Abstract
Within Myxococcus xanthus biofilms, cells actively move and exchange their outer membrane (OM) lipoproteins and lipids. Between genetically distinct strains, OM exchange can regulate recipient cell behaviors, including gliding motility and development. Although many different proteins are thought to be exchanged, to date, only two endogenous OM lipoproteins, CglB and Tgl, are known to be transferred. Protein exchange requires the TraAB proteins in recipient and donor cells, where they are hypothesized to facilitate OM fusion for transfer. To better understand the types of proteins exchanged, we identified the genes for the remaining set of cgl gliding motility mutants. These mutants are unique because their motility defect can be transiently restored by physical contact with donor cells that encode the corresponding wild-type protein, a process called stimulation. Similar to CglB and Tgl, the cglC and cglD genes encode type II signal sequences, suggesting that they are also lipoproteins. Surprisingly, the cglE and cglF genes instead encode type I signal sequences, suggesting that nonlipoproteins are also exchanged. Consistent with this idea, the addition of exogenous synthetic CglF protein (71 amino acids) to a cglF mutant rescued its motility defect. In contrast to a live donor cell, stimulation with purified CglF protein occurred independently of TraA. These results also indicate that CglF may localize to the cell surface. The implications of our findings on OM exchange are discussed.
INTRODUCTION
All cells interact with their environment and other cells. Although cell-cell interactions are fundamental, our understanding of cell-cell contact-dependent interactions within the bacterial kingdom is surprisingly limited. One key constraint on our understanding is how microbes actually interact in their native habitat. Myxobacteria represent one exception to this problem because complex cell-cell behaviors found in nature can be recapitulated under laboratory conditions. In particular, the iconic behavior of fruiting body formation, for example, on environmental dung pellets, is recapitulated in the laboratory on agar plates in response to starvation (42). In this developmental program, thousands of cells assemble to build fruits in which vegetative cells differentiate into environmentally resistant spores. Central to this process is the ability of myxobacteria to coordinate their gliding movements, a process powered by two systems called adventurous (A) and social (S) motility (11, 21, 34).
How myxobacteria coordinate their cell movements to elicit multicellular behaviors is complex. Recent progress has helped unravel these behaviors, such as fruiting body development and rippling, but our understanding is still incomplete (38, 42). One of the first myxobacterial cell-cell interactions studied at a genetic level involved gliding motility. In these early studies, a small subset of the gliding motility mutants were found to be rescued by mixing mutants with strains that contained the corresponding wild-type gene (9, 10). This extracellular complementation phenomenon, termed stimulation, was transient and involved only phenotypic changes, as cells remained genetically the same. Stimulation was originally interpreted by two non-mutually exclusive models in which (i) myxobacterial cells exchange certain motility components or (ii) cells exchange signals through contacts. Recently, the former hypothesis was demonstrated, and elements of the latter hypothesis also appear correct (22, 23a, 36).
Stimulation is beginning to be understood at the molecular and cellular levels. Upon cell-cell contact on a hard surface, where structured biofilms form, myxobacteria exchange their outer membrane (OM) lipoproteins and lipids (23, 23a, 36). Strikingly, transfer is efficient as recipient cells accumulate approximately the same quantities of transferred lipoproteins as donor cells. OM exchange requires gliding motility; however, this requirement is not specific to donor or recipient cells. Instead, cell motility plays an indirect role to presumably align cells to facilitate OM fusion and protein exchange (33, 36). The minimum cis requirement to allow heterologous protein transfer simply involves a type II signal sequence for OM localization (36). Two proteins named TraA and TraB were recently shown to be required for stimulation/transfer in both donor and recipient cells. Bioinformatic and experimental data suggest that TraA is a cell surface receptor that mediates OM fusion between adjoining cells (23a). Cell contact-dependent exchange is hypothesized to serve as a mechanism to facilitate social behaviors. In support of this, cell contact exchange can regulate motility and development behaviors between genetically distinct strains that were derived from the same parent (23a). In their ecological niche, Myxococcus xanthus populations are genetically diverse, meaning that some cells are siblings while others are distant relatives (32). Given mixed environmental populations, the ability of kin cells to distinguish themselves from distant relatives may play an important role in coordinating cellular behaviors and in their social fitness (30).
Our recent findings suggest that many OM lipoproteins, involved in a variety of cellular functions, are transferred (36). However, to date, only two proteins endogenous to myxobacteria have been studied. These proteins, CglB and Tgl, contain type II signal sequences for lipoproteins and are required for A and S motility, respectively (25, 26, 29). Whether other types of proteins are transferred is unknown. Thus, to better understand the types of proteins exchanged, we sought to identify new protein candidates involved in transfer. To do this, we again turned to stimulation as it provides a powerful phenotypic screen for protein transfer that other biological processes do not readily afford. Specifically we sought to identify the genes for the remaining mutants that are stimulatable. These genes, called cglC, cglD, cglE, and cglF (contact or conditional gliding), are required for A motility (9). Here we report their identification and characterization and discuss the implications of our findings on the mechanism of OM exchange.
MATERIALS AND METHODS
Strains and media.
The bacterial strains used in this work are listed in Table 1. Unless stated otherwise, M. xanthus was grown in the dark at 33°C in CTT medium (1% Casitone, 1 mM KH2PO4, 8 mM MgSO4, 10 mM Tris-HCl, pH 7.6) supplemented, when necessary, with kanamycin (Km; 50 μg/ml) or oxytetracycline (15 μg/ml). In [1/2] CTT, the Casitone concentration was reduced to 0.5%. For gliding motility assays, the agar concentration was 1.0 or 1.5%. TPM buffer contains 10 mM Tris, 1 mM KH2PO4, and 8 mM MgSO4, pH 7.6. For cloning experiments Escherichia coli was grown at 37°C in LB medium supplemented, when necessary, with Km (50 μg/ml) or ampicillin (100 μg/ml) (27).
Table 1.
Strains used in this study
| Strain | Relevant feature(s) | Source or reference |
|---|---|---|
| DH5α | E. coli cloning strain | Lab collection |
| DK1622 | A+ S+; wild-type M. xanthus | 35 |
| DK360 | A− S−; cglE1 pilQ1 | 10 |
| DK367 | A− S−; cglC4 pilQ1 | 28 |
| DK381 | A− S−; cglC5 pilQ1 | 28 |
| DK391 | A− S−; cglD1 pilQ1 | 9 |
| DK1210 | A− S−; cglD1 cglC7 pilQ1 | Kaiser collection |
| DK1234 | A− S+; cglF1 | 11 |
| DK1240 | A− S+; cglC6 | Kaiser collection |
| DK1633 | A− S−; cglC1 pilQ1633 | 35 |
| DK1912 | A− S−; Ω1905 cglE1 cglF1 pilQ1 Kmr | 28 |
| DK1963 | A− S−; Ω1903 cglC2 pilQ1 Kmr | 28 |
| DK1973 | A− S−; Ω1904 cglD1 pilQ1 Kmr | 28 |
| DK1979 | A− S−; Ω1904 cglD2 pilQ1 Kmr | Kaiser collection |
| DK2610 | A− S+; Ω1903 cglC3 Kmr | Kaiser collection |
| DK2740 | A− S−; Ω1919 cglE4 pilQ1 Kmr | Kaiser collection |
| DK6204 | A− S−; ΔmglBA (markerless) | 33 |
| DK8615 | A+ S−; ΔpilQ | 35 |
| DW704 | A− S−; cglF1 ΔpilA Tcr | 23a |
| DW1405 | A− S−; cglF1 ΔpilA traA::Km Tcr Kmr | 23a |
| DW1420 | A+ S−; DK391(pDP4) Kmr | This study |
| DW1421 | A− S−; DK391(pDP5) Kmr | This study |
| DW1422 | A+ S−; DK1633(pDP6) Kmr | This study |
| DW1423 | A+ S−; DK1633(pDP7) Kmr | This study |
| DW1424 | A− S−; DK1633(pDP8) Kmr | This study |
| DW1425 | A+ S−; DK1633(pDP9) Kmr | This study |
| DW1426 | A− S−; DK1633(pDP10) Kmr | This study |
| DW1427 | A+ S−; DK360(pDP11) Kmr | This study |
| DW1428 | A+ S−; DK360(pDP12) Kmr | This study |
| DW1429 | A+ S−; DW704(pDP13) Kmr Tcr | This study |
| DW1430 | A+ S−; DW704(pDP14) Kmr Tcr | This study |
| DW1431 | A− S−; DW704(pDP15) Kmr Tcr | This study |
| DW1432 | A− S−; DW704(pDP16) Kmr Tcr | This study |
| DW1433 | A− S−; ΔcglD ΔpilQ (markerless) | This study |
| DW1434 | A+ S−; DK8615(pDP18) Kmr | This study |
| DW1435 | A+ S−; DK8615(pDP19) Kmr | This study |
| DW1436 | A− S−; DK8615(pDP20) Kmr | This study |
| DW1437 | A+ S−; DK8615 Ω1903 Kmr | This study |
| DW1438 | A− S−; DK8615 Ω1903 cglC2 Kmr | This study |
| DW1439 | A+ S−; DK8615 Ω1904 Kmr | This study |
| DW1440 | A− S−; DK8615 Ω1904 cglD1 Kmr | This study |
| DW1441 | A− S−; DK360 Ω1931 cglE1 | This study |
| DW1442 | A+ S−; DK8615 Ω1931 Kmr | This study |
| DW1443 | A− S−; DK8615 Ω1931 cglE1 Kmr | This study |
| DW1444 | A+ S−; DK8615 Ω1919 Kmr | This study |
| DW1445 | A− S−; DK8615 Ω1919 cglF1 Kmr | This study |
| DW1446 | A+ S−; DK8615 Ω1903 cglC2sup1 Kmr | This study |
| DW1447 | A+ S−; DK8615 Ω1903 cglC2sup2 Kmr | This study |
| DW1448 | A+ S−; DK8615 Ω1903 cglC2sup4 Kmr | This study |
| DW1449 | A+ S−; DK360 cglE1sup2 | This study |
| DW1450 | A+ S−; DK360 cglE1sup11 | This study |
| DW1451 | A+ S−; DK360 cglE1sup12 | This study |
| DW1452 | A+ S−; DW704 cglF1sup1 Tcr | This study |
| DW1453 | A+ S−; DW704 cglF1sup2 Tcr | This study |
| DW1454 | A+ S−; DW704 cglF1sup3 Tcr | This study |
| DW1455 | A+ S−; DK8615 Ω1919 cglF1sup3A Kmr | This study |
| DW1456 | A− S−; DK8615 cglC::mia82 Kmr | This study |
| DW1457 | A− S−; DK8615 cglE::mia95 Kmr | This study |
| DW1458 | A− S−; ΔcglC ΔpilQ (markerless) | This study |
| DW1459 | A− S−; ΔcglE ΔpilQ (markerless) | This study |
| DW1460 | A− S−; ΔcglF ΔpilQ (markerless) | This study |
| DW1461 | A− S−; Ω1904 cglD2 ΔpilQ Kmr | This study |
Genetic manipulations.
The plasmids used in this work are listed in Table S1 in the supplemental material. The DNA cloning methods used followed routine protocols (27). Chromosomal and plasmid DNA was isolated with UltraClean Microbial DNA or Mini Plasmid isolation kits as described by the manufacturer (MO BIO Laboratories, Inc.). To create insertion mutations, we PCR amplified internal gene fragments with Taq 2× Master Mix (New England BioLabs) and TOPO cloned them into pCR2.1 as described by the manufacturer (Invitrogen). Full-length genes were similarly PCR amplified and cloned for plasmid rescue studies. Table S2 in the supplemental material lists the primers used in this study. Plasmid constructs were confirmed by restriction digestion analysis and/or DNA sequencing. Verified plasmids were transformed by electroporation into M. xanthus, where they cannot replicate, and integrated into the genome by homologous recombination by selecting antibiotic resistance. Generalized transduction with bacteriophage Mx4 or Mx8 was used for strain construction (28). Strain verification was done by phenotypic analysis and/or genotyping by diagnostic PCR and/or DNA sequencing.
Mapping of cgl mutations.
The linkage between cgl mutations and Tn5 insertions was derived from published Mx8 cotransduction frequencies (28). The size of the Mx8 genome was estimated to be 49 kbp (17). Thus, the physical distance between markers was determined from Wu's formula as follows: (39). The precise location of the Tn5 insertions was determined by an inverse-PCR-based method (27). Purified genomic DNA was treated in single digestions with a panel of restriction enzymes (HindIII, NcoI, NotI, and/or PstI), followed by DNA circularization and ligation. Each of these enzymes cuts within the Tn5 element and an unknown distance away in the chromosome. Each ligated genomic DNA pool was then used as the template in a PCR with outward-facing Tn5 primers (see Table S2 in the supplemental material). For each Tn5 insertion, at least one restriction enzyme digestion/ligation treatment yielded a PCR product. To test whether products were valid, we used a second set of confirmatory Tn5 primers in a PCR to verify that the products predictably changed sizes. Confirmed PCR products were gel purified and sequenced. These DNA sequences were then used in NCBI BLAST searches against the M. xanthus genome to identify precise Tn5 insertion sites (1). Candidate cgl open reading frames (ORFs) were then determined from Tn5 insertion sites and mapping calculations.
A plasmid rescue strategy was used to genetically determine which M. xanthus ORFs contain the respective cgl genes (35). Candidate cgl ORFs were PCR amplified from DK1622 genomic DNA and cloned into pCR2.1. The resulting plasmids were transformed into A− S− strains that contained the respective cgl mutations. Transformants arise by homologous recombination of plasmids into the genome, resulting in gene duplications. If the plasmid encodes the corresponding wild-type cgl gene, then at least some of the transformants will show A motility. The frequency of A-motile transformants depends on where the cgl mutation resides with respect to the cloned fragment. This method assumes that the original mutation was not dominant. Plasmid rescue results were readily scored as a swarming colony on the original transformation plate.
cgl deletion mutations.
To construct the cglD deletion cassette, regions upstream and downstream of the cglD gene were PCR amplified from the DK1622 chromosome, digested with appropriate restriction enzymes, and cloned into plasmid pBJ114. The primers used and their corresponding engineered restriction sites are listed in Table S2 in the supplemental material. Plasmids with cloned deletion cassettes for cglC, cglE, and cglF were kindly provided by Beiyan Nan and Daniela Keilberg. All of these plasmids contain the positive-negative Kan-galK cassette to screen for plasmid insertion, followed by counterselection of plasmid excision to create markerless deletions (31). Counterselection was conducted on 1% galactose CTT agar plates. Strain constructs were confirmed by PCR with primers flanking the deletion site.
Motility assays.
For swarm assays, log-phase M. xanthus cultures were concentrated by centrifugation to a calculated turbidity of 1,000 Klett units (∼3 × 109 CFU ml−1) in TPM buffer and 5-μl spots were placed on [1/2] CTT agar pads (0.5 ml on microscope slides) or plates containing 3 mM CaCl2 (added after autoclaving) (23a, 36). For stimulation assays, donor and recipient strains were similarly prepared and mixed at a 1:1 ratio prior to spotting. All plates/pads were incubated for the times indicated in a humid chamber at 33°C, and colonies were photographed with either an Olympus SZX10 stereo microscope (whole colony) or a Nikon E800 (10× objective) phase-contrast microscope (colony edge) coupled to digital imaging systems (36). To track single-cell movements, we grew M. xanthus strains in CTT to a turbidity of 100 Klett units, centrifuged them, and diluted them to a calculated turbidity of 25 Klett units in TPM buffer. A 5-μl volume of cell suspension was then pipetted onto [1/2] CTT–1% agar pads. The drop of cell suspension was allowed to dry, and pads were incubated for 2 h. To minimize agar pad drying, we placed a humidifier in a small microscopy room. Single-cell movements were tracked by time-lapse microscopy with a 10× phase-contrast objective lens at room temperature. Digital micrographs were captured on a Hamamatsu charge-coupled device camera and digitally processed with Image-Pro Plus software (Media Cybernetics, Bethesda, MD). Pictures were taken every 30 s for 30 min.
Suppressor isolation.
To isolate cgl suppressors, strains were grown to a turbidity of ∼100 Klett units (∼3 × 108 CFU ml−1) in CTT, centrifuged, and resuspended to a calculate turbidity of 100 Klett units in TPM buffer. A 1-ml cell suspension of each strain was placed in a petri dish and mutagenized with UV light, which resulted in ∼99% killing. Immediately after mutagenesis, multiple 5-μl spots (for each strain) were placed on [1/2] CTT–1% agar plates and incubated. Plates were inspected daily for emergent flares that were streaked to obtain single colonies. Purified suppressors were characterized in the described motility assays. A select number of cgl suppressors were sequenced in their respective cgl loci to test for intragenic suppression.
Stimulation with synthetic CglF peptide.
Full-length mature CglF protein (71 amino acids) was synthesized and purified to >85% purity by a commercial vendor (GeneScript). Lyophilized CglF peptide was resuspended in phosphate-buffered saline (pH 7.4) at 10 mg/ml and stored in aliquots at −80°C. For stimulation assays, cells were grown and concentrated to a calculated turbidity of 1,000 Klett units. To test the CglF peptide for stimulation, we mixed 2 μl (20 μg) of freshly thawed CglF peptide with 3 μl of cglF mutant cells. A positive-control nonmotile (ΔmglBA) donor strain was similarly mixed at a 1:1 ratio on a [1/2] CTT–1% agar plate with 3 mM CaCl2. Stimulation of swarm expansion was microscopically examined at various times.
Bioinformatic analysis.
cgl ORFs were derived from the DK1622 genome (6), and homology searches were done with BLAST against the NCBI nonredundant database. SignalP 4.0 and LipoP 1.0 web tools were used to search for signal sequences (12, 24). Domain homology searches against the NCBI Conserved Domain Database were conducted (18). Domain and protein repeats searches were also done with SMART (15). Large repeat sequences were initially found in CglD with SMART. Subsequent visual inspection identified two smaller and more numerous repeat sequences within the larger repeats. Protein sequences were aligned with MUSCLE, and conserved residues were visualized with LOGO (3, 5).
RESULTS
Genetic mapping and characterization of the cgl genes.
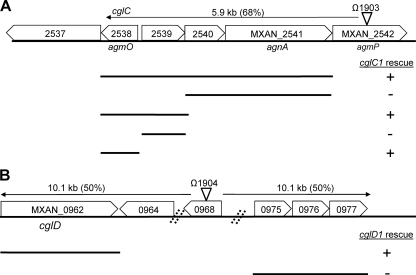
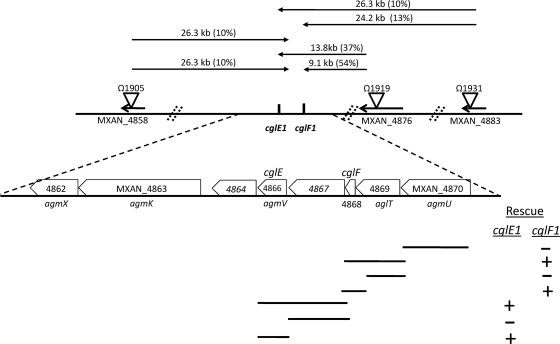
Hodgkin and Kaiser isolated many point mutations in cgl genes (9, 10). Sodergren and Kaiser subsequently isolated Tn5 transposon insertions linked to all of the cgl genes and determined their cotransduction frequencies (28). Thus, to map the remaining cgl genes, we identified relevant Tn5 insertion sites by a PCR-based method. By determining the precise Tn5 insertion sites (Fig. 1 and 2), candidate cgl genes were mapped on the fully sequenced and annotated DK1622 genome (6). The approximate physical distance between the Tn5 insertions and the cgl mutations was calculated (Fig. 1 and 2). For cglC and cglD genes, only one linked Tn5 insertion site was determined. Therefore, mapping data from these two-point crosses did not discern whether these cgl genes were located upstream or downstream of the respective Tn5 insertions (Fig. 1). The cglE and cglF genes were linked, and three independent Tn5 insertions sites were determined (Fig. 2) (28). Based on multicross analysis, the relative gene order and approximate map position were ascribed to cglE and cglF (Fig. 2). In three cases, the Tn5 insertions mapped near known A motility genes and this information was used to assist with gene identification (40, 41). To genetically determine which M. xanthus ORFs contained specific cgl genes, we employed a suicide plasmid rescue strategy that results in homologous recombination and tandem duplications in the genome (35). These results are summarized in Fig. 1 and 2. From these findings, we identified the following locus tags for the cgl genes: cglC, MXAN_2538; cglD, MXAN_0962; cglE, MXAN_4866; cglF, MXAN_4868.
Fig 1.
Genetic maps of cglC and cglD regions. The locations of Tn5 transposon insertions are indicated by triangles and omega numbers. The precise insertion site of Ω1903 in the DK1622 genome was at bp 2,958,528, and that of Ω1904 was at bp 1,117,552. The calculated physical distances between the cgl mutations and Tn5 insertions derived from phage Mx8 cotransduction frequencies are shown. The ability of plasmids containing the indicated genes to rescue cgl mutations are listed. Locus tags are shown as MXAN numbers. (A) cglC gene cluster. Plasmid rescue was conducted against strain DK1633. (B) cglD gene cluster. Plasmid rescue was conducted against strain DK391. The images are not to scale.
Fig 2.
Genetic map of cglE-and-cglF region. See Fig. 1 legend for details. The precise insertion site of Ω1905 in the DK1622 genome was at bp 6,077,315, that of Ω1919 was at bp 6,107,659, and that of Ω1931 was at bp 6,116,470. Plasmid rescue experiments were conducted against strains DK360 (cglE1) and DW704 (cglF1). The image is not to scale.
We note that the Ω1903 insertion resides within MXAN_2542, an ORF previously identified as an A motility gene named agmP (40). Phenotypic characterization of the Ω1903 insertion, which resides in the middle of the MXAN_2542 ORF, in an A+ S− genetic background revealed no overt motility defect, suggesting that MXAN_2542 was not a bona fide A motility gene. The other four Tn5 insertion mutants (Ω1904 in MXAN_0968, Ω1905 in MXAN_4858, Ω1919 in MXAN_4876, and Ω1931 in MXAN_4883) also exhibited no overt defects with respect to cell motility and growth (Fig. 1 and 2).
cgl mutations.
To confirm the above mapping results and to define the molecular lesions, all of the cgl alleles available in our strain collection were PCR amplified from genomic DNA and directly sequenced (Table 2). In the case of the cglC, cglE, and cglF mutations, they all resulted in nonsense (NS) or frameshift (FS) mutations, typically located near the 5′ end of the respective genes. Consequently, all or most of these mutations likely represent null alleles. The cglC6 and cglC7 alleles were identical and thus might be siblings. In contrast, the two cglD alleles contain missense mutations, suggesting that they might not be null alleles. Taken together, these results confirm that the correct cgl genes were identified.
Table 2.
cgl mutations used in this study
| Strain | Allele | Locus tag | Mutation(s) | Amino acid change(s)a |
|---|---|---|---|---|
| DK1633 | cglC1 | MXAN_2538 | 155A insertion | 52I→FS |
| DK1963 | cglC2 | MXAN_2538 | 237C→T (silent), 238C→T | 80Q→NS |
| DK2610 | cglC3 | MXAN_2538 | 482C→A | 161S→NS |
| DK367 | cglC4 | MXAN_2538 | 313C deletion | 105P→FS |
| DK381 | cglC5 | MXAN_2538 | 327G insertion | 110G→FS |
| DK1240 | cglC6 | MXAN_2538 | 61G→T | 21E→NS |
| DK1210 | cglC7 | MXAN_2538 | 61G→T | 21E→NS |
| DK391 | cglD1 | MXAN_0962 | 2568G→A (silent), 2569G→A, 2572G→A | 857E→K, 858D→N |
| DK1979 | cglD2 | MXAN_0962 | 1965G→A (silent), 1966G→A | 656D→N |
| DK360 | cglE1 | MXAN_4866 | 63C→T (silent), 64C→T | 22Q→NS |
| DK2740 | cglE4 | MXAN_4866 | 126C deletion | 43I→FS |
| DK1234 | cglF1 | MXAN_4868 | 103T deletion, 105C→T (silent) | 35Y→FS |
FS, frameshift; NS, nonsense.
Phenotypic analysis.
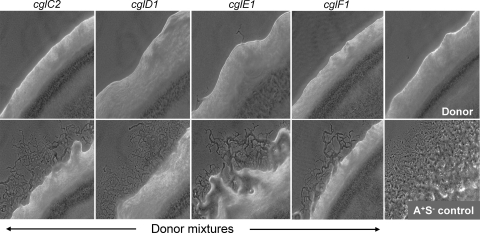
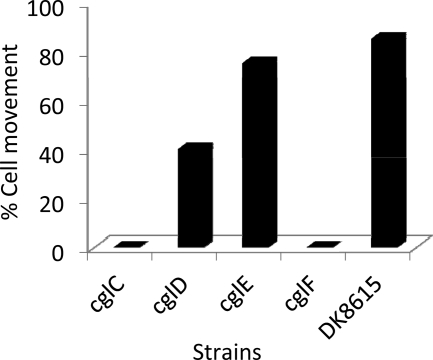
Isogenic mutant strain sets were constructed for the four cgl genes in an S motility mutant background (A+ S−; ΔpilQ) to unambiguously define their phenotypes. These strains were first tested for gliding motility. As shown in the top panels of Fig. 3, all of the cgl mutants exhibited severe swarming defects. In no cases were flares detected for cglC or cglF mutants. In contrast, early flares (1 day) occasionally emerged, and over prolonged incubations, flares inevitably emerged from cglD or cglE mutants incubated at 33°C, a clear indication of residual A motility. In addition, the swarming activity of cglD and cglE mutants was more pronounced at lower temperatures (∼25°C), indicating a temperature-sensitive phenotype (10). Time-lapse microscopy was employed to examine single-cell movements. Consistent with the swarm edge findings, cglC and cglF mutants never exhibited cell movement, while cglD and cglE mutants frequently moved (Fig. 4; see Movies S1 to S3 in the supplemental material). More specifically, single isolated cglD1 mutant cells rarely moved while cells within close proximity or groups frequently moved. In contrast, cglE mutant cells, whether isolated or in groups, moved at frequencies similar to those of the A+ S− control (Fig. 4). The frequencies at which cglD and cglE mutants reversed their direction of movement were similar to those of the A+ S− control. Prior studies established that the swarm rates for A motility were enhanced by cell-cell contact, suggesting that cell contacts regulate motility (14, 19). The CglD1 phenotype underscores this difference; isolated cells fail to move, but upon physical contact with other cglD1 cells, a function was apparently provided that partly restored motility.
Fig 3.
Motility and stimulation phenotypes of cgl mutants. The top panels show the colony edge swarming properties of the indicated isogenic cgl mutants. These strains contain a ΔpilQ mutation that abolishes S motility. The strains used were DW1438 (cglC2 mutant), DW1440 (cglD1 mutant), DW1443 (cglE1 mutant), and DW1445 (cglF1 mutant). The nonmotile donor (DK6204 [ΔmglBA mutant]) and reference A+ S− strain (DK8615; bottom right panel) colony edges are shown. Indicated bottom panels were 1:1 mixtures of the donor strain mixed with the indicated cgl mutants. Phase-contrast micrographs (10× objective) were taken after 2 days of incubation at 33°C.
Fig 4.
Single-cell motility analysis of cgl mutants. Time-lapse microscopy was conducted for 30 min with micrographs taken every 30 s. For each strain at least 50 individual cells were visually tracked. A single discernible cell movement over the duration of the 30 min movie was sufficient for a positive score. Isogenic strain sets contain a ΔpilQ mutation and strain numbers are listed in Fig. 3 legend.
The ability of isogenic cgl mutants to be stimulated was assessed next. For easy observation of stimulation, the donor cell was phenotypically A− S− and nonstimulatable (ΔmglBA) while the recipient was also A− S− (cgl ΔpilQ). As shown in the bottom panels of Fig. 3, the four cgl mutants were stimulated to swarm when mixed with a nonmotile donor strain. Swarm stimulation was relatively robust for the cglC, cglD, and cglE mutants. Although the cglD and cglE mutants exhibit a “leaky” phenotype, stimulation was clear. From our experience, cglF stimulation was weaker and emergent flares were few and small.
The phenotypes reported above for cglC, cglE, and cglF mutants were nearly identical for any of the mutant alleles listed in Table 2 or for their respective markerless deletion alleles (Table 1). Thus, as predicted, all of these point mutations behave as null alleles. We further note that an isogenic strain containing a ΔcglF allele exhibited a stimulation phenotype that was more robust than the cglF1 allele. A prior transposon mutagenesis screen for A motility mutants conducted by Youderian and Hartzell isolated Mariner insertions in cglC (agmO) and cglE (agmV) (8, 40). To test phenotypes in an isogenic background, we transduced the insertion mutations into a ΔpilQ strain (DK8615). As expected the cglC::mia82 and cglE::mia95 mutants lacked A motility. However, the cglE::mia95 mutant was not stimulatable and did not exhibit the leaky A motility phenotype found for other three cglE alleles (data not shown). In addition, unlike the seven cglC point mutation allele and the markerless deletion allele strains, the cglC::mia82 mutant was not stimulatable (data not shown). A possible explanation for why the insertion alleles elicited different phenotypes was that they had polar effects on other A motility genes. To address this possibility, we constructed insertion mutations in the respective downstream genes. However, gene disruptions in MXAN_2537 and MXAN_4864 (Fig. 1 and 2) resulted in no A motility defects in regard to swarm expansion or colony edge morphology (data not shown). Thus, the reason why strains with cglC and cglE insertion alleles behave differently remains unclear, but may be (i) the presence of dominant negative alleles, (ii) the presence of tightly linked second-site mutation(s), and/or (iii) polar effects on other neighboring A motility genes (Fig. 1 and 2). Lastly, we note that MXAN_2537 encodes a predicted secreted protein with a von Willebrand factor A (VWA) domain, which is similarly found in the CglB and CglD proteins (see below).
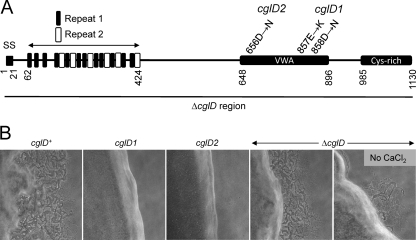
The two sequenced alleles of cglD contain missense mutations, and thus, they might not represent null alleles (Fig. 5A). To unequivocally define the null phenotype, we constructed a deletion mutation that removed the entire cglD ORF. As expected, the ΔcglD mutation resulted in an A motility swarming defect (Fig. 5B). However, the swarming defect of a ΔcglD mutant was less severe than that of isogenic cglD1 and cglD2 mutants (Fig. 5B). We further note that the addition of 2 mM CaCl2 partly reversed the ΔcglD motility defect (Fig. 5B), an addition that was inconsequential for the cglD1 or cglD2 mutants (data not shown). When the ΔcglD mutant was mixed with a donor strain, the size of the swarm increased, thus showing that this mutant was stimulated (data not shown). The observation that cglD1 and cglD2 missense mutants exhibit a different phenotype than a ΔcglD mutant indicates that the former alleles were not nulls. The cglD1 and cglD2 mutations were apparently not dominant negative alleles, because these mutants were complemented extracellularly by a cglD+ strain (Fig. 3). Instead, the cglD missense mutations may represent altered or gain-of-function alleles.
Fig 5.
CglD domain structure and phenotypes. (A) Relevant features include type II signal sequence (SS), repeat elements 1 and 2, VWA domain (VWA) and C-terminal cysteine-rich domain. CglD amino acid substitutions and the ΔcglD deleted region (black bar) are shown for indicated mutants. (B) Colony edge morphologies of cglD strains in isogenic ΔpilQ background. Strains used were DK8615 (cglD+), DW1440 (cglD1), DW1461 (cglD2) and DW1433 (ΔcglD). Micrographs (10× objective) were taken after 1 day incubation on [1/2] CTT 1.5% agar supplemented with 2 mM CaCl2, except as noted.
Bioinformatic analysis.
A central question we sought to address was whether the CglC, CglD, CglE, and CglF proteins contained type II signal sequences like CglB and Tgl. These and other salient features of the Cgl proteins are summarized in Table 3. As expected, CglC and CglD protein sequences contain lipoboxes within their signal sequences, LGGC (17 to 20) and LTGC (19 to 22), respectively, suggesting that they encode lipoproteins. Surprisingly, CglE and CglF contain type I signal sequences, suggesting that they were not lipoproteins but would nevertheless be transported to the cell envelope. The finding that CglE/F contained type I signal sequences suggests that nonlipoproteins were exchanged.
Table 3.
cgl genes identified in this study and their salient features
| Gene | Locus tag | Other name(s) | No. of amino acids | Signal sequence type | Domain | Paralog | Distribution |
|---|---|---|---|---|---|---|---|
| cglC | MXAN_2538 | agmO, gltK | 172 | II | Hypothetical | Myxobacteria | |
| cglD | MXAN_0962 | 1130 | II | von Willebrand | MXAN_5598 | Wide | |
| cglE | MXAN_4866 | agmV, gltH | 209 | I | Hypothetical | MXAN_3378 | Myxobacteria |
| cglF | MXAN_4868 | gltF | 89 | I | Hypothetical | MXAN_3376 | Myxobacteria |
The predicted mature Cgl proteins ranged in size from 71 to 1,109 amino acids (Table 3), suggesting that there are no practical size constraints for protein transfer between cells.
Sequence analysis of CglC, CglE, and CglF found no significant homologies to known protein domains. Furthermore, BLAST searches found that orthologs of CglC, CglE, and CglF were phylogenetically restricted to myxobacteria (Table 3). The narrow phylogenetic distribution of Cgl proteins likely reflects the fact that A motility represents a specialized function within closely related species. Although orthologs were restricted, the DK1622 genome nevertheless contains paralogs of CglE and CglF. Interestingly, these paralogs were located in a gene cluster with other A motility paralogs that likely encode nongliding motility functions (Table 3) (16).
Sequence analysis of CglD revealed that it contains multiple domains (Fig. 5A). First, we identified within the N-terminal region 12 degenerative repeats consisting of 12 amino acids with a consensus sequence of DtDgDGlpDgvE (see Fig. S1A and B in the supplemental material). This repeat, named 1, contained conserved aspartic acid residues and shows similarity to the EF-hand-like motifs involved in calcium binding (7). Therefore, CglD might bind calcium. Within this same region, a second longer repeat, dubbed repeat 2, was identified and consists of 16 amino acids with a consensus sequence of DxNrNGrvDpGETDPr (see Fig. S1C and D in the supplemental material). Repeat 2 was found six times within CglD, and a BLAST search with this repeat found one hypothetical ORF with at least 38 repeats (GenBank accession no. AEE49992.1) (Fig. 5A). Residues 648 to 896 contain a region with low homology to the VWA domain. The putative CglD VWA domain includes a MIDAS motif (DxSxS; 656 to 560) that is characteristic of many VWA domains (37). VWA domains are frequently found in eukaryotic and bacterial extracellular matrix proteins involved in cell adhesion (37), a function that seems plausible for CglD. As previously mentioned, CglB contains a putative VWA domain and resides in the OM (13). Interestingly, the two cglD mutant alleles both result in amino acid substitutions within the VWA domain (Fig. 5A). In the case of CglD2, the amino acid substitution specifically changes the conserved Asp residue within the MIDAS motif (Fig. 5A). These findings highlight the importance of the VWA domain for CglD function in A motility. Finally, the C terminus of CglD contains a cysteine-rich domain that contains 28 cysteines within a 128-amino-acid region. This region includes three CXCXC repeats but otherwise does not show significant homology to other known domains. We note that cysteine residues, which are likely oxidized to form disulfide bonds upon secretion, are frequently found in extracellular matrix proteins. The closest paralog of CglD was MXAN_5598 (E value, 8 × 10−66), a giant protein with a predicted length of 2,699 amino acids that also contains OmpA and MYXO-CTERM domains, which are similarly found in TraB and TraA, respectively (23a). The function of MXAN_5598 was unknown, but this ORF resides within a large cluster of genes predicted to function in cell wall biosynthesis and cell division.
Synthetic CglF peptide restores A motility.
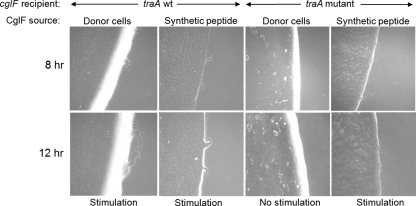
The cglF gene encodes a predicted ORF of 89 amino acids, which includes a type I signal sequence. Following signal sequence cleavage, the CglF protein was predicted to be 71 amino acids. As CglF was unusually small, we tested whether exogenous addition of CglF protein could reverse the motility defect of a ΔcglF mutant. To do this, we synthesized the CglF peptide, purified it, and added it to a cglF mutant. As shown in Fig. 6, synthetic CglF peptide (20 μg) reversed the motility defect of the cglF mutant. Stimulation was clearly observed at 8 h and became more prominent at 12 h. Over this time, the degree of ΔcglF stimulation elicited by the peptide was comparable to that of a live cglF+ donor (ΔmglBA) control (Fig. 6). Detectable, though reduced, cglF stimulation was also found when 5 or 10 μg of peptide was added (data not shown). However, unlike mixing with a live donor strain, prolonged incubations of the cglF mutant with CglF peptide did not result in further stimulation. Instead, by 24 h, no detectable flares were observed with the CglF peptide, while, in contrast, a live donor provides prolonged stimulation (Fig. 3). Presumably, over extended incubations, the exogenous CglF peptide was fully utilized and/or degraded and thus stimulation ceased. In contrast, live donor cells continuously make CglF protein for extended stimulation.
Fig 6.
Addition of synthetic CglF peptide stimulates a cglF mutant in a TraA-independent manner. CglF synthetic peptide (20 μg) was mixed with nonmotile (A− S−) cglF mutants and incubated as shown. Strains used were DW1460 (ΔcglF), DW1405 (cglF1 traA::km) and the nonmotile donor strain was DK6204.
Recently we showed that the TraA protein was absolutely required in donor and recipient cells for stimulation of all cgl/tgl mutants and for lipoprotein and lipid exchange (23a). Next, we tested whether exogenous addition of CglF peptide requires TraA for stimulation. Here, a cglF1 traA mutant (A− S−) was mixed with synthetic CglF peptide and assayed. Interestingly, stimulation of the traA mutant strain was as robust as that of the traA+ strain (Fig. 6). As a control, the cglF1 traA mutant strain was mixed in parallel with a nonmotile cglF+ donor (ΔmglBA) and, as expected, no stimulation occurred (Fig. 6) (23a). We therefore conclude that, in contrast to that of live donors/recipients, exogenous addition of CglF peptide bypasses the requirement of TraA in stimulation.
Suppressor analysis.
To help ascertain the role Cgl proteins play in A motility, we sought to identify suppressors that restore gliding motility. This was a powerful approach, as large numbers of cells (e.g., >109) were easily screened for suppressors that restore A motility, which were detected by emergent motile flares from mutagenized pools. This screen was conducted with all four cgl genes whose mutant alleles contained base pair substitutions or a single base deletion. Suppressors identified by UV mutagenesis were first tested for reversion or intragenic suppression by PCR amplification and DNA sequencing of the respective cgl alleles. In the case of cglC2, cglE1, and cglF1, all characterized suppressors were indeed intragenic and those sequencing results are summarized in Table 4. These suppressors restored A motility swarming to nearly wild-type levels (see Fig. S2, S3, and S4 in the supplemental material). The five sequenced intragenic suppressors of the nonsense cglC2 mutation (80Q→NS) all resulted in the conversion of the codon 80 nonsense mutation to missense mutations (non-wild-type alleles). Similarly, four cglE1 (22Q→NS) suppressors converted the codon 22 nonsense mutation to missense mutations or were true revertants. The cglF1 allele contains a nucleotide deletion at position 103 (103ΔT) that results in a frameshift mutation. All six suppressors restored the cglF reading frame by inserting one nucleotide or deleting five nucleotides at various positions spanning a 43-nucleotide region (Table 4). These cglF1 intragenic suppressors provide structure-function information when mapped on multiple sequence alignments of CglF homologs (see Fig. S5 in the supplemental material). Specifically, the poorly conserved N-terminal region of the processed CglF protein, spanning a 15-amino-acid region, was amendable to dramatic amino acid substitutions and a reduced length that still provided function.
Table 4.
Intragenic suppressors isolated in this study
| Original mutation | Suppressor | Suppressor mutation(s) | Amino acid change(s)a |
|---|---|---|---|
| cglC2 | cglC2sup1 | 240G→C | 80 NS→Y |
| 237C→T (silent) | cglC2sup2 | 238T→A (isolated 3 times) | 80 NS→K |
| 238C→T | cglC2sup4 | 239A→G | 80 NS→W |
| cglE1 | cglE1sup2 | 65A→C | 22 NS→S |
| 63C→T (silent) | cglE1sup11 | 64T→G | 22 NS→E |
| 64C→T | cglE1sup12 | 64T→C (isolated 2 times) | 22 NS→Q (revertant) |
| cglF1 | cglF1sup1 | 72A insertion, 75C→T | 24N→FS (restores reading frame) |
| cglF1sup2 | 72C→A, 78G insertion (isolated 2 times), 74C→T | 24N→K, 25P→L, 27G→FS (restores reading frame) | |
| 103 T deletion | cglF1sup3 | 93C deletion, 94A deletion, 95A deletion, 96T deletion, 97G deletion (isolated 2 times) | 32N→FS (restores reading frame) |
| 105C→T (silent) | cglF1sup3A | 115A insertion | 39P→FS (restores reading frame) |
FS, frameshift; NS, nonsense.
In the case of cglD1, only one suppressor was isolated and no compensatory mutation was found within the cglD1 locus. Therefore, the cglD1 suppressor was extragenic. Although this suppressor was not mapped, we noted that the suppressor exhibited a “sticky” phenotype, a property where cells tightly adhere to each other. This phenotype suggests that the suppressor mutation may reside in the stk locus (MXAN_3474; DnaK paralog) (8). This interpretation is also consistent with a previous report by Dana and Shimkets that a stk Tn5 transposon insertion mutation suppresses a cglD1 motility defect (4). If this interpretation was correct, the stk suppressor might bind cells together and allow them to move in groups, a condition that facilitates cglD1 mutant motion (see Movie S2 in the supplemental material).
DISCUSSION
Thirty-five years ago, Hodgkin and Kaiser described the isolation of tgl and cgl gliding motility mutants whose defects could be transiently restored by cell-cell contact with appropriate donor strains (9). Here we report the identification of four of the six known stimulatable motility genes, cglC, cglD, cglE, and cglF. A fundamental goal of this investigation was to identify the type of gene products that were stimulated and thus likely exchanged. Although we did not show it directly, we argue these Cgl proteins are transferred because (i) their motility defect was complemented extracellularly and (ii) similar to Tgl and CglB, stimulation requires the TraAB proteins in donor and recipient cells (23a). Our working model suggests that TraAB functions as a cell surface receptor that allows juxtaposed cells to transiently fuse their OMs and exchange envelope material through lateral diffusion. Consistent with prior findings with Tgl, CglB, and SSOM-mCherry, the CglC and CglD proteins also contain type II signal sequences, suggesting that they also encode lipoproteins. To our surprise, the CglE and CglF proteins instead contain type I signal sequences, suggesting that they are similarly transported across the cytoplasmic membrane by the Sec pathway but are not modified into lipoproteins. These results thus suggest that the repertoire of exchanged myxobacterial proteins includes nonlipoproteins. This new class of cargo proteins may include soluble periplasmic proteins and/or proteins associated with the OM. Integral OM proteins may also be transferred; however, one argument against this possibility is that pilQ mutants, which encode an integral OM secretin, cannot be complemented when mixed with PilQ+ cells (data not shown). Inner membrane and cytoplasmic proteins are not exchanged (36). We also report that the size of cargo proteins can apparently vary from ≤71 to >1,100 amino acids.
Interestingly, the motility defect of a cglF mutant was rescued by the addition of synthetic CglF peptide (Fig. 6). This result suggests, similar to CglB and Tgl stimulation, that the CglF protein is transferred during the stimulation experiment (22). However, in contrast to CglF peptide, prior attempts to add the purified CglB or Tgl protein to its respective mutant strain were unsuccessful at restoring motility (unpublished data). This failure could result from a number of reasons, including misfolding of recombinant protein or the inability of these larger proteins to cross the OM and properly localize in the cell envelope. The ability of exogenous CglF protein to rescue the cglF motility defect suggests that it localizes to the cell surface. Moreover, the observation that exogenous CglF restores motility in a TraA-independent manner suggests that the mechanism of CglF delivery fundamentally differs between live donor cells and exogenous addition. A possible interpretation of this result is that CglF is tightly bound on the cell surface; thus, live donors cannot transfer CglF unless the TraAB system is functional for OM fusion (23a). In contrast, exogenous CglF addition bypasses TraAB function because it can directly bind the OM. An alternative explanation is that the small CglF protein may have an intrinsic or facilitated ability to cross the OM for proper cell envelope localization. Further experiments are needed to test these possibilities. Last, it is noteworthy that a CglF mutant does not move at the single-cell level and that all suppressor mutations were rare intragenic mutations that restored the short CglF reading frame (Fig. 4 and Table 4; see Fig. S4 and S5 in the supplemental material), suggesting that CglF activity provides an essential A motility motor function that cannot be bypassed. For these reasons, we think it is unlikely that CglF functions as a signal, even though it is a small protein.
cglD is a new A motility gene. The unusually “leaky” motility phenotype of the cglD null mutant may explain why this large gene (3.4 kb) eluded previous transposon-based screens to identify A motility mutants (40, 41). Strikingly, the cglD missense mutations have significantly stronger A motility defects than the null mutant (Fig. 5B). One explanation for this unexpected difference is that CglD may have multiple functions in A motility. In simple terms, one domain may activate A motility, while another domain inhibits A motility. Thus, a null mutant removes both positive and negative functions, while a missense mutation might specifically inactivate a positive function. Consistent with this idea, CglD clearly contains multiple domains and the cglD1 and cglD2 missense mutations, which severely block A motility, both reside within the VWA domain (Fig. 5A), suggesting that this domain plays a critical A motility function. Prior proteomic studies found the CglD lipoprotein (MXAN_0962) in the inner membrane or OM and OM vesicles (OMVs) (2, 13). These different findings may result from complex cell fractionation properties for a large protein that likely binds multiple factors. Our stimulation results and the lack of a clear IM retention signal suggest that CglD may, in fact, reside in the OM and OMVs (Fig. 3) (2, 36).
While this report was in preparation, the Zusman and Mignot groups reported the characterization of genes within the cglE-cglF gene cluster (16, 20). These reports also state that cglF (MXAN_4868, gltF) null mutants have an A motility defect but their phenotypes varied from partial to complete loss of A motility. Our findings with a number of strains and alleles clearly show that cglF null mutants completely lack A motility. For cglE (agmV, gltH) null mutants, both groups report a severe A motility defect but noticed, similar to our findings, residual A motility. Luciano et al. further performed time-lapse microscopy of a cglE mutant and found “jerky” motions (16). In contrast, our time-lapse microscopy observations of a cglE mutant found fairly robust gliding at the single-cell level, though swarming was severely blocked (Fig. 3; see Movie S3 in the supplemental material). We suspect that this subtle but important phenotypic difference was the result of different assay conditions, as medium changes, for example, can significantly alter motility behavior. Based on our analysis, we suggest that CglE plays a nonessential role in single-cell gliding but does play an important role in coordinated group movements in swarm expansion. Interestingly, Nan et al. reported that a MXAN_4864 deletion mutant was partially defective in A motility, a defect we did not observe with an insertion mutation (20). It is possible that our mutation was not a null mutation, and thus, further work needs to delineate the role of MXAN_4864 in A motility. Last, we suggest that the cgl nomenclature be used in lieu of other gene names because (i) the genes were originally named cgl (9, 10), (ii) therefore these are the names most frequently used and recognized in the literature, (iii) they designate the stimulatable phenotype and hence protein transfer, and (iv) they provide consistency between the multinamed cglC, cglE, and cglF genes with the single-named cglB and cglD genes.
Cell fractionation and proteomic experiments found that CglE (MXAN_4866) localizes to the OM and OMVs (13, 16). OM localization is consistent with our findings that CglE mutants can be stimulated in a TraAB-dependent manner (23a, 36). Although CglE is not a lipoprotein, some algorithms predict that it contains beta-barrel structure and thus might be an integral OM protein. In addition, Luciano et al. constructed a CglF-mCherry fusion and found it localized to the cell periphery and colocalized with “focal adhesion complexes” thought to power A motility (16). Crude cell fractionation experiments with this fusion found that it resided in soluble and membrane fractions. Although not definitive, these studies are consistent with our interpretation that CglF likely associates with the OM, perhaps on the cell surface. In sum, in M. xanthus, the CglB, CglC, CglD, CglE, and CglF proteins are readily exchange between cells and yet they also constitute critical components of the A motility machinery (16).
Supplementary Material
ACKNOWLEDGMENTS
We thank Xueming Wei for helpful suggestions and Dale Kaiser, Patricia Hartzell, David Zusman, Beiyan Nan, Lotte Søgaard-Andersen, and Daniela Keilberg for providing strains and/or plasmids used in this study.
This work was support by a grant to D.W. from the National Science Foundation (MCB-0848141) and by the University of Wyoming.
Footnotes
Published ahead of print 17 February 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389– 3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhat S, Zhu X, Patel RP, Orlando R, Shimkets LJ. 2011. Identification and localization of Myxococcus xanthus porins and lipoproteins. PLoS One 6: e27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res. 14: 1188– 1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dana JR, Shimkets LJ. 1993. Regulation of cohesion-dependent cell interactions in Myxococcus xanthus. J. Bacteriol. 175: 3636– 3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792– 1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldman BS, et al. 2006. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. U. S. A. 103: 15200– 15205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grabarek Z. 2006. Structural basis for diversity of the EF-hand calcium-binding proteins. J. Mol. Biol. 359: 509– 525 [DOI] [PubMed] [Google Scholar]
- 8. Hartzell P, Shi W, Youderian P. 2008. Gliding motility in Myxococcus xanthus, p 103– 122 In Whitworth DE. (ed), Myxobacteria: multicellularity and differentiation. ASM Press, Washington, DC [Google Scholar]
- 9. Hodgkin J, Kaiser D. 1977. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. U. S. A. 74: 2938– 2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodgkin J, Kaiser D. 1979. Genetics of gliding motility in Myxococcus xanthus (Mycobacterales): genes controlling movement of single cells. Mol. Gen. Genet. 171: 167– 176 [Google Scholar]
- 11. Hodgkin J, Kaiser J. 1979. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol. Gen. Genet. 171: 177– 191 [Google Scholar]
- 12. Juncker AS, et al. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12: 1652– 1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahnt J, et al. 2010. Profiling the outer membrane proteome during growth and development of the social bacterium Myxococcus xanthus by selective biotinylation and analyses of outer membrane vesicles. J. Proteome Res. 9: 5197– 5208 [DOI] [PubMed] [Google Scholar]
- 14. Kaiser D, Crosby C. 1983. Cell movements and its coordination in swarms of Myxococcus xanthus. Cell Motil. 3: 227– 245 [Google Scholar]
- 15. Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40: D302– 305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luciano J, et al. 2011. Emergence and modular evolution of a novel motility machinery in bacteria. PLoS Genet. 7: e1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magrini V, et al. 1997. Temperate Myxococcus xanthus phage Mx8 encodes a DNA adenine methylase, Mox. J. Bacteriol. 179: 4254– 4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marchler-Bauer A, et al. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39: D225– 229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mauriello EM, Astling DP, Sliusarenko O, Zusman DR. 2009. Localization of a bacterial cytoplasmic receptor is dynamic and changes with cell-cell contacts. Proc. Natl. Acad. Sci. U. S. A. 106: 4852– 4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nan B, Mauriello EM, Sun IH, Wong A, Zusman DR. 2010. A multi-protein complex from Myxococcus xanthus required for bacterial gliding motility. Mol. Microbiol. 76: 1539– 1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nan B, Zusman DR. 2011. Uncovering the mystery of gliding motility in the myxobacteria. Annu. Rev. Genet. 45: 21– 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nudleman E, Wall D, Kaiser D. 2005. Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science 309: 125– 127 [DOI] [PubMed] [Google Scholar]
- 23. Nudleman E, Wall D, Kaiser D. 2006. Polar assembly of the type IV pilus secretin in Myxococcus xanthus. Mol. Microbiol. 60: 16– 29 [DOI] [PubMed] [Google Scholar]
- 23a. Pathak DT, et al. Cell contact-dependent outer membrane exchange in myxobacteria: genetic determinants and mechanism. PLoS Genet., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8: 785– 786 [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez-Soto JP, Kaiser D. 1997. Identification and localization of the Tgl protein, which is required for Myxococcus xanthus social motility. J. Bacteriol. 179: 4372– 4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez AM, Spormann AM. 1999. Genetic and molecular analysis of cglB, a gene essential for single-cell gliding in Myxococcus xanthus. J. Bacteriol. 181: 4381– 4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, third ed. Cold Spring Harbor Press, Cold Spring Harbor, NY [Google Scholar]
- 28. Sodergren E, Kaiser D. 1983. Insertions of Tn5 near genes that govern stimulatable cell motility in Myxococcus. J. Mol. Biol. 167: 295– 310 [DOI] [PubMed] [Google Scholar]
- 29. Spormann AM. 1999. Gliding motility in bacteria: insights from studies of Myxococcus xanthus. Microbiol. Mol. Biol. Rev. 63: 621– 641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strassmann JE, Gilbert OM, Queller DC. 2011. Kin discrimination and cooperation in microbes. Annu. Rev. Microbiol. 65: 349– 367 [DOI] [PubMed] [Google Scholar]
- 31. Ueki T, Inouye S, Inouye M. 1996. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene 183: 153– 157 [DOI] [PubMed] [Google Scholar]
- 32. Vos M, Velicer GJ. 2009. Social conflict in centimeter- and global-scale populations of the bacterium Myxococcus xanthus. Curr. Biol. 19: 1763– 1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wall D, Kaiser D. 1998. Alignment enhances the cell-to-cell transfer of pilus phenotype. Proc. Natl. Acad. Sci. U. S. A. 95: 3054– 3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wall D, Kaiser D. 1999. Type IV pili and cell motility. Mol. Microbiol. 32: 1– 10 [DOI] [PubMed] [Google Scholar]
- 35. Wall D, Kolenbrander PE, Kaiser D. 1999. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J. Bacteriol. 181: 24– 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei X, Pathak DT, Wall D. 2011. Heterologous protein transfer within structured myxobacteria biofilms. Mol. Microbiol. 81: 315– 326 [DOI] [PubMed] [Google Scholar]
- 37. Whittaker CA, Hynes RO. 2002. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13: 3369– 3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitworth DE. (ed). 2008. Myxobacteria: multicellularity and differentiation. ASM Press, Washington DC [Google Scholar]
- 39. Wu TT. 1966. A model for three-point analysis of random general transduction. Genetics 54: 405– 410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Youderian P, Burke N, White DJ, Hartzell PL. 2003. Identification of genes required for adventurous gliding motility in Myxococcus xanthus with the transposable element mariner. Mol. Microbiol. 49: 555– 570 [DOI] [PubMed] [Google Scholar]
- 41. Yu R, Kaiser D. 2007. Gliding motility and polarized slime secretion. Mol. Microbiol. 63: 454– 467 [DOI] [PubMed] [Google Scholar]
- 42. Zusman DR, Scott AE, Yang Z, Kirby JR. 2007. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 5: 862– 872 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.