SUMMARY
Transgenesis is an essential tool for assessing gene function in any organism, and it is especially crucial for parasitic nematodes given the dwindling armamentarium of effective anthelmintics and the consequent need to validate essential molecular targets for new drugs and vaccines. Two of the major routes of gene delivery evaluated to date in parasitic nematodes, bombardment with DNA-coated microparticles and intragonadal microinjection of DNA constructs, draw upon experience with the free-living nematode Caenorhabditis elegans. Bombardment has been used to transiently transfect Ascaris suum, Brugia malayi and Litomosoides sigmodontis with both RNA and DNA. Microinjection has been used to achieve heritable transgenesis in Strongyloides stercoralis, S. ratti and Parastrongyloides trichosuri and for additional transient expression studies in B. malayi. A third route of gene delivery revisits a classic method involving DNA transfer facilitated by calcium-mediated permeabilization of recipient cells in developing B. malayi larvae and results in transgene inheritance through host and vector passage. Assembly of microinjected transgenes into multi-copy episomal arrays likely results in their transcriptional silencing in some parasitic nematodes. Methods such as transposon-mediated transgenesis that favour low-copy number chromosomal integration may remedy this impediment to establishing stable transgenic lines. In the future, stable transgenesis in parasitic nematodes could enable loss-of-function approaches by insertional mutagenesis, in situ expression of inhibitory double-stranded RNA or boosting RNAi susceptibility through heterologous expression of dsRNA processing and transport proteins.
Keywords: Transgenesis, transfection, parasitic nematode, biolistics, microinjection, transposon
INTRODUCTION
Over three decades following the first reports of transgenesis in the free-living nematode Caenorhabditis elegans (Stinchcomb et al. 1985), this methodology has found only limited applications in studies of the biology of parasitic nematodes. Nevertheless, some recent successes with heritable transgenesis in the Strongyoididae (Grant et al. 2006a; Li et al. 2006; Junio et al. 2008) and filariae (Xu et al. 2011) and some creative applications of transient nucleic acid transfection in studies of gene regulation in ascarids (Cohen et al. 2004; Cheng et al. 2007) and filariae (Higazi et al. 2002, 2004, 2005; Shu et al. 2003; Higazi and Unnasch, 2004; Higazi et al. 2005; Liu et al. 2007; Oliveira et al. 2008; Liu et al. 2010a; Liu et al. 2010b; Bailey et al. 2011) highlight the potentialities of even the limited methods for parasitic nematodes and set the stage for more rapid progress in the near future. This review will examine the methods and routes of nucleic acid transfer explored so far in parasitic nematodes and briefly recount applications of these in studies of molecular, cellular or developmental biology of these organisms. It will also explore the alternative modes of transgene inheritance – chromosomal or episomal – and discuss the gene transfer methods, both present and proposed, that bias transgene fate towards one of these modes or the other. It will discuss some problems and pitfalls associated with transgenesis in parasitic nematodes. Finally, the review will project general future applications of transgenesis in parasitic nematodes, including one suggested by studies in C. elegans (Winston et al. 2007) that could boost RNAi sensitivity in parasitic nematodes.
For purposes of this review, the term transfection will be used to denote the transient, non-heritable transfer of nucleic acids into cultured parasitic nematode cells or embryos or into localized cells or tissues of whole worms and their transient expression there. Transgenesis will denote genetic transformation of whole, live parasitic nematodes accompanied by promoter-regulated, tissue-appropriate transgene expression, and heritable transgenesis will refer to this same type of genetic transformation that is stably inherited and expressed from one parasitic nematode generation to the next. In discussions of non-heritable and heritable transgenesis in this review, the parental or P0 generation will be understood to be the generation of parasitic nematodes into which genes are initially transferred. The F1 and subsequent generations will represent the progeny of these.
ROUTES AND MODES OF NUCLEIC ACID DELIVERY
Genetic modification of parasitic nematodes has been accomplished by delivering DNA or RNA constructs using biolistics (Davis et al. 1999; Jackstadt et al. 1999; Higazi et al. 2002), gonadal microinjection (Lok and Massey, 2002; Grant et al. 2006a; Li et al. 2006) and recently, chemical transfection (Xu et al. 2011) (Summarized in Table 1). Biolistics and gonadal microinjection have been used for both transient transfection with DNA or RNA and for DNA transgenesis. While most definitive for gonadal microinjection and chemical transfection, there is evidence that DNA transfer by any of these methods can result in heritable transgenesis in certain parasitic nematodes. Selection of one method over another will generally be based on the parasite species in question, the availability of stages in that species that can be cultured in vitro or ex vivo and the numbers of parental transformants that are required for establishment of stable transgenic lines. The mode of DNA delivery into nematodes also appears to affect the relative frequencies of episomal transgene incorporation or integration into the chromosome (Mello and Fire, 1995; Praitis et al. 2001; Praitis, 2006).
Table 1.
Summary of gene delivery methods used to date in parasitic nematodes with notes on resulting transgene fates and technical advantages and disadvantages of each
| Gene delivery method | Episomal arrays formed? | Relative frequency of spontaneous chromosomal integration | Heritable transgenesis achieved? | Stable transgenic lines derived? | Technical advantages | Technical disadvantages | Key references |
|---|---|---|---|---|---|---|---|
| Gonadal microinjection | Yes | Low | Yes | Yes | C. elegans methods easily adapted to Strongyloides and Parastrongyloides | Specialized equipment required; learning curve for microinjection technique | Li et al. 2006; Grant et al. 2006a; Junio et al. 2008 |
| Microparticle bombardment | Yes | High | ? | No | DNA transfer protocols are rapid and easy. Potential to transfect large numbers of parasites in a single bombardment | Specialized equipment required. Risk of physical damage to bombarded parasites. Selectable co-transformation markers not yet available | Jackstadt et al. 1999; Davis et al. 1999; Higazi et al. 2002; Praitis et al. 2001, 2006 |
| Chemical transformation | Unkown | Unknown | Yes | No | DNA transfer is easy | Maximum gene transfer efficiency in Brugia requires administration during larval moulting | Xu et al. 2011 |
Biolistic transfer of nucleic acids
Transfection or transformation of living tissues or organisms may be achieved by bombardment with nucleic acid-coated tungsten, glass or, most often, gold microparticles driven by pressurized helium. Biolistics was first used in nematodes for DNA transformation of C. elegans (Jackstadt et al. 1999; Wilm et al. 1999) and for transient transfection of Ascaris suum embryos with both DNA and RNA constructs (Davis et al. 1999). The work employing biolistic transfer of DNA and RNA in Ascaris provides an excellent example of how well planned applications of transient transfection can yield important new insights into mechanisms of gene regulation in parasites. Specifically, these studies revealed the nature of a minimal splice leader promoter in Ascaris (Davis et al. 1999) and provided important new insights into aspects of pre-mRNA processing (trans-splicing and capping) that affect translation efficiency (Cohen et al. 2004).
The filariae Litomosoides sigmodontis (Jackstadt et al. 1999) and Brugia malayi (Higazi et al. 2002) are also susceptible to transformation by biolistic DNA transfer. In the case of L. sigmodontis, DNA transferred by bombardment was apparently spread systemically in parental adult worms and expressed in promoter-regulated fashion in specific tissues of male and female worms. Intriguingly, small numbers of F1 microfilariae expressing the lacZ reporter transgene were recovered following implantation of bombarded L. sigmodontis adults into rodent hosts (Mastomys coucha) (Jackstadt et al. 1999). The ability of these microfilariae to cycle through vector mites and rodent hosts was not ascertained, and further work involving transgenesis in L. sigmodontis has not been forthcoming to date. Biolistic transfection has been used to transfer DNA into embryos of Brugia malayi, and this system has yielded a wealth of information on the characteristics of minimal and optimal promoters for a number of genes in this parasite (Shu et al. 2003; Higazi et al. 2005; Bailey et al. 2011), the primary and secondary structural characteristics of trans-splicing elements (Higazi and Unnasch, 2004; Liu et al. 2010a) and the existence of operons in Brugia and their structural and functional characteristics (Liu et al. 2010b). This approach has also demonstrated the presence of an intact ecdysone signaling system in Brugia in which the ecdysone receptor homologue interacts in vivo with response elements in transfected gene constructs to upregulate reporter expression, thus underscoring the potential for ecdysteroid regulation of moulting or reproduction in this parasite (Tzertzinis et al. 2010). Thus, biolistic transfection, when used to induce transient expression in embryos, has proven a highly successful system for basic studies of gene regulation in Brugia. However, heritable transformation of parasitic nematodes by the biolistic route has not been observed. Culture conditions that allow development of transfected Brugia embryos to developmentally competent microfilariae have not yet been identified, and attempts to transfect later larval stages via the biolistic route have resulted in such severe damage to the cuticles of these forms as to render them non-viable (Higazi et al. 2002; Xu et al. 2011). As indicated above, transgenic microfilarial progeny of L. sigmodontis transfected by microparticle bombardment were not assessed for their ability to undergo further development in vectors and hosts and to transmit the transgenes to their progeny (Jackstadt et al. 1999).
In contrast to the results to date with parasitic nematodes, biolistic DNA transfer results in a high frequency of heritable transformation in C. elegans, albeit with high mortality in the P0 generation (Jackstadt et al. 1999; Wilm et al. 1999). Unlike gonadal microinjection, which favours assembly of transgenes into high-copy number episomal arrays in C. elegans (Mello and Fire, 1995), microparticle bombardment appears inherently to favour low-copy number chromosomal insertions (Praitis et al. 2001) and also may result in a significant frequency of homologous recombination (Berezikov et al. 2004) (Table 1). As noted by Praitis and colleagues, the ability of bombardment to integrate relatively small numbers of transgene copies into the C. elegans chromosome mitigates against the confounding physiological effects of transgene overexpression in experimental settings, this being particularly important with transgenes whose products are toxic when expressed at high levels. In demonstrating stable integrative transformation following biolistic DNA transfer, Praitis et al. (2001) also used a strategy of unc-119 mutant rescue to select transformants from among large populations of bombarded worms. This strategy, which is now standard in methodology for C. elegans (Evans, 2006) involves gene transfers (bombardments) in the unc-119 mutant background and incorporation an unc-119 rescuing plasmid as a co-transformation marker with the transgene of interest. Rescue of the motility-deficit phenotype in unc-119 worms, conferring normal progressive motility, greatly facilitates isolation of viable transformants from the large excess of non-transformed worms following bombardment.
In summary, the biolistic route of nucleic acid transfer has been used for transient transfections to study aspects of gene regulation and mRNA processing in parasitic nematodes. It has not, however, been used successfully to generate stable, heritable DNA transformation in these organisms. In general, this has been attributed to the inability to identify bombardment conditions that yield developmentally competent parasite stages for establishment in animal hosts. Nevertheless, modern biolistic systems allow for some flexibility in modifying conditions under which microparticles are propelled into recipient cells or animal tissues, and with suficient study, such conditions might be identified for some parasitic nematodes. The rewards for such an achievement could be significant given the advantages of biolistic gene transfer in C. elegans. First, this method results in a high frequency of spontaneous, low-copy transgene insertions into the chromosomes of recipient worms, giving transgenic lines that ‘breed true’ and are not subject to the confounding effects of high-copy incorporation into episomal arrays that are the predominant result of gonadal microinjection. Second, the potential of biolistics to transfer DNA into small stages such as embryos or developing larvae could provide an entré for transgenesis in the majority of parasitic nematode species in which adult worms are essentially inaccessible within the host.
Gonadal microinjection
The syncytial nature of the C. elegans gonad and the overall transparency of this worm, allowing easy visualization of this region by differential interference contrast (DIC) microscopy, has made gonadal microinjection the most widely used approach to transgenesis in this model organism (Mello and Fire, 1995; Evans, 2006). The presence of free-living females in the life cycles of Strongyloides and related genera of parasitic nematodes and the similarity of these free-living females to C. elegans hermaphrodites in terms of size, cuticular transparency and the syncytial character of their gonads (Schad, 1989; Viney and Ashford, 1990; Viney, 1999; Grant et al. 2006b; Viney and Lok, 2007), has facilitated adaptation of gonadal microinjection as a method for gene transfer in these parasites (Table 1). Expression of reporter constructs in embryos of S. stercoralis following intragonadal microinjection was achieved almost a decade ago (Lok and Massey, 2002). However, it was not until these constructs were modified to contain an S. stercoralis-derived 3′ UTR that promoter-regulated, tissue-specific expression of transgene constructs was attained in F1 progeny of parental S. stercoralis transformed by gonadal microinjection (Li et al. 2006). Subsequently, an expanded number of additional fluorescent reporter constructs was developed, each incorporating a promoter with a unique tissue-specific pattern of activation, and the 3′ UTR from the S. stercoralis gene Ss-era-1 as a standard, multifunctional terminator (Junio et al. 2008). These have been collected, along with promoterless vectors encoding either red or green fluorescent protein in all possible reading frames, into a small vector kit that is available to the research community through AddGene at http://www.addgene.org/pgvec1?identifier=Lok&f=c&cmd=searchpl&x=28&y=12. This mode of transgenesis was recently applied in a functional study that demonstrated the requirement for Ss-daf-16 (previously denoted fktf-1) function in normal morphogenesis of infective third-stage larvae (L3i) in S. stercoralis (Castelletto et al. 2009). Ss-daf-16 encodes the orthologue of a synonymous insulin-regulated transcription factor that brings about dauer larval development in C. elegans (Lin et al. 1997; Ogg et al. 1997; Massey et al. 2003). Recently, we showed that vector constructs designed for S. stercoralis are also expressed in F1 larvae of Strongyloides ratti derived from parental free-living females transformed by gonadal microinjection (Li et al. 2011). Anatomical expression patterns of these reporter constructs are virtually identical in the two parasites.
Parastrongyloides trichosuri, a parasite of the Australian brush-tailed possum, is also capable of undertaking generations of free-living development, and its free-living females may also be transformed by intragonadal microinjection of DNA vectors (Grant et al. 2006a). A reporter construct encoding a fusion of GFP and β-galactosidase under the Pt-hsp-1 promoter was expressed in progeny of microinjected free-living females and in parasitic females explanted from the host. Although production of transgenic F1 larvae from microinjected parasitic females was inefficient and limited by the transient viability of the parental worms in culture this latter finding is significant because it may point to a feasible protocol for DNA transformation of parasitic nematodes, such as hookworms and trichostrongyles, that do not form free-living adults.
Unlike S. stercoralis, and most other Strongyloides spp., which can undergo only one generation of free-living development before reverting to parasitism, P. trichosuri may undergo an apparently unlimited number of free-living generations under optimum conditions, and yet retain the capacity to form parasitic L3i and invade the host under conditions of crowding or food depletion. Broadly viewed, this property gives P. trichosuri the potential to fulfill the role of a genetic model for the study of parasitic nematode biology (Grant et al. 2006b). With specific regard to transgenesis, it has allowed stable transgenic lines to be derived by in vitro passage whereas this process requires alternating rounds of host and culture passage in S. stercoralis.
The filaria Brugia malayihas been transiently transformed by intragonadal microinjection of plasmid vectors (Higazi et al. 2002). Although it was impossible in the context of this study to identify a gonadal syncytium in B. malayi, adult worms microinjected in several locations along the uterus expressed both GFP- and luciferase-encoding vectors. Localization studies revealed that, whereas biolistic gene transfer resulted in expression restricted to hypodermal cells of bombarded adults, embryos proximal to points of intrauterine microinjection took up and expressed GFP-encoding vector constructs, leaving open the possibility of heritable transformation via this route. Exploring this possibility would require that explanted, microinjected adult B. malayi be reared either in vitro under conditions supporting embryonic development to the microfilarial stage or by re-implantation into susceptible hosts such as Mongolian jirds. To date, the dificult experiments required to make this assessment have not been undertaken. Recent results on successful chemically mediated transformation of B. malayi (Xu et al. 2011) may obviate this process.
Chemically mediated transformation
One of the primary barriers to uptake of exogenous DNA into cells is the hydrophobicity of cell membranes relative to this polar molecule. For almost four decades it has been standard procedure to render bacterial cell membranes permeable to exogenous naked DNA by pretreating with high concentrations of calcium (Mandel and Higa, 1970; Cohen et al. 1972). Similarly, eukaryotic cells may take up exogenous naked DNA directly when it is presented as a precipitate with CaPO4 (Sambrook et al. 1989). Recently, this simple approach was shown to mediate heritable DNA transformation of B. malayi (Xu et al. 2011) (Table 1). This was accomplished by co-injecting infective B. malayi larvae and DNA/CaCl2 precipitates into the peritoneal cavities of susceptible Mongolian jirds. A large proportion of developing parasites took up the DNA constructs as indicated by detectable transgene expression in over 60% of adults recovered from jirds treated in this way. Most significantly, these transgenic adults produced F1 transgenic microfilariae that survived cryopreservation and, upon thawing, demonstrated developmental competency by infecting mosquitoes at a frequency equal to that of non-transformed cryopreserved microfilariae. While the frequency of germline transformation among these F1 progeny remains to be determined, this finding represents a highly significant advancement towards a stable DNA transformation in B. malayi, which represents a complex of lymphatic-dwelling filariae that cause disease in over 200 million persons. Moreover, it demonstrates the utility of a long-standing method for cell transfection in transforming whole parasitic nematodes and may indicate a simpler method of DNA transfer into parental worms of many species than either microparticle bombardment or gonadal microinjection.
POSSIBLE FATES AND MODES OF INHERITANCE OF ADMINISTERED TRANSGENES IN PARASITIC NEMATODES
Based upon experience with C. elegans and other nematodes for which transgenesis has been achieved, the fate of transgene sequences administered as plasmid DNA depends to a great degree upon the route of DNA transfer. While transgenes may be stably transmitted as extrachromosomal elements, we submit that single- or low-copy chromosomal integration of transgenes is the goal of efforts towards transgenesis in parasitic nematodes.
Formation of episomal arrays
In up to 90% of C. elegans transformed with plasmid-encoded transgenes introduced by gonadal microinjection, DNA is simply partitioned among somatic cells, expressed transiently and not inherited. In the remaining 10% of transformants, the great majority have transgenes assembled into tandem, multi-copy episomal arrays (Mello and Fire, 1995). These have been aptly described as behaving like mini-chromosomes that may be stably transmitted, though not in Mendelian fashion, through multiple generations in C. elegans (Schlager et al. 2009). It is assumed, but has not been rigorously proven, that microinjected transgene sequences are also assembled into repetitive episomal arrays in parasitic nematodes (Grant et al. 2006a; Junio et al. 2008). There are several disadvantages to maintenance of transgenes as episomal arrays. First, the arrays themselves may be lost after repeated generations of passage (Schlager et al. 2009) or transcriptionally silenced, especially in the germline (Kelly et al. 1997). Second, the highly repetitive nature of these arrays may result in levels of overexpression that are either toxic to transformed worms or exert non-physiological effects in them (Praitis et al. 2001).
Although transient transfection and transgenesis have found application in a number of studies of gene function and regulation in parasitic nematodes (Unnasch et al. 1999; Higazi et al. 2002, 2005; Higazi and Unnasch, 2004; Oliveira et al. 2008; Castelletto et al. 2009; Liu et al. 2010b; Tzertzinis et al. 2010; Bailey et al. 2011), transgenesis will only become a robust functional genomic tool when it becomes generally practical to derive stable transgene-expressing lines of parasitic nematodes. This goal has been technically accomplished in P. trichosuri which, by virtue of its ability to cycle continuously in free-living culture, has allowed stable transgene-expressing lines to be established by culture passage and transferred to the possum host for future study (Grant et al. 2006a). By contrast, consolidation of recent successes with production of F1 transgenic S. stercoralis, S. ratti (Li et al. 2006; Junio et al. 2008; Li et al. 2011) and B. malayi (Xu et al. 2011) by establishment of transgenic lines will require alternating generations of host and culture passage for Strongyloides spp. and serial rounds of passage in both vector and host for B. malayi. It is anticipated that this will also be true for other medically important parasitic nematodes for which transgenesis is achieved in the future.
In the strict sense, stable transgenesis was achieved some years ago in S. stercoralis when F1 transgenic larvae, derived by gonadal microinjection of circular plasmid vector encoding GFP under the Ss-act-2 promoter, were subjected to four alternating passages in susceptible gerbils and free-living culture to an F5 generation. Transgene (gfp)-specific sequences were detected in each generation by PCR, indicating stable transformation, but GFP expression, as evidenced by visual detection of the fluorescent protein or by transcription of specific mRNA, could be detected only in the F1 generation (Junio et al. 2008). This silencing may be due to epigenetic mechanisms incited by some aspect of the configuration of transgene DNA in the parasite.
It is assumed that, like C. elegans, parasitic nematodes assemble gonadally microinjected transgene sequences, especially those delivered in circular plasmid vectors, into multi-copy episomal arrays. Although this assumption has not been rigorously proven in Strongyloides spp. or Parastrongyloides trichosuri, there is indirect evidence in support of it. First, transgenic parasitic female S. stercoralis that are derived from free-living parents transformed by gonadal microinjection transmit transgene sequences to less than 100% of their progeny (Junio et al. 2008). This pattern of inheritance is also seen in serial culture passage of transgenic P. trichosuri (Grant et al. 2006a) and is typical of C. elegans carrying transgenes in episomal arrays (Mello and Fire, 1995). Second, inverse PCR analyses conducted to date on transgenic S. stercoralis derived from parents microinjected with circular plasmids have revealed only stretches of vector sequence flanking reporter transgenes and never genomic sequence from the parasite (Lok et al. unpublished). Similarly, Southern blots of total DNA from transgenic S. stercoralis only show transgene sequences that co-migrate with the vector.
Apparent transcriptional silencing of transgenes in S. stercoralis and P. trichosuri may also be related to their assembly into repetitive episomal arrays. The induction of epigenetic silencing by tandem repeat sequences in mammalian genes (Soragni et al. 2008), leaves open the possibility that parasitic nematodes have a high propensity, relative to C. elegans, to silence coding sequences in tandem repetitive arrays by epigenetic mechanisms. The problem of transcriptional silencing and loss of episomal transgene arrays has been discussed in the context of efforts to establish stable transgenesis in the necromenic nematode Pristionchus pacificus (Schlager et al. 2009). In this case, a retrospective analysis of various protocols for transforming P. pacificus by microinjection of DNA into the gonadal syncytium revealed that coinjection of linearized plasmid vectors with digests of genomic DNA from the worm with complementary restriction ends resulted in the highest frequency of stable transgene-expressing lines of several protocols tested, including syncytial injection of circular plasmid. The logical interpretation of this result was that the highlighted protocol resulted in interspersing of transgene sequences with significant stretches of gDNA from Pr. pacificus, thus reducing the repetitive character of the episomal arrays and rendering them less susceptible to epigenetic silencing or elimination (Schlager et al. 2009). This finding has been reviewed and the potential of such heterogenous arrays to inhibit transgene silencing in medically important parasitic nematodes discussed (Lok, 2009).
Chromosomal integration
Beyond their likely repetitive character, the episomal location of microinjected transgene sequences per se may also be a deciding factor in their silencing by parasitic nematodes. For this reason, methods of gene transfer that favour low-copy chromosomal integration may be the keys to establishment of parasite lines that inherit and express transgenes stably. The standard route of microinjecting plasmid-based constructs into the gonadal syncytium of C. elegans strongly favours formation of episomal transgene arrays, and spontaneous chromosomal integrations are exceedingly rare (Mello and Fire, 1995). By contrast, injection of such constructs into individual oocyte nuclei results in 0·1–0·5% of C. elegans with transgene integrations (Fire, 1986; Mello and Fire, 1995). Similarly, microinjecting linear transgene constructs into the gonadal syncytium in the presence of an excess of random 80-base oligonucleotides appears to promote recombination and results in approximately 1% of worms with chromosomal integrations (Mello et al. 1991). Other approaches to integration of transgenes in C. elegans involve exploiting DNA repair mechanisms to drive transgene incorporation into chromosomes at breaks induced by gamma (Mello and Fire, 1995; Evans, 2006) or UV irradiation (Evans, 2006). Although they possess the advantage of having been standardized into reliable, published ‘bench protocols’ for C. elegans (Evans, 2006), these variations on microinjection technology all have disadvantages as practical alternatives for parasitic nematodes. For example, starting material for the methods involving integration of transgenes at induced chromosomal breaks is always a stable episomal line. With the exception of Parastrongyloides trichosuri (Grant et al. 2006a), no such episomal line has been derived in any parasitic nematode. Also, the frequency of integrations, even in the facilitated system outlined by Mello (Mello et al. 1991), is less than 1%, suggesting that the chances of encountering an integrant among the F1 progeny of a microinjected parasitic nematode are remote. As has been discussed above, microparticle bombardment inherently favours integrative transformation of C. elegans relative to microinjection of transgene sequences, and the proportion of bombardments that results in an integrated transgenic line may be as high as 35% (Praitis et al. 2001). However, even in this case, the practicality of the system for parasitic nematodes must be assessed in light of the fact that in C. elegans, transformants constitute only about 0·05% of the bombarded population. To the extent that these efficiencies may be extrapolated to parasitic nematodes, they combine to predict that production of a single chromosomal integrant individual would require bombardment of about 6,000 parasites. The fact that using such integrants to establish integrated transgenic lines would require passage in a susceptible host further diminishes the likelihood of success. Clearly, the ‘arithmetic’ dictates that alternative means of integrating transgene sequences into the chromosomes of parasitic nematodes be explored. In other systems, vectors encoding elements such as transposons or retroviruses with intrinsic capability to integrate into chromosomal DNA have provided a means of directly and efficiently incorporating transgenes into the genomes of recipient cells, tissues or whole organisms. Both of these approaches have been employed with a high degree of success in schistosomes (Mann et al. 2007) (see also Beckmann and Grevelding, in this special issue of Parasitology) and should be examined for their feasibility in parasitic nematodes.
Transposable elements and retroviruses as vectors for integrative transformation
The development of transposon-mediated transgenesis in schistosomes provides an appropriate model for development of this approach in parasitic nematodes. A valuable background for this work is the increasingly substantial literature on mobile genetic elements that occur naturally in the genome of Schistosoma mansoni (Brindley et al. 2003; Mann et al. 2007). These endogenous elements are generally thought to have been rendered inactive by accumulating mutations, but it has been pointed out (Brindley et al. 2003; Mann et al. 2007) that such degenerate sequences can, like the Sleeping Beauty Tc1/Mariner-class transposon of salmon (Plasterk et al. 1999), be repaired by deliberate ‘back mutation’ and recovered as active elements for functional genomic study. While this avenue has not yet been explored in schistosomes, a transposon of insect origin, piggyBac, has been used to achieve integrative transgenesis in S. mansoni (Morales et al. 2007). This successful effort involved transfection of schistosomules with a linearized donor plasmid encoding a luciferase reporter gene flanked by the piggyBac-specific terminal inverted repeats and with in vitro-transcribed mRNA encoding the piggyBac transposase. Schistosomules transduced with these elements expressed active luciferase, and Southern hybridization and retrotransposon-anchored PCR (RAP) confirmed chromosomal integration of the reporter transgene. Sequencing of RAP products indicated that integrations were specific for TTAA insertion sites and occurred in at least 19 locations in the S. mansoni genome. It is also noteworthy that the piggyBac transposon system has formed the basis of a robust method of integrative transgenesis and a powerful functional genomic tool in Plasmodium spp. (Balu et al. 2005, 2009; Balu and Adams, 2006; Fonager et al. 2011). In addition to its applicability to highly diverse taxa, the piggyBac transposon has the advantage of its ability to transpose large inserts or ‘cargoes’ relative to other transposons (Nakazawa et al. 2009).
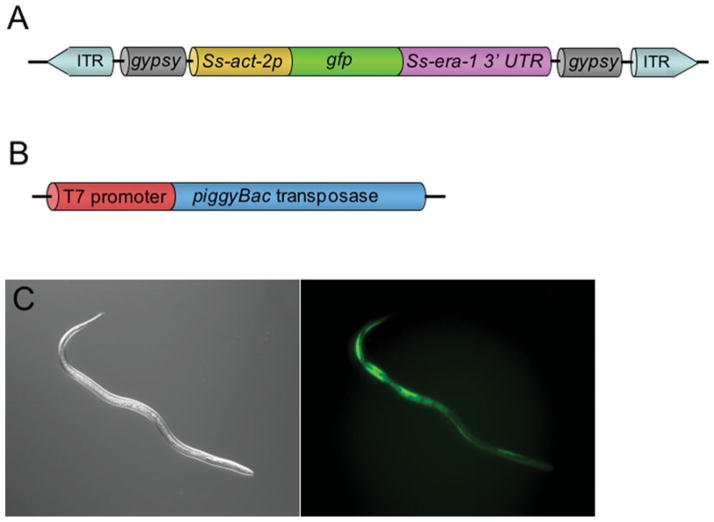
The success of transposon-mediated transgenesis in Schistosoma sp. and Plasmodium sp. argues strongly for efforts along similar lines in parasitic nematodes. Endogenous transposons have been discovered in a number of parasitic nematode species (Brindley et al. 2003; Laha et al. 2006, 2007). The bandit transposon of Ancylostoma caninum is particularly fascinating in that its close sequence homology to human Mariner-like elements suggests the likelihood of lateral gene transfer between host and parasite (Laha et al. 2007). Successful systems for transfection or transgenesis are prerequisites for testing a transposon-mediated system and their existence in Strongyloides stercoralis, S. ratti, Parastrongyloides trichosuri, Brugia malayi, Litomosoides sigmodontis and Ascaris suum argues strongly for a concerted effort to achieve transposon-mediated integrative transgenesis in these parasitic nematodes. Its successful application in parasite taxa as diverse as Schistosoma and Plasmodium make a strong case for the piggyBac system as an opportune subject for this effort. To this end our laboratory has recently deployed the piggyBac transposon system as a possible means of integrative transgenesis in S. stercoralis and S. ratti (Shao et al., unpublished). A donor vector encoding GFP expressed under the promoter for Ss-act-2, which encodes a cellular actin principally localized in the body wall, and flanked by the piggyBac-specific inverted terminal repeats (Fig. 1A) was injected into the gonads of free-living S. stercoralis or S. ratti females along with capped mRNA transcribed in vitro from a plasmid encoding the piggyBac transposase under the T7 promoter (Fig. 1B). It should also be noted that in the donor construct (Fig. 1A), the Ss-act-2::gfp sequence was flanked by the gypsy retroviral insulator sequences from Drosophila as a precaution against transcriptional silencing of the transgene (Markstein et al. 2008). GFP-positive F1 larvae derived in culture from the microinjected female worms were reared to infective third-stage larvae (L3i) and were inoculated into susceptible gerbils (S. stercoralis) or rats (S. ratti) and F2 transgenic parasites were collected from the faeces of the animals at patency. Experiments to ascertain chromosomal integration of the transgenes in this study are still underway but, for both parasites tested, this combination of constructs yielded the first GFP-expressing F2 transgenics observed by us to date (Fig. 1C; Table 2). It is significant that in C. elegans such F2 transgenics serve as the founders of stable transgenic lines (Mello and Fire, 1995; Evans, 2006). The yield of transgene-expressing F2 individuals as a proportion of inoculated F1 transgenic L3i was almost eight-fold higher in S. ratti than in S. stercoralis (Table 2), probably owing to the higher infection efficiency of S. ratti in the rat. This fact underscores the advantage of S. ratti as a model with which to move forward with integrative transgenesis and establishment of stable transgenic lines.
Fig. 1.
Transgenesis in Strongyloides stercoralis and S. ratti mediated by the piggyBac transposon. A. Donor plasmid containing the coding sequence of gfp linked to the Ss-era-1 3′ UTR and expressed under the promoter for the S. stercoralis cellular actin Ss-act-2. Coding sequences are flanked by the gypsy retroviral insulator sequences from Drosophila and by the piggyBac inverted tandem repeats (ITR). B. Construct for in vitro transcription of mRNA encoding the piggyBac transposase. C. DIC (left) and fluorescence images (right) of an F2 transgenic S. stercoralis L3i expressing GFP. These and similar S. ratti larvae represent the first transgene-expressing individuals of the F2 generation observed to date for these parasites.
Table 2.
Preliminary findings on heritable transposon-mediated transformation and the first sustained transgene expression in the F2 generations of Strongyloides stercoralis and S. ratti
| Parasite | Construct | No. passage attempts | Total no. GFP+ F1 L3i inoculated | Host | Total no. (%) parasitic females recovered | No. (%) GFP+ parasitic females recovered | No. (%) GFP+ F2 larvae recovered |
|---|---|---|---|---|---|---|---|
| S. stercoralis | * piggyBac/gypsy Ss-act-2p::gfp | 4 | 448 | Gerbil | 12 (2·67) | 4 (0·89) | 2 (0·44) |
| S. ratti | * piggyBac/gypsy Ss-act-2p::gfp | 3 | 88 | Rat | 8 (9·09) | 1 (1·13) | 3 (3·39) |
The potential of retroviral vectors for transgenesis in parasitic nematodes is indicated by their successful application to transgenesis in schistosomes (Kines et al. 2006; Mann et al. 2007; Yang et al. 2010) (see also Beckmann and Grevelding, in this special issue of Parasitology). A vesicular stomatitis glycoprotein pseudotyped Maloney murine leukemia virus was used to transform schistosomules of S. mansoni (Kines et al. 2006) and S. japonicum (Yang et al. 2010). Schistosomules transformed in this manner express active luciferase (S. mansoni) or human telomerase reverse transcriptase (S. japonicum) from reporter transgenes. Moreover, the VSVG pseudo-typed leukemia virus vector serves to integrate transgenes into the chromosomes of S. mansoni (Kines et al. 2008). As with the piggyBac transposon system, transgene integration was confirmed by Southern hybridization and integration site boundaries were detected and sequenced by RAP. These analyses identified 16 integration sites of the retrovirus-encoded luciferase reporter in the S. mansoni genome with a common gGATcc motif at each. These results also call for evaluation of pseudotyped retroviruses as vectors for integrative transgenesis in parasitic nematodes.
FUTURE DIRECTIONS IN DEVELOPMENT AND APPLICATION OF TRANSGENESIS IN PARASITIC NEMATODES
The application of transient transfection in parasitic nematodes to studies of transcriptional gene regulation has already been actively pursued and the many substantial findings resulting from these studies have been discussed earlier in this review (Cohen et al. 1972; Davis et al. 1999; Higazi et al. 2002, 2005; Cohen et al. 2004; Higazi and Unnasch, 2004; Liu et al. 2007, 2009, 2010a,b; Tzertzinis et al. 2010; Bailey et al. 2011). Transient transfection serves satisfactorily for the experiments outlined in these studies, and it is likely and highly appropriate that these approaches continue to be used where possible for further studies of gene regulation in parasitic nematodes, especially in species that do not lend themselves to the manipulations necessary for achieving stable transgenesis. The following are applications for transient transfection or transgenesis that can presently be applied in Brugia spp. and in Strongyloides and related parasites and that will be extended to other medically important species as technical advances allow.
Analyzing spatial patterns of gene expression and trafficking of gene products in living parasitic nematodes
Constructs linking gene promoters to fluorescent, antigenic or chromogenic reporters have provided information about the patterns of gene expression in cells, tissues and organs of many organisms, including C. elegans, and these techniques will certainly translate to studies of the anatomical pattens of gene expression in parasitic nematodes as transgenesis is attained in these important pathogens. Reporter constructs incorporating promoters with high levels of site specificity have been reported in P. trichosuri (Grant et al. 2006a), S. stercoralis (Li et al. 2006; Junio et al. 2008; Castelletto et al. 2009) and S. ratti (Li et al. 2011), and consistent anatomical patterns of reporter gene expression have been seen in transiently, as well as stably, transformed individuals. A caveat in interpreting such results is the putative nature of the promoters involved. In fact, the characteristics of minimal and optimal promoters have been rigorously defined in only one parasitic nematode to our knowledge, Brugia malayi (Shu et al. 2003; Higazi et al. 2005; Bailey et al. 2011), and the majority of other studies employing reporter transgenes in parasitic nematodes have assumed that one to three kilobases of 5′ flanking sequence from the coding region of a gene contains an authentic promoter. The caveat notwithstanding, the overall similarities of gene expression patterns deduced by this approach in parasitic nematodes to patterns reported for orthologous genes in C. elegans (compare, for example, findings by Castelletto et al. (2009) and Lee et al. (2001) on daf-16 orthologues in S. stercoralis and C. elegans) bolster confidence that this assumption is valid.
Elegant and comprehensive studies of organellar dynamics (Dzierszinski et al. 2004; Nishi et al. 2008) and secretory trafficking (Joiner and Roos, 2002) in the protozoan Toxoplasma gondii illustrate the potentialities of a robust system for transgenesis in the basic cell biology of a medically important parasite. Avenues such as these, which draw heavily upon the ability to express organelle-specific fluorescent reporters, will be opened for parasitic nematodes when stable transgenesis is achieved. Indeed, a transient transformation system in which a GFP-tagged Ss-DAF-16 was expressed in Strongyloides stercoralis, has already been used to find that nuclear versus cytoplasmic localization of this FOXO-class transcription factor is determined by phosphorylation of consensus AKT sites on the molecule (Castelletto et al. 2009). Stable transgenesis will enable a wide range of similar experimentation on parasitic nematodes in the future.
Facilitating RNAi as a functional genomic tool in parasitic nematodes
Given that the use of RNAi as a tool for functional genomic study was pioneered in C. elegans and that, with some reservations, the technique has been applied effectively in schistosomes, it is disappointing that RNAi has failed thus far to be widely applicable to studies in parasitic nematodes of animals (Knox et al. 2007). Very promising results have been obtained with filariae (Aboobaker and Blaxter, 2003; Lustigman et al. 2004; Ford et al. 2005, 2009; Pfarr et al. 2006; Tachu et al. 2008) and there have been some positive technical advances recently (Song et al. 2010; Samarasinghe et al. 2011). However, overall, a relatively small number of RNAi- sensitive targets have been interrogated successfully, and a relatively low efficiency in target knockdown and the consequent infrequency of informative phenotypes have hampered application of RNAi in many species of animal parasitic nematodes.
A system for stable transgenesis might go far towards surmounting the obstacles that hamper application of RNAi methodology in some parasitic nematodes. To the extent that anatomical barriers to uptake or cell-to-cell spread of exogenously applied dsRNA are limiting, vector-based RNAi, whereby dsRNA, short interfering RNA (siRNA) or short hairpin RNA (shRNA) is expressed from a transgene construct, could provide an answer for parasitic nematodes. Robust vector-based RNAi has been achieved in C. elegans (Tavernarakis et al. 2000) and adapted for large-scale screening approaches (Johnson et al. 2005), providing a model for the way forward in parasitic nematodes. As discussed by Beckmann and Grevelding (in this special issue of Parasitology), vector-based RNAi has been achieved in schistosomes (Zhao et al. 2008; Tchoubrieva et al. 2010; Ayuk et al. 2011), and this fact should make in vivo expression of interfering RNAs a top priority once stable transgenesis in parasitic nematodes is achieved.
Deficiencies in key protein components of the processing ‘machinery’ for exogenously applied dsRNA have been proposed as a cause of low or highly variable RNAi sensitivity in parasitic nematodes (Knox et al. 2007). However, a recent transcriptomic survey (Dalzell et al. 2011) reveals that although parasitic nematodes generally have a less diverse repertoire of RNAi processing or transport proteins than C. elegans, they have conserved the major classes of them such that all are qualitatively represented. This study concludes that the low RNAi sensitivity that characterizes parasitic nematodes overall is not associated with any specific deficiency in RNAi processing or transport proteins and is more likely due to morphological barriers to dsRNA uptake or dissemination or to allelic differences in parasite populations studied to date. Nevertheless, while major classes of RNAi effectors such as the proteins affecting uptake and spreading of dsRNA or RISC silencing complex components such as the Argonaut proteins are conserved, individual proteins in these classes are conspicuous by their absence in certain parasitic nematode species (Dalzell et al. 2011). Therefore, the overall conclusions of Dalzell et al. (2011) notwithstanding, it may be rational to attempt heterologous complementation of some of these specific deficiencies by expressing such RNAi effectors from C. elegans in parasitic nematodes on a case-by-case basis as stable transgenesis is achieved. It is noteworthy in this regard that such heterologous expression of C. elegans sid-2 in Caenorhabditis briggsae rendered that nematode susceptible to environmental RNAi (i.e. soaking) (Winston et al. 2007).
Studies of gene function using transgene constructs with dominant mutations
Absent a robust system for functional genomic study based on RNAi, transgenesis provides an alternative approach based on expression of transgene constructs encoding dominant loss-of-function mutations. In general, such ‘dominant-negative’ constructs would be designed to compete with endogenously expressed targets for binding sites or response elements but lack or actively suppress the function of the endogenous target. Such an approach has been used in transgenic parasites to demonstrate a role for the Ss-DAF-16 transcription factor (then designated FKTF-1) in morphogenesis of infective third-stage larvae of Strongyloides stercoralis (Castelletto et al. 2009). Similarly, overexpression of transgene constructs with activating mutations could, by virtue of their overexpression relative to the endogenous gene, achieve a dominant gain of function whose phenotypic effects could also shed light on gene function.
Forward genetics
In addition to their utility as vectors for integration of transgenes into parasite genomes, transposons hold great promise as agents of gene disruption through insertional mutagenesis in functional genomic studies. Transposon-based insertional mutagenesis approaches to ascertaining gene function are now standard in Drosophila, mouse and human genetic systems (Thibault et al. 2004), and have recently been explored as a forward genetic tool to great effect in Plasmodium (Balu et al. 2005, 2009; Balu and Adams, 2006; Fonager et al. 2011). Fully acknowledging the challenges involved with mutagenesis as a functional genomic tool in parasitic nematodes (lack of in vitro systems for parasite propagation most notably) it is worth considering that a significant phenotype, ivermectin resistance, was selected in a chemically mutagenized population of Strongyloides ratti (Viney et al. 2002), supporting the feasibility of such forward genetic approaches in parasitic nematodes. As it does in the area of transgenesis, the piggyBac transposon has significant advantages as an agent for insertional mutagenesis. Unlike the P elements commonly used in Drosophila that tend to insert into 5′ regulatory sequences, piggyBac favours insertion into the 5′ ends of coding sequences (Thibault et al. 2004; Balu and Adams, 2006), thus increasing the likelihood of functional disruption of genes. Combined with this factor, piggyBac inserts randomly into recipient genomes at multiple sites, enhancing its efficiency as a mutagen. Documentation of at least 19 piggyBac insertion sites in S. mansoni (Morales et al. 2007) underscores the potential of this transposon system as a forward genetic tool in parasitic helminths and argues for its exploration in this context in parasitic nematodes.
Genetically targeted cell ablation
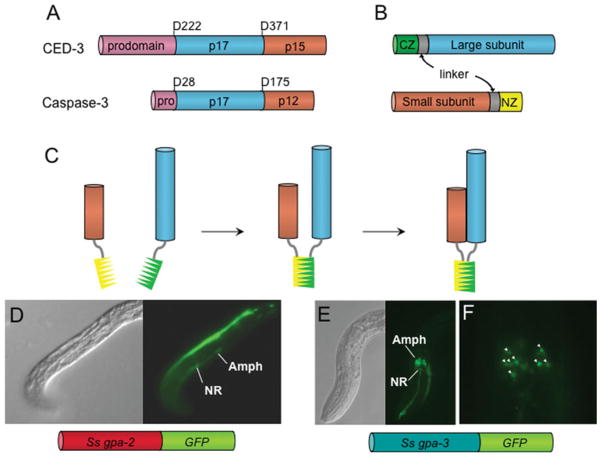
In aspects of cell biology, and particularly in neurobiology, the functions of individual cells have been deduced by ablating them and observing resulting effects on the organism in question. This approach, in which neuronal cell bodies are killed by microlaser surgery, has been widely adopted in C. elegans biology to deduce the neuron function (Bargmann and Avery, 1995; Gray et al. 2005; Bargmann, 2006) and was translated to the study of sensory neurobiology in parasitic nematodes by Schad and colleagues (Ashton et al. 1999, 2007; Lopez et al. 2000; Bhopale et al. 2001; Forbes et al. 2004; Ketschek et al. 2004). Although powerful, the technique of microlaser surgery requires a high degree of technical skill and is limited in the number of successfully operated nematodes that can be produced. Genetically targeted cell ablation, involving expression of lytic or other cytotoxic factors under the control of promoters with specificity for single or small subsets of cells, has been proposed as an alternative approach in C. elegans (Harbinder et al. 1997), with the most contemporary approach being the use of caspases reconstituted from subunits expressed under the control of promoters with specific patterns of expression in overlapping sets of cells (Fig. 2A–C) (Chelur and Chalfie, 2007). This system obviates the use of promoters with single-cell specificity to target lytic factors to individual cells. We are now attempting to use nascent systems for transgenesis to apply this system to cell ablation in Strongyloides spp. To this end, we have identified the promoter of Ss-gpa-3, a gene encoding a Gα protein orthologous to one essential for sensing the dauer pheromone in C. elegans, as one that can drive reporter expression in 8 amphidial neurons (including ASK) and some phasmidial neurons in transgenic S. stercoralis (Fig. 2E, F) (Junio et al. 2008). This pattern is virtually identical to the expression of Ce-gpa-3 in C. elegans (Zwaal et al. 1997). Like its C. elegans orthologue, the promoter for another Gα protein-encoding gene, Ss-gpa-2, drives reporter expression in a single amphidial neuron (Fig. 2D) (Ranjit and Lok, unpublished). We have not conclusively identified the specific neuron in which this reporter is expressed, but based on reported expression patterns of Ce-gpa-2, we hypothesize that it is ASK. The neuronal patterns of expression of C. elegans Gα protein genes are such that the subsets of amphidial neurons in which Ce-gpa-2 and Ce-gpa-3 are expressed overlap in a single neuron ASK (Table 3). Based on the strong similarities between these patterns and those observed for Ss-gpa-2 and Ss-gpa-3 (Fig. 2D–F), we hypothesize that the reconstituted caspase system (Chelur and Chalfie, 2007) could be used to kill ASK and, by extrapolation, other sensory neurons in transgenic S. stercoralis. As proof of principle, we are currently engineering vectors to express individual CED-3 caspase subunits under control of the Ss-gpa-2 and -3 promoters and intend to ascertain their ability to bring about killing of ASK-class neurons when co-expressed in first stage-larval S. stercoralis.
Fig. 2.
Cell ablation by expression of reconstituted caspases: a potential application of transgenesis in Strongyloides sp. A. Schematics of the C. elegans CED-3 and caspase-3 procaspases showing prodomains (pink), large subunits (blue) and small subunits (orange). B. Expression constructs encoding the large and small subunits of a caspase fused to one of two antiparallel leucine zipper domains (CZ, green; NZ, yellow) and an intervening linker sequence (gray). These would be expressed under separate promoters with multi-cell specificities that overlapped in a single cell or cell type. C. In the single cells where the subunit constructs were co-expressed, the complementary leucine-zippers would bind, bringing the large and small subunits into proximity, reconstituting the active caspase and destroying the cell. D – F depict expression of neuron-specific reporter constructs in apparently overlapping subsets of amphidial neurons in transgenic S. stercoralis. D. DIC (left) and fluorescence images (right) of an F1 transgenic first-stage larva expressing GFP under the promoter of the Gα protein-encoding gene Ss-gpa-2. Note that a single amphidial neuron, which we hypothesize is ASK, expresses the reporter. The cell body of this neuron, labeled ‘Amph’, and its dendritic process track along the lower margin of the worm, posterior to the nerve ring (NR) in this image. E. DIC (left) and fluorescence images (right) of an F1 transgenic first-stage larva expressing GFP under the promoter of the Gα protein-encoding gene Ss-gpa-3 (Amph – amphidial cell bodies; NR – nerve ring). F. High-magnification fluorescence image of amphidial cell bodies (arrow heads) expressing GFP under the Ss-gpa-3 promoter. Note that up to eight amphidial neurons express the construct and that based on comparison to C. elegans we hypothesize that these include ASK. Promoters with these cell specificities would theoretically allow ablation of ASK-class neurons by transgene-based expression of reconstituted caspases. Panels A–C are redrawn from Chelur, D. S. and Chalfie, M. (2007). Targeted cell killing by reconstituted caspases. Proceedings of the National Academy of Sciences of the United States of America, 104, 2283–2288, Copyright (2007) National Academy of Sciences, U.S.A., with permission.
Table 3.
Specificity of Ce-gpa-2 and Ce-gpa-3 promoter activation in ampidial neurons. Note that expression overlaps only in ASK class neurons (gray shading), providing a potential unicellular target of genetic cell ablation by expression of reconstituted caspases
| Amphidial neurons
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Promoter | ADE | ADF | ADL | ASE | ASG | ASH | ASI | ASJ | ASK | AFD | AIA | AIZ | ALA | ALN | AQR | AVM | AWA | AWB | AWC |
| Ce-gpa-2 | X | X | X | X | X | X | X | X | X | ||||||||||
| Ce-gpa-3 | X | X | X | ||||||||||||||||
Studies of the host immune response to infection with parasitic nematodes
Stable transgenesis in parasitic nematodes would greatly facilitate studies on the host immune response to these potential pathogens. Among the many possible applications of transgenic nematodes in such studies is their use in elucidating the manner in which polarized T helper cell responses conferring either immunity or chronic infection in nematode parasitism are incited by the parasite and then further differentiate. Antigens of microbial pathogens are sufficiently well characterized to have allowed dominant epitopes to be associated with receptors on specific T cell populations, but due to their greater complexity, such studies in more complex eukaryotic pathogens such as the parasitic protozoa or, to a greater degree, parasitic helminths have been slower to emerge (Lok and Artis, 2008). For example, the manner and anatomical locations in which parasite antigens stimulate populations of CD4+ T cells elaborating type 2 (Th2) cytokines to differentiate during a protective response to intestinal nematode infection are poorly understood (Lok and Artis, 2008; Maizels et al. 2009; Saenz et al. 2010). Transgenic parasites expressing standard model antigens such as avian ovalbumin (OVA) coupled with transgenic OVA-specific T cells have allowed the mechanisms of antigen presentation and CD8+ T cell differentiation and trafficking to be elucidated in Toxoplasma gondii infection (Pepper et al. 2004; Dzierszinski et al. 2007; Jordan et al. 2009, 2010; Tait et al. 2010). This work provides a model for how transgenic parasites expressing a model antigen like OVA could be employed in similar studies of how the Th2-polarized CD4+ T cell response develops in parasitic nematode infection.
CONCLUSION
Although transgenesis in parasitic nematodes has been slow to develop, recent advances in these worms and in parallel studies with schistosomes suggest that the long-term goal of stable transgenesis may be within reach in the near future. Among these advances are vector modifications for transformation of Strongyloides spp. that employ a transposon-mediatiated system for chromosomal integration and incorporation of retroviral sequences to resist epigenetic transgene silencing, allowing heritable transgenesis with stable expression and a highly creative approach involving revival of classic chemically mediated system for gene transfer into the filaria B. malayi and subsequent heritable transgenesis in that important parasite and laboratory model. Establishment of these methods for species of gastrointestinal and tissue-dwelling parasitic nematodes would be a major advancement allowing myriad studies in modern biology, not least of which would be the validation of new drug and vaccine targets and the elucidation of protective and pathological immune responses to host infection.
Acknowledgments
Collaborations and fruitful discussion with E. J. Pearce, P. J. Brindley, R. E. Davis, M. V. Sundaram, J. M. Hawdon, R. Greenberg and the late G. A. Schad as well as discussion of unpublished data with H. Shao, X. Li and N. Ranjit are greatly appreciated by the author.
FINANCIAL SUPPORT
This work received support from the US National Institutes of Health (grant numbers AI82548, AI50668 and AI22662).
References
- Aboobaker AA, Blaxter ML. Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. Molecular and Biochemical Parasitology. 2003;129:41–51. doi: 10.1016/S0166-6851(03)00092-6. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes. Veterinary Parasitology. 1999;84:297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Zhu X, Boston R, Lok JB, Schad GA. Strongyloides stercoralis: Amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Experimental Parasitology. 2007;115:92–97. doi: 10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuk MA, Suttiprapa S, Rinaldi G, Mann VH, Lee CM, Brindley PJ. Schistosoma mansoni U6 gene promoter-driven short hairpin RNA induces RNA interference in human fibrosarcoma cells and schistosomules. International Journal for Parasitology. 2011;41:783–789. doi: 10.1016/j.ijpara.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M, Chauhan C, Liu C, Unnasch TR. The role of polymorphisms in the spliced leader addition domain in determining promoter activity in Brugia malayi. Molecular and Biochemical Parasitology. 2011;176:37–41. doi: 10.1016/j.molbiopara.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu B, Adams JH. Functional genomics of Plasmodium falciparum through transposon-mediated mutagenesis. Cellular Microbiology. 2006;8:1529–1536. doi: 10.1111/j.1462-5822.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- Balu B, Chauhan C, Maher SP, Shoue DA, Kissinger JC, Fraser MJ, Jr, Adams JH. piggyBac is an effective tool for functional analysis of the Plasmodium falciparum genome. BMC Microbiology. 2009;9:83. doi: 10.1186/1471-2180-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu B, Shoue DA, Fraser MJ, Jr, Adams JH. Highefficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proceedings of the National Academy of Sciences, USA. 2005;102:16391–16396. doi: 10.1073/pnas.0504679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI WormBook. Chemosensation in C. elegans. The C. elegans Research Community) WormBook. 2006 doi: 10.1895/wormbook.1.123.1. http://www.wormbook.org. [DOI]
- Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods in Cell Biology. 1995;48:225–250. doi: 10.1016/s0091-679x(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezikov E, Bargmann CI, Plasterk RH. Homologous gene targeting in Caenorhabditis elegans by biolistic transformation. Nucleic Acids Research. 2004;32:e40. doi: 10.1093/nar/gnh033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopale VM, Kupprion EK, Ashton FT, Boston R, Schad GA. Ancylostoma caninum: the finger cell neurons mediate thermotactic behavior by infective larvae of the dog hookworm. Experimental Parasitology. 2001;97:70–76. doi: 10.1006/expr.2000.4575. [DOI] [PubMed] [Google Scholar]
- Brindley PJ, Laha T, McManus DP, Loukas A. Mobile genetic elements colonizing the genomes of metazoan parasites. Trends in Parasitology. 2003;19:79–87. doi: 10.1016/S1471-4922(02)00061-2. [DOI] [PubMed] [Google Scholar]
- Castelletto ML, Massey HC, Jr, Lok JB. Morphogenesis of Strongyloides stercoralis infective larvae requires the DAF-16 ortholog FKTF-1. PLoS Pathogens. 2009;5:e1000370. doi: 10.1371/journal.ppat.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur DS, Chalfie M. Targeted cell killing by reconstituted caspases. Proceedings of the National Academy of Sciences, USA. 2007;104:2283–2288. doi: 10.1073/pnas.0610877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Cohen L, Mikhli C, Jankowska-Anyszka M, Stepinski J, Darzynkiewicz E, Davis RE. In vivo translation and stability of trans-spliced mRNAs in nematode embryos. Molecular and Biochemical Parasitology. 2007;153:95–106. doi: 10.1016/j.molbiopara.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Mikhli C, Friedman C, Jankowska-Anyszka M, Stepinski J, Darzynkiewicz E, Davis RE. Nematode m7GpppG and m3(2,2,7)GpppG decapping: activities in Ascaris embryos and characterization of C. elegans scavenger DcpS. RNA. 2004;10:1609–1624. doi: 10.1261/rna.7690504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SN, Chang AC, Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proceedings of the National Academy of Sciences of the United States of America. 1972;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalzell JJ, McVeigh P, Warnock ND, Mitreva M, Bird DM, Abad P, Fleming CC, Day TA, Mousley A, Marks NJ, Maule AG. RNAi Effector Diversity in Nematodes. PLoS Neglected Tropical Diseases. 2011;5:e1176. doi: 10.1371/journal.pntd.0001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Parra A, LoVerde PT, Ribeiro E, Glorioso G, Hodgson S. Transient expression of DNA and RNA in parasitic helminths by using particle bombardment. Proceedings of the National Academy of Sciences, USA. 1999;96:8687–8692. doi: 10.1073/pnas.96.15.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierszinski F, Nishi M, Ouko L, Roos DS. Dynamics of Toxoplasma gondii differentiation. Eukaryotic Cell. 2004;3:992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierszinski F, Pepper M, Stumhofer JS, LaRosa DF, Wilson EH, Turka LA, Halonen SK, Hunter CA, Roos DS. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in non-professional and professional antigen-presenting cells. Infection and Immunity. 2007;75:5200–5209. doi: 10.1128/IAI.00954-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TC WormBook. Transformation and microinjection. The C. elegans Research Community) WormBook. 2006 doi: 10.1895/wormbook.1.108.1. http://www.wormbook.org. [DOI]
- Fire A. Integrative transformation of Caenorhabditis elegans. EMBO Journal. 1986;5:2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonager J, Franke-Fayard BM, Adams JH, Ramesar J, Klop O, Khan SM, Janse CJ, Waters AP. Development of the piggyBac transposable system for Plasmodium berghei and its application for random mutagenesis in malaria parasites. BMC Genomics. 2011;12:155. doi: 10.1186/1471-2164-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes WM, Ashton FT, Boston R, Zhu X, Schad GA. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Veterinary Parasitology. 2004;120:189–198. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Ford L, Guiliano DB, Oksov Y, Debnath AK, Liu J, Williams SA, Blaxter ML, Lustigman S. Characterization of a novel filarial serine protease inhibitor, Ov-SPI-1, from Onchocerca volvulus, with potential multifunctional roles during development of the parasite. Journal of Biological Chemistry. 2005;280(49):40845–40856. doi: 10.1074/jbc.M504434200. [DOI] [PubMed] [Google Scholar]
- Ford L, Zhang J, Liu J, Hashmi S, Fuhrman JA, Oksov Y, Lustigman S. Functional analysis of the cathepsin-like cysteine protease genes in adult Brugia malayi using RNA interference. PLoS Neglected Tropical Diseases. 2009;3:e377. doi: 10.1371/journal. pntd.0000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WN, Skinner SJM, Howes JN, Grant K, Shuttle worth G, Heath DD, Shoemaker CB. Heritable transgenesis of Parastrongyloides trichosuri: a nematode parasite of mammals. International Journal for Parasitology. 2006a;36:475–483. doi: 10.1016/j.ijpara.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Grant WN, Stasiuk S, Newton-Howes J, Ralston M, Bisset SA, Heath DD, Shoemaker CB. Parastrongyloides trichosuri, a nematode parasite of mammals that is uniquely suited to genetic analysis. International Journal for Parasitology. 2006b;36:453–466. doi: 10.1016/j.ijpara. 2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proceedings of the National Academy of Sciences, USA. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M. Genetically targeted cell disruption in Caenorhabditis elegans. Proceedings of the National Academy of Sciences, USA. 1997;94:13128–13133. doi: 10.1073/pnas.94.24.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higazi TB, Deoliveira A, Katholi CR, Shu L, Barchue J, Lisanby M, Unnasch TR. Identification of elements essential for transcription in Brugia malayi promoters. Journal of Molecular Biology. 2005;353:1–13. doi: 10.1016/j.jmb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Higazi TB, Merriweather A, Shu L, Davis R, Unnasch TR. Brugia malayi: transient transfection by microinjection and particle bombardment. Experimental Parasitology. 2002;100:95–102. doi: 10.1016/S0014-4894(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Higazi TB, Shu L, Unnasch TR. Development and transfection of short-term primary cell cultures from Brugia malayi. Molecular and Biochemical Parasitology. 2004;137:345–348. doi: 10.1016/j.molbiopara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Higazi TB, Unnasch TR. Intron encoded sequences necessary for trans splicing in transiently transfected Brugia malayi. Molecular and Biochemical Parasitology. 2004;137:181–184. doi: 10.1016/j.molbiopara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Jackstadt P, Wilm TP, Zahner H, Hobom G. Transformation of nematodes via ballistic DNA transfer. Molecular and Biochemical Parasitology. 1999;103:261–266. doi: 10.1016/S0166-6851(99) 00089-4. [DOI] [PubMed] [Google Scholar]
- Johnson NM, Behm CA, Trowell SC. Heritable and inducible gene knockdown in C. elegans using Wormgate and the ORFeome. Gene. 2005;359:26–34. doi: 10.1016/j.gene.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Joiner KA, Roos DS. Secretory traffic in the eukaryotic parasite Toxoplasma gondii: less is more. The Journal of cell biology. 2002;157:557–563. doi: 10.1083/jcb.200112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan KA, Dupont CD, Tait ED, Liou HC, Hunter CA. Role of the NF-kappaB transcription factor c-Rel in the generation of CD8+ T-cell responses to Toxoplasma gondii. International Immunology. 2010;22:851–861. doi: 10.1093/intimm/dxq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan KA, Wilson EH, Tait ED, Fox BA, Roos DS, Bzik DJ, Dzierszinski F, Hunter CA. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infection and Immunity. 2009;77:3894–3901. doi: 10.1128/IAI.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junio AB, Li X, Massey HC, Jr, Nolan TJ, Todd Lamitina S, Sundaram MV, Lok JB. Strongyloides stercoralis: cell- and tissue-specific transgene expression and co-transformation with vector constructs incorporating a common multifunctional 3′ UTR. Experimental Parasitology. 2008;118:253–265. doi: 10.1016/j.exppara.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek AR, Joseph R, Boston R, Ashton FT, Schad GA. Amphidial neurons ADL and ASH initiate sodium dodecyl sulphate avoidance responses in the infective larva of the dog hookworm Anclyostoma caninum. International Journal for Parasitology. 2004;34:1333–1336. doi: 10.1016/j.ijpara.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Kines KJ, Mann VH, Morales ME, Shelby BD, Kalinna BH, Gobert GN, Chirgwin SR, Brindley PJ. Transduction of Schistosoma mansoni by vesicular stomatitis virus glycoprotein-pseudotyped Moloney murine leukemia retrovirus. Experimental Parasitology. 2006;112:209–220. doi: 10.1016/j.exppara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kines KJ, Morales ME, Mann VH, Gobert GN, Brindley PJ. Integration of reporter transgenes into Schistosoma mansoni chromosomes mediated by pseudotyped murine leukemia virus. FASEB Journal. 2008;22:2936–2948. doi: 10.1096/fj.08-108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox DP, Geldhof P, Visser A, Britton C. RNA interference in parasitic nematodes of animals: a reality check? Trends in Parasitology. 2007;23:105–107. doi: 10.1016/j.pt.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Laha T, Kewgrai N, Loukas A, Brindley PJ. The dingo non-long terminal repeat retrotransposons from the genome of the hookworm, Ancylostoma caninum. Experimental Parasitology. 2006;113:142–153. doi: 10.1016/j.exppara.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Laha T, Loukas A, Wattanasatitarpa S, Somprakhon J, Kewgrai N, Sithithaworn P, Kaewkes S, Mitreva M, Brindley PJ. The bandit, a new DNA transposon from a hookworm-possible horizontal genetic transfer between host and parasite. PLoS Neglected Tropical Diseases. 2007;1:e35. doi: 10.1371/journal.pntd. 0000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin like signalling pathway. Current Biology. 2001;11:1950–1957. doi: 10.1016/S0960(01) 00595-4. [DOI] [PubMed] [Google Scholar]
- Li X, Massey HC, Nolan TJ, Schad GA, Kraus K, Sundaram M, Lok JB. Successful transgenesis of the parasitic nematode Strongyloides stercoralis requires endogenous non-coding control elements. International Journal for Parasitology. 2006;36:671–679. doi: 10.1016/j.ijpara.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Li X, Shao H, Junio AB, Nolan TJ, Massey HC, Jr, Pearce EJ, Viney ME, Lok JB. Transgenesis in the parasitic nematode Strongyloides ratti. Molecular and Biochemical Parasitology. 2011;179:114–119. doi: 10.1016/jmolbiopara.2011.06.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the lifespan of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Liu C, de Oliveira A, Higazi TB, Ghedin E, DePasse J, Unnasch TR. Sequences necessary for trans-splicing in transiently transfected Brugia malayi. Molecular and Biochemical Parasitology. 2007;156:62–73. doi: 10.1016/j.molbiopara.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chauhan C, Katholi CR, Unnasch TR. The splice leader addition domain represents an essential conserved motif for heterologous gene expression in B. malayi. Molecular and Biochemical Parasitology. 2009;166:15–21. doi: 10.1016/j.molbiopara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chauhan C, Unnasch TR. The role of local secondary structure in the function of the trans-splicing motif of Brugia malayi. Molecular and Biochemical Parasitology. 2010a;169:115–119. doi: 10.1016/j.molbiopara.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Oliveira A, Chauhan C, Ghedin E, Unnasch TR. Functional analysis of putative operons in Brugia malayi. International Journal for Parasitology. 2010b;40:63–71. doi: 10.1016/j.ijpara. 2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok JB. Transgenesis in parasitic nematodes: building a better array. Trends in Parasitology. 2009;25:345–347. doi: 10.1016/j.pt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok JB, Artis D. Transgenesis and neuronal ablation in parasitic nematodes: revolutionary new tools to dissect host-parasite interactions. Parasite Immunology. 2008;30(4):203–214. doi: 10.1111/j.1365-3024.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok JB, Massey HC., Jr Transgene expression in Strongyloides stercoralis following gonadal microinjection of DNA constructs. Molecular and Biochemical Parasitology. 2002;119:279–284. doi: 10.1016/S0166-6851(01)00414-5. [DOI] [PubMed] [Google Scholar]
- Lopez PM, Boston R, Ashton FT, Schad GA. The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. International Journal for Parasitology. 2000;30:1115–1121. doi: 10.1016/S0020-7519(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Lustigman S, Zhang J, Liu J, Oksov Y, Hashmi S. RNA interference targeting cathepsin L and Z-like cysteine proteases of Onchocerca volvulus confirmed their essential function during L3 molting. Molecular and Biochemical Parasitology. 2004;138:165–170. doi: 10.1016/j.molbiopara.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. Journal of Experimental Medicine. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M, Higa A. Calcium-dependent bacteriophage DNA infection. Journal of Molecular Biology. 1970;53:159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mann VH, Morales ME, Kines KJ, Brindley PJ. Transgenesis of schistosomes: approaches employing mobile genetic elements. Parasitology. 2007;134:1–13. doi: 10.1017/S0031182007003824. [DOI] [PubMed] [Google Scholar]
- Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nature Genetics. 2008;40:476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey HC, Jr, Nishi M, Chaudhary K, Pakpour N, Lok JB. Structure and developmental expression of Strongyloides stercoralis fktf-1, a proposed ortholog of daf-16 in Caenorhabditis elegans. International Journal for Parasitology. 2003;33:1537–1544. doi: 10.1016/S0020-7519(03)00205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods in Cell Biology. 1995;48:451–482. [PubMed] [Google Scholar]
- Mello C, Kramer JM, Stinchcomb DT, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. The EMBO Journal. 1991;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales ME, Mann VH, Kines KJ, Gobert GN, Fraser MJ, Jr, Kalinna BH, Correnti JM, Pearce EJ, Brindley PJ. piggyBac transposon mediated transgenesis of the human blood fluke, Schistosoma mansoni. FASEB Journal. 2007;21:3479–3489. doi: 10.1096/fj.07-8726com. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Huye LE, Dotti G, Foster AE, Vera JF, Manuri PR, June CH, Rooney CM, Wilson MH. Optimization of the piggyBac transposon system for the sustained genetic modification of human T lymphocytes. Journal of Immunotherapy. 2009;32:826–836. doi: 10.1097/CJI.0b013e3181ad762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Hu K, Murray JM, Roos DS. Organellar dynamics during the cell cycle of Toxoplasma gondii. Journal of Cell Science. 2008;121:1559–1568. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Oliveira A, Katholi CR, Unnasch TR. Characterization of the promoter of the Brugia malayi 12 kDa small subunit ribosomal protein (RPS12) gene. International Journal for Parasitology. 2008;38:1111–1119. doi: 10.1016/j.ijpara.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infection and Immunity. 2004;72:7240–7246. doi: 10.1128/IAI.72.12.7240-7246. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr K, Heider U, Hoerauf A. RNAi mediated silencing of actin expression in adult Litomosoides sigmodontis is specific, persistent and results in a phenotype. International Journal for Parasitology. 2006;36:661–669. doi: 10.1016/j.ijpara.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Plasterk RH, Izsvak Z, Ivics Z. Resident aliens: the Tc1/mariner superfamily of transposable elements. Trends in Genetics. 1999;15:326–332. doi: 10.1016/S0168-9525(99)01777-1. [DOI] [PubMed] [Google Scholar]
- Praitis V. Creation of transgenic lines using microparticle bombardment methods. Methods in Molecular Biology. 2006;351:93–107. doi: 10.1385/1-59745-151-7:93. [DOI] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends in Immunology. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Samarasinghe B, Knox DP, Britton C. Factors affecting susceptibility to RNA interference in Haemonchus contortus and in vivo silencing of an H11 aminopeptidase gene. International Journal for Parasitology. 2011;41:51–59. doi: 10.1016/j.ijpara.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. pp. 16.30–16.40. [Google Scholar]
- Schad GA. Morphology and life history of Strongyloides stercoralis. In: Grove DI, editor. Strongyloidiasis: a Major Roundworm Infection of Man. Taylor and Francis Inc; Philadelphia: 1989. pp. 85–104. [Google Scholar]
- Schlager B, Wang X, Braach G, Sommer RJ. Molecular cloning of a dominant roller mutant and establishment of DNA-mediated transformation in the nematode Pristionchus pacificus. Genesis. 2009;47:300–304. doi: 10.1002/dvg.20499. [DOI] [PubMed] [Google Scholar]
- Shu L, Katholi CR, Higazi T, Unnasch TR. Analysis of the Brugia malayi HSP70 promoter using a homologous transient transfection system. Molecular and Biochemical Parasitology. 2003;128:67–75. doi: 10.1016/S0166-6851(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Song C, Gallup JM, Day TA, Bartholomay LC, Kimber MJ. Development of an in vivo RNAi protocol to investigate gene function in the filarial nematode, Brugia malayi. PLoS Pathogens. 2010;6:e1001239. doi: 10.1371/journal.ppat.1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soragni E, Herman D, Dent SY, Gottesfeld JM, Wells RD, Napierala M. Long intronic GAA*TTC repeats induce epigenetic changes and reporter gene silencing in a molecular model of Friedreich ataxia. Nucleic Acids Research. 2008;36:6056–6065. doi: 10.1093/nar/gkn604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Molecular and Cellular Biology. 1985;5:3484–3496. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachu B, Pillai S, Lucius R, Pogonka T. Essential role of chitinase in the development of the filarial nematode Acanthocheilonema viteae. Infection and Immunity. 2008;76:221–228. doi: 10.1128/IAI.00701-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait ED, Jordan KA, Dupont CD, Harris TH, Gregg B, Wilson EH, Pepper M, Dzierszinski F, Roos DS, Hunter CA. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. Journal of Immunology. 2010;185:1502–1512. doi: 10.4049/jimmunol. 0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Wang SL, Dorovkov M, Ryazanov A, Driscoll M. Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nature Genetics. 2000;24:180–183. doi: 10.1038/72850. [DOI] [PubMed] [Google Scholar]
- Tchoubrieva EB, Ong PC, Pike RN, Brindley PJ, Kalinna BH. Vector-based RNA interference of cathepsin B1 in Schistosoma mansoni. Cellular and molecular life sciences : CMLS. 2010;67:3739–3748. doi: 10.1007/s00018-010-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, Ryner L, Cheung LM, Chong A, Erickson C, Fisher WW, Greer K, Hartouni SR, Howie E, Jakkula L, Joo D, Killpack K, Laufer A, Mazzotta J, Smith RD, Stevens LM, Stuber C, Tan LR, Ventura R, Woo A, Zakrajsek I, Zhao L, Chen F, Swimmer C, Kopczynski C, Duyk G, Winberg ML, Margolis J. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nature Genetics. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Tzertzinis G, Egana AL, Palli SR, Robinson-Rechavi M, Gissendanner CR, Liu C, Unnasch TR, Maina CV. Molecular evidence for a functional ecdysone signaling system in Brugia malayi. PLoS Neglected Tropical Diseases. 2010;4(3):e625. doi: 10.1371/journal.pntd.0000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnasch TR, Bradley J, Beauchamp J, Tuan R, Kennedy MW. Characterization of a putative nuclear receptor from Onchocerca volvulus. Molecular and Biochemical Parasitology. 1999;104:259–269. doi: 10.1016/S0166-6851(99)00152-8. [DOI] [PubMed] [Google Scholar]
- Viney ME. Exploiting the life cycle of Strongyloides ratti. Parasitology Today. 1999;15:231–235. doi: 10.1016/S0169-4758(99)01452-0. [DOI] [PubMed] [Google Scholar]
- Viney ME, Ashford RW. The ultrastructure of the peri-vulval region of Strongyloides cebus. Journal of Helminthology. 1990;64:23–28. doi: 10.1017/s0022149x00011834. [DOI] [PubMed] [Google Scholar]
- Viney ME, Green LD, Brooks JA, Grant WN. Chemical mutagenesis of the parasitic nematode Strongyloides ratti to isolate ivermectin resistant mutants. International Journal for Parasitology. 2002;32:1677–1682. doi: 10.1016/S0020-7519(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Viney ME, Lok JB WormBook. Strongyloides spp. The C. elegans Research Community) WormBook. 2007 doi: 10.1895/wormbook.1.141.1. http://www.wormbook.org. [DOI]
- Wilm T, Demel P, Koop HU, Schnabel H, Schnabel R. Ballistic transformation of Caenorhabditis elegans. Gene. 1999;229:31–35. doi: 10.1016/s0378-1119(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10565–10570. doi: 10.1073/pnas.0611282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Liu C, Tzertzinis G, Ghedin E, Evans CC, Kaplan R, Unnasch TR. In vivo transfection of developmentally competent Brugia malayi infective larvae. International Journal for Parasitology. 2011;41:355–362. doi: 10.1016/j.ijpara.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Brindley PJ, Zeng Q, Li Y, Zhou J, Liu Y, Liu B, Cai L, Zeng T, Wei Q, Lan L, McManus DP. Transduction of Schistosoma japonicum schistosomules with vesicular stomatitis virus glycoprotein pseudotyped murine leukemia retrovirus and expression of reporter human telomerase reverse transcriptase in the transgenic schistosomes. Molecular and Biochemical Parasitology. 2010;174:109–116. doi: 10.1016/j.molbiopara.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZR, Lei L, Liu M, Zhu SC, Ren CP, Wang XN, Shen JJ. Schistosoma japonicum: inhibition of Mago nashi gene expression by shRNA-mediated RNA interference. Experimental Parasitology. 2008;119:379–384. doi: 10.1016/j.exppara.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans dauer-inducing pheromone. Genetics. 1997;145:715–727. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]