overnight at 37°C, and the cells were then scraped off and resuspended in 400 μl of 10% glycerol; appropriate dilutions were plated onto Simmons citrate agar supplemented with gentamicin (100 μg/ml). Putative merodiploid derivatives were maintained on LB agar supplemented with gentamicin (100 μg/ml), verified by PCR analysis, plated at high density onto LB agar supplemented with 6% sucrose, and incubated at 37°C for counterselection of the pJTOOL-3-borne sacB (32).
Antimicrobial susceptibility testing and MIC determination.
Iso-Sensitest agar (Oxoid, Basingstoke, United Kingdom) was used for disc diffusion susceptibility and Etest-based MIC assays which were performed according to British Society for Antimicrobial Chemotherapy (BSAC) guidelines (3). All results shown are based on a minimum of three replicates. Antibiotic discs were purchased from Oxoid (Basingstoke, United Kingdom), while Etest strips were obtained from AB Biodisk (Solna, Sweden).
Nucleotide sequence accession number.
The sequence of TnAbaR23 of A. baumannii strain A424 has been deposited in GenBank under accession number JN676148.
RESULTS AND DISCUSSION
Targeted en bloc deletion of the TnAbaR23 transposon from Acinetobacter baumannii strain A424.
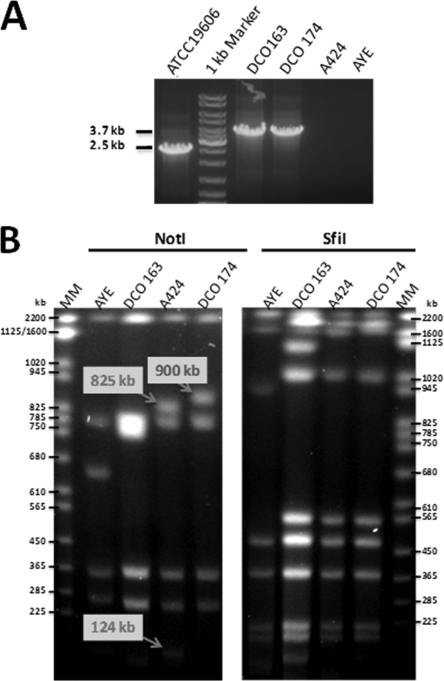
PCR analysis of the multiantibiotic-resistant A. baumannii strain A424 using primer pairs 2F/2R and 4F/4R which targeted the well-conserved comM-TnAbaR junctions (29) demonstrated that A424 harbored a typical TnAbaR-like transposon in the comM gene. This element was designated TnAbaR23. A pDS132-based suicide plasmid, pJTAG, carrying the characteristic TnAbaR-flanking 5′ and 3′ comM-associated targeting sequences interrupted by an aacC1 gentamicin resistance-encoding cassette, was introduced into strain A424 by conjugation (see Fig. S1 in the supplemental material). Two independent single-crossover mutants were then subjected to a sucrose selection step to identify likely double-crossover mutants that had lost the entire TnAbaR transposon. Candidate double-crossover mutants were analyzed by PCR using primers AbaRUF2 and AbaRDR2 that anneal to regions just outside those on the pJTAG targeting sequences (Fig. S1). Two TnAbaR deletant mutants, A424ΔTnAbaR23-163 (DCO163) and A424ΔTnAbaR23-174 (DCO174), each derived from an independent single-crossover mutant, were identified by the production of an expected 3.7-kb amplicon. As predicted, no amplicons were produced for strains A424 and AYE, while strain ATCC 19606, which harbors an uninterrupted comM gene, yielded an expected 2.5-kb product (Fig. 1A). The deleted comM-borne transposon in strain A424 has henceforth been referred to as TnAbaR23.
Fig 1.
Analysis of the putative TnAbaR23-minus mutants of Acinetobacter baumannii A424. (A) PCR amplification across the targeted deleted locus in DCO163 and DCO174 mutant strains using the primers AbaRUF2 and AbaRDR2 yielded the expected ∼3.7-kb product, while failing to amplify across the A424 TnAbaR23 element. The sequenced strains ATCC 19606 and AYE that lacked and harbored an island within the comM gene were used as positive (2.5-kb band expected) and negative PCR controls, respectively. (B) Pulsed-field gel electrophoresis analysis of NotI- and SfiI-digested A424, DCO163, DCO174, and AYE high-molecular-weight genomic DNA. The three bands with arrows and labels indicate the only observed NotI profile differences between A424 and DCO174. Subsequently available sequence data showed that TnAbaR23 harbors two very closely spaced NotI sites, while the replacement cassette lacks a NotI site. Hence, the observed loss of two A424 bands (825 kb plus 124 kb) and the appearance of a new DCO174-specific band (900 kb), with the difference between the two being the size of TnAbaR23 (48.3 kb). Lanes MM contain molecular size markers.
High-molecular-weight DNA purified from strain A424 and its TnAbaR23-minus isogenic mutants, DCO163 and DCO174, was digested separately with NotI and SfiI and examined by PFGE analysis (Fig. 1B). Despite both mutants being constructed identically and being confirmed to be indistinguishable from strain A424 by analysis of six sequence-polymorphic A. baumannii genes (ABAYE3778, filE, ompA, lldD, ABAYE3780, and filB), both the NotI and SfiI profiles of DCO163 and DCO174 exhibited minor band discrepancies. The NotI banding patterns of A424 and DCO174 were consistent with targeted deletion of TnAbaR23 alone (Fig. 1B), while that of DCO163 could be explained only by a further genomic rearrangement event, potentially triggered by conjugation and/or allelic exchange. Indeed, based on summation of the observed DCO163 NotI fragments, it would appear that this strain had lost a further ∼50 kb of DNA by comparison with DCO174. Nevertheless, it should be noted that despite repeated subculture, PFGE genomic profiles of the two mutants have remained unchanged from those detected originally, strongly supporting the notion that the described genome configurations are stable and not prone to further spontaneous undefined genome rearrangements.
Use of a novel combined subtractive amplification-PCR mapping strategy to define the genetic composition and organization of TnAbaR23.
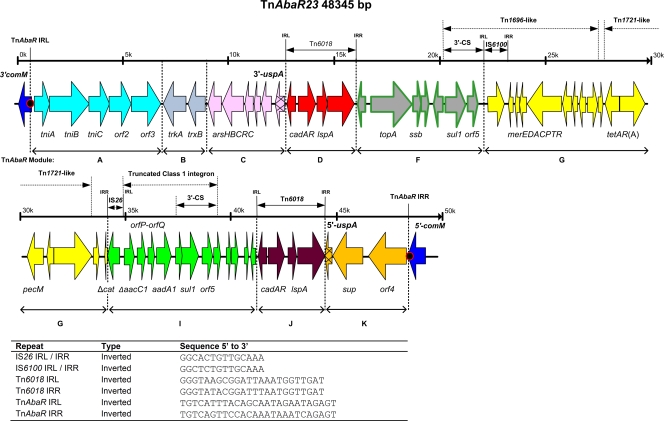
PCR primer pairs targeting 52 genes/loci selected empirically from those found within TnAbaR3 to TnAbaR4 and/or associated directly with resistance in A. baumannii were designed. The parent strain A424 and its TnAbaR23 deletant derivatives were subjected to PCR assays specific to each of these targets. Examination of this “subtractive amplification” data set allowed for firm conclusions as to genes/loci that mapped specifically to TnAbaR23 (see Table S2 in the supplemental material). A series of conventional short- and long-range PCR mapping assays informed by these gene content data and the recognized organization of previously characterized TnAbaR elements were then performed to completely map TnAbaR23 (Table S3). Whole-genome sequencing of strain A424 yielded ∼120,000 reads with an average length of 450 bp. Almost all (97.7%) of the reads were aligned into 715 contigs with a peak depth of eight and an estimated genome size of ∼3.9 Mb. These data have been submitted to the Sequence Read Archive under accession number SRA045827.2. Further examination of the contigs allowed definition of the entire 48.3-kb sequence of TnAbaR23 (GenBank accession number JN676148) (Fig. 2).
Fig 2.
Schematic map showing the genetic organization of TnAbaR23. Genes are colored by module (see Fig. 3 for details) with key genes labeled. The locations of identified repeat sequences (inverted repeat left [IRL] and inverted repeat right [IRR]) are indicated (positions are shown in thousands[k] from 0k to 50k), and full sequence details are shown in the graph at the bottom of the figure. The red circles shown at both TnAbaR23 ends indicate the 5-bp direct repeat sequences (5′-ACCGC-3′) which flank this element. Study-defined module boundaries are shown as dotted lines (see text and the legend to Fig. 3 for details). This figure is drawn to scale. Full annotation details for TnAbaR23 are available in GenBank (accession number JN676148). Minor annotation changes represented in this figure are listed in Table S4 in the supplemental material. 3′-CS, 3′ conserved sequence.
Genetic organization of TnAbaR23 and modular mosaicism of TnAbaR transposons.
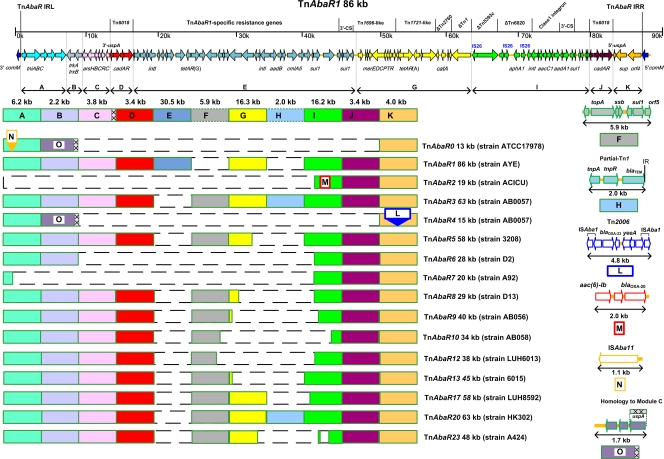
The genetic organization and modular composition of TnAbaR23 were systematically defined through comparative genomic analysis against 15 other TnAbaR elements chosen empirically to represent major known variants. For consistency, we used broad module definitions previously proposed by Adams et al. (2) who compared five TnAbaR islands (TnAbaR0 to TnAbaR4) in four A. baumannii strains but refined these to define precise module boundaries based on the TnAbaR1 sequence and other secondary reference sequences where necessary. One each of a TnAbaR2-specific insert and a TnAbaR4-specific insert previously identified by Adams et al. (2) and an additional two TnAbaR-associated segments have now been assigned module names and included in our updated comparative schematic (Fig. 3). Based on identification of repeat sequences, four modules (D, J, L, and N) undoubtedly represented genuine genetic modules, while all others have been inferred solely by comparative means. We emphasize that the modules we describe are a reflection of the set of sequences compared and the original Adams et al. (2) module definitions and that these do not necessarily reflect individual genetic units. Indeed, a Tn1696-like unit is found to span adjacent modules E and G in TnAbaR1 and modules F and G in eight of the other elements analyzed. While the modular schema we have presented offer the advantage of simplicity and intuitive interpretation, particularly when analyzing large numbers of genome sequences, we direct interested readers to detailed studies pioneered by Post et al. (24) and subsequently extended by others that have attempted to deconstruct TnAbaR elements through precise identification and definition of boundaries of transposon-, IS element-, and integron-related sequences (1, 18, 19, 25). We have captured some but not all of these finer-level data in Fig. 2 and 3. Furthermore, based on the hypothesized path of evolution of TnAbaR transposons proposed by Post et al. (25), modules C and K would have been contiguous in an ancestral transposon, designated Tn6019 by Post et al. Separation of modules C and K was proposed to have followed insertion of a Tn6018-flanked compound transposon into uspA. Intriguingly, all seven European clone II strains studied by Post et al. (25) possessed an intact uspA gene, while the reverse was true for all known European clone I strains, perhaps with the exception of TnAbaR4. TnAbaR23, like most defined TnAbaR elements possesses intact versions of the terminal modules A and K. The former carries the left terminal inverted repeat and the tniA-tniB-tniC-orf2-orf3 tandem cluster (previously designated orf1-tniA-tniB-orf2-orf3 [27]), members of which show protein-level homology to Tn7 transposition-associated partners and/or are postulated to partake in transposition (27), while the latter carries the cognate right terminal inverted repeat. Hence, like most other elements in this family, TnAbaR23 would appear to possess the genetic machinery required for active transposition. In contrast, TnAbaR2 in strain ACICU appears to have arisen from a one-ended deletion that has led to the total loss of its transposition module, while TnAbaR7 retains only a short fossil remnant of module A which carries an intact tniA gene alone (Fig. 3).
Fig 3.
Comparison of the modular compositions of 15 previously characterized A. baumannii TnAbaR transposons and that of TnAbaR23. A schematic representation of TnAbaR1 drawn to scale with genes colored by module and module boundaries as indicated is shown in the map at the top of the figure; selected genes are labeled. Details of six further modules not represented within TnAbaR1 are shown below the map to the right. Full annotation details of TnAbaR1 and the remaining modules are available via GenBank; accession numbers are listed below or in Table S5 in the supplemental material. Minor annotation changes represented in this figure are listed in Table S4. Modules A to K are shown as equal-sized rectangles when intact or as terminally or internally truncated boxes, as appropriate, to highlight incomplete modules. Rectangles outlined by large dashes represent deleted and/or absent modules. Modules N and L are inserted at sites as shown, while module M in TnAbaR2 occurs in place of a part of module I. Module O is restricted to TnAbaR0 (previously designated Tn6021) and TnAbaR4 and possess an ∼500-bp segment that exhibits high-level identity to a matching segment of the highly conserved module C; corresponding homologous sequences are shown crosshatched. The size to the nearest kilobase and host strain information are as shown. TnAbaR4 is the only characterized member of this family which is not inserted into the comM gene. Additional genetic features are shown where particularly pertinent. The TnAbaR transposons shown above and the GenBank accession numbers of the transposons or associated genomes (shown in parentheses) are as follows: TnAbaR0 (CP000521), TnAbaR1 (CT025832), TnAbaR2 (CP000863), TnAbaR3 (CP001182), TnAbaR4 (CP001182), TnAbaR5 (FJ172370), TnAbaR6 (GQ406245), TnAbaR7 (GQ406246), TnAbaR8 (HM590877), TnAbaR9 (project accession ADGZ01000000), TnAbaR10 (project accession ADHA01000000), TnAbaR12 (JF262168), TnAbaR13 (JF262169), TnAbaR17 (JF262173), TnAbaR20 (HM357806), and TnAbaR23 (JN676148).
In common with most other characterized TnAbaR elements, TnAbaR23 also carries intact versions of modules B, C, D, F, and J, where modules D and J comprise one each of two directly orientated Tn6018 elements (commonly designated ISPpu12), at least one copy of which can be found in all but TnAbaR0 and the sole characterized non-comM-resident member of this family, TnAbaR4 (Fig. 3). Compared with TnAbaR1 and TnAbaR3, the first two well-described members of this family, TnAbaR23 lacks module E and module H. Module E is a 30.6-kb TnAbaR1-specific segment that contains 20 resistance genes and components of three distinct class 1 integrons, while module H is a 2.0-kb blaTEM-bearing truncated version of Tn1 unique to TnAbaR3 (4). In addition, TnAbaR23 contains substantially truncated versions of modules G and I, two frequently contiguous resistance gene-bearing modules. Importantly, the TnAbaR23 module I remnant lacks a class 1 integron-associated intI1 gene and an intact attI1 site but contains a truncated aacC1 proximal cassette that abuts onto an IS26 element and at least two other resistance cassettes. The IS26 element may have also contributed to the partial deletion of a second resistance gene as it adjoins on the other flank the 126-bp 3′ remnant of cat (Fig. 2).
In addition to the classical resistance genes identified on TnAbaR23 [sul1, ΔaacC1, aadA1, tetAR(A), and ΔcatA], this element carries genes that code for components of several putative or likely efflux systems that could contribute toward the wider resistance phenotype of strain A424. These include the five-gene arsCRCBH operon that is predicted to confer resistance to arsenic and antimony and the cadmium resistance-associated Tn6018-borne cadAR genes (8, 28, 35). ArsB serves as an antiporter which effluxes arsenite ions (6, 23), while cadA and cadR code for a putative heavy metal-associated cation efflux transporter and a heavy metal resistance regulator (21), respectively. CadR contains a characteristic N-terminal helix-turn-helix domain and belongs to the PbrR transcription regulator family that also includes CadR of Pseudomonas aeruginosa and PbrR of Ralstonia metallidurans that regulate the expression of cognate cadmium and lead resistance operons, respectively. These latter proteins share an N-terminal DNA binding domain with other transcription regulators of the MerR superfamily (5, 7, 21). Module G carries the Tn1696 merEDACPTR gene cluster which codes for resistance to mercury (24). Based on similarities to Tn21 counterparts, MerA is predicted to function as a mercuric reductase, while MerE and MerT (inner membrane proteins) and MerP (periplasmic protein) are hypothesized to constitute key structural components of the mercury transport system itself (30). The 4.0-kb module K carries the annotated genes sup and orf4 and the 5′ remnant of uspA. Orf4 is an A. baumannii-specific uncharacterized protein, while Sup is a predicted MFS superfamily sulfate permease that exhibits either 100% identity or ∼89% identity to counterparts encoded by all other TnAbaR elements. Sup also bears striking homology with many other MFS family putative sulfate permeases from a wide range of bacteria. In addition, given that topA and ssb code for a predicted topoisomerase and single-stranded DNA binding protein, respectively, and that gyrase A and topoisomerase IV are known to interact with fluoroquinolones (20, 22), it would be intriguing if the TnAbaR23 topA and/or ssb genes contributed to the unusual ciprofloxacin-associated high-level resistance phenotype observed in mutant strain DCO174 following deletion of TnAbaR23 (details below).
As shown in Fig. 3, TnAbaR1 at ∼86 kb in size and with 45 likely resistance genes remains the largest TnAbaR element identified thus far. At the other extreme, the ∼13-kb TnAbaR element found in strain ATCC 17978, which had originally been isolated in the 1950s, contains only one likely resistance-associated gene, sup. Based on these and previous comparative genomics data and our hypothesis regarding the core TnAbaR components required for transposition (27), the ATCC17978 element almost certainly comprises or closely resembles the ancestral prototype of the TnAbaR transposon family. At this time, we have designated the ATCC17978 element as TnAbaR0 to reflect its presence in the earliest known A. baumannii isolate harboring such an element. Recently, an ∼63-kb element which we designated TnAbaR20 was identified in A. baumannii strain HK302 and reported to be indistinguishable from TnAbaR3 by PCR mapping. Strain HK302 was originally isolated from the respiratory tract of a patient in an intensive care unit in Switzerland in 1977 (19). The discovery of TnAbaR20 emphasized the earlier than initially presumed origins of “well-armed” versions of these elements and the wide geographic dissemination of these entities, as its “PCR-identical twin,” TnAbaR3, was identified in AB0057, a strain originally isolated from the bloodstream of a patient in Washington, DC, only in 2004. However, consistent with the postulated plasticity of TnAbaR elements, TnAbaR3 and TnAbaR20 represent the only two examples sharing an indistinguishable modular makeup (Fig. 3).
Contribution of TnAbaR23 to the resistance phenotype of Acinetobacter baumannii A424.
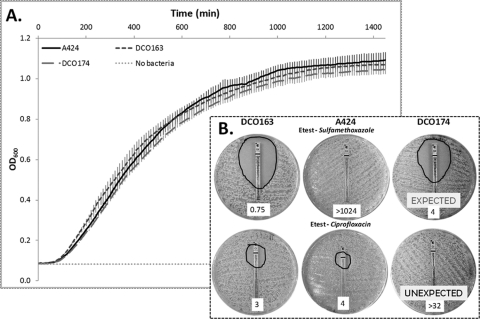
Antibiotic susceptibility profiles of the wild-type strain A424 and its isogenic TnAbaR23-minus mutants, DCO163 and DCO174, were determined (Table 2). Disc diffusion-based assays were performed for 16 antibiotics, and with the exception of sulfamethoxazole and trimethoprim-sulfamethoxazole, no major and/or consistent (matching results for both mutants) differences were observed between the results for wild-type strain A424 and its isogenic TnAbaR23-minus mutants. Consistent with TnAbaR23 carrying two copies of sul1, both TnAbaR23-minus mutants were significantly more sensitive to sulfamethoxazole than was the parent strain (Table 2). Zone of inhibition diameters for tetracycline also suggested that both mutants were less resistant to this agent than A424, although yet again DCO163 appeared to be even more susceptible than DCO174. In addition, both mutants exhibited slightly increased resistance to streptomycin, amikacin, and tobramycin and markedly increased resistance to gentamicin most probably as a result of acquisition of the gentamicin resistance-encoding aacC1 cassette that was used to replace TnAbaR23. Intriguingly, for the 10 antibiotics with mutant-to-mutant variation in zone of inhibition diameters, DCO163 consistently yielded larger zones than DCO174, except for rifampin where the measured discrepancy was very small (11.5 mm compared to 13.0 mm, respectively) and the reverse was true. As PFGE data suggested that DCO163 carries an additional undefined large deletion and as this mutant consistently exhibited increased susceptibility compared to DCO174, we postulated that DCO163 may display a growth defect relative to DCO174. However, growth curve analysis using LB medium and shaking incubation at 37°C failed to reveal any significant differences in growth kinetics between DCO163, DCO174, and the parent strain, A424 (Fig. 4A).
Table 2.
Antimicrobial susceptibility of Acinetobacter baumannii strain A424 and its TnAbaR23-minus mutants
| Strain | Zone of inhibition diam (MIC) of strain to the following antibiotica: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TET (10 μg) | IMPc (10 μg) | CAR (100 μg) | AMP (25 μg) | TIM (75 μg) | CTX (30 μg) | CIPc (5 μg) | STR (10 μg) | TOB (10 μg) | AMK (30 μg) | GENc (10 μg) | TMP (2.5 μg) | SUL (25 μg) | SXT (25 μg) | CHL (10 μg) | RIF (2 μg) | |
| AYEb | 6.0 | 28.0 (0.38) | 6.0 | 6.0 | 6.0 | 6.0 (>256) | 6.5 (>32) | 9.0 | 13.0 | 17.0 | 8.5 (16) | 6.0 | 6.0 (>1,024) | 6.0 | 6.0 | 6.0 |
| A424 | 6.0 | 15.0(4) | 6.0 | 6.0 | 6.0 | 6.0 (>256) | 14.0 (4) | 16.0 | 26.0 | 16.0 (12) | 23.0 (0.75) | 6.5 | 6.0 (>1,024) | 6.0 | 6.0 | 11.5 |
| DCO163 | 15.0 | 18.0(2) | 6.0 | 6.0 | 6.0 | 6.0 (>256) | 16.0 (3) | 11.0 | 23.0 | 11.0 (48) | 9.0 (16) | 10.0 | 31.0 (0.75) | 30.5 | 6.0 | 11.5 |
| DCO174 | 9.5 | 15.0(4) | 6.0 | 6.0 | 6.0 | 6.0 (>256) | 6.0 (>32) | 9.0 | 23.0 | 9.0 (96) | 6.0 (48) | 6.0 | 23.5 (4) | 21.5 | 6.0 | 13.0 |
| NCTC12241b | 20.0 | 35.0 | 18.0 | 13.0 | NA | 35.0 | 30.0 | 17.0 | 21.0 | 24.0 (1.5) | 23.0 | 26.0 | 6.0 (>1,024) | 29.0 | 20.0 | 6.0 |
Antibiotic abbreviations: TET, tetracycline; IMP, imipenem; CAR, carbenicillin; AMP, ampicillin; TIM, ticarcillin-clavulanate; CTX, cefotaxime; CIP, ciprofloxacin; STR, streptomycin; TOB, tobramycin; AMK, amikacin; GEN, gentamicin; TMP, trimethoprim; SUL, sulfamethoxazole; SXT, sulfamethoxazole-trimethoprim; CHL, chloramphenicol; RIF, rifampin. The mass of antibiotic is given in parentheses after the antibiotic. NA, not available.
AYE data were in accordance with previously published resistance data (Adams et al. [2] and references cited therein). E. coli strain NCTC12241 was used as a BSAC reference control.
BSAC guidelines (3) on zone diameter breakpoints were as follows: for IMP, resistant (R) ≤ 13 mm, susceptible (S) ≥ 25 mm, and intermediate (I) = 14 to 24 mm; for CIP, R ≤ 20 mm and S ≥ 21 mm. BSAC (3) guidelines on MIC breakpoints for Acinetobacter species: for GEN, R > 4 mg/liter and S ≤ 4 mg/liter; for CIP, R > 1 mg/liter and S ≤ 1 mg/liter; for IMP, R > 8 mg/liter, S ≤ 2 mg/liter, and I = 4 to 8 mg/liter. Numbers shown in boldface type and underlined indicate resistant and intermediate, respectively, by BSAC interpretive criteria. MIC was tested using Etest strips (AB bioMérieux, Sweden), and the MIC results for antibiotics tested are shown in parentheses in the table. Each assay was repeated at least three times. Zone diameters are shown to the nearest 0.5 mm, while MIC is shown in milligrams per liter.
Fig 4.
Growth curve and Etest analyses of Acinetobacter baumannii A424 and its TnAbaR23-minus mutants. (A) The wild-type strain A424 and DCO163 and DCO174 mutants were grown in 200 μl antibiotic-free LB medium at 37°C with shaking in 96-well plates from standardized overnight cultures and monitored in a Multiskan GO instrument to determine the optical density at 600 nm (OD600) measurements at 10-min intervals. The data shown represent the mean values ± standard deviations (error bars) for four independent wells for each strain. No significant difference in growth dynamics of the strains was observed. (B) Images of Etest assays for sulfamethoxazole and ciprofloxacin MICs on A424, DCO163, and DCO174 to highlight both an expected and an unexpected resistance phenotype of DCO174 associated with the loss of TnAbaR23. The edges of the zones of inhibition have been outlined, and the measured MIC values (in micrograms per milliliter) are shown on the white labels at the bottom of the plate image. The switch to sulfamethoxazole susceptibility observed in both DCO163 and DCO174 was entirely predictable from the defined gene content of TnAbaR23 and the PCR-informed absence of a sul1 gene elsewhere on the A424 chromosome. In contrast, the paradoxical changes in ciprofloxacin MIC values observed in DCO163 and DCO174 relative to that of A424 were entirely unexpected.
Etests were used to determine the MICs of a subset of these antibiotics (Table 2). As suggested through disc susceptibility analysis, the gentamicin MICs of both mutants were markedly elevated than that of wild-type strain A424 (16 μg/ml [DCO163] and 48 μg/ml [DCO174] compared to 0.75 μg/ml [A424]). Similarly, the amikacin MICs of the mutants were at least 4-fold that of A424. The largest observed shifts corresponded to sulfamethoxazole MICs, with both mutants exhibiting MICs much less than 1/200th that of the wild-type strain (0.75 μg/ml [DCO163] and 4 μg/ml [DCO174] compared to >1,024 μg/ml [A424]) (Fig. 4B).
Given the roles of gyrA and parC point mutations in fluoroquinolone resistance in many bacterial species, we amplified and sequenced the quinolone resistance-determining regions (QRDRs) of these genes from strain A424 and the two TnAbaR23-minus mutants. We had postulated that the markedly increased ciprofloxacin MIC values exhibited by DCO174 (>32 μg/ml) compared to A424 (4 μg/ml) or DCO163 (3 μg/ml) would be due to a distinguishing coincidental QRDR-associated mutation(s) in DCO174 (Fig. 4B). However, all three strains contained identical sequences over the available overlapping 289-bp and 326-bp segments wholly containing the QRDRs of gyrA and parC, respectively (see Fig. S2 in the supplemental material). Nevertheless, consistent with the elevated ciprofloxacin MIC levels of all three strains and previous reports on ciprofloxacin resistance-associated mutations in A. baumannii and other Gram-negative bacteria (34, 36), a mutation associated with the common Ser83Leu substitution in GyrA was detected. However, an accompanying Ser80Leu-associated mutation in parC was not observed, and no other gyrA or parC polymorphisms were detected within the QRDRs of these genes. A PCR screen for qnrA, a gene coding for plasmid-mediated ciprofloxacin resistance, was also negative for all three strains. Hence, the basis of the >8-fold increase in ciprofloxacin MIC of DCO174 relative to that of DCO163 and A424 remains elusive.
Summary.
Despite the long list of TnAbaR elements identified thus far, there have been no reports on the experimental characterization of these elements, though much has been inferred from sequence annotation information. Using a classical genetic strategy, we attempted to plug this gap by deleting TnAbaR23 from the clinically derived multidrug-resistant A. baumannii strain A424. In contrast to expectations, the two independently derived TnAbaR23-minus mutants exhibited distinct but related PFGE profiles and discordant susceptibility patterns, despite both being conclusively shown to be isogenic with the wild-type parent strain. Most remarkably, the DCO174 mutant exhibited a >8-fold increase in its ciprofloxacin MIC, while the MIC of the second mutant, DCO163, closely resembled that of A424, despite all three strains sharing identical gyrA and parC QRDR sequences. Furthermore, as the NotI PFGE profile of DCO174 revealed no other incidental genomic changes, this mutant may well constitute a genuine single-mutation derivative of A424. If so, these data would suggest that loss of TnAbaR23 alone resulted in the markedly elevated ciprofloxacin MIC exhibited by DCO174. Alternatively, a cryptic secondary mutation, potentially affecting an efflux mechanism, may explain this finding. Experiments to define the basis of this intriguing phenomenon, potentially suggestive of a paradoxical role for TnAbaR23 in promoting sensitivity as opposed to resistance to ciprofloxacin, are under way. The nature of the additional genomic rearrangement and/or deletion in strain DCO163 is also being actively pursued, as the observed increased sensitivity to a broad range of antimicrobials without impairment of growth in antibiotic-free medium exhibited by DCO163 would suggest that this TnAbaR23-minus mutant has lost a further yet-to-be-defined resistance-associated function. This study highlights the importance of experimental scrutiny of the function of TnAbaR elements in A. baumannii and challenges the assumption that resistance phenotypes can be predicted from the genetic makeup of large resistance gene assemblages alone.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nutan Sapkota, Shirley Tang, and Ansie Martin for technical assistance and are grateful to Kevin Towner for the donation of a large A. baumannii strain collection and to Alessandra Carratolli and Mark Adams and Robert Bonomo for strains ACICU and AB0057, respectively.
This work was funded by a British Society for Antimicrobial Chemotherapy (BSAC) research grant to K.R. H.-Y.O. was supported by the National Natural Science Foundation of China, the Program for New Century Excellent Talents in University, MOE, China (NCET-10-0572), and the Program for Chen Xing Scholars for Shanghai Jiaotong University. Genome sequencing was supported by a Medical Research Council grant (G0901717) to M.P. and C.C.
ADDENDUM IN PROOF
Since the submission of this manuscript, two further members of the TnAbaR family have been described. AbaR21 is present in A. baumannii strain RUH875 (A297), a strain that has frequently served as a European clone I type strain (S. J. Nigro, V. Post, and R. M. Hall, J. Antimicrob. Chemother. 66:1928–1930). By contrast, AbaR22 is found in multidrug-resistant A. baumannii strain MDR-ZJ06, belonging to European clone II, which is widely disseminated in China (H. Zhou et al., Antimicrob. Agents Chemother. 55:4506–4512). Accordingly, we have named the presently reported element TnAbaR23.
Footnotes
Published ahead of print 30 January 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Adams MD, Chan ER, Molyneaux ND, Bonomo RA. 2010. Genomewide analysis of divergence of antibiotic resistance determinants in closely related isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams MD, et al. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrews J. 2010. BSAC methods for antimicrobial susceptibility testing. Version 9.1. March 2010 British Society for Antimicrobial Chemotherapy, Birmingham, United Kingdom: http://www.bsac.org.uk/Resources/BSAC/Version_9.1_March_2010_final.pdf [Google Scholar]
- 4. Bailey JK, Pinyon JL, Anantham S, Hall RM. 2011. Distribution of the blaTEM gene and blaTEM-containing transposons in commensal Escherichia coli. J. Antimicrob. Chemother. 66:745–751 [DOI] [PubMed] [Google Scholar]
- 5. Borremans B, Hobman JL, Provoost A, Brown NL, van Der Lelie D. 2001. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 183:5651–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Branco R, Chung AP, Morais PV. 2008. Sequencing and expression of two arsenic resistance operons with different functions in the highly arsenic-resistant strain Ochrobactrum tritici SCII24T. BMC Microbiol. 8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brocklehurst KR, Megit SJ, Morby AP. 2003. Characterisation of CadR from Pseudomonas aeruginosa: a Cd(II)-responsive MerR homologue. Biochem. Biophys. Res. Commun. 308:234–239 [DOI] [PubMed] [Google Scholar]
- 8. Cai J, Salmon K, DuBow MS. 1998. A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology 144:2705–2713 [DOI] [PubMed] [Google Scholar]
- 9. Chaudhuri RR, et al. 2008. xBASE2: a comprehensive resource for comparative bacterial genomics. Nucleic Acids Res. 36:D543–D546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi KH, Schweizer HP. 2006. Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat. Protoc. 1:153–161 [DOI] [PubMed] [Google Scholar]
- 11. Damier-Piolle L, Magnet S, Brémont S, Lambert T, Courvalin P. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Lorenzo V, Timmis KN. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386–405 [DOI] [PubMed] [Google Scholar]
- 13. Dorsey CW, Tomaras AP, Actis LA. 2002. Genetic and phenotypic analysis of Acinetobacter baumannii insertion derivatives generated with a transposome system. Appl. Environ. Microbiol. 68:6353–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fournier PE, et al. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison EM, et al. 2010. Pathogenicity islands PAPI-1 and PAPI-2 contribute individually and synergistically to the virulence of Pseudomonas aeruginosa strain PA14. Infect. Immun. 78:1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrero M, de Lorenzo V, Timmis KN. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iacono M, et al. 2008. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 52:2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krizova L, Dijkshoorn L, Nemec A. 2011. Diversity and evolution of AbaR genomic resistance islands in Acinetobacter baumannii strains of European clone I. Antimicrob. Agents Chemother. 55:3201–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krizova L, Nemec A. 2010. A 63 kb genomic resistance island found in a multidrug-resistant Acinetobacter baumannii isolate of European clone I from 1977. J. Antimicrob. Chemother. 65:1915–1918 [DOI] [PubMed] [Google Scholar]
- 20. Laponogov I, et al. 2009. Structural insight into the quinolone-DNA cleavage complex of type IIA topoisomerases. Nat. Struct. Mol. Biol. 16:667–669 [DOI] [PubMed] [Google Scholar]
- 21. Lee SW, Glickmann E, Cooksey DA. 2001. Chromosomal locus for cadmium resistance in Pseudomonas putida consisting of a cadmium-transporting ATPase and a MerR family response regulator. Appl. Environ. Microbiol. 67:1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madurga S, Sánchez-Céspedes J, Belda I, Vila J, Giralt E. 2008. Mechanism of binding of fluoroquinolones to the quinolone resistance-determining region of DNA gyrase: towards an understanding of the molecular basis of quinolone resistance. ChemBioChem 9:2081–2086 [DOI] [PubMed] [Google Scholar]
- 23. Muller D, et al. 2007. A tale of two oxidation states: bacterial colonization of arsenic-rich environments. PLoS Genet. 3:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Post V, Hall RM. 2009. AbaR5, a large multiple-antibiotic resistance region found in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:2667–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Post V, White PA, Hall RM. 2010. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1162–1170 [DOI] [PubMed] [Google Scholar]
- 26. Rajakumar K, Sasakawa C, Adler B. 1997. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect. Immun. 65:4606–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rose A. 2010. TnAbaR1: a novel Tn7-related transposon in Acinetobacter baumannii that contributes to the accumulation and dissemination of large repertoires of resistance genes. Bioscience Horizons 3:40–48 [Google Scholar]
- 28. Rosenstein R, Peschel A, Wieland B, Götz F. 1992. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J. Bacteriol. 174:3676–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaikh F, et al. 2009. ATPase genes of diverse multidrug-resistant Acinetobacter baumannii isolates frequently harbour integrated DNA. J. Antimicrob. Chemother. 63:260–264 [DOI] [PubMed] [Google Scholar]
- 30. Sone Y, Pan-Hou H, Nakamura R, Sakabe K, Kiyono M. 2010. Role played by MerE and MerT in the transport of inorganic and organic mercury compounds in Gram-negative bacteria. J. Health Sci. 56:123–127 [Google Scholar]
- 31. Srinivasan VB, Rajamohan G, Gebreyes WA. 2009. Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:5312–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Aartsen JJ, Rajakumar K. 2011. An optimized method for suicide vector-based allelic exchange in Klebsiella pneumoniae. J. Microbiol. Methods 86:313–319 [DOI] [PubMed] [Google Scholar]
- 33. Vila J, Martí S, Sánchez-Céspedes J. 2007. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 59:1210–1215 [DOI] [PubMed] [Google Scholar]
- 34. Vila J, Ruiz J, Goñi P, Jimenez de Anta T. 1997. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J. Antimicrob. Chemother. 39:757–762 [DOI] [PubMed] [Google Scholar]
- 35. Williams PA, Jones RM, Shaw LE. 2002. A third transposable element, ISPpu12, from the toluene-xylene catabolic plasmid pWW0 of Pseudomonas putida mt-2. J. Bacteriol. 184:6572–6580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wisplinghoff H, et al. 2003. Mutations in gyrA and parC associated with resistance to fluoroquinolones in epidemiologically defined clinical strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:177–180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.