Abstract
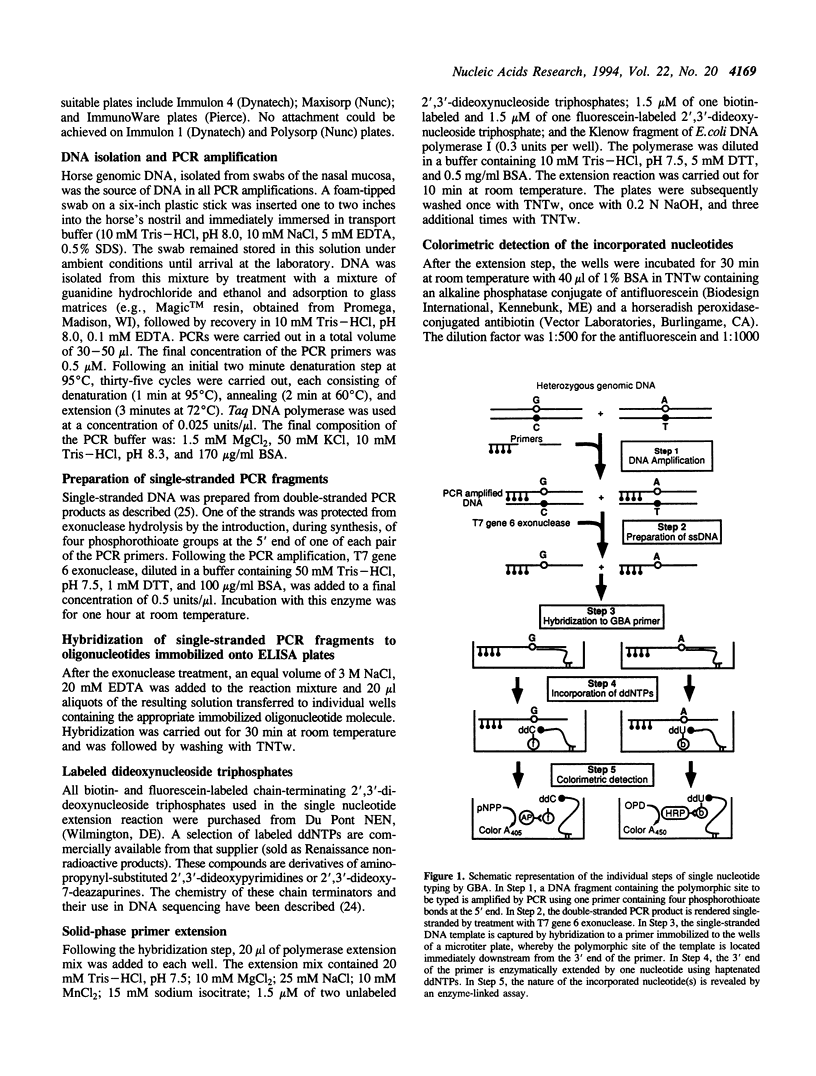
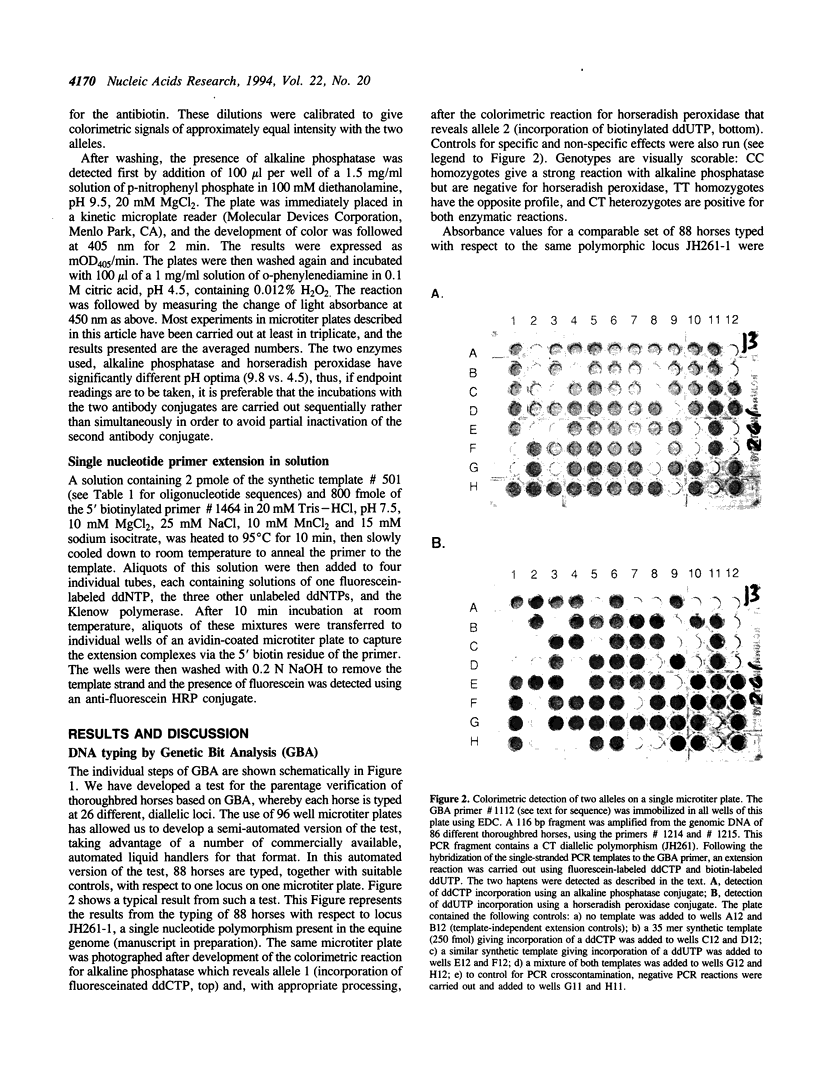
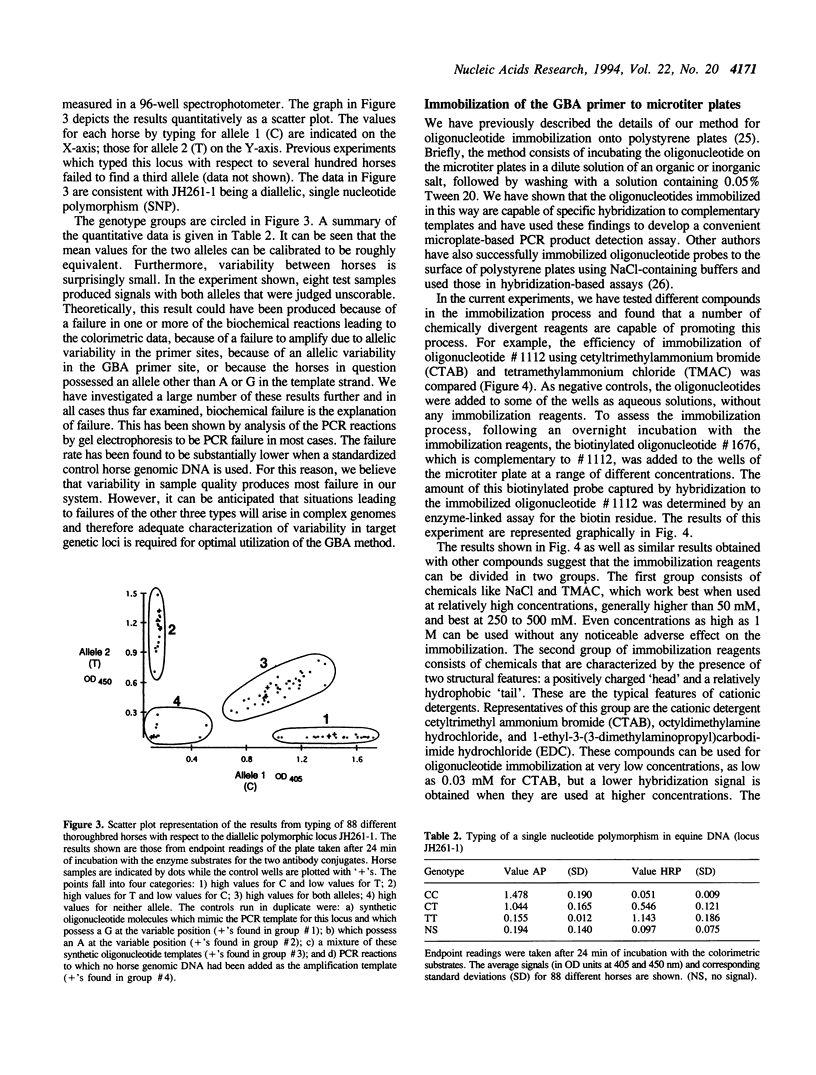
A new method for typing single nucleotide polymorphisms in DNA is described. In this method, specific fragments of genomic DNA containing the polymorphic site(s) are first amplified by the polymerase chain reaction (PCR) using one regular and one phosphorothioate-modified primer. The double-stranded PCR product is rendered single-stranded by treatment with the enzyme T7 gene 6 exonuclease, and captured onto individual wells of a 96 well polystyrene plate by hybridization to an immobilized oligonucleotide primer. This primer is designed to hybridize to the single-stranded target DNA immediately adjacent from the polymorphic site of interest. Using the Klenow fragment of E. coli DNA polymerase I or the modified T7 DNA polymerase (Sequenase), the 3' end of the capture oligonucleotide is extended by one base using a mixture of one biotin-labeled, one fluorescein-labeled, and two unlabeled dideoxynucleoside triphosphates. Antibody conjugates of alkaline phosphatase and horseradish peroxidase are then used to determine the nature of the extended base in an ELISA format. This paper describes biochemical features of this method in detail. A semi-automated version of the method, which we call Genetic Bit Analysis (GBA), is being used on a large scale for the parentage verification of thoroughbred horses using a predetermined set of 26 diallelic polymorphisms in the equine genome.
Full text
PDF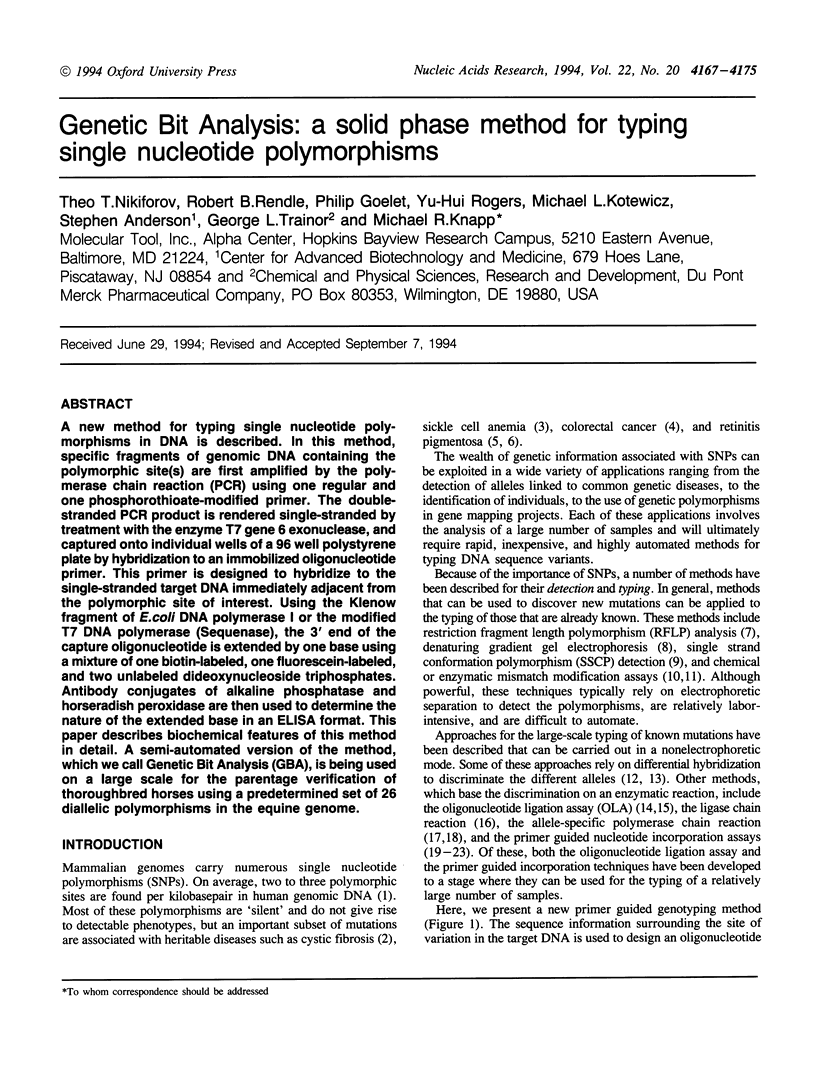

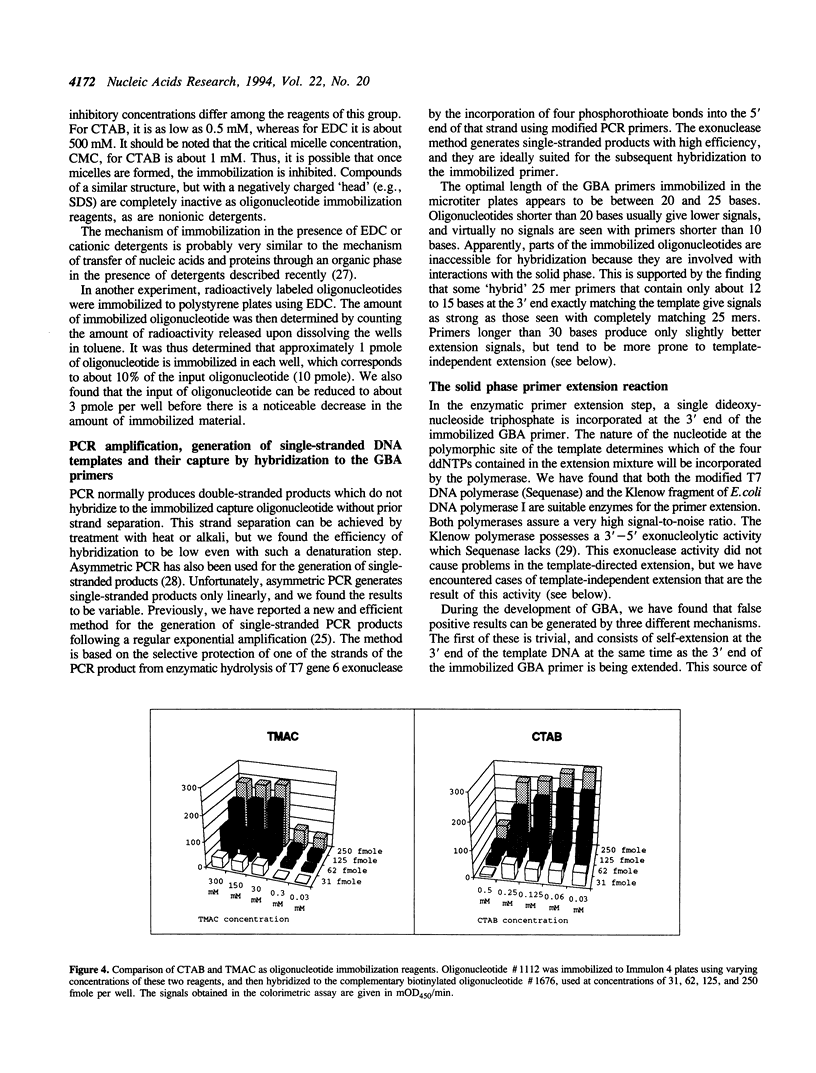
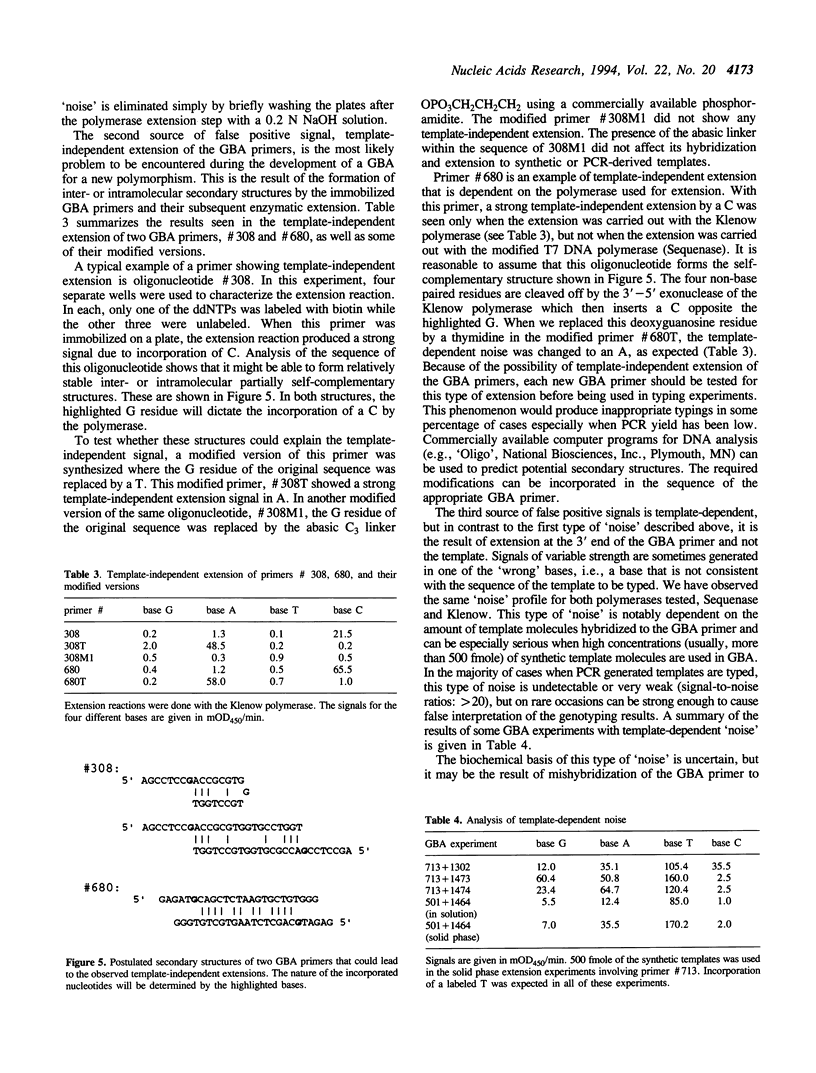


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaguer P., Térouanne B., Allibert P., Cros P., Boussioux A. M., Mandrand B., Nicolas J. C. Detection of single base substitutions in polynucleotides by capture with immobilized oligonucleotides. Mol Cell Probes. 1993 Apr;7(2):155–159. doi: 10.1006/mcpr.1993.1022. [DOI] [PubMed] [Google Scholar]
- Barany F. Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):189–193. doi: 10.1073/pnas.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Bromberg L. E., Klibanov A. M. Detergent-enabled transport of proteins and nucleic acids through hydrophobic solvents. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):143–147. doi: 10.1073/pnas.91.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner B. J., Reyes A. A., Morin C., Itakura K., Teplitz R. L., Wallace R. B. Detection of sickle cell beta S-globin allele by hybridization with synthetic oligonucleotides. Proc Natl Acad Sci U S A. 1983 Jan;80(1):278–282. doi: 10.1073/pnas.80.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Smith B. A., Cooke H. J., Niemann S., Schmidtke J. An estimate of unique DNA sequence heterozygosity in the human genome. Hum Genet. 1985;69(3):201–205. doi: 10.1007/BF00293024. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Goodman M. F., Creighton S., Bloom L. B., Petruska J. Biochemical basis of DNA replication fidelity. Crit Rev Biochem Mol Biol. 1993;28(2):83–126. doi: 10.3109/10409239309086792. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K. PCR-SSCP: a simple and sensitive method for detection of mutations in the genomic DNA. PCR Methods Appl. 1991 Aug;1(1):34–38. doi: 10.1101/gr.1.1.34. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy M. N., Hoffmann J. W., Kasper C. K., Spitzer S. G., Groce S. L., Bajaj S. P. Single nucleotide primer extension to detect genetic diseases: experimental application to hemophilia B (factor IX) and cystic fibrosis genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1143–1147. doi: 10.1073/pnas.88.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U., Kaiser R., Sanders J., Hood L. A ligase-mediated gene detection technique. Science. 1988 Aug 26;241(4869):1077–1080. doi: 10.1126/science.3413476. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson D. A., Kaiser R., Lappin S., Stewart J., Hood L., Landegren U. Automated DNA diagnostics using an ELISA-based oligonucleotide ligation assay. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8923–8927. doi: 10.1073/pnas.87.22.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov T. T., Rendle R. B., Kotewicz M. L., Rogers Y. H. The use of phosphorothioate primers and exonuclease hydrolysis for the preparation of single-stranded PCR products and their detection by solid-phase hybridization. PCR Methods Appl. 1994 Apr;3(5):285–291. doi: 10.1101/gr.3.5.285. [DOI] [PubMed] [Google Scholar]
- Nyrén P., Pettersson B., Uhlén M. Solid phase DNA minisequencing by an enzymatic luminometric inorganic pyrophosphate detection assay. Anal Biochem. 1993 Jan;208(1):171–175. doi: 10.1006/abio.1993.1024. [DOI] [PubMed] [Google Scholar]
- Prober J. M., Trainor G. L., Dam R. J., Hobbs F. W., Robertson C. W., Zagursky R. J., Cocuzza A. J., Jensen M. A., Baumeister K. A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science. 1987 Oct 16;238(4825):336–341. doi: 10.1126/science.2443975. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Walsh P. S., Levenson C. H., Erlich H. A. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6230–6234. doi: 10.1073/pnas.86.16.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Aalto-Setälä K., Harju L., Kontula K., Söderlund H. A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics. 1990 Dec;8(4):684–692. doi: 10.1016/0888-7543(90)90255-s. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Sajantila A., Lukka M. Identification of individuals by analysis of biallelic DNA markers, using PCR and solid-phase minisequencing. Am J Hum Genet. 1993 Jan;52(1):46–59. [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Selective inactivation of the exonuclease activity of bacteriophage T7 DNA polymerase by in vitro mutagenesis. J Biol Chem. 1989 Apr 15;264(11):6447–6458. [PubMed] [Google Scholar]
- Ugozzoli L., Wahlqvist J. M., Ehsani A., Kaplan B. E., Wallace R. B. Detection of specific alleles by using allele-specific primer extension followed by capture on solid support. Genet Anal Tech Appl. 1992 Aug;9(4):107–112. doi: 10.1016/1050-3862(92)90049-b. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Marotta C. A., Forget B. G., Weissman S. M. Structure of human hemoglobin messenger RNA and its relation to hemoglobinopathies. Trans Assoc Am Physicians. 1977;90:117–126. [PubMed] [Google Scholar]
- Wu D. Y., Ugozzoli L., Pal B. K., Wallace R. B. Allele-specific enzymatic amplification of beta-globin genomic DNA for diagnosis of sickle cell anemia. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2757–2760. doi: 10.1073/pnas.86.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]



