Abstract
Research suggests individuals possess multifaceted cognitive representations of various diseases. These illness representations consist of various beliefs, including causal attributions for the disease, and are believed to motivate, guide, and shape health-related behavior. As little research has examined factors associated with beliefs about cancer causation, the present study examined the relationship between personal and family history of cancer and beliefs about the causes and prevention of malignant disease. Data was obtained from 6369 adult respondents to the 2003 Health Information National Trends Survey (HINTS), a national population-based survey. Information about personal and family history of cancer and beliefs regarding cancer causation and prevention was obtained. Results showed both a personal and family history of cancer were associated with differences in beliefs about the causes of cancer. In general, a personal history of cancer was not significantly linked to causal attributions for cancer relative to those without a personal history. In contrast, a family history of cancer tended to increase the likelihood a respondent viewed a particular cause as increasing cancer risk. Thus, personal and vicarious experience with cancer had dramatically diverging influences on attributions of cancer causation, which may be due to differing self-protection motives. Results support the belief that illness representations, in this case the causal belief component, are influenced by both personal and vicarious experience with a disease and also suggest illness representations may influence receptivity to messages and interventions designed to increase appropriate cancer risk reduction behavior.
Keywords: cancer, oncology, causal attributions, attribution theory, illness representations, prevention
Individuals possess cognitive representations of various diseases and illnesses [1–3]. These illness representations, also known as common sense models of illness, are specific to a particular disease or illness. The representation consists of beliefs about the causes, symptoms, timeline, consequences, and treatment of a given disease or illness. Importantly, the set of beliefs subsumed by the illness representation motivates, guides, and shapes an individual’s health-related behaviors [3–4]. Beliefs about a particular disease can influence whether and how an individual takes steps to reduce the risk for developing that disease or minimizing risk associated with ongoing disease. Consequently, the study of these disease-related beliefs is important for the development of interventions targeting risk-reducing health behaviors.
Attribution theory asserts that humans have a need to understand, give meaning to, and ascribe causation to life events [5–6]. This helps explain why individuals are motivated to assign causal beliefs to diseases in illness representations. In addition, attribution theory suggests causal attributions are centrally important in a person’s understanding of the world and, therefore, are important determinants of his/her interactions with that world [5–6]. Most attribution theories assert there is a dimensional structure underlying causal attributions and that categorizing attributions into dimensions allows for increased understanding of causal beliefs. Weiner [7] posits causal attributions vary on three primary dimensions: locus (internal/external), stability (stable/unstable), and controllability (controllable/uncontrollable). Given causal attributions can significantly impact behavior, specific beliefs about the cause of a particular disease likely strongly influence risk reduction behaviors relevant to that disease. Thus, understanding these causal beliefs, as well as factors that shape these beliefs, becomes a significant research goal. It is assumed that illness representations can be modified or shaped by exposure to various sources of information, as well as by personal and vicarious experience with a particular disease [8–11].
Previous research has examined causal beliefs regarding malignant disease. In general, this research has focused upon two related, but different, questions. Research has examined beliefs which cancer patients and survivors have regarding the cause of their own cancer [12–18]. Often, this research has sought to link personal causal beliefs to psychological adjustment in cancer patients and survivors [14, 18]. Other research has examined beliefs about the causes of cancer, in general. This research has studied causal beliefs held by both the general public [19–22] and individuals with a personal cancer history [11, 23–25]. Research regarding causal beliefs serves two purposes. First, this research can help identify links between specific causal beliefs and the practice of specific health behaviors, such as the practice of complementary anticancer therapies [23–24]. Second, this research can provide an empirical foundation for public health efforts to educate the public about causes of cancer and appropriate risk reduction behaviors. For example, causal beliefs of cancer survivors have been found to differ substantially from those of cancer experts [25], with cancer survivors underestimating the role of behavioral factors, such as physical inactivity and poor diet, and overestimating the role of environmental pollutants and stress. This research suggests that enhanced educational efforts are needed to ensure survivors are appropriately aware of cancer risk factors and to identify inappropriate causal beliefs requiring modification.
Little attention has been devoted to identification of factors associated with causal beliefs, even though identification of factors associated with specific causal beliefs could shed light upon the development of these beliefs. In particular, little research has examined whether a personal or family history of cancer might be linked to causal beliefs about cancer. As both personal and vicarious experience with a particular disease can influence illness representations or causal attributions, a personal or family history of cancer could be expected to be associated with different causal beliefs. While no research has examined the impact of family history of cancer on causal attributions for cancer, multiple studies have compared the causal beliefs of individuals with and without a personal cancer history. Linn et al. [11] compared causal beliefs of late stage cancer patients to matched controls and found cancer patients consistently reported weaker beliefs about the role various causes played in cancer development, even when causes, such as smoking, were probably associated with the development of their own cancer. Similarly, Buick [26] found cancer patients held weaker beliefs about the role of internal and chance factors than did physically healthy women. Anagnostopoulos & Spanea [27] found breast cancer patients reported weaker beliefs about the role of environmental factors (e.g., pollution, chemical exposure) compared to controls without a history of breast cancer. Here, patients reported stronger beliefs about the role of chance and did not differ in the role of internal factors, such as personality or anxiety, when compared with controls. Thus, while specific findings regarding the influence of personal cancer history on causal attributions have been mixed, the overall pattern suggests a personal history of cancer tends to be associated with weaker causal beliefs.
The weakening of causal beliefs, especially “controllable” attributions such as behavior or lifestyle factors, in cancer patients or survivors may be due to the need to defend oneself against blame from self or others as a means of coping with illness [11]. This is consistent with the notion that individuals tend to maintain causal beliefs that result in self-enhancement or beliefs of personal control. This is evidenced by research demonstrating individuals attribute causes for negative events externally and that people may attribute responsibility for negative events to event victims (consistent with the “just world hypothesis”), respectively [6]. As research has demonstrated breast cancer patients can be “double stigmatized,” both for having cancer and for causing their own cancer [26], it is not surprising that cancer patients might be motivated to protect themselves against blame for their own cancer.
Using data from a large, population-based national survey, the present study examines the relationship between personal and family history of cancer and beliefs about the causes of cancer. Based on previous research, and consistent with the notion that causal attributions can play a self-protective role, we hypothesize individuals with a personal history of cancer will report weaker beliefs about the role played by specific causes in cancer development. Additionally, we hypothesize a family history of cancer, alone or in combination with a personal cancer history, should influence beliefs about the causes of cancer. A directional hypothesis is not made at this time due to lack of previous investigations and the diverging directionality that would be predicted by attributions designed to protect close others (weaker beliefs) or to maintain beliefs in personal control (stronger beliefs). Given the potential link between causal beliefs and subsequent cancer risk reduction behaviors, basic research identifying how causal attributions vary as a function of personal and/or family history of cancer could assist future public health efforts to target cancer risk reduction messages to appropriate audiences.
Method
Study Sample and Procedures
The data for this study was provided by the 2003 Health Information National Trends Survey (HINTS). The HINTS was commissioned by the National Cancer Institute and used random digit dialing techniques to obtain a national probability sample of U.S. adults, 18 years of age or older. Complete information regarding HINTS study design, data collection procedures and response rate is reported elsewhere [28].
Study Measures
Demographic Information
All participants provided information regarding sex, age, educational attainment, partner status, and race/ethnicity.
History of Malignant Disease
Family history of cancer was assessed by one item, “Have any of your brothers, sisters, parents, children or other close family members ever had cancer?” Personal history of cancer was assessed by one item, “Have you ever been told by a doctor that you had cancer?” Individuals who answered “yes” to this item then indicated the type of cancer they were diagnosed with. Respondents were also asked their age at initial cancer diagnosis. This information was coupled with age at interview to determine number of years since initial cancer diagnosis at interview (≤ 1 year, 1–5 years; 6–10 years, ≥ 11 years).
Cancer Causal and Prevention Beliefs
To assess beliefs about specific causes of cancer, respondent were asked, “Do you think that _________ increase(s) a person’s chances of cancer a lot, a little, or not at all, or do you have no opinion?” Fifteen potential causes of cancer were assessed. These causes were divided into two groups, and respondents were randomly assigned to report beliefs regarding Group A (smoking, pesticides/food additives, stress, not eating much fiber, being hit in the breast, family history of cancer, not getting much exercise) or Group B (eating a high-fat diet, exposure to the sun, not eating many fruits and vegetables, drinking a lot of alcoholic beverages, having many sexual partners, being a particular race or ethnicity, pollution, radon). Only women responded to the items “being hit in the breast” and “having many sexual partners.” Thus, participants responded to 6–8 of the 15 cancer causes assessed.
Respondents were asked to indicate their agreement with three statements regarding general beliefs regarding cancer causation and prevention: “It seems like almost everything causes cancer,” “There’s not much people can do to lower their chances of getting cancer,” and “There are so many different recommendations about preventing cancer, it’s hard to know which ones to follow.” Response options included “strongly agree,” “somewhat agree,” “somewhat disagree,” “strongly disagree,” and “no opinion.”
Data Preparation
Each of the 15 causes of cancer assessed were characterized as either a “controllable” or an “uncontrollable” cause. A panel of six raters was convened, each with post-baccalaureate training in oncology nursing or clinical psychology. Each rater independently categorized each cause as “controllable” or “uncontrollable.” Complete agreement among raters was achieved for 12 causes. For the remaining three causes (sun exposure, pesticides/food additives, stress) agreement was evident for 5 of 6 raters. Based on these ratings, nine causes were characterized as controllable (smoking, high fat diet, sun exposure, not eating fiber, not eating fruits/vegetables, stress, drinking alcohol, having many sexual partners, not getting exercise), and six causes were characterized as uncontrollable (pesticides/food additives, being hit in the breast, family history of cancer, race/ethnicity, pollution, radon). A Controllable Cause composite index was created by counting how many of the nine controllable causes a person indicated having some positive belief of increasing chances of getting cancer (responses of “a lot” or “a little”) and then dividing by the total number of these nine controllable causes responded to (as not all persons responded to each of the nine items). A similar procedure was used to calculate an Uncontrollable Cause index using the six potential causes characterized as uncontrollable. Scores on both the Controllable Cause and Uncontrollable Cause indices ranged from 0 to 1 and represented the proportion of individual items comprising the Controllable and Uncontrollable Cause indices a respondent felt increased cancer risk.
A series of hierarchical logistic regression analyses was used to examine the relationship between beliefs about cancer causation and personal and family history of cancer. Dependent variables were the 15 items assessing beliefs about potential causes of cancer. For each item, responses were dichotomized to compare respondents who indicated having some positive belief that a particular cause increased a person’s chances of getting cancer (responses of “a lot” or “a little”) versus negative (not at all) or no opinion responses. Independent variables entered in the logistic regression analyses at step one included personal cancer history (yes vs. no) and family history of cancer (yes vs. no). An interaction term representing the combination of personal and family history of cancer was entered at step two in the analysis. The interaction term was retained in the final model only if it accounted for a significant increment in variance beyond that accounted for by the two main effects (i.e., personal and family history of cancer). A similar set of hierarchical logistic regression analyses were performed to examine the relationship between personal and family history of cancer and agreement with the three statements of general belief about cancer causation and prevention. For each statement, responses were dichotomized to compare respondents who indicated some level of agreement with the statement (agreeing either “strongly” or “somewhat”) to those who reported a negative belief (disagreed “strongly” or “somewhat) or no opinion. Age, gender, and education were included as covariates in all logistic regression analyses.
Results
6369 individuals responded to the HINTS survey. Of these, 763 respondents indicated they had been diagnosed with cancer, with 140 reporting being diagnosed with skin cancer (non-melanoma). The 140 respondents with a history of skin cancer were compared to the remaining 623 respondents with any other cancer diagnosis with regard to our primary dependent variables (beliefs regarding specific potential causes of cancer, general beliefs regarding cancer causation and prevention, Controllable and Uncontrollable Cause composite indices. Significant differences (p < .05) between cancer survivors with and without skin cancer were present for 3 of these 20 variables. Due to the relatively small number of differences between these two groups, individuals with a history of skin cancer were included in the larger group of individuals considered to have a personal history of cancer (n = 763).
Most respondents with a personal history of cancer had received a single cancer diagnosis (n = 703; 92.1%) while a minority had been diagnosed on two or more occasions (n = 60; 7.9%). A variety of diagnoses were present, with the most common initial diagnoses being gynecologic (20.2%), skin (18.3%), and breast cancers (14.7%). Additional cancers included genitourinary cancers (11.5%), melanoma (7.9%), and gastrointestinal (7.5%) hematologic (3.1%), head and neck (3.1%), thyroid (2.1%), lung (1.7%), musculoskeletal (0.4%) and other cancers (4.6%). Time since initial cancer diagnosis varied with most cancer survivors being ≥ 11 years post-diagnosis (40.0%), followed by 6 to 10 years post-diagnosis (21.5%), 1 to 5 years post-diagnosis (22.4%), and ≤ 1 year post-diagnosis (15.1%). Time since cancer diagnosis was unavailable for 8 survivors (1%).
The final study sample consisted of 6369 respondents (763 with a personal history of cancer). Mean age was 47.7 years (SD = 17.4, range 18–95), and the majority of respondents were female (60.4%) and currently married or in a stable relationship (54.2%). The majority of respondents were white, non-Hispanic/Latino (67.6%) with other groups represented as follows: Black/African American, (11.9%), white, Hispanic/Latino (5.3%), American Indian/Alaskan native (3.1%), Asian (2.1%), Native Hawaiian/Pacific Islander (0.5%). Information on race/ethnicity was not available for 9.5% of respondents. Educational attainment was: < high school (11.7%), high school or equivalent (28.7%), some college or technical school (25.7%), college graduate or more (30.3%). Information on educational level was not available for 3.6% of respondents. Annual household income was: < $25,000 (26.8%), $25000 – $49,999 (27.4%), $50000 – $74999 (15.0%), ≥ $75000 (19.1%). Information on income was not available for 11.7% of respondents. A family history of cancer was acknowledged by 62.3% of respondents.
Results of the logistic regression analyses examining the relationship between personal and family history of cancer and beliefs about cancer causation are shown in Table 1. Using a significant model X2 as criterion (i.e., p for model ≤ .05), the combination of personal and family history of cancer was significantly associated with beliefs about cancer causation for 8 of the 15 items. Of the 8 items with a significant overall model X2, a significant interaction between family and personal history of cancer was obtained for one item: having many sexual partners (OR = 0.53; p <.05). Thus, the combination of both a personal and family history of cancer reduced the likelihood a respondent believed having many sexual partners increased cancer risk by about 40–50%, relative to those not possessing both a personal and family history of cancer (i.e., those possessing a history of neither or only one).
Table 1.
Logistic Regression Analysis of Relationship Between Personal and Family History of Cancer and Beliefs About Cancer Causation
| Personal Cancer Hxa | Family Cancer Hx a | Personal x Family Hx b | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Controllable Causes | ||||||
| Smoking | 0.93 | 0.60 – 1.47 | 1.43 | 1.06 – 1.95 * | ||
| Eating a High Fat Diet | 1.02 | 0.80 – 1.28 | 1.20 | 1.03 – 1.40 * | ||
| Exposure to Sun | 1.03 | 0.66 – 1.60 | 1.27 | 0.95 – 1.69 | ||
| Not Eating Much Fiber | 0.85 | 0.66 – 1.10 | 1.07 | 0.91 – 1.26 | ||
| Not Eating Many Fruits/Vegetables | 0.99 | 0.76 – 1.29 | 1.12 | 0.94 – 1.33 | ||
| Stress | 1.14 | 0.88 – 1.48 | 1.19 | 1.01 – 1.39 * | ||
| Drinking a Lot of Alcoholic Beverages | 0.77 | 0.61 – 0.98 * | 1.19 | 1.01 – 1.40 * | ||
| Not Getting Much Exercise | 0.88 | 0.68 – 1.14 | 1.25 | 1.05 – 1.49 * | ||
| Having Many Sexual Partners b | 2.40 | 0.89 – 6.52 | 2.55 | 1.30 – 5.01 * | 0.53 | 0.30 – 0.94 * |
| Uncontrollable Causes | ||||||
| Pesticides/Food additives | 0.99 | 0.74 – 1.33 | 1.29 | 1.07 – 1.55 ** | ||
| Being Hit in the Breast b | 1.06 | 0.81 – 1.40 | 0.95 | 0.77 – 1.16 | ||
| Pollution | 1.07 | 0.75 – 1.53 | 1.21 | 0.96 – 1.54 | ||
| Radon | 0.83 | 0.65 – 1.05 | 1.10 | 0.93 – 1.30 | ||
| Having a Family History of Cancer | 1.06 | 0.64 – 1.77 | 1.92 | 1.38 – 2.68 *** | ||
| Being a Particular Race or Ethnicity | 1.07 | 0.85 – 1.34 | 0.97 | 0.83 – 1.13 | ||
Note. Responses for each cancer cause coded as 1 = increases chances of getting cancer “a lot” or “a little”; 0 = “not at all” or “no opinion.” All analyses control for age, gender, and education as covariates.
coded as 1 = no; 2 = yes;
final logistic regression model includes personal history x family history interaction term only if interaction term is significant;
asked of females only
p ≤ .05;
p ≤ .01;
p ≤ .001
The remaining 7 items with a significant overall model X2 yielded significant main effects for either a personal or family history of cancer, or both. A significant main effect for personal history of cancer was present for only one potential cause: drinking a lot of alcoholic beverages (OR = 0.77; p < .05). A personal history of cancer was related to an approximate 20% lesser likelihood of indicating drinking a lot of alcoholic beverages increased cancer risk (relative to a respondent without a personal history of cancer). In contrast, a significant main effect for family history was present for seven of the eight potential cancer causes: smoking (OR = 1.43; P < .05), eating a high fat diet (OR = 1.20; p < .05), stress (OR = 1.19; p < .05), drinking a lot of alcoholic beverages (OR = 1.19, p <.05), not getting much exercise (OR = 1.25, p < .05), pesticides/food additives (OR = 1.29, p < .01, and family history of cancer (OR = 1.92; p <.001). On average, a family history of cancer was linked to a 20% to 40% greater likelihood of indicating a specific factor was associated with increased cancer risk (relative to a respondent without a family history of cancer). The lone exception was “having a family history of cancer.” For this cause, a family history of cancer was associated with about a 90% greater likelihood of believing this factor increased cancer risk.
Results of the logistic regression analyses examining the relationship between personal and family history of cancer and agreement with the three statements of general belief about cancer causation and prevention are shown in Table 2. A significant overall model X2 was obtained for two items. A significant interaction between personal and family history of cancer was found for the item, “Everything causes cancer” (OR = 0.64; p <.05). Here, possessing both a personal and family history of cancer was associated with an approximate 35% lesser likelihood of agreeing with this statement, relative to respondents without both a personal and family history of cancer. Additionally, a family history of cancer was associated with agreement with the statement, “There are so many different recommendations about preventing cancer, it’s hard to know which ones to follow” (OR = 1.16; p <.05). Here, a family history of cancer was associated with an approximate 15% greater likelihood of agreement, relative to a respondent without a family history of cancer.
Table 2.
Logistic Regression Analysis of Relationship Between Personal and Family History of Cancer and Beliefs About Cancer Prevention
| Personal Cancer Hxa | Family Cancer Hx a | Personal x Family Hx | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| “Everything Causes Cancer” | 2.24 | 1.24 – 4.06** | 2.04 | 1.38 – 3.02*** | 0.64 | 0.46 – 0.90* |
| “Not Much Can Do To Prevent Cancer” | 1.09 | 0.91 – 1.30 | 0.95 | 0.84 – 1.07 | ||
| “Too Many Recommendations…” | 1.14 | 0.95 – 1.37 | 1.16 | 1.03 – 1.30* | ||
Note. Note. Responses for each cancer prevention belief coded as 1 = “strongly agree” or “somewhat agree”; 0= “somewhat disagree” or “strongly disagree” or “no opinion.” All analyses control for age, gender, and education as covariates.
coded as 1 = no; 2 = yes;
final logistic regression model includes personal history x family history interaction term only if interaction term is significant
p ≤ .05;
p ≤ .01;
p ≤ .001
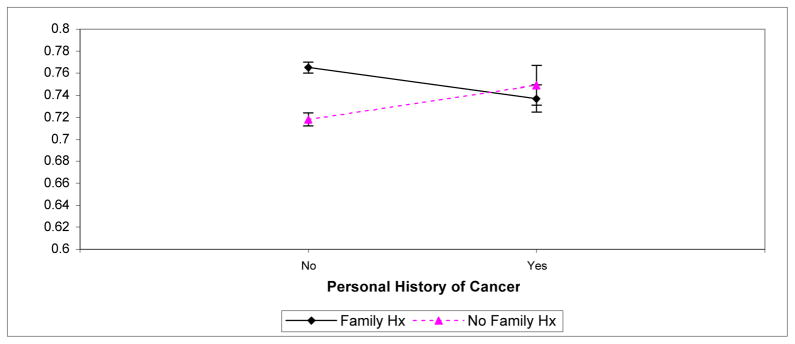
Finally, a pair of 2 × 2 analyses of variance (ANOVA) were performed to examine the relationship between personal and family history of cancer and the Controllable and Uncontrollable Cause indices. The two factors used in the ANOVA’s were personal cancer history (yes or no) and family history of cancer (yes or no). Age, gender, and education were included as covariates in the analyses. No significant main or interaction effects were obtained for the Uncontrollable Cause index. However, for the Controllable Cause index, a significant interaction effect was obtained (F(1, 6113) = 4.82; p <.05). This interaction is shown in Figure 1. For respondents with a personal history of cancer (i.e., cancer survivors), whether they also possessed a family history of cancer did not affect the tendency to believe controllable factors increased cancer risk. In contrast, for respondents without a personal history of cancer, a family history of cancer markedly affected the tendency to believe controllable factors increased cancer risk. Specifically, individuals with no family or personal history of cancer were least likely to believe controllable factors increased cancer risk. Individuals with a family history of cancer (but no personal history of cancer) were most likely to believe controllable factors increased cancer risk. Figure 1 suggests respondents with both a personal and family history of cancer also evidenced a low tendency to endorse controllable factors as cancer causes.
Figure 1.
Scores on the Controllable Cause Composite Index as a Function of Personal and Family History of Cancer.
Discussion
Our results suggest personal and family history of cancer are associated with different beliefs about cancer causation. Thus, our results are consistent with the belief that illness representations, specifically the causal belief component, are influenced by both personal and vicarious experience [1–3]. Of personal and family history of cancer, our results suggest a family history of cancer is more strongly linked to cancer causal beliefs. In fact, a personal history of cancer was only weakly linked to causal beliefs about cancer. The latter is consistent with our hypothesis, based upon prior research demonstrating those with a personal history of cancer evidence significantly weaker cancer causal beliefs than those without a personal history of cancer [11, 26–27]. Other than beliefs that cancer is due to chance or God’s will, prior research has shown beliefs that specific factors cause cancer are associated with increased distress in cancer survivors [13]. Thus, for those with a personal history of cancer (i.e., cancer survivors), espousing weaker beliefs about cancer causation, particularly, downplaying of the influence of personal choice and behavior in cancer causation, may serve a self-protective function. However, as cancer survivors are at greater risk than the general population for developing second malignancies [29–32], the practice of appropriate cancer risk reduction behaviors is still important for cancer survivors [33]. Thus, as performance of appropriate cancer risk reduction behaviors are at least partially determined by causal beliefs, determination of why cancer survivors possess generally weaker causal beliefs about cancer than the general public should be a significant research goal.
A family history of cancer was linked to stronger causal beliefs about cancer. Specifically, a family history tended to increase the likelihood of agreeing a specific factor caused cancer. Furthermore, those with a family history of cancer were particularly likely to acknowledge the potential impact of various “controllable” causes of cancer, particularly when no personal history of cancer was present. The tendency for those with a family history of cancer to possess stronger beliefs about potential causes of cancer, especially controllable causes, is noteworthy in two respects. First, as a family history of cancer is objectively associated with increased risk for some cancers [34], these individuals should be a particular focus of clinical and public health recommendations regarding risk reduction behaviors. Our results suggest these recommendations are likely to fall on fertile ground, as they are consistent with already strong beliefs among those with a family history regarding potentially controllable causes of cancer. Second, while distancing oneself from personal responsibility for cancer genesis may be anxiety-reducing and protective for cancer survivors, aligning oneself with controllable factors may serve a similar anxiety-reducing function for those possessing a family history. Relative to those without a family history, those with a family history of cancer were almost twice as likely to endorse family history as a cancer cause (Table 1). The belief one can make behavioral changes now to prevent cancer in the future may enable those with a family history to reduce any anxiety associated with the strong belief that their family history places them at risk for cancer. This interpretation appears consistent with the notion of attribution theory that individuals want to maintain belief that they have effective control over their lives, which may cause them to attribute cancer to controllable factors that they themselves can avoid to prevent cancer.
While the use of a large, national, population-based sample was a strength of this study, some limitations should be acknowledged. First, both cancer survivors and those with a family history of cancer were grouped together regardless of cancer type or length of time since cancer diagnosis/treatment. Important differences may exist in illness representations based on cancer type or other factors [13]. Second, a couple of commonly reported perceived causes of cancer, chance and/or God’s will, were not examined in the present study. Third, other factors which might influence causal beliefs about cancer, such as cultural information which can affect the social construction of cancer, were not examined. As such cultural information has been linked to distorted or inaccurate beliefs about cancer [35], the link between cultural information and social constructions of cancer and causal beliefs about cancer merits investigation. Finally, the present study examined the empirical link between personal and family history of cancer and cancer causal beliefs. Future research should be designed to elucidate why personal and family history of cancer are differentially linked to beliefs about cancer causation.
In sum, our results have both conceptual and clinical significance. Conceptually, they demonstrate the influence of personal and vicarious experience in the development of the causal component of cancer illness representations. Clinically, they suggest potential differences between those with a personal and family history of cancer in receptivity to messages and interventions designed to increase appropriate cancer risk reduction behavior. Since causal attributions can influence health behaviors [3, 36–37], the lack of strong causal beliefs about cancer among cancer survivors may reduce the likelihood they will spontaneously engage in appropriate cancer risk reduction behaviors [38]. On the other hand, survivors’ lack of existing strong causal beliefs about cancer may enhance the likelihood of success for intervention efforts to enhance risk-reducing behaviors. After all, it is likely easier to instill appropriate causal beliefs about cancer when no previous beliefs exist than it is when inappropriate beliefs already exist. On the other hand, those with a family history of cancer appear more likely to embrace the notion that controllable factors can cause cancer. This suggests a greater likelihood of accepting behavior change messages or interventions to reduce cancer risk among those with a family history. However, those with a family history of cancer were more likely to also believe “there are too many recommendations” about reducing cancer risk. So while those with a family history of cancer might hold causal beliefs that make them good targets for clinical and public health interventions to reduce risk, care must be taken to avoid overwhelming them with risk reduction recommendations or encouraging their counterproductive belief that “everything causes cancer.” Those with a family history of cancer might benefit most from highly focused messages targeting specific cancers and specific risk reduction recommendations [39].
Acknowledgments
This research was supported by grant K05 CA096558 from the National Institutes of Health
References
- 1.Lau RR, Hartmann KA. Common sense representations of common illnesses. Health Psychol. 1983;2:167–185. doi: 10.1037//0278-6133.8.2.195. [DOI] [PubMed] [Google Scholar]
- 2.Leventhal H, Leventhal EA, Cameron L. Representation, procedures, and affect in illness self-regulation: A perceptual, cognitive model. In: Baum A, Revenson T, Singer JE, editors. Handbook of Health Psychology. Larence Earlbaum Associates; Hillsdale, NJ: 2001. pp. 19–47. [Google Scholar]
- 3.Leventhal H, Meyer D, Nerenz D. The common sense representation of illness danger. In: Rachman S, editor. Medical Psychology. Vol. 2. Permagon Press; New York: 1980. pp. 7–30. [Google Scholar]
- 4.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cogn Ther Res. 1992;16:143–163. [Google Scholar]
- 5.Kelley HH. The processes of causal attribution. Am Psychol. 1973;28:107–128. [Google Scholar]
- 6.Kelley HH, Michela JL. Attribution theory and research. Ann Rev Psychol. 1980;31:457–501. doi: 10.1146/annurev.ps.31.020180.002325. [DOI] [PubMed] [Google Scholar]
- 7.Weiner B. An attribution theory of achievement motivation and emotion. Psychol Rev. 1985;92:548–573. [PubMed] [Google Scholar]
- 8.Abood DA, Black DR, Feral D. Nutrition education worksite intervention for university staff: Application of the Health Belief Model. J Nutr Educ Behav. 2003;35:260–267. doi: 10.1016/s1499-4046(06)60057-2. [DOI] [PubMed] [Google Scholar]
- 9.Boling W, Laufman L, Lynch GR, Weinberg AD. Increasing mammography screening through inpatient education. J Canc Educ. 2005;20:247–250. doi: 10.1207/s15430154jce2004_14. [DOI] [PubMed] [Google Scholar]
- 10.Cappelli M, Surh L, Walker M, Korneluk Y, Humphreys L, Verma S, Hunter A, Allanson J, Logan D. Psychological and social predictors of decisions about genetic testing for breast cancer in high-risk women. Psychol Health Med. 2001;6:321–333. [Google Scholar]
- 11.Linn MW, Linn BS, Stein SR. Beliefs about causes of cancer in cancer patients. Soc Sci Med. 1982;16:835–839. doi: 10.1016/0277-9536(82)90236-2. [DOI] [PubMed] [Google Scholar]
- 12.Arman M, Backman M, Carlsson M, Hamrin E. Women’s perceptions and beliefs about the genesis of their breast cancer. Canc Nurs. 2006;29:142–148. doi: 10.1097/00002820-200603000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Costanzo ES, Lutgendorf SK, Bradley SL, Rose SL, Anderson B. Cancer attributions, distress, and health practices among gynecologic cancer survivors. Psychosom Med. 2005;67:972–980. doi: 10.1097/01.psy.0000188402.95398.c0. [DOI] [PubMed] [Google Scholar]
- 14.Faller H, Schilling S, Lang H. Causal attribution and adaptation among lung cancer patients. J Psychosom Res. 1995;39:619–627. doi: 10.1016/0022-3999(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 15.Mumma C, McCorkle R. Causal attribution and life-threatening disease. Int J Psychiatr Med. 1982/83;12:1311–1319. doi: 10.2190/ejeh-5wkw-ftpn-fbyg. [DOI] [PubMed] [Google Scholar]
- 16.Stewart DE, Cheung AM, Duff S, Wong S, McQuestion M, Cheng T, Purdy L, Bunston T. Attributions of cause and recurrence in long-term breast cancer survivors. Psycho Oncol. 2001a;10:179–183. doi: 10.1002/pon.497. [DOI] [PubMed] [Google Scholar]
- 17.Stewart DE, Duff S, Wong F, Melancon C, Cheung AM. The views of ovarian cancer survivors on its cause, prevention, and recurrence. Medsc Wom Health. 2001b;6:5. [PubMed] [Google Scholar]
- 18.Taylor SE, Lichtman RR, Wood JV. Attributions, beliefs about control, and adjustment to breast cancer. J Pers Soc Psychol. 1984;46:489–502. doi: 10.1037//0022-3514.46.3.489. [DOI] [PubMed] [Google Scholar]
- 19.Breslow RA, Sorkin JD, Frey CM, Kessler LG. Americans’ knowledge of cancer risk and survival. Prev Med. 1997;26:170–177. doi: 10.1006/pmed.1996.0136. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Iwasaki M, Otani T, Sasazuki S, Tsugane S. Public awareness of risk factors for cancer among the Japanese general population: A population-based survey. BMC Publ Health. 2006;6:2–7. doi: 10.1186/1471-2458-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols HB, Trentham-Dietz A, Newcomb P, Yanke L, Remington P, Love RR. What causes cancer? Reports from sixth-grade girls. J Canc Educ. 2006;21:142–146. doi: 10.1207/s15430154jce2103_11. [DOI] [PubMed] [Google Scholar]
- 22.Wardle J, Waller J, Brunswick N, Jarvis MJ. Awareness of risk factors for cancer among British adults. Public Health. 2001;115:173–174. doi: 10.1038/sj/ph/1900752. [DOI] [PubMed] [Google Scholar]
- 23.Maskarinec G, Gotay CC, Tatsumura Y, Shumay DM, Kakai H. Perceived cancer causes: use of complementary and alternative therapy. Canc Pract. 2001;9:183–190. doi: 10.1046/j.1523-5394.2001.94006.x. [DOI] [PubMed] [Google Scholar]
- 24.Risberg T, Wist E, Bremnes RM. Patients’ opinion and use of non-proven therapies related to their view on cancer aetiology. Anticancer Res. 1998;18:499–506. [PubMed] [Google Scholar]
- 25.Wold KS, Byers T, Crane LA, Ahnen D. What do cancer survivors believe causes cancer? (United States) Canc Causes Contr. 2005;16:115–123. doi: 10.1007/s10552-004-2414-0. [DOI] [PubMed] [Google Scholar]
- 26.Buick DL. Illness representations and breast cancer: coping with radiation and chemotherapy. In: Petrie KJ, Weinman JA, editors. Perceptions of health and illness. Harwood Academic Publishers; Amsterdam: 1997. pp. 379–409. [Google Scholar]
- 27.Anagnostopoulos F, Spanea E. Assessing illness representations of breast cancer: A comparison of patients with health and benign controls. J Psychosom Res. 2005;58:327–334. doi: 10.1016/j.jpsychores.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Nelson DE, Kreps GL, Hesse BW, Croyle RT, Willis G, Arora NK, Rimer BK, Viswanath KV, Weinstein N, Alden S. The Health Information National Trends Survey (HINTS): Development, design, and dissemination. J Health Comm. 2004;9:443–460. doi: 10.1080/10810730490504233. [DOI] [PubMed] [Google Scholar]
- 29.Bassal M, Mertens AC, Taylor L, Neglia JP, Greffe BS, Hammond S, Ronckers CM, Friedman DL, Stovall M, Yasui YY, Robison LL, Meadows AT, Kadan-Lottick NS. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 30.Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, van Leeuwen FE, Holowaty EJ, Andersson M, Wiklund T, Joensuu T, van’t Veer MB, Stovall M, Gospodarowicz M, Travis LB. Second malignant neoplasms among long-term survivors of Hodgkin’s Disease: A population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Henderson TO, Whitton J, Stovall M, Mertens AC, Mitby P, Friedman D, Strong LC, Hammond S, Neglia JP, Meadows AT, Robison L, Diller L. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Natl Canc Inst. 2007;99:300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis LB, Fossa SD, Schonfeld SJ, McMasater ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD, Jr, Dores GM, Gilbert ES. Second cancers among 40,756 testicular cancer patients: Focus on long-term survivors. J Nal Canc Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 33.Marriotto AB, Rowland JH, Ries LAG, Scoppa S, Feuer EJ. Multiple cancer prevalence: A growing challenge in long-term survivorship. Canc Epidemiol Biomarkers Prev. 2007;16:566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 34.Kerber R, O’Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103:1906–1915. doi: 10.1002/cncr.20989. [DOI] [PubMed] [Google Scholar]
- 35.Thorne SE, Murray C. Social constructions of breast cancer. Health Care Women Int. 2000;21:141–159. doi: 10.1080/073993300245221. [DOI] [PubMed] [Google Scholar]
- 36.Martin R, Lemos K. From heart attacks to melanoma: do common sense models of somatization influence symptom interpretation for female victims? Health Psychol. 2002;21:25–32. [PubMed] [Google Scholar]
- 37.Stillman MJ. Women’s health beliefs about cancer and breast self-examination. Nurs Res. 1977;26:121–127. doi: 10.1097/00006199-197703000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Niederdeppe J, Gurmankin Levy A. Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer Epidemiol Biomarkers Prev. 2007;16:998–1003. doi: 10.1158/1055-9965.EPI-06-0608. [DOI] [PubMed] [Google Scholar]
- 39.Glanz K, Steffen AD, Taglialatela LA. Effects of colon cancer risk counseling for first-degree relatives. Cancer Epidemiol Biomarkers Prev. 2007;16:1485–1491. doi: 10.1158/1055-9965.EPI-06-0914. [DOI] [PubMed] [Google Scholar]