Abstract
Earthworms emit denitrification-derived nitrous oxide and fermentation-derived molecular hydrogen. The present study demonstrated that the earthworm Eudrilus eugeniae, obtained in Brazil, emitted methane. Other worms displayed a lesser or no capacity to emit methane. Gene and transcript analyses of mcrA (encoding the alpha subunit of methyl-CoM reductase) in gut contents of E. eugeniae suggested that Methanosarcinaceae, Methanobacteriaceae, and Methanomicrobiaceae might be associated with this emission.
TEXT
Earthworms can be a dominant soil fauna and can greatly influence the structure and fertility of soils (1, 3, 7). The high microbial diversity in the gut of the earthworm largely reflects the high microbial diversity of the ingested soil (i.e., ingested substrate) (6, 8, 21). The low availability of molecular oxygen (O2) and high availability of saccharides in the gut of the earthworm can stimulate ingested microbes capable of anaerobiosis (6, 13, 14, 32, 33). This stimulation leads to the emission of denitrification-derived nitrous oxide and fermentation-derived molecular hydrogen (H2) during gut passage (15, 20, 25, 27, 32, 33). Previous studies have failed to detect the emission of methane from earthworms, and the methanogenic capacities of gut contents and feces of earthworms appear to be insignificant (6, 16, 19, 30). However, those studies were restricted to a limited number of earthworm species. The objective of the present study was to evaluate the capacity of native and nonnative earthworms in Brazil to emit methane and to assess the potential occurrence of methanogens in gut contents by gene and transcript analyses of mcrA and mrtA (encoding the alpha subunit of methyl-CoM reductase and its isoenzyme, respectively).
Field sites, earthworms, and earthworm substrates.
The seven different earthworm substrates are outlined in Table 1. In March 2011, Amynthas gracilis (Megascolecidae; not native to Brazil [18]) was collected from the organic layer and upper 5 cm of and Pontoscolex corethrurus (Glossoscolecidae; native to Brazil [18]) from a 5- to 30-cm depth of grassland soil within the Esalq campus in Piracicaba, state of São Paulo, Brazil (22°42′22″S, 47°38′02″W), along with their grassland soil (substrate 4). Glossoscolex paulistus (Glossoscolecidae; native to Brazil [18]) was collected from a pasture near the district of Assistência, Rio Claro, state of São Paulo, Brazil (22°30′47″S, 47°36′55″W), along with its soil (substrate 5), and Glossoscolex sp. (Glossoscolecidae) was collected from a neighboring swampy meadow (22°30′36″S, 47°36′41″W), along with its soil (substrate 6). In addition, Rhinodrilus alatus (Glossoscolecidae; native to Brazil [18], collected near Paraopeba, state of Minas Gerais, Brazil) was obtained from local distributors in the district of Assistência along with its soil (substrate 7). Eudrilus eugeniae (Eudrilidae; not native to Brazil [18]) and Perionyx excavatus (Megacsolecidae) were obtained from the earthworm distributor Minhobox along with their substrate (substrate 1), which was commercially composted cow manure. The composting process involves the periodic wetting and daily turning of cow manure under aerated conditions for several weeks prior to introducing earthworms to it. This process removes urine and urea and yields a substrate that is odorless and has the appearance of a rich soil.
Table 1.
Origin of earthworm substrates in Brazil
| Substrate | Type | Earthworm speciesa | Origin | Mo of sampling in 2011 |
|---|---|---|---|---|
| S1 | Composted cow manure | E. eugeniae, P. excavatus | Minhobox | March, September |
| S2 | Processed sugarcane residue | E. eugeniae | Earthworm distributor | September |
| S3 | Processed sugarcane residue | E. eugeniae, E. andrei | Earthworm distributor | September |
| S4 | Grassland soil | A. gracilis, P. corethrurus | Piracicaba, São Paulo, Brazil | March, September |
| S5 | Pasture soil | G. paulistus | Assistência district, São Paulo, Brazil | March |
| S6 | Soil from a swampy meadow | Glossoscolex sp. | Assistência district, São Paulo, Brazil | March |
| S7 | Soil obtained with worms | R. alatus | Paraopeba, Minas Gerais, Brazil | March |
Earthworms were originally obtained on the indicated substrates. See text and Fig. 1 for information on which worms were subjected to different substrate regimens.
In September 2011, E. eugeniae and P. excavatus were obtained from Minhobox along with their substrate (substrate 1) (see above). R. alatus and E. eugeniae were obtained from a private distributor near Boituva, state of São Paulo, Brazil; R. alatus was in diapause (i.e., the alimentary canal was empty) when collected by the distributor and remained in diapause until obtained. E. eugeniae was obtained together with its substrate (substrate 2), which consisted of residues of commercially processed sugarcane that had been stored for several weeks and wetted for several days prior to introducing earthworms to it. Eisenia andrei (Lumbricidae; not native to Brazil [18]) and, again, E. eugeniae were obtained from a distributor in Vinhedo, state of São Paulo, Brazil, along with their substrate (substrate 3), which consisted of residues of commercially processed sugarcane (see above). Substrate 4, the grassland soil, was obtained in the September 2011 sampling as described for the March 2011 sampling. Unless otherwise indicated, all worms were kept on their substrate or their natural soil at 16°C in the dark until use. The general properties of substrates 1 to 4 (see Table S1 in the supplemental material) were determined by the Soil Analysis Laboratory of the University of São Paulo (http://www.solos.esalq.usp.br).
Microcosms and analytical techniques.
Earthworms were washed in sterile water, dried with tissue paper, weighed, and placed into sterile gas-tight 120-ml serum vials. Emission of methane by living earthworms (single individuals or, in the case of E. andrei, two individuals) and earthworm substrates (10 g) were assessed under (i) an air atmosphere or (ii) an air atmosphere with 1.5% H2 and 0.4% CO2 at room temperature (approximately 25°C) in the dark.
Earthworms were put on substrates different from their original substrates for 60 h. Ingestion of the new substrate was verified by determining that newly produced casts displayed the same color as that of the new substrate.
Gut contents of E. eugeniae raised on substrate 1 were carefully squeezed out of washed earthworms and homogenized while being subjected to 100% argon to minimize exposure of gut contents to O2. Gut content (0.35 g) was placed into gas-tight serum vials that were previously and subsequently flushed with 100% argon. Vials were supplemented with (i) 0.5 ml sterile anoxic water, (ii) 0.5 ml sterile anoxic water with 1.5% H2 and 0.4% CO2 in the headspace, or (iii) 1.5% H2 and 0.4% of CO2 with 0.5 ml of an anoxic solution of bromoethanesulfonate (BES; a metabolic inhibitor of methanogenesis (10), yielding a final concentration of 30 mM BES. Incubation was at room temperature (approximately 25°C) in the dark. Methane was determined by gas chromatography (22).
mcrA phylogenetic analyses.
Substrate 1 and gut content of E. eugeniae raised on substrate 1 (both from the September 2011 sampling) were put in RNAlater RNA stabilization reagent (Qiagen, Hilden, Germany) to stabilize nucleic acids for subsequent analyses. After three washing steps with RNase-free 1× phosphate-buffered saline (PBS) buffer (centrifugation at 10,000 × g for 15 min), DNA and RNA were coextracted from pellets by bead-beating lysis, organic solvent extraction, and precipitation (9). Reverse transcription of RNA (DNA was removed with DNase I [Fermentas, St. Leon-Rot, Germany] according to the manufacturer's protocol) into cDNA was performed with SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol but for 120 min instead of 60 min at 50°C. Analyses of mcrA and mrtA, including the determination of operational taxonomic units (OTUs), were as previously described (17). DNA and cDNA were amplified with the following primer sets: mcrAf (5′-TAYGAYCARATHTGGYT-3′) and mcrAr (5′-ACRTTCATNGCRTARTT-3′) for mcrA (29). Phylogenic trees were calculated with neighbor-joining (Dayhoff correction) (26), maximum-likelihood (Jukes-Cantor or Dayhoff correction), and maximum-parsimony methods. Trees used a 100% similarity filter and 131 valid amino acid positions between positions 98 and 227 of mcrA of Methanocella paludicola SANAE.
qPCR.
The quantification of mcrA and mrtA genes in gut content of E. eugeniae raised on substrate 1 and of substrate 1 was performed with an iQ5 quantitative PCR (qPCR) cycler (Bio-Rad, Germany). Extracted DNA was diluted 200-fold to minimize potential PCR inhibition, and 5 μl of the diluted DNA was used as the template in a 20-μl reaction mixture containing 1-fold SensiMix, 3 mM MgCl2 (Bioline, Germany), BSA (0.75 μg/μl), 1,250 nM (each) mcrAf and mcrAr primers (29), and sterilized deionized water. Initial denaturation was at 95°C for 8 min, followed by 45 cycles of denaturation at 95°C for 45 s, annealing at 62°C for 45 s, and elongation at 72°C for 45 s, when the fluorescence signal was recorded. The final PCR elongation step was at 72°C for 5 min. Melting curve analyses were performed from 55°C to 95°C with increments of 0.5°C per cycle. Agarose gel electrophoreses of the qPCR products displayed single bands of the expected size. Gene copy numbers were calculated according to the standard curve and were corrected for potential PCR inhibition (35). Values are representative of triplicate analyses.
Emission of methane by earthworms and earthworm substrates.
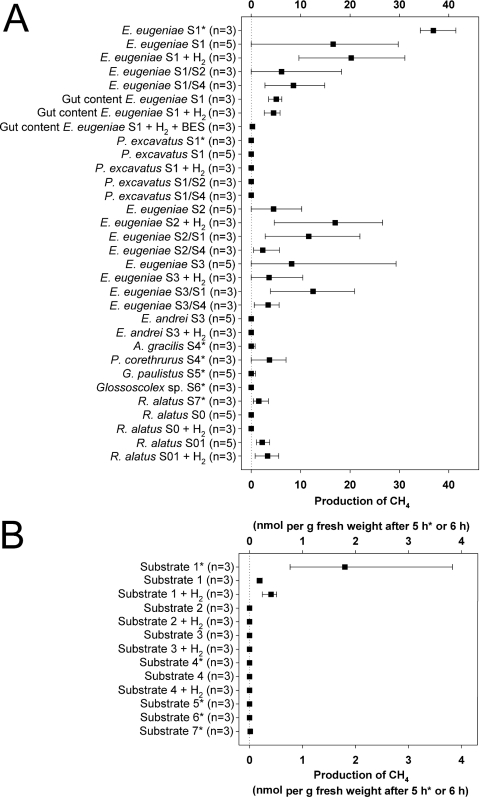
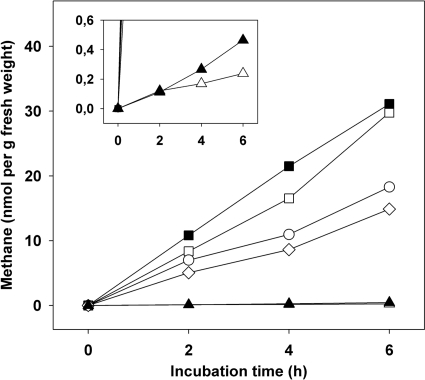
E. eugeniae displayed the highest propensity to emit methane of all earthworm species evaluated (Fig. 1A). E. eugeniae emitted various amounts of methane when raised on substrate 1, 2, or 3 and yielded up to 41 and 30 nmol of methane per g fresh weight after 5 and 6 h of incubation, respectively, when raised on substrate 1 and up to 29 nmol of methane per g fresh weight after 6 h of incubation when raised on substrate 3 (Fig. 1A). The emission of methane was relatively linear (Fig. 2). Emission rates observed with E. eugeniae raised on substrate 1 approximated 5 nmol of methane g (fresh weight)−1 h−1 (Fig. 1A and 2). Rates for the emissions of nitrous oxide and H2 by various earthworms approximated 1 and 6 nmol g (fresh weight)−1 h−1, respectively (6, 32). Numerous other invertebrates have been observed to emit methane, and the emission of methane by E. eugeniae was approximately 1 order of magnitude less than that reported for certain species of cockroaches and termites and similar to that reported for millipedes (11, 30).
Fig 1.
Emission of methane. (A) Living earthworms and gut content; (B) substrates. Results marked with an asterisk are from the sampling in March 2011 and a 5-h incubation. Results not marked with an asterisk are from the sampling in September 2011 and a 6-h incubation. Filled squares indicate mean values, and lines indicate lowest and highest values. Codes: S, substrate; first number after S, substrate on which worms were raised and maintained (e.g., S1 is substrate 1); second S and accompanying number, the substrate to which worms were transferred and maintained for 60 h prior to assay (e.g., S1/S2 indicates that worms raised on substrate 1 were transferred to and maintained on substrate 2). H2 indicates that the headspace contained 1.5% H2 and 0.4% CO2; BES indicates that assays were supplemented with BES; S0 indicates that worms were received in diapause without gut content; S01 indicates that worms were received in diapause without gut content and incubated on substrate 1 for 60 h.
Fig 2.
Emission of methane by representative specimens of E. eugeniae and substrate 1. Symbols: squares, E. eugeniae raised and maintained on substrate 1; circles, E. eugeniae raised on substrate 1 and transferred to substrate 2 for 60 h prior to assay; diamonds, E. eugeniae raised on substrate 1 and transferred to substrate 4 for 60 h prior to assay; triangles, substrate 1; empty symbols, headspace was air; filled symbols, headspace was air supplemented with 1.5% H2 and 0.4% CO2.
Although most specimens of E. eugeniae emitted methane, some did not. Such variability also occurs for the emission of nitrous oxide by earthworms (25). Gut contents of E. eugeniae raised on substrate 1 produced methane when incubated under anoxic conditions, and the production of methane by gut contents was inhibited by BES (Fig. 1A). The reduced rates at which methane was produced by gut contents in comparison to living earthworms may have been due to the temporary exposure of gut contents to O2 during the preparation of gut contents, which was performed under not strictly anoxic conditions.
Supplemental H2 did not stimulate the in vivo production of methane by E. eugeniae raised on substrate 1 or gut contents of E. eugeniae under anoxic conditions (Fig. 1A and 2), suggesting (i) that hydrogenotrophic methanogenesis was not the primary source of methane or (ii) that methanogenesis was either substrate saturated or impaired such that supplemental H2 did not augment methane production. In contrast, the emission of methane by E. eugeniae raised on substrate 2 appeared to be slightly stimulated by H2 (Fig. 1A).
E. andrei and P. excavatus did not emit methane, although E. eugeniae raised on the same substrates (i.e., substrate 1 for P. excavatus and substrate 3 for E. andrei) did (Fig. 1A). P. corethrurus and R. alatus obtained in March 2011 emitted small amounts of methane. R. alatus obtained in September 2011 had an empty alimentary canal and did not emit methane; however, specimens placed on substrate 1 for 60 h emitted small amounts of methane. Supplemental H2 did not significantly enhance the minimal capacity of R. alatus to emit methane. A. gracilis, G. paulistus, and Glossoscolex sp. did not emit methane (Fig. 1).
Under the aerated conditions used to assess the in vivo emission of methane by earthworms, substrate 1 yielded very small amounts of methane (approximately 90- and 20-fold less than the average capacity of E. eugeniae determined on the basis of fresh weight in grams in August and March 2011, respectively); all the other substrates displayed no capacity to emit methane under these conditions (Fig. 1B and 2). This finding suggested that the methanogenic capacity of substrate 1 might be associated with the in vivo capacity of E. eugeniae to emit methane. However, most specimens of E. eugeniae raised on substrates that did not yield methane (i.e., substrates 2 and 3) nonetheless emitted methane (Fig. 1A). Furthermore, P. excavatus and E. andrei, which were maintained on the same substrates as E. eugeniae (i.e., substrates 1 and 3), did not emit methane. In addition, E. eugeniae raised on substrate 1 had a reduced capacity to emit methane when maintained for 60 h on an alternative substrate (i.e., substrate 2 or 4) that displayed no capacity to emit methane (Fig. 1A).
Identification of methanogenic taxa in gut contents of E. eugeniae.
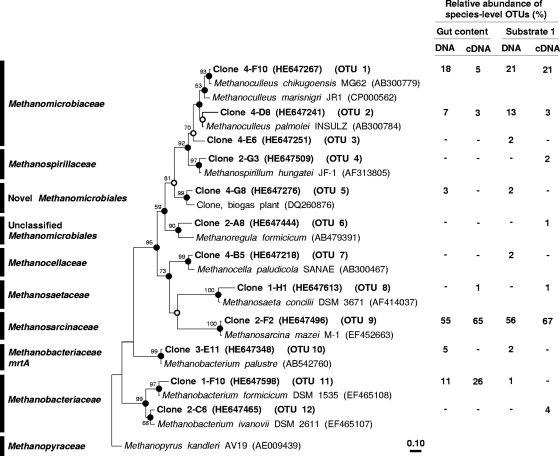
Gut contents of E. eugeniae raised on substrate 1 were evaluated for the presence of mcrA and mrtA to resolve methanogenic taxa potentially associated with the emission of methane. A total of 94 gene sequences (including 5 mrtA sequences) and 94 transcript sequences were obtained from gut contents of E. eugeniae (Fig. 3; see also Fig. S1 in the supplemental material). A total of 87 gene sequences (including 2 mrtA sequences) and 92 transcript sequences were obtained from substrate 1. The coverage of each of the four gene libraries was greater than 97% at the species level. A total of 12 species-level OTUs were detected (Fig. 3).
Fig 3.
Phylogenic neighbor-joining tree of representative species-level amino acid sequences encoded by mcrA or mrtA retrieved from E. eugeniae (substrate 1) and of reference sequences. Values next to the branches represent the percentages of replicate trees (>50%) in which the associated taxa clustered together in the bootstrap test (10,000 bootstraps). Dots at nodes indicate the confirmation of the tree topology by all maximum-likelihood and maximum-parsimony calculations with the same data set. Empty circles indicate the confirmation of the tree topology by 3 of 4 calculations. Sequences in the tree are mcrA sequences, if not otherwise indicated. The bar indicates a 0.1 estimated change per amino acid.
Detected mcrA and mrtA sequences were affiliated with Methanosarcinaceae, Methanomicrobiaceae, Methanobacteriaceae, Methanocellaceae, and a novel Methanomicrobiales (Fig. 3). Methanosarcinaceae and Methanomicrobiaceae were the main mcrA-affiliated taxa of species detected in both gut contents and substrate; these two taxa accounted for approximately 56% and 30%, respectively, of the analyzed sequences. Detected Methanobacteriaceae-affiliated mcrA and mrtA sequences had a substantially higher relative abundance in gut contents than in substrate 1. Methanocellaceae-affiliated mcrA sequences were detected only in substrate 1.
McrA transcripts detected in E. eugeniae gut contents were mainly affiliated with Methanosarcinaceae, Methanobacteriaceae, and Methanomicrobiaceae (Fig. 3). Methanosarcinaceae-affiliated transcripts were similarly abundant in gut contents of E. eugeniae and in substrate 1. Methanomicrobiaceae-affiliated transcripts were more abundant in substrate 1 than in gut contents. In contrast, Methanobacteriaceae-affiliated transcripts were more abundant in gut contents than in substrate 1. Methanosaetaceae-, Methanospirillaceae-, and Methanoregula formicicum-affiliated sequences had very low relative abundances and were detected only at the transcript level.
McrA OTU 5 was the most novel phylotype detected. This phylotype shares 72% to 84% similarity with its next closest cultured relatives, Methanosphaerula palustris (NCBI accession no. EU296536; 83% to 84% mcrA sequence similarity), Methanoculleus palmolei (AB300784; 79% to 84% mcrA sequence similarity), and M. formicicum (AB479391; 72% to 77% mcrA sequence similarity).
Gene copy numbers.
On the basis of level per gram of fresh weight, the combined numbers of mcrA and mrtA genes detected in substrate 1 and in gut contents of E. eugeniae raised on substrate 1 approximated (4.17 ± 0.00) × 104 and (2.64 ± 0.02) × 105, respectively, which was approximately a 6-fold-higher number for gut contents. On the basis of DNAs per microgram, the combined number of mcrA and mrtA genes detected in substrate 1 and in gut contents of E. eugeniae raised on substrate 1 approximated (6.69 ± 0.00) × 103 and (3.50 ± 0.03) × 104, respectively, which was approximately a 5-fold-higher number for gut contents.
Conclusions and future perspectives.
The capacity of earthworms to emit nitrous oxide and H2 appears to be due to ingested denitrifiers and ingested fermenters, respectively, rather than endogenous gut microbiota (13–15, 19, 25, 27, 33, 34). The present study demonstrated that certain earthworms, in particular, E. eugeniae, can emit methane, and the considerations discussed above with respect to denitrifiers and fermenters suggest that methane emission was not linked to endogenous methanogens but rather to the stimulation of ingested methanogens. Indeed, most of the methanogenic species detected in gut contents of E. eugeniae were phylogenetically similar to those detected in the substrate on which E. eugeniae was maintained. The differences between the taxa of detected methanogenic species of gut contents and the taxa of detected methanogenic species of substrate 1 suggested that ingested methanogens might not have responded uniformly to the in situ conditions of the gut during gut passage. Similar observations have been reported for ingested nitrate-reducing bacteria (5). The finding of higher numbers of detected mcrA genes in the earthworm gut of E. eugeniae compared to its substrate (i.e., substrate 1) is also indicative of an activation of ingested methanogens.
Maximal in vivo emission of methane occurred with E. eugeniae raised on a substrate (i.e., substrate 1) rich in organic material (see Table S1 in the supplemental material) that was derived from composted cow manure, a potential source of methanogens. However, E. eugeniae raised on a substrate not derived from mammalian fecal material (i.e., substrate 2 or 3) or subjected to a diet of grassland soil (substrate 4) also emitted methane (Fig. 1A). Furthermore, two different species (E. eugeniae and P. excavatus) that were maintained on substrate 1 displayed dissimilar capacities to emit methane. The amount of organic matter in the substrate was not strictly correlated with the capacity of earthworms to emit methane, since E. eugeniae raised on substrate 3 displayed a higher propensity to emit methane than E. eugeniae raised on substrate 2, which had a smaller amount of organic matter than substrate 3 (Fig. 1A; see also Table S1 in the supplemental material).
McrA transcripts detected in gut contents suggested that Methanosarcinaceae, Methanobacteriaceae, and (to a lesser extent) Methanomicrobiaceae are methanogenic taxa that might be associated with the emission of methane by E. eugeniae. Collectively, these methanogenic taxa are known to be capable of acetoclastic, hydrogenotrophic, and methylotrophic methanogenesis (12, 23), suggesting that acetate, H2, and methanol might have been drivers of methanogenesis in the alimentary canal of E. eugeniae.
Different fermentations occur during gut passage in Lumbricus terrestris, with H2-forming butyrate fermentation apparently occurring during the middle to later stages of gut passage (32, 34). Methanogenesis is very O2 sensitive, and the anoxic conditions of the earthworm gut could be postulated to stimulate methanogenesis. However, average redox potentials in the core of the alimentary canal of L. terrestris approximate 150 mV (27), a value that is far from optimal for methanogenesis, since the standard redox potential of the carbon dioxide-methane half-cell reaction is −240 mV (24). One could postulate that the redox potential of the gut of E. eugeniae might be more negative than that of the gut of L. terrestris and thus more favorable for methanogenesis.
The considerations discussed above suggest that ingested methanogens might be the source of methane emitted by E. eugeniae. However, we cannot rule out the possibility that methanogens might also be associated with the alimentary canal. In this regard, E. eugeniae maintained its ability to emit methane when incubated on grassland soil (substrate 4) that was limited in organic materials and did not emit methane (Fig. 1A; see also Table S1 in the supplemental material). In addition, mcrA transcripts of OTU 11 (Methanobacteriaceae) were relatively abundant in and exclusive to gut contents (Fig. 3). It has been shown that symbiotic bacteria colonize the excretion organs of earthworms (4) and that gut tissue harbors microbes that might be opportunistically attached to it (2, 28, 31).
In conclusion, the origin of methane that is emitted by E. eugeniae remains unresolved. Current studies are focused on this issue and on understanding how the nature of the substrate and in situ factors of the gut might stimulate methanogenic taxa in the alimentary canal and lead to the in vivo emission of methane.
Nucleotide sequence accession numbers.
Sequences obtained in this study are available from the EMBL nucleotide sequence database under accession numbers HE647204 to HE647384 for genes and HE647438 to HE647623 for transcripts.
Supplementary Material
ACKNOWLEDGMENTS
We thank Marcus A. Horn, University of Bayreuth, for helpful discussion.
Support for this study was provided in Germany by the Deutsche Forschungsgemeinschaft (DFG) (DR310/4-1 and DR310/7-1) and the University of Bayreuth and in Brazil by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Embrapa Florestas, and the University of São Paulo.
Footnotes
Published ahead of print 17 February 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Bouché MB. 1977. Strategies lombriciennes, p 122–132 In Lohm U., Persson T. (ed), Soil organisms as components of ecosystems, vol 25 Ecological Bulletins, Stockholm, Sweden [Google Scholar]
- 2. Byzov BA, et al. 2009. Culturable microorganisms from the earthworm digestive tract. Mikrobiologiya 78:360–368 [PubMed] [Google Scholar]
- 3. Darwin C. 1881. The formation of vegetable mould through the action of worms, with observations of their habits. Murray, London, United Kingdom [Google Scholar]
- 4. Davidson SK, Stahl DA. 2006. Transmission of nephridial bacteria of the earthworm Eisenia fetida. Appl. Environ. Microbiol. 72:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Depkat-Jakob PS, Hilgarth M, Horn MA, Drake HL. 2010. Effect of earthworm feeding guilds on ingested dissimilatory nitrate reducers and denitrifiers in the alimentary canal of the earthworm. Appl. Environ. Microbiol. 76:6205–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drake HL, Horn MA. 2007. As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Annu. Rev. Microbiol. 61:169–189 [DOI] [PubMed] [Google Scholar]
- 7. Edwards CA, Bohlen PJ. 1996. Biology and ecology of earthworms, 3rd ed Chapman and Hall, London, United Kingdom [Google Scholar]
- 8. Furlong MA, Singleton DR, Coleman DC, Whitman WB. 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 68:1265–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griffiths RI, Whiteley AS, O'Donnell AG, Bailey MJ. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunsalus RP, Romesser JA, Wolfe RS. 1978. Preparation of coenzyme-M analogs and their activity in methyl coenzyme-M reductase system of Methanobacterium thermoautotrophicum. Biochemistry 17:2374–2377 [DOI] [PubMed] [Google Scholar]
- 11. Hackstein JHP, Stumm CK. 1994. Methane production in terrestrial arthropods. Proc. Natl. Acad. Sci. U. S. A. 91:5441–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hedderich R, Whitman WB. 2006. Physiology and biochemistry of the methane-producing Archaea, p 1050–1079 In Dworkin MM, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E. (ed), The prokaryotes, 3rd ed, vol 2 Springer, New York, NY [Google Scholar]
- 13. Horn MA, Schramm A, Drake HL. 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl. Environ. Microbiol. 69:1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horn MA, Drake HL, Schramm A. 2006. Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Appl. Environ. Microbiol. 72:1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horn MA, Mertel R, Kästner M, Gehre M, Drake HL. 2006. In vivo emission of dinitrogen by earthworms via denitrifying bacteria in the gut. Appl. Environ. Microbiol. 72:1013–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hornor SG, Mitchell MJ. 1981. Effect of the earthworm Eisenia foetida (Oligochaeta) on fluxes of volatile carbon and sulphur compounds from sewage sludge. Soil Biol. Biochem. 13:367–372 [Google Scholar]
- 17. Hunger S, et al. 2011. Competing formate- and carbon dioxide-utilizing prokaryotes in an anoxic methane-emitting fen soil. Appl. Environ. Microbiol. 77:3773–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. James SW, Brown GG. 2006. Earthworm ecology and diversity in Brazil, p 56–116 In Moreira FMS, Siqueira JO, Brussaard L. (ed), Soil biodiversity in Amazonian and other Brazilian ecosystems. CAB International, Wallingford, United Kingdom [Google Scholar]
- 19. Karsten GR, Drake HL. 1995. Comparative assessment of the aerobic and anaerobic microfloras of earthworm guts and forest soils. Appl. Environ. Microbiol. 61:1039–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karsten GR, Drake HL. 1997. Denitrifying bacteria in the earthworm gastrointestinal tract and in vivo emission of nitrous oxide (N2O) by earthworms. Appl. Environ. Microbiol. 63:1878–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knapp BA, Podmirseg SM, Seeber J, Meyer E, Insam H. 2009. Diet-related composition of gut microbiota of Lumbricus rubellus as revealed by a molecular fingerprinting technique and cloning. Soil Biol. Biochem. 41:2299–2307 [Google Scholar]
- 22. Kusel K, Drake HL. 1995. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl. Environ. Microbiol. 61:3667–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Whitman WB. 2008. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 1125:171–189 [DOI] [PubMed] [Google Scholar]
- 24. Madigan M, Martinko J, Stahl D, Clark D. 2012. Brock biology of microorganisms, 13th ed Pearson Education, Inc., San Francisco, CA [Google Scholar]
- 25. Matthies C, Griesshammer A, Schmittroth M, Drake HL. 1999. Evidence for involvement of gut-associated denitrifying bacteria in emission of nitrous oxide (N2O) by earthworms obtained from garden and forest soils. Appl. Environ. Microbiol. 65:3599–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saitou N, Nei M. 1987. The neighbor-joining method—a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 27. Schmidt O, et al. 2011. Novel [NiFe]- and [FeFe]-hydrogenase gene transcripts indicative of active facultative aerobes and obligate anaerobes in earthworm gut contents. Appl. Environ. Microbiol. 77:5842–5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singleton DR, Hendrix PF, Coleman DC, Whitman WB. 2003. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta). Soil Biol. Biochem. 35:1547–1555 [Google Scholar]
- 29. Springer E, Sachs MS, Woese CR, Boone DR. 1995. Partial gene sequences for the A subunit of methyl-coenzyme M reductase (mcrI) as a phylogenetic tool for the family Methanosarcinaceae. Int. J. Syst. Bacteriol. 45:554–559 [DOI] [PubMed] [Google Scholar]
- 30. Šustr V, Šimek M. 2009. Methane release from millipedes and other soil invertebrates in Central Europe. Soil Biol. Biochem. 41:1684–1688 [Google Scholar]
- 31. Thakuria D, Schmidt O, Finan D, Egan D, Doohan FM. 2010. Gut wall bacteria of earthworms: a natural selection process. ISME J. 4:357–366 [DOI] [PubMed] [Google Scholar]
- 32. Wüst PK, Horn MA, Drake HL. 2009. In situ hydrogen and nitrous oxide as indicators of concomitant fermentation and denitrification in the alimentary canal of the earthworm Lumbricus terrestris. Appl. Environ. Microbiol. 75:1852–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wüst PK, Horn MA, Henderson GPH, Janssen Rehm BH, Drake HL. 2009. Gut-associated denitrification and in vivo emission of nitrous oxide by the earthworm families Megascolecidae and Lumbricidae in New Zealand. Appl. Environ. Microbiol. 75:3430–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wüst PK, Horn MA, Drake HL. 2011. Clostridiaceae and Enterobacteriaceae as active fermenters in earthworm gut content. ISME J. 5:92–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaprasis A, Liu Y-J, Liu S-J, Drake HL, Horn MA. 2010. Abundance of novel and diverse tfdA-like genes, encoding putative phenoxyalkanoic acid herbicide-degrading dioxygenases, in soil. Appl. Environ. Microbiol. 76:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.