Abstract
HIV elite controllers (EC) are a rare group of HIV-infected patients who are able to maintain undetectable viral loads during a long period of time in the absence of antiretroviral treatment. Adaptive immunity and host genetic factors, although implicated, do not entirely explain this phenomenon. On the other hand, plasmacytoid dendritic cells (pDCs) are the principal type I interferon (IFN) producers in response to viral infection, and it is unknown whether pDCs are involved in the control of HIV infection in EC. In our study, we analyzed peripheral pDC levels and IFN-α production by peripheral blood mononuclear cells (PBMCs) in EC compared to other groups of HIV-infected patients, the ability of pDCs to reduce HIV production in vitro, and the mechanisms potentially involved. We showed preserved pDC counts and IFN-α production in EC. We also observed a higher capacity of pDCs from EC to reduce HIV production and to induce T cell apoptosis, whereas pDCs from viremic patients barely responded without previous Toll-like receptor 9 (TLR-9) stimulus. The preserved functionality of pDCs from EC to reduce viral production may be one of the mechanisms involved in the control of HIV viremia in these subjects. These results demonstrate the importance of innate immunity in HIV pathogenesis, and an understanding of pDC mechanisms would be helpful for the design of new therapies.
INTRODUCTION
HIV elite controllers (EC) are a rare group of HIV-infected patients who are able to maintain undetectable viral loads during a long period of time in the absence of antiretroviral treatment (ART) (2, 9). Mechanisms responsible for this spontaneous control have been studied with the hope of developing a therapeutic vaccine against HIV.
Preserved T cell functionality has been described for EC (16, 22, 24); however, the innate immunity response needs to be characterized. Plasmacytoid dendritic cells (pDCs) are innate immune cells that respond to viral infections, producing up to 1,000-fold more alpha interferon (IFN-α) than other cell types (31) through the stimulation of Toll-like receptor 7 (TLR-7) and TLR-9. The IFN-α produced by pDCs not only participates in the inhibition of viral replication but also has an adjuvant effect on different immune cells types, like monocytes (20), natural killer cells (14), and T cells (10), providing a link between innate and adaptive immunity. In the viral infection scenario, HIV stimulation of pDCs induces high levels of IFN-α production and the rapid expression of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), transforming them into IFN-producing killer pDCs (iKpDCs) (5). TRAIL was shown previously to be involved in the selective induction of apoptosis of uninfected CD4+ T cells (12). Furthermore, TRAIL-expressing iKpDCs were shown previously to be in close proximity to apoptotic CD4+ T cells in tonsils of HIV-infected viremic patients (30).
In a clinical setting, the number of pDCs has been shown to be preserved in long-term nonprogressor subjects and to be decreased in patients progressing to AIDS (29). Highly active antiretroviral therapy (HAART) induced the recovery of pDC numbers and IFN-α production earlier and at a higher level than CD4+ T cell recovery (27, 28). This increase in the number of pDCs during HAART correlates with a lower virus set point after structure therapy interruption (21).
All this knowledge prompted us to hypothesize whether pDCs could be involved in the spontaneous control of HIV viremia experienced in EC. The aim of this study was to quantify pDC levels and to analyze pDC functionality in relation to the spontaneous control of HIV viremia observed for HIV elite controllers.
MATERIALS AND METHODS
Study subjects.
The study was performed at the Infectious Diseases Services of the Virgen del Rocío University Hospital (Seville, Spain) and the CNRS-Unite Mixte de Recherche UMR-8147 at the Necker Hospital (Paris, France). HIV-infected patients came from the Infectious Diseases Services HIV-infected patient cohort (26) and from the French ANRS CO18 HIV controller cohort. Buffy coat from HIV-1- and hepatitis C virus (HCV)-seronegative blood bank donors was obtained from the Centro Regional de Transfusión Sanguínea de Sevilla-Huelva y Banco de Tejidos (Seville, Spain) and from the Etablissement Français du Sang (Paris, France). In Spain, samples from patients were kindly provided by the HIV BioBank, integrated within the Spanish AIDS Research Network (RIS).
In this study, we included 14 EC, who were defined as HIV-infected subjects with undetectable viral loads (<40 HIV RNA copies/ml) in at least three determinations during the last year in the absence of any antiretroviral therapy. The EC were compared with 13 relative controllers (RC), defined as HIV-infected subjects without any antiretroviral therapy and with viral loads of between 40 and 1,000 HIV RNA copies/ml in at least the last three determinations during the last year. There were also 19 treated subjects (HAART), defined as HIV-infected subjects on HAART and with undetectable viral loads at least during the last year; 25 viremic subjects (VIR), defined as HIV-infected subjects with high viral loads (>10,000 HIV RNA copies/ml) and naive for any antiretroviral therapy; and 22 HIV- and HCV-seronegative donors (HD). All participants gave written informed consent prior to blood sampling. Experimental procedures with human blood have been approved by the Virgen del Rocío, Bicêtre, and Necker Hospital Ethical Committees for human research and were done according to European Union guidelines and the Declaration of Helsinki.
Laboratory measurements.
Absolute CD4 T cell numbers in fresh blood were determined with an Epics XL-MCL flow cytometer (Beckman Coulter). Plasma HIV-1 RNA levels were measured by quantitative PCR (Cobas Ampliprep/Cobas TaqMan HIV-1 test; Roche Molecular Systems) according to the manufacturer's instructions. The detection limit was 40 HIV RNA copies/ml. HCV RNA was detected with a commercially available PCR procedure (Cobas Amplicor; Roche Diagnosis) with a detection limit of 15 IU/ml.
Isolation and culture of blood leukocytes.
In vitro experiments were performed with peripheral blood mononuclear cells (PBMCs) freshly isolated by density gradient centrifugation. Primary CD4+ T cells were negatively isolated (purity of >90%) from whole blood (RosetteSep human CD4+ T cell enrichment cocktail). Fresh pDCs (purity of >90%) were isolated from 450 ml of whole blood after density gradient centrifugation by use of an EasySep Human Plasmacytoid DC enrichment kit (StemCell) according to the manufacturer's instructions. All cells were cultured in RPMI 1640 (Invitrogen) containing 10% fetal bovine serum (HyClone) and 1% penicillin-streptomycin-glutamine (Invitrogen).
IFN-α production by PBMCs.
Freshly isolated PBMCs (1.5 × 106 cells) were cultured in a 48-well plate overnight and stimulated with 1 μM CpG ODN 2216 (Invivogen), a TLR-9 ligand. The amount of IFN-α in the supernatants was assessed by an IFN-α multisubtype enzyme-linked immunosorbent assay (ELISA) kit (PBL Interferon Source) according to the manufacturer's instructions.
Primary CD4+ T cell infections.
Purified CD4+ T cells were stimulated during 3 days with phytohemagglutinin (PHA) (5 μg/ml). CD4+ T cells (106 cells/ml) were infected with HIV-1 BaL, a CCR5-tropic strain, at a multiplicity of infection (MOI) of 0.01 in 6-well plates by spinoculation at 2.5 krpm for 2 h at room temperature (19). After challenge, the cells were washed and cultured during 6 days in 5 ml of culture medium containing interleukin-2 (IL-2) (100 U/ml). Viral replication was measured by quantitative PCR (Cobas Ampliprep/Cobas TaqMan HIV-1 test; Roche Molecular Systems) according to the manufacturer's instructions. Levels of virus production in the supernatant after 6 days of infection ranged from 103 to 106 HIV RNA copies/ml, depending on the donor.
pDC-mediated suppression and apoptosis assays.
Purified pDCs (effectors cells) were incubated overnight with or without 1 μM CpG ODN 2216 (Invivogen). The endosomal acidification inhibitor chloroquine diphosphate salt (CQ) at 1 μM (Sigma-Aldrich) and 10 μg/ml of anti-IFN-α antibody (R&D Systems) were used. In a 96-well plate, 50 × 103 pDCs per well were cocultivated with the chronically HIV-infected H9 T cell line (23, 25) at a 2:1 ratio of effector cells/target cells. After 5 days of coculture, the supernatants were collected to assess p24 (Innogenetic) and IFN-α levels by an ELISA (PBL Interferon Source). To analyze the ability of pDCs to suppress viral production, we calculated the index of suppression in the supernatants [index of suppression = log HIV p24 (T cells) − log HIV p24 (T cells + pDCs)]. Apoptosis determined by annexin V/Topro-III staining and intracellular p24-positive (p24+) cells were measured by flow cytometry with H9 T cells of the coculture. To analyze the antiviral effect of IFN-α, in a different experiment, we cultured HIV-infected H9 T cells alone and in the presence of recombinant IFN-α (R&D Systems); after 1 and 5 days of culture, p24+ H9 T cell percentages were assessed by flow cytometry. In a different experiment, HIV-infected primary autologous CD4+ T cells were used as target cells and cultured in a 96-well plate in the presence of 50 × 103 unstimulated and CpG-stimulated pDCs per well at a ratio 1:2 (effector cells/target cells). After 24 h of coculture, the cells were washed with annexin buffer, and HIV-infected primary autologous CD4+ T cell apoptosis rates were analyzed by annexin V/Topro-III staining.
Flow cytometry.
Freshly isolated PBMCs were incubated for 20 min at 4°C with fluorescein isothiocyanate (FITC)-conjugated anti-BDCA2 (Miltenyi Biotec) and phycoerythrin (PE)-conjugated anti-CD123 (BD Bioscience) antibodies. pDCs were defined as BDCA2+ CD123+. This analysis was performed with a Cytomics FC500 flow cytometer, and data were analyzed by use of CXP software (Beckman Coulter). To measure apoptosis rates, cocultured cells were washed with annexin buffer (BD Bioscience) and incubated for 15 min at 4°C with FITC-conjugated anti-annexin V (BD Bioscience), PE-conjugated anti-CD123 (BD Bioscience), and allophycocyanin (APC)-conjugated anti-Topro-III (Invitrogen) antibodies. For intracellular p24 detection, after extracellular staining with PE-conjugated anti-CD123 antibodies, cells were incubated in permeabilization buffer containing 1% saponin with monoclonal anti-p24 (FITC-KC57; Beckman Coulter) or control isotype antibodies. Annexin V/Topro-III or intracellular p24 was measured in H9 T cells and in HIV-infected autologous CD4+ T cells defined as being CD123 negative. Fluorescence-activated cell sorter (FACS) analysis was performed on a FACS Canto 7 color flow cytometer using FACS Diva software (BD Bioscience). FlowJo software (Treestar, Ashland, OR) was used to analyze flow cytometry data.
Three-dimensional (3D) microscopy.
Purified pDCs from HD, VIR, and EC subjects were plated onto poly-l-lysine-coated slides (Sigma) and then fixed in 4% paraformaldehyde, quenched with 0.1 M glycine. Cells were incubated in permeabilizing buffer containing 1% saponin with mouse anti-TLR-7 (Cliniscience) and Alexa 547-labeled anti-CD4 (BD Bioscience) antibodies. The nucleus was stained by using 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes). Mounted slides were scanned with a Nikon Eclipse 90i upright microscope (Nikon Instruments Europe, Badhoevedorp, Netherlands) using a 100× Plan Apo VC Piezo objective (numerical aperture [NA], 1.4) and Chroma Bloc filters (ET-DAPI, ET-green fluorescent protein [GFP], and ET-Cy3) and were subsequently deconvoluted with a Meinel algorithm and 8 iterations and analyzed by using Metamorph (MDS Analytical Technologies). The TLR-7/DAPI/CD4/overlay/confocal plane was made by using ImageJ software (NIH, Bethesda, MD).
Statistical analysis.
Statistical analyses were performed by using Statistical Package for the Social Sciences software (SPSS 17.0; SPSS Inc., Chicago, IL). The Spearman test was used to analyze the correlation between continuous variables. Differences between groups were analyzed by Mann-Whitney U tests. The Wilcoxon test was used to analyze related samples. All differences with a P value of <0.05 were considered statistically significant.
RESULTS
Quantification of peripheral pDCs and IFN-α production.
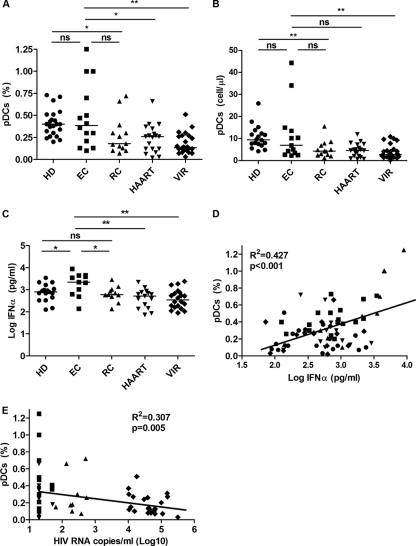
Characteristics of the study subjects are summarized in Table 1. pDC percentages were assessed for 14 EC, 13 RC, 19 HAART, 25 VIR, and 22 HD subjects (Fig. 1A). We observed preserved pDC levels in EC (0.38%) compared to HD (0.4%) subjects and pDC higher levels than those of noncontroller groups (Fig. 1A). These results were similar when absolute pDC number were analyzed (Fig. 1B); the only difference was that EC presented a trend of higher pDC numbers than HAART subjects (6.9 pDCs/μl and 4.5 pDCs/μl, respectively; P = 0.065). Interestingly, when both controller groups were analyzed, we observed a trend of higher pDC percentages in EC than in RC subjects (P = 0.076), and no differences were observed when RC subjects were compared to HAART and VIR subjects (P = 0.788 and P = 0.175, respectively) (Fig. 1A). We also analyzed IFN-α production by PBMCs after TLR-9 stimulation (Fig. 1C). The level of IFN-α production was significantly higher in EC patients than in all other groups, even HD and RC subjects (P = 0.038 and P = 0.030, respectively). The level of production of IFN-α was not statistically different for other groups. As previously described (29), we also observed that peripheral pDC percentages correlated positively with IFN-α production (r = 0.427; P < 0.001) (Fig. 1D) and negatively with viral load (r = −0.307; P = 0.005) (Fig. 1E).
Table 1.
Characteristics of the study subjectsa
| Parameter | Value for group |
||||
|---|---|---|---|---|---|
| HD (n = 22) | EC (n = 14) | RC (n = 13) | HAART (n = 19) | VIR (n = 25) | |
| No. (%) of female subjects | 9 (41) | 8 (57) | 5 (38) | 8 (42) | 10 (40) |
| Median age (yr) (interquartile range) | 40 (35–50) | 45 (40–48) | 42 (33–44) | 46 (40–49) | 38 (30–44) |
| Median time from diagnosis (yr) (interquartile range) | NA | 18 (8–21) | 9 (2–19) | 13 (7–16) | 2 (1–5) |
| No. (%) of naive patients | NA | 7 (50) | 7 (54) | 0 | 25 (100) |
| Median no. of CD4+ cells/μl (interquartile range) | NA | 583 (408–938) | 522 (397–741) | 572 (355–706) | 409 (235–520) |
| No. (%) of HCV+ PCR+ subjectsb | NA | 4 (29) | 6 (46) | 4 (21) | 4 (16) |
| Median no. of HIV RNA copies/ml (interquartile range) | NA | <40 | 201 (130–360) | <40 | 41,600 (15,534–65,000) |
| No. (%) of patients with risk | |||||
| IDU | 7 (50) | 3 (23) | 9 (47) | 2 (8) | |
| Sexual | 5 (36) | 10 (77) | 9 (47) | 15 (60) | |
| Others | 2 (14) | 0 | 1 (5) | 8 (32) | |
NA, not applicable; IDU, injection drug use.
Percentage of subjects with antibodies who were PCR positive for hepatitis C virus.
Fig 1.
Quantification of peripheral pDCs and IFN-α production. (A and B) pDC quantification. Percentages (A) and absolute numbers (B) of pDCs (BDCA2+ CD123+) from 22 healthy donors (HD), 14 elite controllers (EC), 13 relative controllers (RC), 19 HIV-treated patients (HAART), and 25 viremic subjects (VIR) were determined. (C) IFN-α production measured in the supernatants of PBMC cultures, in the presence of a TLR-9 ligand (CpG ODN 2216), from 18 HD, 11 EC, 11 RC, 15 HAART, and 22 VIR subjects. The level of IFN-α production by PBMCs cultured in medium alone was in all the cases below the detection limits (data not shown). (D) Correlation between numbers of peripheral pDCs and HIV RNA copies/ml (log10) in 11 EC, 11 RC, 15 HAART, and 22 VIR subjects. (E) Correlation between numbers of peripheral pDCs and IFN-α produced by CpG-stimulated PBMCs from 18 HD, 11 EC, 11 RC, 15 HAART, and 22 VIR subjects. P values were determined by using a Mann-Whitney U test. ns, not significant (a P value of <0.05 was considered statistically significant); *, P < 0.05; **, P < 0.005.
Preserved pDC-mediated HIV suppression in EC.
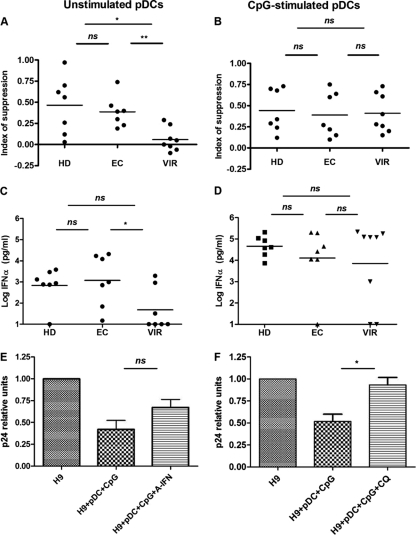
In order to analyze the capability of pDCs to reduce HIV production, we designed a coculture system using pDCs from 7 EC, 8 VIR, and 7 HD subjects as effector cells and HIV-infected H9 T cells (23, 25) as target cells. The EC included in these experiments were all naive for ART and had a time from diagnosis of over 5 years. First, we analyzed unstimulated pDCs (Fig. 2A) cocultured with H9 T cells. Because H9 T cells produce HIV-1 particles, pDCs should be activated during the 5 days of coculture. We observed that pDCs from EC subjects were able to induce levels of viral suppression similar to those of pDCs from HD subjects, while pDCs from VIR subjects were not able to reduce viral production in H9 T cells. Interestingly, when pDCs were previously stimulated with CpG (Fig. 2B), we did not observed any difference among the groups. pDCs from VIR subjects responded as well as pDCs from HD or EC subjects by inducing similar levels of viral suppression.
Fig 2.
pDC-mediated suppression of HIV production in H9 T cells. Purified pDCs from 7 HD, 7 EC, and 8 VIR subjects were plated overnight at 5 × 104 pDCs/well with or without CpG and cocultured in the presence of HIV-infected T cells. After 5 days of coculture, amounts of p24 and IFN-α in the supernatants were measured by an ELISA. (A and B) Effects of unstimulated (A) and CpG-stimulated (B) pDCs on HIV-infected T cell line virus production. The index of suppression by pDCs is expressed as the difference of log HIV p24 production between the culture containing T cells alone and the coculture of T cells and pDCs [index of suppression = log HIV p24 (T cells) − log HIV p24 (T cells + pDCs)]. (C and D) IFN-α production measured in the supernatants of the coculture. (E) Implication of IFN-α in pDC-mediated viral suppression. IFN-α produced by pDCs was partially neutralized by the addition of an anti-IFN-α antibody. Data are representative of 5 different experiments (pDCs from 3 HD and 2 EC subjects). (F) Blocking of endocytosis by the addition of CQ at 1 μM to wells of CpG-stimulated pDCs. Data are representative of 7 different experiments (pDCs from 2 HD, 1 EC, and 4 VIR subjects). P values were determined by using a Mann-Whitney U test. ns, not significant (a P value of <0.05 was considered statistically significant); *, P < 0.05; **, P < 0.005.
These data are in accordance with IFN-α production (Fig. 2C); pDCs from HD and EC subjects produced 100-fold more IFN-α than did pDCs from VIR subjects when pDCs were not previously stimulated. On the other hand, there were no differences in IFN-α production by pDCs among the different groups when pDCs were previously stimulated with TLR-9 (Fig. 2D).
We also observed that anti-IFN-α antibodies partially reverted the reduction of p24 production induced by CpG-stimulated pDCs (Fig. 2E). We also analyzed whether endocytosis was involved in this process. We observed a restoration of p24 levels when chloroquine (CQ) was used in the coculture. The levels of p24 in CQ-treated cultures were similar to those in H9 T cells alone, demonstrating a full recovery of p24 (Fig. 2F). These data show that the antiviral effect of pDCs is at least in part due to IFN-α and that endocytosis is necessary for pDC responses and IFN-α production.
In an attempt to reproduce the results found with the supernatants and to examine the mechanisms involved in viral suppression, we analyzed by flow cytometry p24+ H9 T cell percentages in the coculture and the antiviral effect of IFN-α by adding recombinant IFN-α to H9 T cells (see Fig. S1 in the supplemental material). We used CD123 staining to distinguish between the two cell types (see Fig. S1A in the supplemental material). We observed a reduction in numbers of p24+ H9 T cells when all cells were gated; this effect was abolished when pDCs were treated with CQ (see Fig. S1B in the supplemental material). In contrast, when we gated on live cells, we did not observe any change in p24+ H9 T cell percentages, suggesting that the reduction of viral production was possibly due to the apoptosis of H9 T cells (see Fig. S1C in the supplemental material). In order to analyze the antiviral effect of IFN-α, HIV-infected H9 T cells were cultured with and without recombinant IFN-α. After 1 day of culture with recombinant IFN-α, we observed a reduction of p24+ H9 T cell percentages, as we observed when H9 T cells were cocultured with pDCs. However, this effect disappeared after 5 days, because no additional recombinant IFN-α was added to the culture, showing the transient effect of IFN-α (see Fig. S1D in the supplemental material). These results strongly suggested that IFN-α is responsible for the suppression of p24, probably due to the apoptosis of target cells.
Preserved capability of pDCs from EC to induce HIV-infected T cell apoptosis.
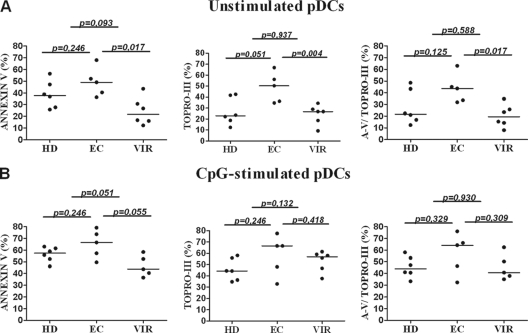
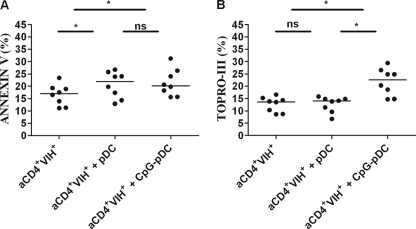
To confirm that pDC-mediated apoptosis was involved in the reduction in p24 levels, we investigated the efficiency of pDCs to induce H9 T cell apoptosis. First, by analyzing cocultures of unstimulated pDCs and H9 T cells (Fig. 3A), we observed that pDCs from EC subjects induced similar annexin V-positive T cell percentages and a tendency to induce higher Topro-III-positive T cell percentages than pDCs from HD subjects. When EC and VIR pDCs were compared, a greater capacity of pDCs from EC to induce T cell apoptosis was observed (Fig. 3A). However, when CpG-stimulated pDCs were analyzed, no differences were observed among the groups; CpG-stimulated pDCs from EC, HD, and VIR subjects induced quite similar levels of H9 T cell apoptosis. It should be noted that there was a tendency for pDCs from EC to induce greater levels of annexin V-positive T cells than those from VIR subjects (Fig. 3B). We also analyzed the ability of pDCs to induce HIV-infected primary autologous CD4+ T cell apoptosis in 8 healthy donors (Fig. 4). Both unstimulated and CpG-stimulated pDCs induced HIV-infected autologous CD4+ T cell apoptosis.
Fig 3.
pDC-mediated H9 T cell apoptosis. Unstimulated (A) and CpG-stimulated (B) pDC-induced apoptosis was assessed on H9 T cells from the cocultures of the suppression assays. The data represent data from 6 HD, 5 EC, and 6 VIR subjects. Left panels represent annexin V (A-V)-positive T cells, middle panels represent Topro-III-positive T cells, and right panels represent annexin V-positive/Topro-III-positive T cells. P values were determined by using a Mann-Whitney U test. A P value of <0.05 was considered statistically significant.
Fig 4.
pDC-mediated HIV-infected primary autologous CD4+ T cell apoptosis. Isolated primary autologous CD4+ T cells from 8 healthy donors were infected for 6 days with HIV BaL. HIV-infected primary autologous CD4+ T cells (aCD4+ HIV+) were cultured in the presence of purified pDCs at a ratio of 1:2. After 24 h, annexin V-positive CD4+ T cells (A) and Topro-III-positive CD4+ T cells (B) were assayed by flow cytometry. Differences were analyzed by using a Wilcoxon test. A P value of <0.05 was considered statistically significant. ns, not statistically significant; *, P < 0.05.
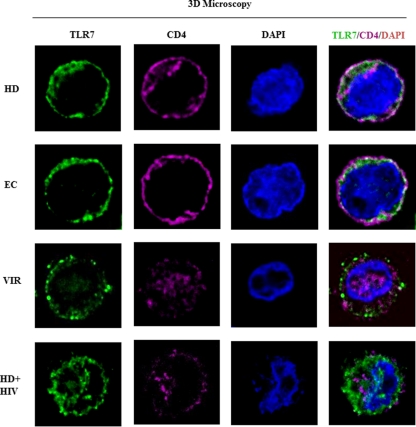
Reduced response of pDCs from VIR subjects is associated with CD4 expression.
According to the results of suppression and apoptosis assays, unstimulated pDCs from VIR subjects showed low degrees of responses to HIV. We also showed by the addition of CQ that the endocytosis pathway is needed for pDC activation. HIV binding to the CD4 expressed by pDCs is essential to activate the endocytosis pathway (7). Thus, we studied CD4 expression on pDCs from HD, EC, and VIR subjects using 3D microscopy (Fig. 5). CD4 was homogeneously expressed on the cell surface of pDCs from EC and HD subjects. In contrast, pDC from VIR subjects showed low levels of membrane CD4. We also observed intracellular CD4 expression in pDCs from VIR subjects, contrasting with pDCs from HD or EC subjects. When pDCs from HD subjects were exposed to HIVMN overnight, we observed the intracellular expression of CD4, as we observed for pDCs from VIR subjects. We also wanted to corroborate these results by flow cytometry; a decrease in CD4 cell surface expression levels in pDCs from VIR compared to HD subjects was observed (see Fig. S2 in the supplemental material). This finding suggests that continuous exposure to HIV in VIR subjects is associated with the internalization of CD4 in pDCs.
Fig 5.
Microscopy analysis of CD4 distribution in pDCs. Shown is three-dimensional microscopy of the CD4 distribution in pDCs. pDCs from HD, EC, and VIR subjects and HIV-activated pDCs from HD (HD+HIV) subjects were stained with anti-CD4 antibody (pink), anti-TLR-7 antibody (green), and DAPI (blue). pDC stainings (green, pink, and blue) were merged and analyzed by 3D microscopy.
DISCUSSION
In the present study, we show the preserved frequency and functionality of pDCs in relation with the capacity to control HIV infection by EC. The decreased peripheral pDC count observed for VIR subjects was described previously (29) and is possibly due to the migration of these cells to lymph nodes (11, 13). We observed that after successful HAART, the loss of pDC numbers in the chronic phase of HIV infection seems not to be restored entirely, similar to data from a previous study (3). We also observed preserved IFN-α production in PBMCs from EC at even higher levels than in PBMCs from HD subjects. Indirectly, we can assume that this IFN-α production is due mainly to pDC stimulation. Indeed, only pDCs and B lymphocytes express TLR-9 (8), and only pDCs express 1,000 times more IFN-α than other cell types, including B lymphocytes (31). This finding was supported by the correlation observed between IFN-α production by PBMCs after TLR-9 stimulation and pDC counts (r = 0.464; P < 0,001). The higher level of IFN-α production by PBMCs through TLR-9 stimulation observed for EC may be due to the preserved pDC levels and not to a better functionality of pDCs in these patients. In fact, when isolated pDCs were stimulated with TLR-9, we observed similar levels of IFN-α production in HD, EC, and VIR subjects (Fig. 2B). However, when we analyzed IFN-α production by purified pDCs after HIV stimulation, we observed comparable amounts of IFN-α between HD and EC subjects and considerably reduced amounts for VIR subjects. These results demonstrate a preserved pDC response to HIV in EC.
On the other hand, when both HIV controller groups were compared, despite the lower difference in viral loads, we showed differences in pDC percentages and IFN-α production (P = 0.076 and P = 0.030, respectively) between them; these data demonstrated how the presence of detectable viral loads, even at lower levels, could affect peripheral pDC levels. Interestingly, when we compared RC and HD subjects, despite the lower pDC levels, PBMCs from RC subjects produced levels of IFN-α similar to those produced by PBMCs from HD subjects. Thus, we can conclude that the preserved pDC numbers are associated with the spontaneous control of HIV viremia.
We demonstrated a preserved capacity of pDCs from EC to suppress viral production in H9 T cells. This result is in agreement with data from a previous study (15), where a higher capacity to suppress HIV replication in autologous CD4+ T cells was found for antiretroviral-naive low-viremic subjects (<12,500 HIV RNA copies/ml) than for treated subjects and high-viremic subjects (≥12,500 HIV RNA copies/ml). However, in our study, we used the same target cells (H9 T cell line) to compare the suppressive faculty of pDCs among the study groups. We demonstrated that pDCs from EC subjects had the same capacity to reduce HIV production as pDCs from HD subjects, whereas pDCs from VIR subjects could scarcely respond. In contrast, when pDCs from all groups were prestimulated with TLR-9, we observed the same behavior for the three groups. These results agree with previous data showing that CpG A- and HIV-stimulated pDCs are not refractory to IFN-α production after restimulation (18). In this work, we show that the magnitude of the response after continuous HIV stimulation is higher in EC than in VIR subjects. This result demonstrates that pDCs from VIR subjects are functional but are not able to be efficiently stimulated by HIV and need to be previously activated by an HIV-independent pathway to display antiviral activity against HIV-infected T cells. This activity was associated with the IFN-α produced by isolated pDCs. These results show that pDCs from EC have preserved functionality to suppress HIV.
The great majority of in vitro studies on pDCs suggested that IFN-α is the principal mechanism of viral suppression (4, 17). A previous report demonstrated that the HIV stimulation of pDCs induces a high level of production of IFN-α and a rapid expression of TRAIL, transforming them into IFN-producing killer pDCs (iKpDCs) (5). It was also shown previously that despite TRAIL expression, pDCs could not induce the lysis of autologous CD4+ T cells (1). However, in the present work, when primary HIV-infected autologous CD4+ T cells were used as target cells, we also observed apoptosis induced by both unstimulated and TLR-9-stimulated pDCs. This discrepancy can be explained because we investigated not lysis but apoptosis using early (annexin V) and late (Topro-III) apoptosis markers, and autologous CD4+ T cells were productively infected and not only exposed to HIV; in addition, cocultures were maintained not for 6 h as in previous work but overnight in the case of autologous CD4+ T cells or for 5 days in the case of H9 T cells. Thus, we demonstrated that besides IFN-α, pDCs exert their antiviral effect by inducing T cell apoptosis. Indeed, when p24+ T cell levels in the coculture were analyzed, we observed a reduction of p24+ T cell percentages. This effect was also observed when recombinant IFN-α was added to H9 T cells. These observations suggest that the pDC-induced viral reduction is due mostly to H9 T cell-induced apoptosis, which is preserved in EC. This finding is in accordance with our results showing that purified pDCs from EC and HD subjects produced large amounts of IFN-α in response to HIV-1 particles produced by the T cell line. In contrast, the level of IFN-α production by pDCs from VIR subjects was very low and barely suppressed or induced T cell apoptosis, confirming the fact that VIR subjects had an impaired HIV-mediated activation of pDCs. Thus, in an attempt to understand why pDCs from VIR subjects were not able to respond to the HIV stimulus, we analyzed CD4 receptor expression on pDCs, which is necessary for HIV binding to pDCs and the subsequent activation of the endocytosis pathway by these cells (6, 7). The very low membrane CD4 expression level on pDCs from VIR subjects could explain the lack of responses when they were cultured in the presence of HIV. However, when pDCs from VIR subjects where stimulated by a CD4-independent activator (CpG), they responded similarly to those from HD or EC subjects. These data also explain the similar behaviors of pDCs among the different groups when a previous stimulation via a CD4-independent pathway, such as the TLR-9 ligand CpG, was performed.
In conclusion, this study shows the qualitative and functional involvement of pDCs in the spontaneous control of HIV viremia. These findings highlight the important role of innate immunity in HIV immunopathogenesis and could have important immunotherapeutic applications.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Redes Telemáticas de Investigación Cooperativa en Salud (RETICS) (2006, Red de SIDA RD06/0006/0021, 2007 to 2010), Consejería de Salud de la Junta de Andalucía (2008, PI-0270), Consejería de Salud de la Junta de Andalucía (2009, PI-0066), Fondo de Investigacion Sanitaria (PS09-00120), and the French National Agency for AIDS Research (ANRS) EP36 V study. E.R.-M. has a grant from Fondo de Investigaciones Sanitarias (CP08/00172). K.M. has a grant from Redes Telemáticas de Investigación Cooperativa en Salud (RETICS) (2006, Red de SIDA RD06/0006/0021, 2007 to 2010). The Biobank of the Spanish AIDS Research Network is supported by Instituto de Salud Carlos III and the Spanish Health Ministry (grant no. RD06/006/0035).
We are grateful to all of the patients who participated in this study; to Manuel Moyano, Maria Teresa Martínez, Angel Ayala, Jesus García, and Juan Manuel Aznar from the Centro Regional de Transfusiones Sanguíneas y Banco de Tejidos Sevilla/Huelva for their support; to Marien Gutierrez, Magdalena Rodriguez, and Paca Cano from the Infectious Diseases Service at HUVR for their clinical support; and to the Biochemistry Department at HUVR for their support. We thank Laurence Meyer, Daniel Séréni, Caroline Lascoux, Olivier Taulera, Jeannine Delgado, David Zucman, Nadège Velazquez, Isabelle Poizot-Martin, and all the other physicians and nurses. The H9 T cell line was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (H9/HTLV-IIIB NIH 1983), from Robert Gallo.
Footnotes
Published ahead of print 8 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Chehimi J, et al. 2010. Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 84:2762–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deeks SG, Walker BD. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406–416 [DOI] [PubMed] [Google Scholar]
- 3. Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. 2004. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J. Clin. Immunol. 24:647–652 [DOI] [PubMed] [Google Scholar]
- 4. Gurney KB, Colantonio AD, Blom B, Spits H, Uittenbogaart CH. 2004. Endogenous IFN-alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J. Immunol. 173:7269–7276 [DOI] [PubMed] [Google Scholar]
- 5. Hardy AW, Graham DR, Shearer GM, Herbeuval JP. 2007. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc. Natl. Acad. Sci. U. S. A. 104:17453–17458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haupt S, et al. 2008. CD4 binding affinity determines human immunodeficiency virus type 1-induced alpha interferon production in plasmacytoid dendritic cells. J. Virol. 82:8900–8905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbeuval JP, Shearer GM. 2006. Are blockers of gp120/CD4 interaction effective inhibitors of HIV-1 immunopathogenesis? AIDS Rev. 8:3–8 [PubMed] [Google Scholar]
- 8. Hornung V, et al. 2002. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537 [DOI] [PubMed] [Google Scholar]
- 9. Lambotte O, et al. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053–1056 [DOI] [PubMed] [Google Scholar]
- 10. Le Bon A, Tough DF. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432–436 [DOI] [PubMed] [Google Scholar]
- 11. Lehmann C, et al. 2010. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS One 5:e11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lichtner M, et al. 2004. HIV type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res. Hum. Retroviruses 20:175–182 [DOI] [PubMed] [Google Scholar]
- 13. Malleret B, et al. 2008. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood 112:4598–4608 [DOI] [PubMed] [Google Scholar]
- 14. Marshall JD, Heeke DS, Abbate C, Yee P, Van Nest G. 2006. Induction of interferon-gamma from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-alpha and tumour necrosis factor-alpha. Immunology 117:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyers JH, et al. 2007. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS One 2:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Migueles SA, et al. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muller-Trutwin M, Hosmalin A. 2005. Role for plasmacytoid dendritic cells in anti-HIV innate immunity. Immunol. Cell Biol. 83:578–583 [DOI] [PubMed] [Google Scholar]
- 18. O'Brien M, et al. 2011. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-α-producing and partially matured phenotype. J. Clin. Invest. 121:1088–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Doherty U, Swiggard WJ, Malim MH. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074–11008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ortaldo JR, et al. 1983. Effects of several species of human leukocyte interferon on cytotoxic activity of NK cells and monocytes. Int. J. Cancer 31:285–289 [DOI] [PubMed] [Google Scholar]
- 21. Pacanowski J, et al. 2004. Early plasmacytoid dendritic cell changes predict plasma HIV load rebound during primary infection. J. Infect. Dis. 190:1889–1892 [DOI] [PubMed] [Google Scholar]
- 22. Pereyra F, et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Popovic M, Sarngadharan MG, Read E, Gallo RC. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497–500 [DOI] [PubMed] [Google Scholar]
- 24. Potter SJ, et al. 2007. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J. Virol. 81:13904–13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ratner L, et al. 1985. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313:277–284 [DOI] [PubMed] [Google Scholar]
- 26. Ruiz-Mateos E, et al. 2010. High levels of CD57+CD28− T-cells, low T-cell proliferation and preferential expansion of terminally differentiated CD4+ T-cells in HIV-elite controllers. Curr. HIV Res. 8:471–481 [DOI] [PubMed] [Google Scholar]
- 27. Schmidt B, Fujimura SH, Martin JN, Levy JA. 2006. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J. Clin. Immunol. 26:55–64 [DOI] [PubMed] [Google Scholar]
- 28. Siegal FP, Fitzgerald-Bocarsly P, Holland BK, Shodell M. 2001. Interferon-alpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS 15:1603–1612 [DOI] [PubMed] [Google Scholar]
- 29. Soumelis V, et al. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98:906–912 [DOI] [PubMed] [Google Scholar]
- 30. Stary G, et al. 2009. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood 114:3854–3863 [DOI] [PubMed] [Google Scholar]
- 31. Swiecki M, Colonna M. 2010. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 234:142–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.