Abstract
A variety of amino acid substitutions, such as K122I and G145R, have been identified around or within the a determinant of hepatitis B surface antigen (HBsAg), impair HBsAg secretion and antibody binding, and may be responsible for immune escape in patients. In this study, we examined how different substitutions at amino acid positions 122 and 145 of HBsAg influence HBsAg expression, secretion, and recognition by anti-HBs antibodies. The results showed that the hydrophobicity, the presence of the phenyl group, and the charges in the side chain of the amino acid residues at position 145 reduced HBsAg secretion and impaired reactivity with anti-HBs antibodies. Only the substitution K122I at position 122 affected HBsAg secretion and recognition by anti-HBs antibodies. Genetic immunization in mice demonstrated that the priming of anti-HBs antibody response was strongly impaired by the substitutions K122I, G145R, and others, like G145I, G145W, and G145E. Mice preimmunized with wild-type HBsAg (wtHBsAg) or variant HBsAg (vtHBsAg) were challenged by hydrodynamic injection (HI) with a replication-competent hepatitis B virus (HBV) clone. HBsAg persisted in peripheral blood for at least 3 days after HI in mice preimmunized with vtHBsAg but was undetectable in mice preimmunized with wtHBsAg, indicating that vtHBsAgs fail to induce proper immune responses for efficient HBsAg clearance. In conclusion, the biochemical properties of amino acid residues at positions 122 and 145 of HBsAg have a major effect on antigenicity and immunogenicity. In addition, the presence of proper anti-HBs antibodies is indispensable for the neutralization and clearance of HBsAg during HBV infection.
INTRODUCTION
Hepatitis B surface antigens (HBsAgs), the envelope proteins of hepatitis B virus (HBV), are the target for viral neutralization by specific anti-HBs antibodies. The epitopes in HBsAg are found mainly within the region comprising amino acid residues 99 to 169 of HBsAg, which is known as the major hydrophilic region (MHR). The MHR of HBsAg forms several loop structures due to complex folding involving cysteine residues in HBsAg. The core part of MHR is termed the a determinant and harbors a cluster of epitopes targeted by neutralizing anti-HBs antibodies. By analyzing HBV isolates from patients, a variety of amino acid substitutions have been identified within or around the HBsAg a determinant. Such HBV isolates with amino acid substitutions in the HBsAg a determinant often emerge in association with diagnostic failure or the breakthrough of HBV infection in patients with anti-HBs antibodies (1). Therefore, such variant HBsAgs (vtHBsAgs) are of great importance for the diagnosis of HBV infection and vaccine development. vtHBsAgs may have a reduced ability to bind anti-HBs antibodies, thus escaping neutralization (3, 6). Such amino acid substitutions in vtHBsAg could affect HBsAg assembly and secretion, virion formation, and HBV infectivity (14).
Among the large number of amino acid substitutions, the substitutions at positions 122 and 145 are of particular interest. The glycine-to-arginine substitution at position 145 (G145R) is a result of a point mutation (G to A) at the nucleotide position 587 and is the best known mutation in immune escape and diagnostic failure (2, 5, 31). The G145R substitution has been identified in a great number of HBV isolates from a variety of patients, such as vaccinated infants with HBV breakthrough and anti-HBV immunoglobulin-treated individuals after transplantation (2, 5, 9). The G145R mutant has been shown to be replication competent, may persist stably over time, and could be transmitted vertically or horizontally (9, 12, 21, 23). Chimpanzees could be successfully experimentally infected with HBV G145R mutants (20). Other substitutions, such as G145A, G145K, and G145T, were also identified but occurred in rare cases (24). The Arg/Lys residue at position 122 is the determinant for HBsAg serotypes d and y (15). The K122I substitution in HBsAg has been frequently identified in chronically infected patients who tested negative for HBsAg (10, 11, 27). The K122M and K122N substitutions were also found in very few cases. Our previous study demonstrated that vtHBsAg with K122I has a significantly reduced ability to bind anti-HBs antibodies and to induce anti-HBs responses in mice (26, 28). Among all of the HBsAg mutants studied, the K122I mutant has the most severe impairment in reactivity with anti-HBs antibodies in immunoassays.
The number of amino acid substitutions found in HBsAg is large. However, this number is far smaller than the possible combinations of positions in the HBsAg a determinant and the number of different amino acid residues available. In the present study, we tested the hypothesis that only a few selected amino acid substitutions would significantly change the conformation of HBsAg MHR and thereby impair the binding of anti-HBs antibodies without reducing the viability of the virus. We introduced a number of different amino acid substitutions at positions 122 and 145 of HBsAg and studied their influence on HBsAg production, secretion, and recognition by anti-HBs antibodies in immunoassays. Further, the ability of vtHBsAgs to induce anti-HBs antibodies was determined by DNA immunization in mice to assess the effect of different amino acid substitutions on the immunogenicity of vtHBsAg. As a novel approach, we performed hydrodynamic injection (HI) with two different replication-competent HBV clones in mice that had been immunized with vtHBsAg. The clearance of HBsAg from peripheral blood of the mice was then monitored. The results suggested that only a few amino acid substitutions at the positions 122 and 145 indeed significantly reduced the binding of anti-HBs antibodies. However, amino acid substitutions that did not significantly reduce the binding of anti-HBs antibodies may impair the induction of anti-HBs antibodies. Finally, wild-type HBsAg (wtHBsAg) could be detected in peripheral blood and persisted for at least 3 days following hydrodynamic injection despite prior immunization with vtHBsAg, indicating severely impaired neutralization and the clearance of HBsAg despite the preprimed vtHBsAg-specific immune responses.
MATERIALS AND METHODS
Construction of plasmids expressing vtHBsAg.
A total of 24 expression vectors of HBsAg variants with amino acid substitutions at position 122 or 145 were constructed (see Table S1 in the supplemental material). The mutations were introduced into the wtHBsAg sequence (HBV genotype B2, HBsAg subtype adw2) in the backbone vector pHBsAg-WT by PCR-based mutagenesis using the primer pairs SP1/T145 or T122 and SP2/G145X-U or K122X-U (X denotes the amino acid replacing G145 or K122), which are listed in Table S2 in the supplemental material, as described previously (28). The plasmids containing the mutated sequences were subjected to sequence analysis to exclude the presence of unintended mutations. As described previously, HBsAg expressed by these vectors carries an amino-terminal hemagglutinin (HA) tandem tag with the sequence MYPYDVPDYANSPYPYDVPDYA, allowing the detection of wtHBsAg and mutated HBsAg by Western blotting using a monoclonal anti-HA antibody (MAb) (Sigma-Aldrich). The detection of HBsAg by the HA tag is necessary, since the mutated HBsAg may be not properly detected by anti-HBs antibodies and conventional HBsAg immunoassays.
HBV replication-competent plasmids pAAV-HBV-GA1.3 and pAAV-HBV-GB1.3 were used for hydrodynamic injection. pAAV-HBV-GA1.3 was constructed based on plasmid pHBV1.3, which contains a 1.3-fold overlength HBV genotype A2 genome (subtype adw2; GenBank accession no. X02763.1) (16). Plasmid pAAV-HBV-GB1.3 was made similarly with a 1.3-fold overlength HBV genotype B2 genome (subtype adw2; GenBank accession no. AY220698.1).
Transient transfection and detection of HBsAg by Western blotting and ELISA.
The expression of HBsAg in Huh7 cells by transient transfection and detection of HBsAg by Western blotting and enzyme-linked immunosorbent assay (ELISA) were performed as described previously (26, 28, 29). Human hepatoma cell line Huh7 (provided by American Type Culture Collection, Manassas, VA) was used for transient transfection to express recombinant proteins. Transient transfection was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as described previously (19). Four micrograms of plasmids was incubated with 10 μl of Lipofectamine 2000 in 100 μl of Opti-MEM (Gibco BRL, Neu-Isenburg, Germany) for 25 min and was given to cells in 1 ml of Opti-MEM for 6 h at 37°C, 5% CO2. Transfected Huh7 cells were maintained for 48 h at 37°C, 5% CO2. wt- and vtHBsAg presence in cell culture supernatant and cell lysates was detected by the Architect system, an HBsAg CMIA kit (Abbott Laboratories, Wiesbaden-Delkenheim, Germany), and an HBsAg enzyme immunoassay (EIA) kit (Kehua, Shanghai, China) according to the manufacturer's instructions.
For Western blotting, a mouse anti-HA MAb (Sigma-Aldrich) was used as the primary antibody. The levels of HBsAg expression were quantified by densitometry, normalized against beta-actin, and expressed in arbitrary units. The relative expression levels of vtHBsAg in supernatants and cell lysates of transfected cells were calculated as a percentage of the level of wtHBsAg expression. The reactivity of wt- and vtHBsAgs in a commercial HBsAg immune assay kit (Abbott Laboratory) was also tested by following the manufacturer's instructions. In addition, three monoclonal anti-HBs antibodies, A11, S11, and S1, produced against HBV subviral particles (kindly provided by Yan Bin), were tested against vtHBsAgs. These MAbs are not reactive with denatured HBsAg, therefore they may recognize conformational epitopes on HBsAg. The fine specificities of the three MAbs to the epitope on HBsAg are not known.
IF staining of transfected cells.
Immunofluorescence (IF) staining of transfected cells was performed as described previously (28). In brief, transfected cells were fixed with 3.7% paraformaldehyde. The expressed wtHBsAg and other recombinant vtHBsAgs were detected by indirect IF staining using mouse monoclonal anti-HBs antibody S1 and a monoclonal antibody to HA tag (Sigma-Aldrich) as primary antibodies and fluorescein isothiocyanate (FITC)- and rhodamine-labeled rabbit antisera to mouse IgG (Novagen and Sigma-Aldrich) as secondary antibodies, respectively. Cell nuclei were stained with Hoechst 33258 (Beyotime, Shanghai, China). The stained cells were analyzed by System Microscope (Olympus BX53; Japan).
DNA immunization of mice by in vivo electroporation.
Female BALB/cJ(H-2d) mice (6 to 8 weeks of age) were kept under standard pathogen-free conditions in the Central Animal Laboratory of Wuhan Institute of Virology, Chinese Academy of Sciences, and treated by following the guidance of the institutional animal ethical standard. In vivo electroporation was performed according to previously described protocols (28, 29). Mice were immunized by the intramuscular injection of 30 μg of plasmids at a concentration of 1 mg/ml into Musculus tibialis anterior. Two-needle array electrodes (BTX, San Diego, CA) were inserted into the muscle immediately after DNA delivery for electroporation. The array was inserted longitudinally relative to the muscle fibers with a distance of 5 mm between the electrodes. The parameters for in vivo electroporation were set as 20-V/mm distance between the electrodes, 50-ms pulse length, 3 pulses with the reversal of polarity after the 3 pulses, 1 pulse/s, and were controlled by a BTX 830 square wave generator (BTX, San Diego, CA). The immunization procedure was repeated three times at intervals of 2 weeks. The control mice received 30 μl of phosphate-buffered saline (PBS) instead of plasmid DNA.
Serological assays.
Anti-HBs antibodies in sera were detected using a commercial enzyme immunoassay AUSAB kit (Abbott Laboratory) by following the manufacturer's instructions as described previously (28). In addition, anti-HBs antibodies were detected by specific ELISAs. The microtiter plates coated with HBsAg were provided by the commercial diagnostic kit (Kehua, Shanghai, China). Fifty μl of 1:10-diluted mouse sera was added to the wells and incubated for 1 h at 37°C. Bound mouse IgG antibodies were detected with a secondary antibody labeled with horseradish peroxidase (HRP) (Novagen) at a dilution of 1:10,000. The development of color occurred at room temperature and was read at 450 nm. The cutoff was set as 2.1 times above the level of negative controls.
HBsAg was detected with serum samples at a 1:10 dilution by the commercial HBsAg EIA kit (Kehua, Shanghai, China) according to the manufacturer's instructions. The EIA values were read at 450 nm.
HBV challenge by hydrodynamic injection of pAAV/HBV1.3 in mice.
At 3 weeks after the last immunization, mice were challenged by the hydrodynamic injection of pAAV-HBV-GA1.3 or pAAV-HBV-GB1.3 as described previously (13, 29). Ten μg of plasmid in a volume of 0.9% NaCl solution equivalent to 0.1 ml/g of the mouse body weight was injected into the tail veins of mice within 8 s.
Detection of liver HBV core-associated DNA by real-time PCR.
HBV DNA was purified from intracellular core particles in liver tissue as described previously (28). In brief, 60 mg liver tissue was homogenized in 1 ml of ice-cold Tris-EDTA (TE) buffer (pH 8.0) with 0.5% NP-40, and the clarified supernatants obtained after centrifugation were treated with 150 U of DNase I (Sigma-Aldrich) to remove free DNA, followed by digestion with 20 mg/ml proteinase K. Finally, HBV DNA was extracted with phenol-chloroform and subjected to real-time PCR. A SYBR green mix kit (Toyobo) was used for quantitative PCR according to the manufacturer's instructions with the primer pair RC-FW/RC-REV (see Table S2 in the supplemental material). PCR parameters were 7 min at 95°C, then 40 cycles of 10 s at 94°C, 10 s at 57°C, and 10 s at 72°C. Additionally, the specificity of the PCR products was verified by melting curve analysis and agarose gel electrophoresis.
ELISpot assay.
Enzyme-linked immunospot (ELISpot) assay was carried out using the mouse gamma interferon (IFN-γ) precoated ELISpot kit (Dakewe, Shenzhen, China) according to the manufacturer's instructions. Briefly, 96-well flat-bottomed microtiter plates were preincubated with the coating antibody (anti-IFN-γ monoclonal antibody) at 4°C overnight and blocked for 2 h at 37°C. Mouse splenocytes at a density of 2 × 105 cells per well were added to wells in triplicate with 10 μg/ml peptides (HBsAg peptides 29 to 38; IPQSLDSWWTSL for H-2Ld-restricted cytotoxic T lymphocytes [CTLs]) separately and incubated at 37°C, 5% CO2 for 24 h. Five μg/ml of concanavalin A (ConA) (Sigma, St. Louis, MO) was used as a positive control. Thereafter cells were removed. Wells were washed 10 times with PBS containing 0.05% Tween 20 (PBST) and incubated with 100 μl of biotinylated anti-IFN-γ antibody for 1 h. The plates were washed again with PBST and incubated with 50 μl HRP-strepto-avidin solution at 37°C for 1 h. Spot-forming cells were counted and analyzed with an ELISpot plate reader (BioReader 4000; Biosys, Germany). Results were presented as spot-forming cells per 2 × 105 cells.
Statistical analysis.
The statistical analysis was carried out using GraphPad (GraphPad Software, San Diego). Differences in multiple comparisons were determined for statistical significance using Student's t test. P < 0.05 was considered statistically significant. Results are presented as means ± standard deviations.
RESULTS
Expression and secretion of wt- and vtHBsAg in transfected mammalian cells.
Western blot analysis was performed to detect wt- and vtHBsAg in culture supernatants and cell lysates of transiently transfected Huh7 cells using a specific antibody targeting the HA tag (Fig. 1). Two bands corresponding to the glycosylated and unglycosylated forms of HBsAg were detected for wtHBsAg and all vtHBsAgs with amino acid substitutions at the G145 and K122 positions, which is consistent with previous results (22, 26, 28). The amount of vtHBsAg with amino acid substitution G145W was greatly reduced in culture supernatant and apparently increased in the intracellular fraction, indicating that this vtHBsAg was secretion deficient (Fig. 1A). Other wtHBsAgs with amino acid substitutions G145L, G145I, G145P, G145N, and G145R showed a slightly reduced efficiency in exporting HBsAg compared to that of wtHBsAg (Fig. 1A). Among vtHBsAgs with substitutions at position K122, only amino acid substitution K122I led to the secretion deficiency of HBsAg (Fig. 1B), as already described (28). Thus, amino acid substitutions at positions 145 and 122 of HBsAg did not prevent the expression but may change the secretion efficiency of HBsAg.
Fig 1.
Expression of wt- and vtHBsAgs with amino acid substitutions at position 145 or 122. Huh7 cells were transfected with the expression plasmids pHBsAgWT, pHBsAgK122X (including 122G, 122M, 122L, 122I, 122P, 122N, 122Q, 122T, 122D, 122E, 122W, and 122H), and pHBsAgG145X (including 145M, 145L, 145I, 145P, 145N, 145Q, 145T, 145D, 145E, 145W, 145H, and 145R). Culture supernatants and lysates of Huh7 cells were collected at 48 h after transfection. HBsAgs were detected by a monoclonal mouse antibody to the HA tag for the expression analysis. Beta-actin was used as a loading control. CK, cells transfected with empty plasmid as a negative control. The levels of HBsAg expression were quantified by densitometry, normalized against beta-actin, and expressed in arbitrary units. The relative expression levels of vtHBsAg in supernatants and cell lysates of transfected cells were calculated as a percentage of the level of wtHBsAg expression.
Reactivity of wt- and vtHBsAgs in commercial ELISA kits.
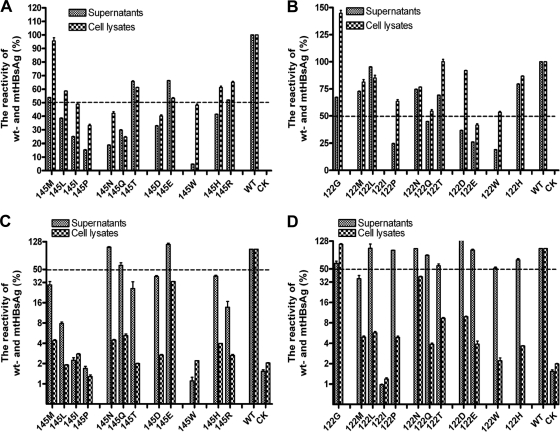
wtHBsAg with an HA tag could be recognized properly in commercial HBsAg immune assays (Fig. 2), and as shown previously, there was no difference from HBsAg without an HA tag (28). The amino acid substitutions G145R and K122I reduced the binding of HBsAg with specific anti-HBs antibodies. Thus, many immunoassays for HBsAg detection failed to recognize such vtHBsAgs (18, 26, 28, 30). We addressed the question of whether other amino acid substitutions at positions 145 and 122 also impair the recognition of vtHBsAg in immunoassays. Although wtHBsAg and vtHBsAgs were expressed at a comparable level inside transfected cells (Fig. 1), the reactivity of vtHBsAgs with amino acid substitutions was different in immunoassays and often reduced to a level of less than 50% of the reactivity of wtHBsAg, as demonstrated by the results of cell lysates (Fig. 2). The majority of amino acid substitutions at position 145, G145L, G145I, G145P, G145T, G145D, G145W, G145H, and G145R, reduced the reactivity of HBsAg to a level of less than 50% of that of wtHBsAg in at least one of the two commercial HBsAg immunoassays (Fig. 2A and C). However, vtHBsAg with amino acid substitution G145W was the only one having markedly lower reactivity value in supernatants than that in cell lysates detected in both commercial HBsAg immunoassays (Fig. 2A and C), suggesting that its low reactivity of supernatants is due to both impaired secretion and recognition by anti-HBs antibodies.
Fig 2.
Reactivity of vtHBsAgs expressed in transient transfection in commercial HBsAg assays corrected against the expression levels in cell lysates. The culture supernatants and cell lysates of transiently transfected cells expressing wt- and vtHBsAgs were collected at 48 h. wt- and vtHBsAgs were detected by the Architect system and HBsAg CMIA kits (Abbott Laboratories, Wiesbaden-Delkenheim, Germany) (A and B) and an HBsAg enzyme immunoassay (EIA) kit (Kehua, Shanghai, China) (C and D) according to the manufacturer's instructions. The reactivity of vtHBsAgs with the amino acid substitutions at position 145 (A and C) or 122 (B and D) was standardized by the relative expression levels of wt- and vtHBsAg based on the Western blotting result (Fig. 1) and is expressed as the percentage of the OD450 of samples compared to that of the wtHBsAg. The average values from 4 replicates were calculated and given as the final reactivity of wt- or vtHBsAgs. CK, cells transfected with empty plasmid as a negative control.
Previously, vtHBsAg with K122I was found to be nonreactive with the majority of HBsAg immunoassays tested (26, 28) (Fig. 2B and D). Although the expression level of vtHBsAg with K122I in cell lysates was about 75% of that of wtHBsAg (Fig. 1B), its reactivity in both commercial HBsAg immunoassays was less than 1% of that of wtHBsAg (Fig. 2B and D). However, other amino acid substitutions at position 122 did not affect the reactivity of HBsAg in at least one of the two commercial HBsAg immunoassays, even the similar amino acid substitution K122L, as demonstrated by the results of cell lysates (Fig. 2B and D). Thus, the amino acid substitution K122I was a unique case that may affect the conformational epitopes of HBsAg for anti-HBs antibodies. Notably, the impaired secretion as well as the reduced recognition by anti-HBs antibodies may contribute to the low reactivity of supernatants in the case of K122I substitution, similarly to the G145W substitution.
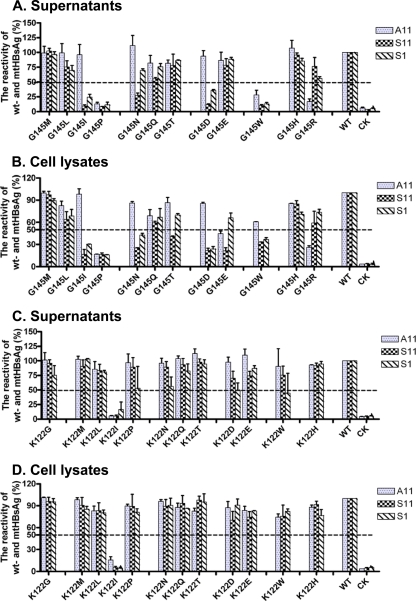
Reactivity of wt- and vtHBsAgs in ELISAs based on other anti-HBs MAbs.
The commercial HBsAg immunoassays use selected anti-HBs antibodies and recognize vtHBsAg with high specificity and sensitivity. Therefore, we further tested the reactivity of vtHBsAgs in ELISAs based on other monoclonal anti-HBs antibodies to judge the effect of the amino acid substitutions at positions G145 and K122 in general. Three monoclonal anti-HBs antibodies, A11, S11, and S1, described in Materials and Methods, were used in this study. Figure 3 shows that the vtHBsAgs with amino acid substitutions at position 145 reacted to the anti-HBs antibodies. The amino acid substitutions of G145I, G145P, and G145W led to a strong reduction of the reactivity of vtHBsAgs in all three ELISAs in either supernatants or cell lysates (Fig. 3A and B). The reactivity of vtHBsAgs with G145N and G145D was normal to A11 but reduced to S11 and S1 (Fig. 3A and B). However, vtHBsAg with G145R could be recognized well by S11 and S1 but not by A11 (Fig. 3A and B). In addition, we tested the reactivity of vtHBsAgs in ELISA based on monoclonal antibodies specifically against vtHBsAg with G145R. Only vtHBsAg with G145R was recognized in this specific ELISA, while wtHBsAg and the other vtHBsAg with the G145 substitution were not reactive (see Fig. S1 in the supplemental material). For the amino acid substitutions at position K122, only vtHBsAg with the K122I substitution showed no reactivity with all monoclonal anti-HBs antibodies (Fig. 3C and D), which is consistent with our previous findings (28). The reactivity of wtHBsAg and other vtHBsAgs with K122 substitutions was comparable in ELISAs with A11, S11, and S1 (Fig. 3C and D).
Fig 3.
Reactivity of vtHBsAg in immunoassays based on three monoclonal anti-HBs antibodies. The culture supernatants (A and C) and cell lysates (B and D) of transiently transfected cells expressing wt- and vtHBsAgs were collected at 48 h. wt- and vtHBsAgs were detected by ELISAs based on three monoclonal anti-HBs antibodies, A11, S11, and S1. The reactivity of vtHBsAgs with the amino acid substitutions at position 145 (A and B) or 122 (C and D) was expressed as the percentage of the OD450 of samples compared to that of the wtHBsAg. The average values from 4 replicates were calculated and given as the final reactivity of wt or vtHBsAgs. CK, cells transfected with empty plasmid as a negative control.
Different subcellular distributions of wt- and vtHBsAg.
The amino acid substitutions in HBsAg may affect the secretion of HBsAg and lead to the accumulation of HBsAg in the endoplasmic reticulum (ER) lumen and Golgi apparatus and the unusual distribution of HBsAg inside cells, which in turn can induce ER stress and contribute to the immunopathogenesis of HBV infection (4, 28). Thus, we examined the subcellular localization of HBsAg by anti-HA antibody and anti-HBs monoclonal antibody S1 (28). Anti-HA antibody stained all HBsAgs expressed in cells while the S1 antibody only recognized HBsAg folded and thereby with the conformational HBsAg a determinant.
The wtHBsAg and all vtHBsAgs, including G145R and K122I, were positively stained by anti-HA antibody (Fig. 4), confirming that wt- and vtHBsAgs were produced at comparable levels in transfected cells (Fig. 1). An even distribution of staining through the cytoplasm of transfected cells was seen for wt- and vtHBsAgs (Fig. 4).
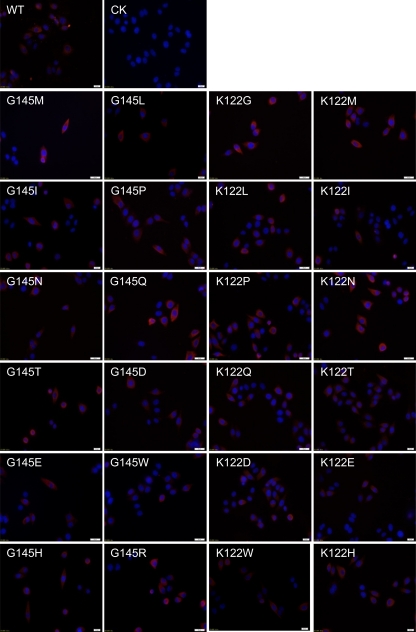
Fig 4.
IF staining of wt- and vtHBsAgs with amino acid substitutions with anti-HA antibody. HeLa cells were transfected with the expression plasmid pHBsAgWT, pHBsAgG145X, or pHBsAgK122X, respectively. Cells were fixed 48 h after transfection and stained with a monoclonal mouse antibody to the HA tag. The nuclei were stained with Hoechst 33258. Magnification, ×400. CK, cells transfected with empty plasmid as a negative control.
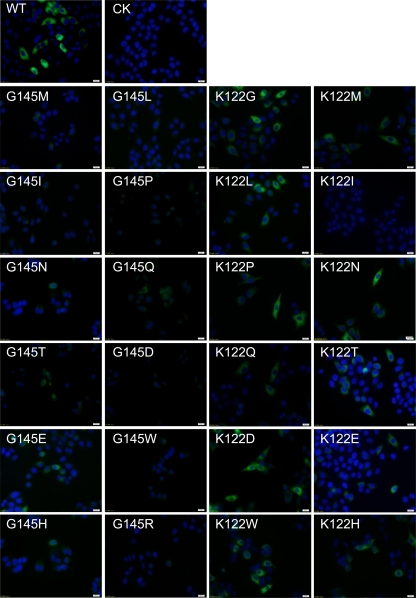
wtHBsAg was stained by anti-HBs antibody S1 and showed a strong and even distribution throughout the cytoplasm of transfected cells, which is consistent with our previous finding (Fig. 5) (28). However, the fluorescence intensity for vtHBsAgs with the amino acid substitutions at position G145 was reduced. In particular, weak staining was observed for vtHBsAgs with G145I, G145P, G145D, G145W, and G145R, suggesting that the binding ability of these vtHBsAgs to anti-HBs antibody S1 was impaired (Fig. 5). Laser confocal scanning microscopy was used to clarify the subcellular localization of vtHBsAgs with reduced fluorescence intensity, and it demonstrated the dot-like distribution of vtHBsAgs with G145I, G145E, and G145W substitutions in cells (see Fig. S2B in the supplemental material). There were some minor differences among HBsAg ELISAs due to the anti-HBs antibodies and the higher concentrations of antibodies used for IF. Consistently with our previous study, vtHBsAg with K122I was not detected by staining with anti-HBs antibody S1 (28). Other vtHBsAgs with amino acid substitutions at position K122 were positively stained like wtHBsAg (Fig. 5).
Fig 5.
IF staining of wt- and vtHBsAgs with amino acid substitutions with anti-HBs monoclonal antibody S1. HeLa cells were transfected with the expression plasmid pHBsAgWT, pHBsAgG145X, or pHBsAgK122X, respectively. Cells were fixed 48 h after transfection and stained with the anti-HBs antibody S1. The nuclei were stained with Hoechst 33258. Magnification, ×400. CK, cells transfected with empty plasmid as a negative control.
Induction of anti-HBs antibodies (against wtHBsAg) by wt-and vtHBsAgs.
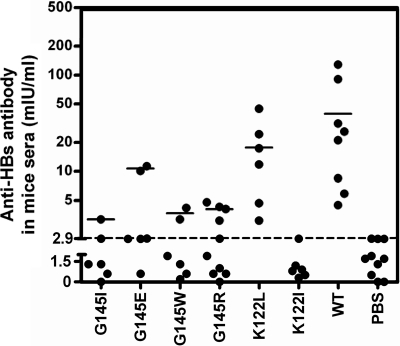
Our previous studies showed that vtHBsAgs with G145R and K122I substitutions had greatly reduced abilities to induce anti-HBs antibody responses (28, 30). This could be one reason that the G145R and K122I substitutions emerged much more frequently than the other possible amino acid substitutions at the two sites in the clinic. Therefore, we asked if the other vtHBsAgs in our studies are able to induce the HBsAg-specific antibody and T-cell responses. DNA immunizations by in vivo electroporation were performed to test the ability of wt- and vtHBsAgs to induce anti-HBs antibody responses in mice at the indicated time points (Fig. 6A). We selected vtHBsAg with G145I, G145E, G145W, G145R, K122L, and K122I substitutions according to their different reactivities in HBsAg immunoassays. Mice were immunized three times with plasmids pHBsAg-WT, pHBsAg-G145I, pHBsAg-G145E, pHBsAg-G145W, pHBsAg-G145R, pHBsAg-K122L, and pHBsAg-K122I. A commercial anti-HBs assay was used to detect the anti-HBs antibody response. Certainly, it is to be noted that the commercial anti-HBs assays are designed to determine antibodies specifically against wtHBsAg and may not recognize antibodies that are reactive only with vtHBsAgs. The result showed that the ability of vtHBsAgs to induce anti-HBs antibodies was well correlated with their reactivity (Fig. 6). Mice immunized with wtHBsAg developed anti-HBs antibodies with a titer of 39 mIU/ml on average (Fig. 6). vtHBsAg with G145E had a reduced reactivity in different immunoassays and had a low ability to induce anti-HBs antibodies (at a titer of 10.7 mIU/ml on average). Mice immunized with the other three vtHBsAgs with G145I, G145W, and G145R showed a strongly impaired antibody response to HBsAg (at a titer below 5.0 mIU/ml on average) (Fig. 6). vtHBsAg with K122L induced anti-HBs antibodies responses in mice with an average titer of 17 mIU/ml, while the group immunized with vtHBsAg with K122I showed no anti-HBs antibody, which is consistent with our previous results (Fig. 6) (28).
Fig 6.
vtHBsAgs with amino acid substitutions at position 145 or 122 had reduced ability to induce anti-HBs antibody response. BALB/c (H-2 Ld) mice were immunized with 30 μg of the expression plasmid pHBsAgWT (n = 8), pHBsAgG145I (G145I) (n = 6), pHBsAgG145E (G145E) (n = 6), pHBsAgG145W (G145W) (n = 6), pHBsAgG145R (G145R) (n = 10), pHBsAgK122L (K122L) (n = 6), pHBsAgK122I (K122I) (n = 6), or with 30 μl of PBS (n = 10) as a control. The immunizations were given three times within 3-week intervals. Sera were collected and tested for anti-HBs antibodies at day 10 after the last immunization. Results are shown as the anti-HBs IgG (mIU/ml) level for each mouse per group. Filled circles represent IgG titers in individual mice. Solid lines represent geometric mean values. The dotted line represents the cutoff, which was assumed to be 2.1-fold the mean value of the negative samples.
The HBsAg expression level in vivo after the injection of naked DNA is low and cannot be detected by Western blotting properly. In a previous publication, we measured HBsAg-specific T-cell responses to judge the expression of HBsAg in vivo (18). It is known that DNA vaccination induces HBsAg-specific T-cell responses in a dose-dependent manner (7). Therefore, we compared the HBsAg-specific T-cell responses induced by different vtHBsAgs and found that the HBsAg-specific T-cell responses in immunized mice were comparable and did not show a significant difference (see Fig. S6 in the supplemental material). These results indicate that vtHBsAgs are able to prime comparable T-cell responses but different antibody responses.
Dynamic of HBsAg and anti-HBs in mice preimmunized with wt- or vtHBsAg.
As vtHBsAg had a reduced ability to induce antibodies cross-reactive with wtHBsAg, it is interesting to examine the impact on secondary challenge with wtHBsAg. The HI method has been used to study HBV clearance after immunizations with different DNA vaccines (29). Here, mice were immunized with wtHBsAg or different vtHBsAgs and then challenged by HI with 10 μg of replication-competent wild-type HBV plasmid pAAV-HBV-GB1.3 at day 14 after the third immunization (Fig. 6A). HBsAg and anti-HBs antibodies in mouse sera were monitored for up to 21 days after HI.
After HI challenge, HBsAg became detectable in sera of all mice of the PBS group from day 1 (see Fig. S5 in the supplemental material). It reached a peak beginning on day 3 and maintained a high level until day 10, and then it declined to an undetectable level at day 14 (Fig. 7A). In contrast, all mice in the group immunized with wtHBsAg remained HBsAg negative for the follow-up period until day 21 (Fig. 7A; also see Fig. S5 in the supplemental material). HBsAg became detectable in some mice of the groups immunized against vtHBsAg on day 1 and reached the peak level on day 3 (Fig. 7A). In particular, 4 of 6 mice immunized against vtHBsAgs with G145E, G145W, G145R, and K122I were strongly positive for HBsAg (see Fig. S5B, C, D, and F in the supplemental material), indicating that vtHBsAgs were not able to prime an efficient protective response to HBV challenge. Nevertheless, HBsAg levels in all mice immunized with HBsAg were below the detection limit from day 5 on (Fig. 7A; also see Fig. S5). Consistently, HBV DNA concentrations in the liver of mice immunized with HBsAg were below the detection limit of quantitative PCR (103 copies/ml) at day 5 (Fig. 7B), while the average load in control mice was 3.4 × 105 copies/ml at day 5 (Fig. 7B), indicating that the preexisting immunity induced by either wtHBsAg or vtHBsAgs leads to an accelerated kinetic of HBV clearance.
Fig 7.
Kinetics of HBsAg and HBV DNA clearance and anti-HBs antibody responses in immunized mice after HI. Hydrodynamic injection with pAAV-HBV-GB1.3 was carried out with immunized mice at day 14 after the last immunization. Sera were collected at 1, 3, 5, 7, 10, 14, 17, and 21 days postinfection, and the anti-HBs antibodies (left y axis) or HBsAgs (right y axis) (A) were detected by ELISA. The HBsAg level was presented as the OD450. The anti-HBs antibody level was presented as the OD450 with a cutoff, which was assumed to be 2.1-fold the mean value of the negative samples. (B) HBV DNA concentrations in mouse sera at 5 days postinfection were determined by real-time PCR. The relative levels of HBV DNA were calculated by the average of HBV DNA concentrations to the average of HBV DNA concentrations of control mice. Control mice developed a level of serum HBV DNA concentrations at 3.4 × 105 copies/ml, which was set as 100%. The hydrodynamic injection with pAAV-HBV GB1.3 was performed with 4 to 7 mice per group as indicated.
The levels of anti-HBs antibodies in mice were determined from day 1 on and up to day 21 after HI challenge. In mice immunized against wtHBsAg, the anti-HBs levels declined slightly at day 3 and then increased steadily to higher levels at day 21, indicating that HI challenge has a boosting effect on the antibody response (Fig. 7A). Similarly, the mice of other groups developed anti-HBs antibodies with different kinetics and became positive for anti-HBs antibodies at day 5. Only the group immunized with vtHBsAg-K122L showed kinetics similar to those of the group immunized against wtHBsAg. The results were consistent with the detection of HBsAg in mouse sera, as the appearance of anti-HBs antibodies coincided with the elimination of HBsAg in sera (Fig. 7A).
Characterization of cellular responses to HBsAg before and after HI.
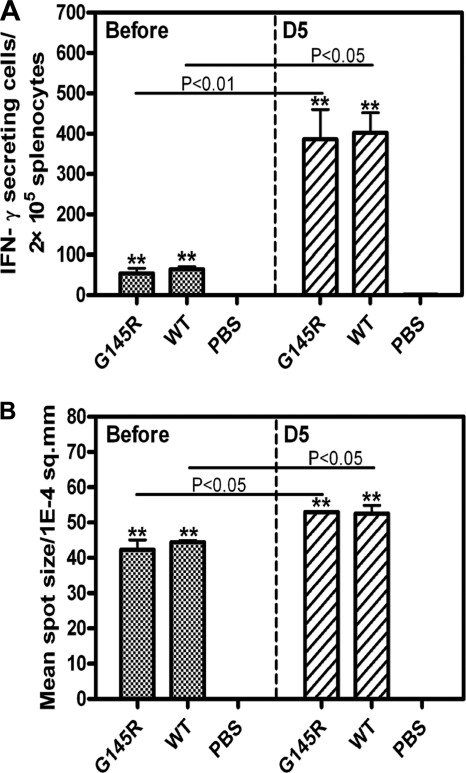
Previously, we have shown that amino acid substitutions in the HBsAg loop did not abolish the ability of HBsAg to prime specific T-cell responses in DNA vaccination (18, 28). However, HBsAg clearance occurred significantly earlier in mice immunized against wtHBsAg, as shown in Fig. 7. Therefore, we addressed the question of whether the specific CTL response to HBsAg by wtHBsAg and vtHBsAgs contributes to the different kinetics of HBsAg clearance. Here, we further addressed the question of whether the G145R substitution could influence the induction of cell-mediated immune responses to HBsAg. The levels of the HBsAg-specific CTL responses to the major H-2Ld-restricted CTL epitope HBsAg amino acids 29 to 38 in mice were assessed by ELISpot assays (28). As shown in Fig. 8A and B, cell-mediated immune responses in mice immunized against wtHBsAg and vtHBsAg with G145R did not differ from each other, with the average frequencies of IFN-γ-producing cells being 64 and 53 in 2 × 105 splenocytes, respectively. The mean spot sizes of HBsAg-specific IFN-γ-producing cells were 42.3 × 10−4 and 44.5 × 10−4/mm2 in mice receiving immunizations against wtHBsAg and vtHBsAg with G145R, respectively. After HI challenge, the numbers and the mean spot sizes of HBsAg-specific T cells increased greatly in all mice of both groups, as measured at day 5 after HI challenge (P < 0.05) (Fig. 8A and B). However, there was no significant difference in either of the numbers or mean spot size of HBsAg-specific IFN-γ-producing cells between the pHBsAg-G145R preimmunized group and wtHBsAg preimmunized group. Thus, G145R substitution in HBsAg did not affect the induction of HBsAg-specific CTLs, which is consistent with previous descriptions (28). In addition, the preexisting immunity may accelerate HBsAg clearance from peripheral blood at day 5 after HBV challenge, coinciding with the rise of HBV-specific antibody and T-cell responses.
Fig 8.
HBsAg-specific T-cell immune responses in immunized mice before or after hydrodynamic injection. The numbers (A) and mean spot sizes (B) of HBsAg-specific IFN-γ-producing cells in 2 × 105 splenocytes from BALB/c mice immunized with pHBsAgWT, pHBsAgG145R (G145R), and PBS are shown. The IFN-γ ELISpot assays were performed at day 1 before hydrodynamic injection or at day 5 after hydrodynamic injection with pAAV-HBV-GB1.3 in the presence of the major H-2Ld-restricted CTL epitope of HBsAg amino acids 29 to 38 (10 μg/ml) for 18 h. Experiments were performed with three mice per group. The data were analyzed by t test, and the differences were statistically significant (**, P < 0.01 compared to the PBS group).
DISCUSSION
Results of the present study demonstrated clearly that only a limited number of amino acid substitutions in HBsAg lead to a significant change of binding of anti-HBs antibodies. At amino acid positions 145 and 122 of HBsAg, the majority of amino acid substitutions did not have a measurable effect on the binding to anti-HBs antibodies in immunoassays. The results are summarized in Table 1. It is obvious that the biochemical properties of amino acid residues at such positions play an important role for the binding of anti-HBs antibodies to HBsAg. The hydrophobicity, the presence of the indolyl group, and the positive charge at position 145 led to reduced HBsAg secretion and impaired reactivity with anti-HBs antibodies, likely resulting in the delayed clearance of HBV (Table 1 and Fig. 6). For example, two vtHBsAgs with G145I and G145P showed a reduced reactivity in HBsAg ELISA with a level no more than 50% of that of wtHBsAg (Fig. 2A and C), although they were expressed at levels in cell lysates corresponding to 80 and 89% of that of wtHBsAg, respectively (Fig. 1A). Thus, G145I and G145P impaired the antibody binding of vtHBsAg. Three amino acid residues with hydrophilic side chains, as well as two amino acid residues with negative charges in the side chain, had only a slight influence on the anti-HBs antibody binding of HBsAg. The well-known G145R substitution, with a positive charge, led to impaired reactivity to anti-HBs antibodies. As far as the G145W substitution was concerned, the presence of the indolyl group in the side chain strongly reduced the antigenicity of HBsAg, as this side chain may cause a significant change in the structure due to its size. Moreover, the strong hydrophobicity due to the presence of the indolyl group may influence the conformation of HBsAg and lead to abnormal assembly and secretion. Thus, the G145W substitution also significantly impaired the secretion of HBsAg. Among the amino acid substitutions at position K122, only the substitution K122I affected HBsAg secretion and recognition by anti-HBs antibodies (Table 1).
Table 1.
Summary of characteristics of vtHBsAgsa
| Group and amino acid change | NTb | SS | Antigenicity | Anti-HBsc | CHd | Molecular size (kDa) | PI | SC |
|---|---|---|---|---|---|---|---|---|
| Glycine | ||||||||
| K122G | GGA | ++++ | ++++ | ND | ND | 75.07 | 5.97 | 4 |
| WT (G145) | GGA | ++++ | ++++ | ++++ | ++++ | 75.07 | 5.97 | 4 |
| HpB | ||||||||
| G145 M | ATG | ++++ | +++ | ND | ND | 149.21 | 5.75 | 1 |
| G145L | TTA | +++ | ++ | ND | ND | 131.17 | 5.98 | 6 |
| G145I | ATA | +++ | + | + | +++ | 131.17 | 6.02 | 3 |
| G145P | CCA | +++ | + | ND | ND | 115.13 | 6.3 | 4 |
| WT (K122) | AAA | ++++ | ++++ | ++++ | ++++ | 146.19 | 9.74 | 2 |
| K122 M | ATG | ++++ | ++++ | ND | ND | 149.21 | 5.75 | 1 |
| K122L | TTA | ++++ | ++++ | +++ | ++++ | 131.17 | 5.98 | 6 |
| K122I | ATA | + | + | + | + | 131.17 | 6.02 | 3 |
| HpL | ||||||||
| G145N | AAC | +++ | +++ | ND | ND | 132.1 | 5.41 | 2 |
| G145Q | CAA | ++++ | +++ | ND | ND | 146.15 | 5.65 | 2 |
| G145T | ACA | ++++ | +++ | ND | ND | 119.12 | 6.53 | 4 |
| K122P | CCA | ++++ | ++++ | ND | ND | 115.13 | 6.3 | 4 |
| K122N | AAT | ++++ | ++++ | ND | ND | 132.1 | 5.41 | 2 |
| K122Q | CAA | ++++ | ++++ | ND | ND | 146.15 | 5.65 | 2 |
| Negative charge | ||||||||
| G145D | GAC | ++++ | +++ | ND | ND | 133.1 | 2.97 | 2 |
| G145E | GAA | ++++ | +++ | ++ | + | 147.13 | 3.22 | 2 |
| K122T | ACA | ++++ | ++++ | ND | ND | 119.12 | 6.53 | 4 |
| K122D | GAT | ++++ | ++++ | ND | ND | 133.1 | 2.97 | 2 |
| K122E | GAA | ++++ | ++++ | ND | ND | 147.13 | 3.22 | 2 |
| Indolyl | ||||||||
| G145W | TGG | + | ++ | + | + | 204.22 | 5.89 | 1 |
| K122W | TGG | +++ | ++++ | ND | ND | 204.22 | 5.89 | 1 |
| Positive charge | ||||||||
| G145H | CAC | ++++ | +++ | ND | ND | 155.16 | 7.59 | 2 |
| G145R | CGA | +++ | +++ | + | ++ | 174.2 | 10.76 | 6 |
| K122H | CAT | ++++ | ++++ | ND | ND | 155.16 | 7.59 | 2 |
HpB, hydrophobicity; HpL, hydrophilicity; NT, nucleotide; SS, secretion of HBsAg; CH, HBsAg clearance; PI, isoelectric point; SC, synonymous codons. ++++, Normal; +++, slightly impaired; +, impaired; ND, not detected.
The nucleotide substitution.
The ability to induce anti-HBs.
The ability to clear wtHBsAg.
The Western blot analysis of HBsAg expressed in transfected cells revealed that the proportion of glycosylated HBsAg generated in cell cultures was less than that in serum-derived HBsAg. It is not known if the reduced glycosylation of HBsAg affects HBsAg assembly and export. Nevertheless, previous studies showed that HBsAg generated in cell cultures was suitable for studies of HBsAg antigenicity.
The presence of vtHBsAgs in clinical samples has been a concern for diagnostic failure for a long time. However, HBsAg immunoassays were improved greatly during recent years. One commercial HBsAg immunoassay uses high-affinity anti-HBs antibodies and therefore failed to detect only the two vtHBsAgs with G145W and K122I (Fig. 2A and B). Another commercial HBsAg immunoassay may miss vtHBsAgs with G145I and G145P substitutions (Fig. 2C). Certainly, wtHBsAg and vtHBsAg samples used in this study were expressed in transient transfection and therefore were at high levels. Some clinical samples may contain vtHBsAgs at lower levels and therefore will not be recognized in HBsAg immunoassays. In addition, the failure of the detection of some HBsAg mutants can be found in recent studies (17). To solve this issue, the development of new kits with MAb pools or polyclonal Abs are urgently needed.
Consistently with these findings, the amino acid substitutions G145R and K122I were found to have a major effect on the induction of anti-HBs antibody response (28, 30). In this study, we compared different vtHBsAgs for their abilities to induce anti-HBs antibody responses. We selected four vtHBsAgs with amino acid substitution at G145 (G145E, G145I, G145W, and the well-known G145R) according to their different reactivities in HBsAg immunoassays. Compared to the immunizations with wtHBsAg, a markedly impaired anti-HBs antibody response was seen not only after immunization with vtHBsAg with G145I and G145W but also for that with G145E. It could be concluded that the priming of the anti-HBs antibody response is more sensitive to impairment by the amino acid substitutions in HBsAg. One of the possible reasons for this observation is that HBsAg was expressed only in a low quantity during DNA vaccination in vivo and therefore may not be effective in the induction of anti-HBs antibodies. In addition, vtHBsAg with G145R has been shown to induce antibodies with changed specificity (30). Thus, vtHBsAg with amino acid substitutions at position G145 may induce antibodies that could not be properly detected by our commercial anti-HBs antibody assay, as only wtHBsAg is used. This issue should be studied in the future if vtHBsAg with different amino acid substitutions induce specific but non-cross-reactive antibodies.
For K122, two vtHBsAgs with the amino acid substitutions K122L and K122I were selected. This is of particular interest because the leucine residue and isoleucine residue differ only slightly by the position of a methyl group in the side chain. However, these two vtHBsAgs also differed greatly in their ability to induce anti-HBs antibody, in correspondence with their reactivity in HBsAg immunoassays. However, the ability of vtHBsAg with K122L to induce anti-HBs antibodies was also reduced compared to that of wtHBsAg.
Until now, there was no suitable in vivo system available to study the clearance of HBsAg and the effect of escape mutations in HBsAg. In this study, we tested HI to study HBsAg clearance in immunized mice. Previously, we used this system to analyze the process of HBV clearance in mice immunized against HBV nucleocapsid (29). Here, we could clearly show that mice immunized with wtHBsAg did not show any detectable HBsAg in peripheral blood after HI challenge, indicating that anti-HBs antibodies present in these mice neutralized and cleared HBsAg immediately. The very interesting point was that the immunization with vtHBsAgs failed to prevent the HBs antigenemia after HI challenge, although HBsAg clearance occurred 3 days later. It is evident that wtHBsAg and vtHBsAgs were able to prime HBsAg-specific T-cell responses. However, the T-cell responses to HBsAg apparently were not sufficient to prevent HBsAg expression during the first 3 days. Moreover, preexisting immunity induced by either wtHBsAg or vtHBsAgs may lead to an accelerated kinetic of HBV clearance, as shown in Fig. 7. These findings emphasize the importance of a proper anti-HBs antibody response for the control of HBV infection. Consistently, mice immunized against HBsAg of genotype B2 (HBsAg subtype adw2) became HBsAg positive after a heterologous challenge with pAAV-HBV-GA1.3 containing 1.3-fold overlength HBV genotype A2 genome (HBsAg subtype adw2) and cleared HBsAg at day 3 (see Fig. S3 in the supplemental material). This result indicated that anti-HBs antibodies are not able to immediately clear heterologous HBsAg. Thus, infection with wild-type HBV isolates of different genotypes or subtypes may occur in patients with a normal anti-HBs antibody response, and these findings have been consolidated by the work of Stramer et al. on vaccine breakthroughs in vaccinated blood donors with heterologous HBV genotypes (25).
The mice immunized with vtHBsAg quickly developed anti-HBs antibodies and cleared HBsAg at day 5. Again, there was a clear inverse correlation between the presence of anti-HBs antibody and HBsAg in wtHBsAg- or vtHBsAg-with-K122L-immunized mice and control mice. Thus, anti-HBs antibody responses could be quickly mounted, presumably due to the helper functions primed by the immunizations with vtHBsAg. Interestingly, the coincidence of low HBsAg (OD450 of <0.5) and low anti-HBs antibody (OD450 < 0.5) were also noted in most mice immunized with vtHBsAg containing G145I, G145W, G145R, or K122I (see Fig. S4 in the supplemental material). The results suggested that mice with a level of anti-HBs antibody above a specific threshold (OD450 of approximately <0.7) will efficiently clear HBsAg in peripheral blood. Only mice with a low anti-HBs antibody titer (OD450 of approximately <0.5) will become HBsAg positive after HI challenge. These results suggest strongly that infection with HBV isolates with mutated HBsAg occurs in patients with a low anti-HBs antibody response (25). The development of antibodies with the correct specificity to mutated HBsAgs and control HBV variants efficiently depends on the presence of specific T-cell responses. Once the T-cell responses are impaired, for example, by immunosuppression, HBV with vtHBsAg may get a chance to persist in patients, since no new antibody to vtHBsAg could be generated.
Taken together, our results indicate that biochemical properties of the side chains of amino acid residues at a given position in HBsAg have a major effect on the conformation of HBsAg, leading to immune escape by limited types of amino acid substitutions. The amino acid substitutions in HBsAg that do not significantly impair the recognition of vtHBsAg in immunoassays may still have an effect on the induction of anti-HBs antibody. Our findings suggest that the presence of anti-HBs antibodies is indispensable for the immediate neutralization and clearance of HBsAg in HBV reinfection.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Bin Yan, Xuefang An, Yuan Zhou, and Xue Hu for excellent technical support. We also thank Wolfram Gerlich and Shuiping Tong for critical reading and helpful suggestions.
This work was supported by the National Basic Research Priorities Program of China (2007CB512901 and 2011CB 106303) and the grants of Deutsche Forschungsgemeinschaft (GRK 1045/2 and TRR60) and the Bundesministerium für Bildung und Forschung (GU0207) to M.L.
Footnotes
Published ahead of print 1 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Carman WF. 1997. The clinical significance of surface antigen variants of hepatitis B virus. J. Viral Hepat. 4(Suppl. 1):11–20 [DOI] [PubMed] [Google Scholar]
- 2. Carman WF, et al. 1990. Vaccine-induced escape mutant of hepatitis B virus. Lancet 336:325–329 [DOI] [PubMed] [Google Scholar]
- 3. Chiou HL, Lee TS, Kuo J, Mau YC, Ho MS. 1997. Altered antigenicity of ‘a’ determinant variants of hepatitis B virus. J. Gen. Virol. 78:2639–2645 [DOI] [PubMed] [Google Scholar]
- 4. Chua PK, et al. 2005. Reduced secretion of virions and hepatitis B virus (HBV) surface antigen of a naturally occurring HBV variant correlates with the accumulation of the small S envelope protein in the endoplasmic reticulum and Golgi apparatus. J. Virol. 79:13483–13496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooreman MP, Leroux-Roels G, Paulij WP. 2001. Vaccine- and hepatitis B immune globulin-induced escape mutations of hepatitis B virus surface antigen. J. Biomed. Sci. 8:237–247 [DOI] [PubMed] [Google Scholar]
- 6. Cooreman MP, van Roosmalen MH, Rte Morsche, et al. 1999. Characterization of the reactivity pattern of murine monoclonal antibodies against wild-type hepatitis B surface antigen to G145R and other naturally occurring “a” loop escape mutations. Hepatology 30:1287–1292 [DOI] [PubMed] [Google Scholar]
- 7. Davis HL, Schirmbeck R, Reimann J, Whalen RG. 1995. DNA-mediated immunization in mice induces a potent MHC class I-restricted cytotoxic T lymphocyte response to the hepatitis B envelope protein. Hum. Gene Ther. 6:1447–1456 [DOI] [PubMed] [Google Scholar]
- 8. El Chaar M, Candotti D, Crowther RA, Allain JP. 2010. Impact of hepatitis B virus surface protein mutations on the diagnosis of occult hepatitis B virus infection. Hepatology 52:1600–1610 [DOI] [PubMed] [Google Scholar]
- 9. Ghany MG, et al. 1998. Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology 27:213–222 [DOI] [PubMed] [Google Scholar]
- 10. Grethe S, Monazahian M, Bohme I, Thomssen R. 1998. Characterization of unusual escape variants of hepatitis B virus isolated from a hepatitis B surface antigen-negative subject. J. Virol. 72:7692–7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hou J, et al. 2001. Prevalence of naturally occurring surface gene variants of hepatitis B virus in nonimmunized surface antigen-negative Chinese carriers. Hepatology 34:1027–1034 [DOI] [PubMed] [Google Scholar]
- 12. Hsu HY, et al. 1997. Surface gene mutants of hepatitis B virus in infants who develop acute or chronic infections despite immunoprophylaxis. Hepatology 26:786–791 [DOI] [PubMed] [Google Scholar]
- 13. Huang LR, Wu HL, Chen PJ, Chen DS. 2006. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. U. S. A. 103:17862–17867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito K, et al. 2010. Impairment of hepatitis B virus virion secretion by single-amino-acid substitutions in the small envelope protein and rescue by a novel glycosylation site. J. Virol. 84:12850–12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kay A, Zoulim F. 2007. Hepatitis B virus genetic variability and evolution. Virus Res. 127:164–176 [DOI] [PubMed] [Google Scholar]
- 16. Lei YC, et al. 2006. Inhibition of hepatitis B and duck hepatitis B virus replication by APOBEC3G. Zhonghua Gan Zang Bing Za Zhi 14:738–741 [PubMed] [Google Scholar]
- 17. Lou SC, et al. 2011. An improved Abbott ARCHITECT assay for the detection of hepatitis B virus surface antigen (HBsAg). J. Clin. Virol. 51:59–63 [DOI] [PubMed] [Google Scholar]
- 18. Lu M, Lorentz T. 2003. De novo infection in a renal transplant recipient caused by novel mutants of hepatitis B virus despite the presence of protective anti-hepatitis B surface antibody. J. Infect. Dis. 187:1323–1326 [DOI] [PubMed] [Google Scholar]
- 19. Lu X, Lee M, Tran T, Block T. 2005. High level expression of apoptosis inhibitor in hepatoma cell line expressing Hepatitis B virus. Int. J. Med. Sci. 2:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogata N, Zanetti AR, Yu M, Miller RH, Purcell RH. 1997. Infectivity and pathogenicity in chimpanzees of a surface gene mutant of hepatitis B virus that emerged in a vaccinated infant. J. Infect. Dis. 175:511–523 [DOI] [PubMed] [Google Scholar]
- 21. Okamoto H, et al. 1992. Mutations within the S gene of hepatitis B virus transmitted from mothers to babies immunized with hepatitis B immune globulin and vaccine. Pediatr. Res. 32:264–268 [DOI] [PubMed] [Google Scholar]
- 22. Peterson DL. 1981. Isolation and characterization of the major protein and glycoprotein of hepatitis B surface antigen. J. Biol. Chem. 256:6975–6983 [PubMed] [Google Scholar]
- 23. Protzer-Knolle U, et al. 1998. Hepatitis B virus with antigenically altered hepatitis B surface antigen is selected by high-dose hepatitis B immune globulin after liver transplantation. Hepatology 27:254–263 [DOI] [PubMed] [Google Scholar]
- 24. Roque-Afonso AM, et al. 2007. Viral and clinical factors associated with surface gene variants among hepatitis B virus carriers. Antivir. Ther. 12:1255–1263 [PubMed] [Google Scholar]
- 25. Stramer SL, et al. 2011. Nucleic acid testing to detect HBV infection in blood donors. N. Engl. J. Med. 364:236–247 [DOI] [PubMed] [Google Scholar]
- 26. Tian Y, et al. 2007. The amino acid residues at positions 120 to 123 are crucial for the antigenicity of hepatitis B surface antigen. J. Clin. Microbiol. 45:2971–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinberger KM, Bauer T, Bohm S, Jilg W. 2000. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J. Gen. Virol. 81:1165–1174 [DOI] [PubMed] [Google Scholar]
- 28. Wu C, et al. 2010. Biological significance of amino acid substitutions in hepatitis B surface antigen (HBsAg) for glycosylation, secretion, antigenicity and immunogenicity of HBsAg and hepatitis B virus replication. J. Gen. Virol. 91:483–492 [DOI] [PubMed] [Google Scholar]
- 29. Yin Y, et al. 2011. DNA immunization with fusion of CTLA-4 to hepatitis B virus (HBV) core protein enhanced Th2 type responses and cleared HBV with an accelerated kinetic. PLoS One 6:e22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng X, et al. 2004. Mutant hepatitis B virus surface antigens (HBsAg) are immunogenic but may have a changed specificity. Virology 329:454–464 [DOI] [PubMed] [Google Scholar]
- 31. Zoulim F, Buti M, Lok AS. 2007. Antiviral-resistant hepatitis B virus: can we prevent this monster from growing? J. Viral Hepat. 14(Suppl. 1):29–36 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.