Abstract
The blistering disorder, lethal junctional epidermolysis bullosa (JEB), can result from mutations in the LAMB3 gene, which encodes laminin 5 β3 (β3). Appropriate expression of LAMβ3 in JEB skin tissue could potentially ameliorate the symptoms of the underlying disease. To explore the utility of this therapeutic approach, primary keratinocytes from six unrelated JEB patients were transduced with a retroviral vector encoding β3 and used to regenerate human skin on severe combined immunodeficient (SCID) mice. Tissue regenerated from β3-transduced JEB keratinocytes produced phenotypically normal skin characterized by sustained β3 expression and the formation of hemidesmosomes. Additionally, β3 gene transfer corrected the distribution of a number of important basement membrane zone proteins including BPAG2, integrins β4/β1, and laminins α3/γ2. Skin produced from β3-negative (β3[−]) JEB cells mimicked the hallmarks of the disease state and did not exhibit any of the aforementioned traits. Therefore, by effecting therapeutic gene transfer to β3-deficient primary keratinocytes, it is possible to produce healthy, normal skin tissue in vivo. These data support the utility of gene therapy for JEB and highlight the potential for gene delivery in the treatment of human genetic skin disease.
Keywords: gene therapy, skin, retrovirus
A number of devastating inherited skin disorders have been characterized at the molecular level. Among these are epidermolysis bullosa (EB) (1) and severe autosomal recessive ichthyoses such as lamellar ichthyosis (LI) (2, 3). EB comprises a group of blistering skin disorders caused by mutations in a variety of genes responsible for epithelial–mesenchymal and intraepidermal cohesion. These genes include keratins 5 and 14 as well as plectin in the superficial simplex EB subtypes, BPAG2 (type XVII collagen/BP180), laminin 5 chains, α6 and β4 integrins in junctional EB, and type VII collagen in deeper, dystrophic EB (4–6). In more severe subtypes of junctional EB (Herlitz JEB) (7), caused by mutations in any of the α3, β3, and γ2 chains of laminin 5, affected children may die in infancy and early childhood (6). Currently, no corrective therapies are available for these disorders.
In epidermis, basal keratinocytes adherent to an underlying basement membrane zone (BMZ) give rise to progeny that migrate outwards, lose contact with the BMZ, and undergo terminal differentiation to form the stratum corneum. Genetic lesions responsible for junctional epidermolysis bullosa (JEB) affect proteins important in adhesion of basal layer cells to the dermis, including laminin 5, BPAG2, and α6/β4 integrin. Laminin 5 is an important adhesion molecule of epithelial origin (8) that, along with α6/β4 integrin, BPAG2, and plectin, contributes to hemidesmosome attachment structures. Loss of any of these components reduces normal epidermal–dermal cohesion and leads to clinical blister formation, as seen in JEB (9–14) and in targeted gene disruption of homologous genes in mice (15–17). Therefore, localization of key proteins at the BMZ is a cardinal feature of healthy stratified epithelium and is necessary for adhesion of basal keratinocytes to the underlying mesenchymal tissue.
Gene transfer represents a potentially effective molecular therapy for severe monogenic human skin disorders where current therapy is only palliative. Application of specific genetic therapies, however, has been delayed by an inability to achieve effective cutaneous gene delivery (7). Significant among current limitations has been the inability to sustain gene expression in cutaneous epithelium, either because of a lack of gene transfer to long-lived stem cells or to promoter inactivation. Recent gene transfer efforts to epidermis have restored normal gene expression to patient skin regenerated in vivo on immune deficient mice, including transglutaminase 1 (TGase1) in LI (18), steroid sulfatase in X-linked ichthyosis (19), and BPAG2 in nonlethal JEB (20). To date, however, successful in vivo genetic correction of lethal JEB has not been reported. Although initial efforts have achieved disease correction at histologic, molecular, and functional levels, the duration of target gene expression failed to extend beyond the 3- to 4-week period estimated for a complete epidermal turnover cycle (7, 18–20). Recent progress in which marker gene expression has been sustained through repeated cycles of epidermal self-renewal (21, 22) has raised hopes that the formerly transient correction of human genetic skin disease tissue can be extended for clinically meaningful periods.
We have used a modified retroviral vector and optimized gene transfer to sustain laminin 5 β3 expression in human JEB skin through multiple cycles of epidermal growth and renewal. Following β3 gene transfer, JEB cells display morphology and growth kinetics more closely resembling that of normal controls than did JEB cells following GFP gene transfer. LAMB3 expression in JEB cells supports the growth of phenotypically normal skin and normalizes the distribution of BPAG2, α6/β4 integrin, and β1 integrin, as well as the α3, β3, and γ2 chains of laminin 5, suggesting a role for laminin 5 in the distribution and maintenance of proteins at the BMZ in stratified epithelial tissues.
Methods
Cells and Cell Culture.
Primary keratinocytes from previously characterized JEB and LI patients as well as normal controls were obtained by skin biopsies by Stanford Institutional Review Board-approved protocols. Cells were isolated and grown in culture in 50% serum-free medium SFM (GIBCO/BRL) and 50% medium 154 (Cascade Biologics, Portland, OR) as described (23). For BrdUrd labeling, keratinocytes were grown in the presence of 0.5 mg/ml BrdUrd in vitro for 5 days until all cells incorporated this nucleoside, as verified by immunofluorescence staining. The efficiency and distribution in the number of proviral integrants in 24 transduced JEB cell clones was determined by semiquantitative PCR as described (24). PCR primers that hybridize to sequences within the MoMuLV psi region were used to amplify 100 ng total genomic DNA. The reaction conditions were as follows: 56°C annealing temperature, 20 cycles of 45 s duration for each step. The primers used were 5′-CCCGCCCCTTGTAAACTTCCCT-22mer, and 3′-CCCCCGGGGTCGGCAGCCTTCACG-24mer. PCR products were electrophoresed in 1% agarose gel containing EtdBr. The intensity of the resultant product band was compared with a DNA standard curve amplified simultaneously by using genomic DNA from a cell line known to contain six copies of a MoMuLV provirus.
Retroviral Expression Vectors and Gene Transfer.
The full-length LAMB3 (gift of R. Burgeson, Harvard University, Boston; ref. 25) and TGM1 (gift of R. Rice, University of California, Davis, CA) cDNAs (26) were subcloned as a blunt fragment and an EcoRI fragment into the BamHI/NotI and NotI sites, respectively, of the previously described backbone vector SIN-IP (22) to generate the IN-β3 and IN-TGase1 vectors. High titer retrovirus was prepared in human 293T packaging cells as described (27). Primary patient and normal control keratinocytes were transduced with retroviral vectors at a multiplicity of infection of 15 in the presence of 5 μg/ml polybrene during centrifugation at 300 × g for 1 h at 32°C. No drug selection was used. Efficiency of gene transfer by this approach was verified at >99% by immunofluorescence microscopy as described (23).
Animal Studies.
Genetically engineered and untreated patient and control skin was regenerated on severe combined immunodeficient (SCID) mice as described (18); human devitalized dermal substrate was used for LI and porcine dermal substrate was used for JEB. In addition, bovine type I collagen was used as a dermal substrate for JEB regeneration efforts as described (21). Approximately 15% of specimens could not be analyzed because of graft failure. At 4, 8, and 12 weeks postgrafting, human skin tissue was excised and subjected to analysis. Because grafts did not survive in appreciable numbers past 8–12 weeks, this latter time point defines the endpoint of the study.
Analysis of Protein Expression and Tissue Ultrastructure.
Murine monoclonal antibodies to human laminin 5 β3 chain (ref. 28; gift of M. P. Marinkovich, Stanford University, Stanford, CA) were used to verify expression of full-length laminin 5 β3 protein on immunoblot analysis. Approximately 20 μg of media extract protein was loaded per lane. Blots were incubated simultaneously with a polyclonal antibody to MMP2 (Chemicon), an additional internal control for protein concentration and efficiency of transfer. For immunohistochemistry, skin cryosections were fixed and immunostained with the mouse monoclonal and rabbit polyclonal antisera as described (29); antibodies were used to BrdUrd (Becton Dickinson), integrins α6 (Chemicon, MAB1378, NKI-GoH3), β4 (Santa Cruz Biotechnology, sc6628), β1 (Santa Cruz Biotechnology, sc9970), laminin γ2 (Santa Cruz Biotechnology, sc7652), and the 233 antibody against BPAG2 (ref. 30; gift of K. Owaribe, Nagoya University, Nagoya, Japan). Sections were also stained and analyzed simultaneously with a monoclonal antibody specific for human filaggrin (BTI), to verify human tissue origin, by using dual color immunofluorescence. A murine monoclonal antibody to human TGase1 (ref. 26; gift of R. Rice) was used to verify TGase1 protein expression.
Results
IN Vector Gene Transfer to Epithelial Progenitor Cells.
In an effort toward developing molecular therapies for severe inherited genetic disorders such as JEB and LI, we focused on lethal JEB as a model disease. Information on the six unrelated lethal JEB patients in this study is summarized in Table 1. All patients exhibited severe generalized blistering accompanied by loss of laminin 5 β3 expression (as measured by immunofluorescence) due to a variety of mutations, the most common of which was the previously identified R635X hotspot (31). None of the patients survived beyond the first 20 months of life because of complications of the disease.
Table 1.
Clinical data for JEB patients A–F
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| Age | 19 mo. | 3 mo. | 6 mo. | 17 mo. | 4.2 mo. | 3.3 mo. |
| Deceased | Y | Y | Y | Y | Y | Y |
| Race | C | C | C | C | C | C |
| Sex | F | M | M | M | M | M |
| Mutation(s) | R635X-p | R635X-m | R635X | ND | M001I | R635X-m |
| C293S-m | Q243X-p | R635X-p |
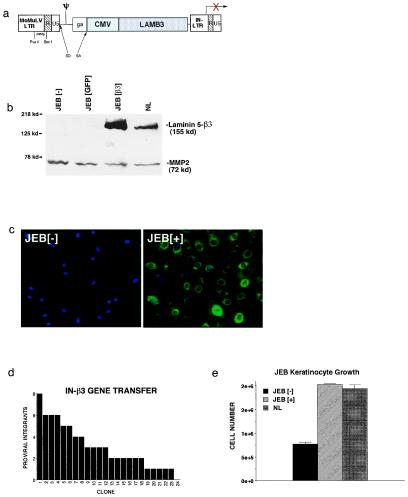
To achieve sustained, corrective gene expression in human epithelial tissue, we generated a β3 expression vector based on a backbone that contains deletions of sequences within the U3 region of the 3′LTR previously implicated in vector inactivity or silencing (22, 32). In the vector backbone, termed IN, expression is driven by an internal cytomegalovirus (CMV) immediate early promoter (IN-β3, Fig. 1a). IN-β3 gene transfer to JEB primary cells restores expression and secretion of full-length β3 protein in cell culture media from undetectable amounts in controls to relatively large quantities in the target cells (Fig. 1b). Gene transfer in vitro was achieved at >99% efficiency in unselected primary cell populations as measured by immunofluorescence staining (Fig. 1c).
Figure 1.
Laminin 5 β3 gene transfer. (a) Retroviral vector (IN-β3) generated for these studies. Expression of the human LAMB3 gene cDNA is driven via the internal cytomegalovirus (CMV) promoter. The IN-β3 vector has deletions of LTR sequences in the U3 region of the 3′ LTR previously implicated in retroviral silencing. (b) β3 protein expression following gene transfer to JEB patient cells. Primary JEB keratinocytes were transduced with either IN-β3 or GFP control vectors. Protein extracts from media were prepared 48 h later and subjected to Western blot analysis with the K140 monoclonal antibody to β3. MMP2 was included as an internal control of protein quality and loading. (c) Immunohistochemistry with β3 antibodies demonstrating efficiency of β3 gene transfer to JEB cells. Note the β3 expression (green) in all JEB cells after gene transfer (JEB[+]) compared with control (JEB[−]). Cell nuclei are counterstained with Hoechst 33342 (blue). (d) Distribution of the number proviral integrants in 24 transduced JEB cell holoclones. JEB cells were cloned at limiting dilution after IN-β3 transduction and 24 holoclones (35) were obtained. The presence and number of proviral genomes is noted for each clone. (e) Growth of JEB cells after β3 gene transfer (JEB[+]) or GFP gene transfer (JEB[−]) in comparison to normal keratinocytes (NL). Cells were plated at identical densities in triplicate and grown on tissue culture plastic for 4 days then counted.
Successful genetic correction in self-renewing tissues, such as stratified epithelia, requires not only that gene transfer to bulk populations be efficient but also that the small subpopulation of long-lived progenitor cells be transduced (7, 21, 33, 34). To test this, transduced JEB cells of high replicative capacity (35) were cloned at limiting dilution, after transduction, and examined by semiquantitative PCR for IN-β3 gene transfer. Of the 24 holoclones isolated, all but one (95.8%) contained the IN-β3 provirus, with an average of 3.2 proviral genomes per cell (Fig. 1d), confirming efficient gene transfer to epithelial progenitor cells. Following β3 or GFP gene transfer, JEB keratinocytes and site-matched normal keratinocytes from three unrelated individuals (NL) were grown on tissue culture plastic in parallel. We found that β3 gene delivery, but not GFP, resulted in the restoration of cellular morphology and short-term growth kinetics to that of normal controls (Fig. 1e and data not shown).
Restoration of Laminin 5 β3 to Patient Skin in Vivo.
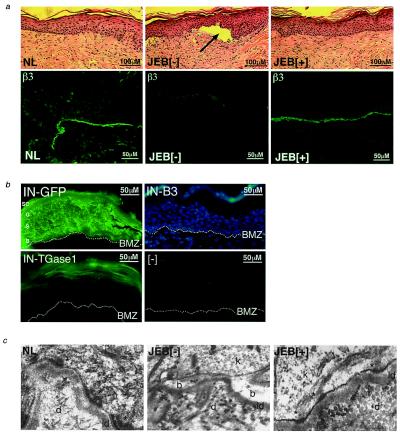
Skin regenerated on SCID mice using IN-β3-transduced JEB cells displays normally localized β3 protein at amounts similar both to skin regenerated from normal keratinocytes and to normal unmanipulated human skin (Fig. 2a and data not shown). This finding is in contrast with the increased quantities of β3 protein produced by IN-β3 transduced cells in culture (Fig. 1b). Normal levels of β3, as well as the other two chains of the laminin 5 heterotrimer, α3 and γ2, are seen in IN-β3-engineered JEB tissue (Figs. 2a and 3c and data not shown). Although these levels appear normal by immunohistochemistry, the precise quantitation of laminin 5 protein in vivo is not possible given the insoluble nature of the complexed protein and the difficulty in extracting it efficiently and consistently from skin (M. P. Marinkovich, personal communication). The apparent normalization of the amount of β3 protein detected in vivo may be due to posttranslational regulation of the subunits of the laminin 5 heterotrimer and to the fact that β3 is not the limiting chain in laminin 5 trimer assembly (36–38). Consistent with the idea that laminin 5 expression and distribution is tightly regulated in vivo is the observation that β3 protein was detected only at the BMZ, although the IN vector backbone directs expression throughout all layers of the epidermis, as demonstrated in IN-GFP-engineered tissue (Fig. 2b).
Figure 2.
Restoration of laminin 5 β3 to JEB skin in vivo. Genetically engineered human JEB skin was excised for examination 4 to 8 weeks after grafting and regeneration on SCID mice. (a) Histology of JEB skin in vivo (Upper)—note the normal histologic appearance with the presence of differentiated cell layers and the blister present in uncorrected skin (arrow in Center). NL, skin graft regenerated from normal keratinocytes; JEB[−], regenerated JEB tissue following GFP gene transfer; JEB[+], regenerated JEB tissue following β3 gene transfer. Restoration of correctly localized β3 protein expression in vivo (Lower). Data are representative of those obtained from tissue regenerated from six patients. (b) IN vector backbone drives gene expression throughout stratified epithelium. Epidermis was regenerated in vivo from JEB[−] keratinocytes transduced with either the IN-GFP or IN-β3 retrovector and fluorescence microscopy performed on unfixed cryosections. Note the GFP expression in all epidermal layers of IN-GFP[+] tissue and the lack of GFP expression in the IN-β3[+] tissue. Dual fluorescence (GFP + Hoechst 33342) staining of IN-β3 transduced tissue is shown to highlight cellular nuclei and the lack of GFP fluorescence. Epidermal layers are labeled: SC, stratum corneum; G, granular layer; S, spinous layer; B, basal layer. When using the same IN-vector backbone to drive expression of TGase-1 in regenerated LI patient skin, TGase-1 is only detectable in the stratum corneum (Lower Left) and absent in the nontransduced LI tissue (Lower Right). (c) Hemidesmosome formation in JEB skin after β3 gene transfer. Transmission electron microscopy was performed on skin regenerated from normal keratinocytes (NL), GFP transduced JEB tissue (JEB[−]), and β3-engineered JEB skin tissue (JEB[+]). Note the ultrastructural evidence of blister formation in uncorrected tissue and the lack of hemidesmosomes in JEB[−] tissue as opposed to the restoration of hemidesmosome structures (denoted by arrows) in JEB[+] tissue; d, dermis; ld, lamina densa; k, keratinocyte; b, blister. (×40,000 magnification.)
Functional laminin 5 trimers are important for hemidesmosome formation and stability (39). Lethal JEB is characterized by an absence of hemidesmosomes with a resultant lack of epithelial–mesenchymal cohesion and associated blistering (6). β3 gene transfer led to correction of ultrastructural blister formation and the reappearance of hemidesmosome structures that were entirely absent in the regenerated β3-negative (β3[−]) tissue (Fig. 2c). These findings indicate that IN-β3-directed gene expression restores appropriately localized β3 protein expression to normal quantities in vivo, and is associated with the correction of ultrastructural features of stratified epithelium.
Sustainability of Corrective Gene Expression.
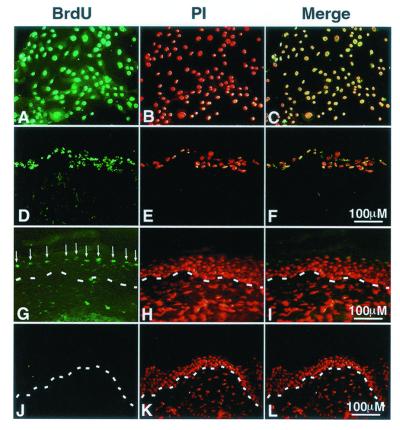
To achieve clinical utility, therapeutic gene expression for the treatment of inherited disease must be sustainable. Self-renewing tissues such as epidermis lose genetically engineered cells within one epidermal turnover cycle unless long-lived progenitors are stably transduced (40). Therefore, we determined the kinetics of epidermal turnover in regenerated tissue by creating grafts from normal keratinocytes labeled with BrdUrd. Loss of BrdUrd-labeled cells was obvious by 3 weeks in vivo, with elimination of all but scattered label-retaining cells complete by 6 weeks (Fig. 4). Our estimate of a 3- to 4-week span for epidermal renewal is consistent with previously reported data derived by other approaches in murine and human epidermis (41).
Figure 4.
Kinetics of BrdUrd turnover in epidermal regeneration. Human keratinocytes were grown in the presence of BrdUrd for 5 days until all cells incorporated this nucleoside. Labeled cells were seeded on dermal substrate in vitro then grafted onto SCID mice in vivo. (A–C) BrdUrd-labeled human keratinocytes in vitro; BrdUrd, immunostaining with FITC labeled antibody to BrdUrd; PI, propidium iodide counterstaining to highlight all of the cells present in the same field; Merge, merged image of identical fields subjected to BrdUrd and PI staining. (D–F) Labeled human keratinocytes on dermal substrate in vitro. (G–I) Skin regenerated from labeled cells at 3 weeks postgrafting. Note the layer of labeled cells in the outer epidermis in G (arrows). (J–L) Skin regenerated from labeled cells at 6 weeks postgrafting. Note the loss of BrdUrd detection by 6 weeks postgrafting, which corresponds to 8 weeks postlabeling. The dashed line in G–L denotes the basement membrane zone.
We examined IN-vector-driven gene expression in regenerated human skin to determine whether it could be sustained through multiple epidermal turnover cycles in vivo. Because immunoreactive human β3 is present on the human dermal substrate used extensively in prior regeneration efforts (data not shown; refs. 18–20), it was not used in the current EB study to avoid artifacts in detection. In place of the human dermis, a porcine dermal substrate was used. Although the porcine dermis has no laminin 5 that is cross-reactive with available antibodies to the human protein, it unfortunately produces less durable grafts that fail after 8 weeks. However, we could detect IN-β3-directed protein expression for the lifespan of the grafts (8 weeks). To extend the duration of our observation period, we also studied IN vector performance in tissue regenerated from patients previously characterized with LI (18, 23, 42). Because dermal cross-reactivity is not a complication with the defective suprabasal TG1 protein in LI epidermis, we conducted these experiments by using the more durable human dermal substrate. IN-vector-driven gene expression was sustained at normal levels throughout the 12 week duration of the experiments (Table 2). These results indicate not only that we had transduced long-lived progenitor cells of the epidermis, but that therapeutic gene expression could be maintained by the IN vector backbone through at least three cycles of epidermal regeneration in vivo.
Table 2.
Summary of durability of IN-vector-delivered gene expression
| Vector | Cell origin | Week analyzed | Therapeutic gene expression | Total no. of mice |
|---|---|---|---|---|
| LTR-LacZ | NL | 4 | Yes | 4 |
| LTR-LacZ | NL | 8 | Yes | 3 |
| LTR-LacZ | NL | 12 | Yes | 3 |
| LTR-LacZ/GFP | JEB(4) LI (2) | 4 | No* | 6 |
| IN β3/TG1 | JEB(8) LI (3) | 4 | Yes | 11 |
| IN β3/TG1 | JEB(2) LI (0) | 6 | Yes | 2 |
| IN β3/TG1 | JEB(2) LI (1) | 8 | Yes | 3 |
| IN β3/TG1 | JEB(0) LI (3) | 12 | Yes | 3 |
Human skin tissue from either JEB or LI patients as well as normal control (NL) was regenerated after gene transfer in vitro with the IN retroviral vector backbone driving expression of laminin 5 β3 (β3) or transglutaminase 1 (TG1), respectively. Xenograft tissue was excised and analyzed at either 4, 6, 8, or 12 weeks after grafting. The total number of mice analyzed at each timepoint is noted with the number of independent xenografts analyzed at each timepoint for each genodermatosis shown in parentheses under the column labeled “Cell origin.” Each entry at each timepoint represents a separately grafted and analyzed mouse. β3 and TGase1 expression was determined by immunohistochemistry as noted in the Methods section. No significant variability in the levels of delivered gene expression were detected; expression was either at levels similar to normal control (Yes) or not detectable (No).
Therapeutic gene expression is defined as expression of β3 protein in JEB tissue or TGase1 protein in LI tissue.
β3 Delivery Restores Normal Distribution of Protein Components of the Epidermal Basement Membrane.
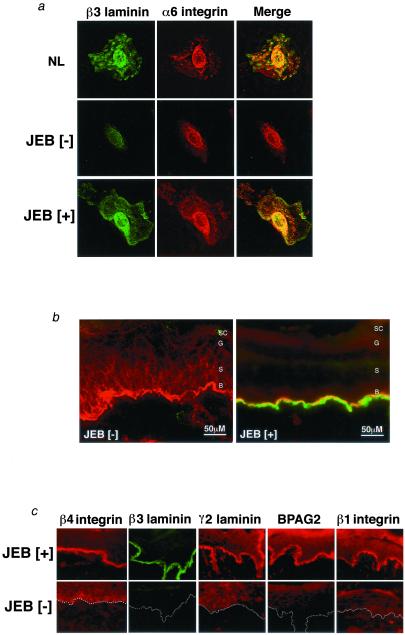
The laminin 5 heterotrimer interacts with a number of important BMZ proteins, including α6/β4 integrin (43) and α3/β1 integrin (44, 45). Because laminin 5 is involved in hemidesmosome formation through binding to the α6/β4 integrin (46), we wished to examine the effects of β3 gene transfer on the distribution of hemidesmosomal components. β3[−] JEB cells exhibit an abnormal, predominantly perinuclear distribution pattern for α6 integrin that is restored to normal after β3 gene transfer (Fig. 3a). In β3[−] JEB tissue regenerated on SCID mice as well as in skin biopsy tissue from JEB patients in vivo, α6 integrin can be detected in all layers of the epidermis (Fig. 3b and data not shown). Following β3 gene transfer, the distribution of α6 integrin is appropriately confined to the BMZ (Fig. 3b). In addition to α6 integrin, other BMZ proteins are also either abnormally localized or present in small quantities in regenerated JEB[−] tissue and patient skin. These include β4 integrin, β1 integrin, BPAG2, and the α3 and γ2 chains of the laminin 5 trimer. However, in tissue regenerated from IN-β3-transduced JEB cells these proteins are restored to their normal distribution patterns (Fig. 3c and data not shown), indicating that β3 expression influences the distribution of a number of adhesion proteins within the tissue.
Figure 3.
Laminin β3 delivery restores polarized distribution of protein components of the epidermal basement membrane. (a) Expression of laminin 5 β3 (green) and α6 integrin (red) was examined in β3-transduced (JEB[+]) and untransduced JEB cells (JEB[−]), as well as normal control (NL) cells by laser confocal immunofluorescence microscopy. Note the diminished presence of α6 integrin with a perinuclear distribution in the JEB[−] cell and the restoration of appropriately distributed expression concomitant with restoration of β3 expression. (b) Expression of laminin 5 β3 (green) and α6 integrin (red) was examined in β3-engineered JEB skin tissue (JEB[+]) and GFP-transduced control (JEB[−]) by laser confocal microscopy. Note the mislocalization of α6 integrin throughout the epidermis in the absence of β3 in JEB[−] tissue and the restoration of strongly polarized distribution to the BMZ with β3 restoration. (c) Localization and expression of basement membrane proteins integrins β4 and α6, laminins β1, β3, and γ2, and BPAG2 were examined as a function of laminin 5 β3 delivery in β3-engineered JEB skin tissue (JEB[+]) and GFP-transduced control (JEB[−]) tissue. Note the decreased and/or mislocalized distribution of all of the BMZ proteins in JEB[−] tissue and the restoration of polarized expression after β3 gene delivery. Data are representative of independently analyzed tissues generated from cells of multiple patients. Dotted lines denote the location of the BMZ in JEB[−] tissue.
Discussion
Our data indicate that expressing β3 from a constitutive viral promoter, which is active throughout all layers of the epidermis, supports appropriately localized laminin 5 β3 protein expression in vivo. Correct localization of β3 is likely due to stabilization and retention of the protein subunit following its association with the limited quantities of the α3 and γ2 chains of laminin 5. Furthermore, although retroviral vector transduced JEB cells express supranormal quantities of β3 protein in culture, within regenerated tissue β3 was detected at amounts indistinguishable from normal. Although this discrepancy is somewhat surprising in the case of laminin 5 protein, similar observations have been made of the delivery of other genes that undergo posttranslational regulation within stratified epithelium, such as TGase1 in suprabasal epidermis (18) and BPAG2 in basal epidermis (20). This result may be accounted for by the fact that β3 is a nonlimiting subunit of a secreted heterotrimeric protein that interacts with multiple ligands. These findings also suggest the existence of homeostatic mechanisms that maintain quantities of key proteins at physiologic levels. Additionally, these mechanisms appear to provide control of localization at the post gene delivery stage rather than requiring complex regulatory elements to be encoded within the vector itself.
Laminin 5 appears to be necessary to achieve the correct localization of important adhesion proteins within regenerated and native epidermis, including integrin β1, integrins α6/β4, and BPAG2. One model for this effect would have laminin 5 serving as a matrix ligand anchor for transmembrane proteins that constrains their distribution by physical association. Consistent with this possibility is the fact that these proteins or their complex partners have been shown to bind directly to laminin 5 (43–45). However, the dynamics of hemidesmosome component assembly are complex and involve multiple interactions (47), making such a model a provisional one.
Clinically useful molecular therapy requires that therapeutic gene expression be sustainable. This is particularly important in self-renewing tissues such as pulmonary, gastrointestinal, and cutaneous epithelia, where delivered gene expression has been observed to cease after one cycle of self-renewal (7, 18–20, 48, 49). Diverse mechanisms may account for this loss in epidermis, including failure to transduce long-lived progenitor cells (21), vector silencing (22), or immune clearing. The relative contributions of each factor may vary depending on the approaches and models used. Although the kinetics of epidermal turnover in mammalian species have been measured by various methods (41), it was important in our studies to analyze this issue in our ex vivo human skin gene transfer model. We found that the vast majority of BrdUrd-labeled cells were lost within 3–4 weeks, thereby corroborating the previously reported estimates for mammalian epidermal turnover. Therefore, because we have observed consistent therapeutic gene expression for a period of 12 weeks in vivo (3–4 turnover cycles), this expression must arise from the progeny of transduced epidermal progenitors. An alternative grafting approach based on bovine type I collagen substrate has been reported to sustain regenerated tissue for time periods longer than 12 weeks. Although we successfully used the bovine collagen technique in this research effort, we were unable to achieve similar graft survival. This was possibly due to the lack of commercial availability of some of the specific reagents used in that study (21).
In these studies, we have used genetically characterized diseased human skin cells and regenerated tissue. This system has the advantage of closely replicating an ex vivo gene transfer strategy that is likely to serve as the basis for the first efforts for genetic therapy of skin disease in humans. Efficient ex vivo transduction of target cells may be of particular value in genetic skin diseases where failure to correct the majority of the cells within the tissue may lead to persistent disease features, such as focal blistering in epidermolysis bullosa, hyperkeratosis and abnormal barrier function in LI, and neoplasia in xeroderma pigmentosum (7). In the treatment of EB, however, the strict requirement for high efficiency may be lessened by the fact that corrected cells are more adherent, have improved growth kinetics, and thus may have a selective advantage in vivo.
Given this, how completely does gene transfer into developmentally mature humans restore cell and tissue features to normal? The data presented here indicate that restoration of LAMB3 gene expression in postnatal JEB cells corrected critical features such as laminin 5 β3 chain expression, hemidesmosome formation, and the correct localization of other BMZ proteins. These findings indicate that healthy skin tissue can be regenerated from β3[−] JEB keratinocytes once they have received retroviral mediated LAMB3 gene transfer. This procedure produced normal cells and tissue from all clinical and histological measurements and, therefore, should have utility in the treatment of JEB and other genetic skin diseases.
Acknowledgments
We thank P. Marinkovich for antibody reagents and advice throughout this effort and L. Taichman and J. Garlick for generous help with the bovine collagen grafting technique and helpful discussions. We thank D. Kohn. M. Kay, G. Nolan, P. Marinkovich, J. McGuire, A. Lane, R. Levy, and E. Bauer for presubmission review; G. S. Herron, G. Meneguzzi, and M. DeLuca for advice; and N. Griffiths and P. Bernstein for expert administrative support. We thank S. Kohler and J. Harvell for help in characterizing patients; I. Glass and D. Murrell for referral of patients; and L. Nall, E. Pfendner, and J. Uitto and the National Epidermolysis Bullosa Registry for assistance with patient records and mutation detection. We thank R. Burgeson for the LAMB3 cDNA, K. Owaribe for 233 antibody, and R. Rice for the TGM1 cDNA. This work was supported by the U.S. Veterans Affairs Office of Research and Development and by National Institutes of Health Grants AR44012, AR45192, and AR43799 (to P.A.K.) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. P.B.R. is a recipient of postdoctoral fellowship support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Abbreviations
- EB
epidermolysis bullosa
- JEB
junctional EB
- SCID
severe combined immunodeficient
- LI
lamellar ichthyosis
- BMZ
basement membrane zone
- TGase1
transglutaminase 1
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Uitto J, Eady R, Fine J D, Feder M, Dart J. J Invest Dermatol. 2000;114:734–737. doi: 10.1046/j.1523-1747.2000.00930.x. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama M. Int J Dermatol. 1998;37:722–728. doi: 10.1046/j.1365-4362.1998.00488.x. [DOI] [PubMed] [Google Scholar]
- 3.Bale S J, Doyle S Z. J Invest Dermatol. 1994;102:49S–50S. doi: 10.1111/1523-1747.ep12388591. [DOI] [PubMed] [Google Scholar]
- 4.Uitto J, Pulkkinen L, McLean W H. Mol Med Today. 1997;3:457–465. doi: 10.1016/s1357-4310(97)01112-x. [DOI] [PubMed] [Google Scholar]
- 5.Marinkovich M P. Dermatol Clin. 1999;17:473–485. doi: 10.1016/s0733-8635(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 6.Marinkovich M P, Herron G S, Khavari P A, Bauer E A. In: Dermatology in General Medicine. 5th Ed. Freedberg I M, Eisen A Z, Wolff K, Austen K A, Goldsmith L A, Katz S I, Fitzpatrick T B, editors. New York: McGraw–Hill; 1999. pp. 690–701. [Google Scholar]
- 7.Khavari P A. J Invest Dermatol. 1998;110:462–467. doi: 10.1038/jid.1998.3. [DOI] [PubMed] [Google Scholar]
- 8.Marinkovich M P, Keene D R, Rimberg C S, Burgeson R E. Dev Dyn. 1993;197:255–267. doi: 10.1002/aja.1001970404. [DOI] [PubMed] [Google Scholar]
- 9.Aberdam D, Galliano M F, Vailly J, Pulkkinen L, Bonifas J, Christiano A M, Tryggvason K, Uitto J, Epstein E H, Jr, Ortonne J P, et al. Nat Genet. 1994;6:299–304. doi: 10.1038/ng0394-299. [DOI] [PubMed] [Google Scholar]
- 10.McGrath J A, Gatalica B, Christiano A M, Li K, Owaribe K, McMillan J R, Eady R A, Uitto J. Nat Genet. 1995;11:83–86. doi: 10.1038/ng0995-83. [DOI] [PubMed] [Google Scholar]
- 11.Pulkkinen L, Christiano A M, Airenne T, Haakana H, Tryggvason K, Uitto J. Nat Genet. 1994;6:293–297. doi: 10.1038/ng0394-293. [DOI] [PubMed] [Google Scholar]
- 12.Pulkkinen L, Kimonis V E, Xu Y, Spanou E N, McLean W H, Uitto J. Hum Mol Genet. 1997;6:669–674. doi: 10.1093/hmg/6.5.669. [DOI] [PubMed] [Google Scholar]
- 13.Ruzzi L, Gagnoux-Palacios L, Pinola M, Belli S, Meneguzzi G, D'Alessio M, Zambruno G. J Clin Invest. 1997;99:2826–2831. doi: 10.1172/JCI119474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal F, Aberdam D, Miquel C, Christiano A M, Pulkkinen L, Uitto J, Ortonne J P, Meneguzzi G. Nat Genet. 1995;10:229–234. doi: 10.1038/ng0695-229. [DOI] [PubMed] [Google Scholar]
- 15.Dowling J, Yu Q C, Fuchs E. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 17.Ryan M C, Lee K, Miyashita Y, Carter W G. J Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choate K A, Medalie D A, Morgan J R, Khavari P A. Nat Med. 1996;2:1263–1267. doi: 10.1038/nm1196-1263. [DOI] [PubMed] [Google Scholar]
- 19.Freiberg R A, Choate K A, Deng H, Alperin E S, Shapiro L J, Khavari P A. Hum Mol Genet. 1997;6:937–933. doi: 10.1093/hmg/6.6.927. [DOI] [PubMed] [Google Scholar]
- 20.Seitz C S, Giudice G J, Balding S D, Marinkovich M P, Khavari P A. Gene Ther. 1999;6:42–47. doi: 10.1038/sj.gt.3300809. [DOI] [PubMed] [Google Scholar]
- 21.Kolodka T M, Garlick J A, Taichman L B. Proc Natl Acad Sci USA. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng H, Lin Q, Khavari P A. Nat Biotechnol. 1997;15:1388–1391. doi: 10.1038/nbt1297-1388. [DOI] [PubMed] [Google Scholar]
- 23.Choate K A, Kinsella T M, Williams M L, Nolan G P, Khavari P A. Hum Gene Ther. 1996;7:2247–2253. doi: 10.1089/hum.1996.7.18-2247. [DOI] [PubMed] [Google Scholar]
- 24.Halene S, Wang L, Cooper R M, Bockstoce D C, Robbins P B, Kohn D B. Blood. 1999;94:3349–3357. [PMC free article] [PubMed] [Google Scholar]
- 25.Gerecke D R, Wagman D W, Champliaud M F, Burgeson R E. J Biol Chem. 1994;269:11073–11080. [PubMed] [Google Scholar]
- 26.Thacher S M, Rice R H, Greenberg C S, Birckbichler P J, Rice R H. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella T M, Nolan G P. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 28.Marinkovich M P, Lunstrum G P, Burgeson R E. J Biol Chem. 1992;267:17900–17906. [PubMed] [Google Scholar]
- 29.Seitz C S, Lin Q, Deng H, Khavari P A. Proc Natl Acad Sci USA. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishizawa Y, Uematsu J, Owaribe K. J Biochem (Tokyo) 1993;113:493–501. doi: 10.1093/oxfordjournals.jbchem.a124072. [DOI] [PubMed] [Google Scholar]
- 31.Kivirikko S, McGrath J A, Pulkkinen L, Uitto J, Christiano A M. Hum Mol Genet. 1996;5:231–237. doi: 10.1093/hmg/5.2.231. [DOI] [PubMed] [Google Scholar]
- 32.Robbins P B, Skelton D C, Yu X J, Halene S, Leonard E H, Kohn D B. Proc Natl Acad Sci USA. 1998;95:10182–10187. doi: 10.1073/pnas.95.17.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy L, Broad S, Zhu A J, Carroll J M, Khazaal I, Peault B, Watt F M. Gene Ther. 1998;5:913–922. doi: 10.1038/sj.gt.3300689. [DOI] [PubMed] [Google Scholar]
- 34.Bickenbach J R, Roop D R. Proc Assoc Am Physicians. 1999;111:184–189. doi: 10.1046/j.1525-1381.1999.99222.x. [DOI] [PubMed] [Google Scholar]
- 35.Barrandon Y, Green H. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsui C, Wang C K, Nelson C F, Bauer E A, Hoeffler W K. J Biol Chem. 1995;270:23496–23503. doi: 10.1074/jbc.270.40.23496. [DOI] [PubMed] [Google Scholar]
- 37.Goldfinger L E, Stack M S, Jones J C. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amano S, Scott I C, Takahara K, Koch M, Gerecke D R, Keene D R, Hudson D L, Nishiyama T, Lee S, Greenspan D S, Burgeson R E. J Biol Chem. 2000;275:22728–22735. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- 39.Baker S E, Hopkinson S B, Fitchmun M, Andreason G L, Frasier F, Plopper G, Quaranta V, Jones J C R. J Cell Sci. 1996;109:2509–2520. doi: 10.1242/jcs.109.10.2509. [DOI] [PubMed] [Google Scholar]
- 40.Ghazizadeh S, Harrington R, Taichman L. Gene Ther. 1999;6:1267–1275. doi: 10.1038/sj.gt.3300956. [DOI] [PubMed] [Google Scholar]
- 41.Dover R, Wright N A. In: Dermatology in General Medicine. 4th Ed. Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austin K F, editors. New York: McGraw-Hill; 1993. pp. 160–171. [Google Scholar]
- 42.Choate K A, Williams M L, Khavari P A. J Invest Dermatol. 1998;110:8–12. doi: 10.1046/j.1523-1747.1998.00070.x. [DOI] [PubMed] [Google Scholar]
- 43.Falk-Marzillier J, Domanico S Z, Pelletier A, Mullen L, Quaranta V. Biochem Biophys Res Commun. 1998;251:49–55. doi: 10.1006/bbrc.1998.9400. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X P, Puzon-McLaughlin W, Irie A, Kovach N, Prokopishyn N L, Laferte S, Takeuchi K, Tsuji T, Takada Y. Biochemistry. 1999;38:14424–14431. doi: 10.1021/bi990323b. [DOI] [PubMed] [Google Scholar]
- 45.Gonzales M, Haan K, Baker S E, Fitchmun M, Todorov I, Weitzman S, Jones J C. Mol Biol Cell. 1999;10:259–270. doi: 10.1091/mbc.10.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones J C, Hopkinson S B, Goldfinger L E. BioEssays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 47.Schaapveld R Q, Borradori L, Geerts D, van Leusden M R, Kuikman I, Nievers M G, Niessen C M, Steenbergen R D, Snijders P J, Sonnenberg A. J Cell Biol. 1998;142:271–284. doi: 10.1083/jcb.142.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson W F. Nature (London) 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 49.Blau H M, Khavari P A. Nat Med. 1997;3:13–14. doi: 10.1038/nm0697-612. [DOI] [PubMed] [Google Scholar]