Abstract
Seven NDM-positive Acinetobacter baumannii isolates of worldwide origin were studied to evaluate the best technique for their identification. Detection of carbapenemase producers based on the measurement of carbapenemase activity by UV spectrophotometry (as for A. baumannii strains producing other types of carbapenemase), or by the modified Hodge test, failed. Inhibition activity using EDTA was a sensitive technique but lacked specificity compared to molecular techniques, which remain the gold standard.
TEXT
The emergence of the metallo-ß-lactamase (MBL) named NDM-1 (New Dehli metallo-ß-lactamase) is one of the latest and most important resistance mechanisms identified in Gram-negative rods (12, 17). The blaNDM-1 gene, identified initially in Klebsiella pneumoniae and Escherichia coli strains mostly from India and Pakistan, was recently identified extensively in other enterobacterial species worldwide (18, 21). Besides spread of the NDM gene in Enterobacteriaceae, NDM producers in A. baumannii are increasingly being identified worldwide (9–11).
Whereas detection of NDM producers in Enterobacteriaceae is easy (16), our preliminary results indicated that detection of NDM-producing A. baumannii strains may be much more complicated. Therefore, we evaluated several techniques for the detection of NDM-producing A. baumannii clinical isolates.
A collection of seven NDM-positive Acinetobacter baumannii isolates of worldwide origin was studied, to identify the best approach to detect the production of NDM-type carbapenemases among clinical isolates. Another collection corresponding to carbapenemase-producing but NDM-negative A. baumannii isolates of worldwide origin was studied for comparison (Table 1). Those strains produced either Ambler class B carbapenemases (IMP-4, VIM-4, or SIM-1), class D carbapenemases (OXA-23, OXA-40, or OXA-58), or a class A carbapenemase (GES-14). Susceptibility testing was performed by Etest (AB bioMérieux, Solna, Sweden) on Mueller-Hinton agar plates at 37°C, and results were classified according to the updated CLSI guidelines (5). The CLSI breakpoints for A. baumannii of imipenem and meropenem were as follows: susceptible (S), ≤4 μg/ml; resistant (R), ≥16 μg/ml (5). The EUCAST breakpoints for imipenem and meropenem were as follows: S, ≤2 μg/ml; R, ≥8 μg/ml. Those for doripenem were as follows: S, ≤1 μg/ml; R, ≥4 μg/ml (13). All NDM-producing A. baumannii clinical isolates were resistant to all β-lactams, including all carbapenems, according to CLSI and EUCAST guidelines (Table 1).
Table 1.
Comparison of techniques for detection of carbapenemase-producing A. baumannii isolates
| Isolated | Acquired carbapenemase(s) | MIC (μg/ml) |
Result of: |
Specific activity (mU/mg)a |
PCR resultc | Reference(s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTX | CAZ | IPM | IPM+EDTA | MEM | DOR | Etest MBL | IPM/IPM+EDTA test (diameter −/+ EDTA) (mm) | Hodge test | IPM | MEM | ERT | ||||
| SLO | NDM-1 | >256 | >256 | >32 | 1 | >32 | >32 | + | + (6/25) | − | 5.1 | 0.6 | 1.4 | + | This study |
| All | NDM-1 | >256 | >256 | >32 | <1 | >32 | >32 | + | + (6/24) | − | 5.9 | 0.9 | 1.4 | + | 9 |
| Ora-1 | NDM-1 | >256 | >256 | >32 | <1 | >32 | >32 | + | + (6/22) | − | 6.4 | 0.9 | 1.1 | + | This study |
| StN | NDM-1 | >256 | >256 | >32 | <1 | >32 | >32 | + | + (6/23) | − | 6.3 | 0.8 | 1.2 | + | This study |
| Gen | NDM-1, OXA-23 | >256 | >256 | >32 | 3 | >32 | >32 | + | + (6/18) | − | 7.2 | 1.1 | 1.5 | + | 20 |
| ML | NDM-2 | >256 | >256 | >32 | <1 | >32 | >32 | + | + (6/23) | − | 4.7 | 0.9 | 1.3 | + | 10 |
| 124 | NDM-2 | >256 | >256 | >32 | <1 | >32 | >32 | + | + (6/23) | − | 4.5 | 0.7 | 1.3 | + | This study |
| IMP | IMP-4 | >256 | >256 | 4 | <1 | 8 | 4 | + | −/+ (14/20) | − | 718.1 | 95.6 | 65.2 | − | 4 |
| A154 | VIM-4 | >256 | >256 | 32 | <1 | 32 | 32 | + | + (8/25) | + | 279.8 | 45.2 | 33.3 | − | 6 |
| SIM | SIM-1 | >256 | >256 | 3 | <1 | 4 | 3 | −/+e | −/+ (21/29) | +b | 58.3 | 12.6 | 23.2 | − | 15 |
| 35 | OXA-23 | >256 | >256 | 32 | 4 | 32 | 24 | −/+ | −/+ (9/17) | − | 2.8 | 1.1 | 0.8 | − | This study |
| Acb2 | OXA-23 | >256 | >256 | 32 | 4 | 32 | 24 | −/+ | −/+ (11/20) | − | 2.1 | 1.1 | 0.8 | − | 2 |
| Acb13 | OXA-23 | >256 | >256 | 24 | 4 | 24 | 16 | −/+ | −/+ (11/18) | − | 1.9 | 1.2 | 0.9 | − | 2 |
| LAH | OXA-40 | >256 | 128 | 32 | 12 | >32 | 32 | −/+ | −/+ (7/18) | +b | 2.1 | 0.5 | 0.5 | − | This study |
| MUZ | OXA-40 | >256 | 256 | 32 | 12 | >32 | 32 | −/+ | −/+ (7/16) | +b | 1.6 | 0.6 | 0.4 | − | This study |
| Acb19 | OXA-58 | >256 | >256 | 4 | 2 | 4 | 4 | − | − (14/18) | − | 3.1 | 1.2 | 1.5 | − | 2 |
| MAY | OXA-58 | 8 | 2 | 3 | 2 | 2 | 1 | − | − (15/17) | − | 2.9 | 1.1 | 0.6 | − | This study |
| MAD | OXA-58 | 256 | 256 | 24 | 16 | 24 | 24 | − | − (11/15) | +b | 3.0 | 1.2 | 0.6 | − | 19 |
| AP | GES-14 | >256 | >256 | 24 | 8 | 16 | 8 | − | − (11/17) | +b | 7.5 | 1.4 | 1.1 | − | 1 |
Standard deviations were within 10% of the means.
Weakly positive.
PCR was performed under standard conditions with NDM-For and NDM-Rev.
All isolates are A. baumannii except isolate A154, which belongs to genomospecies 16.
−/+, indeterminate result.
The Etest MBL strip is one of the recommended tests for detection of MBLs based on inhibition of MBL activity by EDTA (16). This Etest method showed good sensitivity for the detection of MBL production. Susceptibility to imipenem was restored in the presence of EDTA, confirming the significance of MBL as a source of carbapenem resistance among MBL producers. However, several MBL-negative strains producing OXA-23 or OXA-40 gave false-positive results (Table 1). This showed that the intrinsic effect of EDTA on A. baumannii might interfere in the specificity of this test.
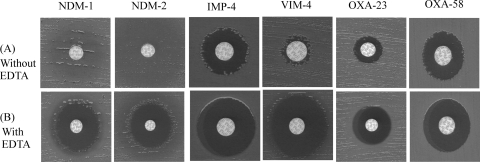
Another technique was therefore evaluated to detect MBL production using the same principle based on inhibition by EDTA. This technique consisted of two imipenem disks with or without 10 μl of 0.5 M EDTA (8). An increase of 10 mm in the inhibition zone diameter in the presence of EDTA was considered a positive result. An increase between 5 and 10 mm was considered doubtful and therefore required further investigation. This technique gave results similar to those obtained with Etest MBL strips, with MBL producers being easily detected, except for an IMP-4-producing isolate possessing low MICs of carbapenems (MIC of imipenem, 4 mg/liter) (Fig. 1). However, OXA-23 and OXA-40 producers gave some false-positive results, as observed with Etest MBL strips (Table 1; Fig. 1).
Fig. 1.
Results of the IPM-EDTA synergy test for carbapenemase-producing A. baumannii clinical isolates. (A) Imipenem disk (10 μg) alone. (B) Imipenem disk supplemented with 10 μl of EDTA at 0.5 M. A significant increase of the inhibition zone in the presence of EDTA was considered positive. From left to right, the panels contain an NDM-1-producing A. baumannii strain (isolate SLO), an NDM-2-producing A. baumannii strain (isolate ML), an IMP-4-producing A. baumannii strain (isolate IMP), a VIM-4-producing Acinetobacter genomospecies 16 strain (isolate A154), an OXA-23-producing A. baumannii strain (isolate Acb2), and an OXA-58-producing A. baumannii (isolate MAY).
The modified Hodge test (MHT) has been widely used for screening of carbapenemase activity because of the direct analysis of the carbapenemase activity. We therefore evaluated the MHT as previously described (14) at a turbidity of 0.5 McFarland, using E. coli ATCC 25922 as the indicator organism, K. pneumoniae BIC (producing the carbapenemase OXA-48) as a positive control, and K. pneumoniae COO (a non-carbapenemase-producing but carbapenem-resistant isolate) as a negative control. This test gave negative results for all tested NDM-producing A. baumannii strains (Table 1). VIM-, IMP- and some OXA-type producers gave weak synergistic images. This test was thus poorly sensitive and specific for detecting the carbapenemase activity of any carbapenemase producers.
Biochemical detection of NDM activity in A. baumannii was then analyzed. Ten ml of overnight broth cultures of A. baumannii isolates was centrifuged at 5,000 × g for 15 min and then sonicated in ice. Specific activities for carbapenems were measured using UV spectrophotometry at a wavelength value of 297 nm for imipenem as described previously (3). Detection of carbapenemase activity by UV spectrophotometry performed with crude culture extracts of NDM producers did not give any significant positive result. The standard mean of specific activities obtained for the NDM producers was evaluated at 5.7 mU/mg of proteins. This value was not significant compared to the value obtained with the reference strain A. baumannii AYE strain (7), which does not possess any acquired carbapenemase gene and is susceptible to imipenem (2.6 mU/mg of proteins, which corresponds to the baseline). Specific activities measured using other carbapenem molecules gave similar results but lower levels of carbapenemase activities (Table 1). Similar low-level activities were observed for carbapenem-hydrolyzing class D β-lactamases (CHDL), whereas a high level of activity was observed for class B ß-lactamases of the types VIM, IMP, and SIM (Table 1). The latter results may be related to stronger promoter or/and plasmid locations of those MBL genes.
Molecular techniques performed as previously described (16), using the primers NDM-For (5′-GGTGCATGCCCGGTGAAATC-3′) and NDM-Rev (5′-ATGCTGGCCTTGGGGAACG-3′) for internal gene amplification (660-bp amplicon) and the primers pre-NDM-for (5′-CACCTCATGTTTGAATTCGCC-3′) and pre-NDM-rev (5′-CTCTGTCACATCGAAATCGC-3′) for amplification of the entire gene sequence (984-bp amplicon), are useful tools for identification of NDM producers and the precise identification of the resistance determinant, respectively. In a single case, as reported previously, two carbapenemase genes were identified that were blaOXA-23 and blaNDM-1 (20). Using these PCR-based techniques, all NDM producers were detected. Multiplex PCR for detecting several carbapenemase genes should be adapted to A. baumannii, since recently developed multiplex PCR schemes focused on detection of carbapenemase genes only in Enterobacteriaceae. One limit of molecular techniques, however, is the failure to detect carbapenemase producers due to unknown carbapenemase genes.
This work indicates that identification of NDM producers may be suspected by comparing results of MIC of carbapenems with and without EDTA. Detection of carbapenemase activity using either the modified Hodge test or UV spectrophotometry is likely to fail to detect NDM producers in A. baumannii, whereas this detection is efficient with members of the Enterobacteriaceae. The discrepancy between A. baumannii and Enterobacteriaceae could be explained by a weak enzyme production in A. baumannii, likely related to the chromosomal location (a single copy) of the blaNDM gene. Considering that many microbiology laboratories do not possess the facilities for molecular detection, carbapenem-resistant A. baumannii should be screened first by using EDTA inhibition-based techniques followed by further PCR-based techniques in a reference laboratory.
These observations may have clinical implications for detection and therefore for controlling the spread of NDM producers in A. baumannii, which is in our opinion one of the most difficult tasks, considering the persistence of multidrug-resistant A. baumannii in many health care facilities.
ACKNOWLEDGMENTS
This work was mostly funded by the INSERM (U914), France, and by the European Community (TEMPOtest-QC, HEALTH-2009-241742).
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Bonnin RA, et al. 2011. Carbapenem-hydrolyzing GES-type extended-spectrum β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonnin RA, Poirel L, Licker M, Nordmann P. 2011. Genetic diversity of carbapenem-hydrolyzing β-lactamases in Acinetobacter baumannii from Romanian hospitals. Clin. Microbiol. Infect. 17:1524–1528 [DOI] [PubMed] [Google Scholar]
- 3. Carrër A, et al. 2010. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob. Agents Chemother. 54:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu YW, et al. 2001. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45:710–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. CLSI document M100–S21 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Figueiredo S, Poirel L, Papa A, Koulourida V, Nordmann P. 2008. First identification of VIM-4 metallo-ß-lactamase in Acinetobacter spp. Clin. Microbiol. Infect. 14:289–290 [DOI] [PubMed] [Google Scholar]
- 7. Fournier PE, et al. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franklin C, Liolios L, Peleg AY. 2006. Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing gram-negative bacilli in the clinical laboratory. J. Clin. Microbiol. 44:3139–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Göttig S, et al. 2010. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect. Dis. 10:828–829 [DOI] [PubMed] [Google Scholar]
- 10. Kaase M, et al. 2011. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J. Antimicrob. Chemother. 66:1260–1262 [DOI] [PubMed] [Google Scholar]
- 11. Karthikeyan K, Thirunarayan MA, Krishnan P. 2010. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J. Antimicrob. Chemother. 65:2253–2254 [DOI] [PubMed] [Google Scholar]
- 12. Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leclercq R, et al. 2011. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect., in press doi:10.1111/j.1469-0691.2011.03703.x [DOI] [PubMed] [Google Scholar]
- 14. Lee K, Lim YS, Yong D, Yum JH, Chong Y. 2003. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 41:4623–4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee K, et al. 2005. Novel acquired metallo-β-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob. Agents Chemother. 49:4485–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordmann P, Poirel L, Carrër A, Toleman MA, Walsh TR. 2011. How to detect NDM-1 producers. J. Clin. Microbiol. 49:718–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordmann P, Poirel L, Walsh TR, Livermore DM. 2011. The emerging NDM carbapenemases. Trends Microbiol. 19:588–595 [DOI] [PubMed] [Google Scholar]
- 18. Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poirel L, et al. 2005. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poirel L, et al. 2011. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 56:1087–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yong D, et al. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]