Abstract
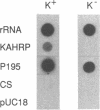
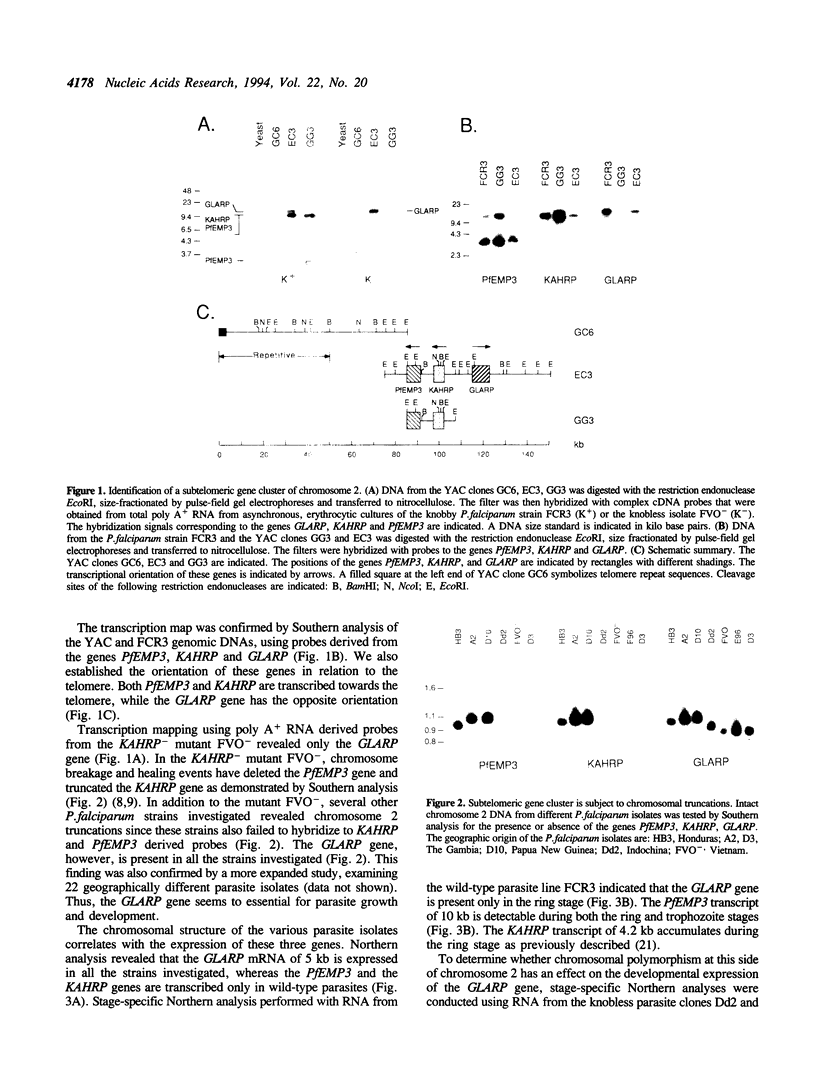
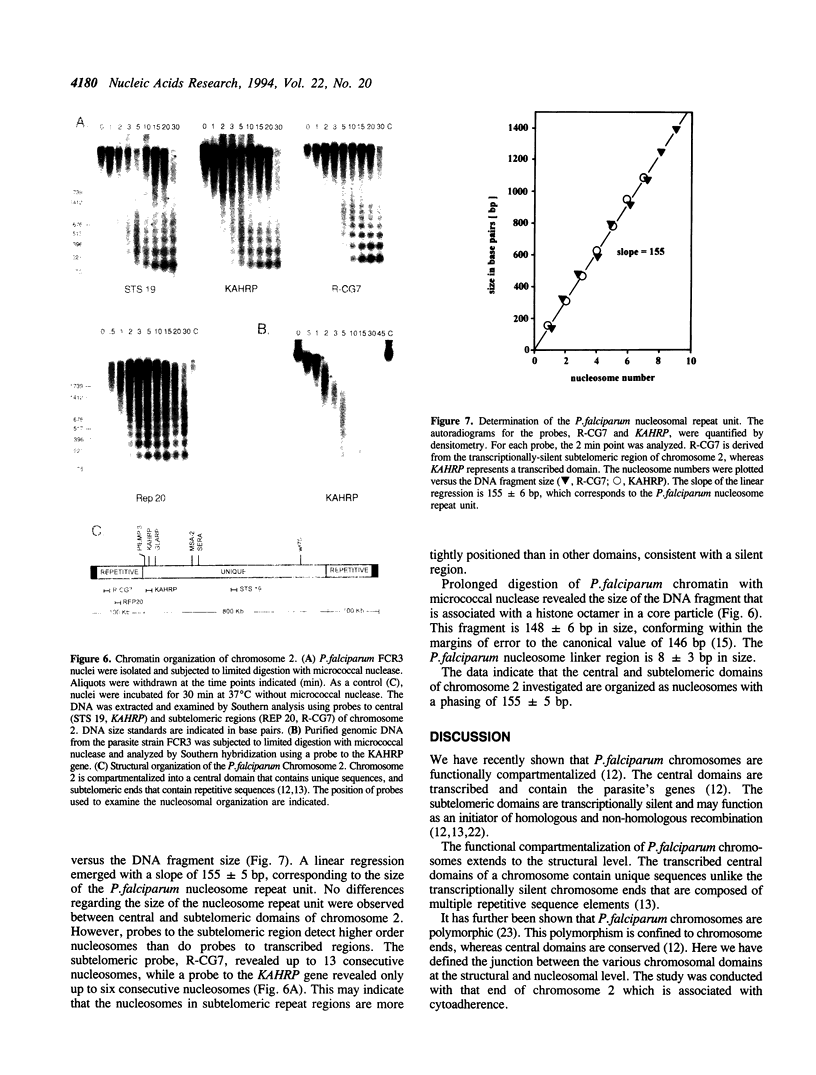
We have recently demonstrated that Plasmodium falciparum chromosomes are compartmentalized into different domains: conserved and polymorphic domains; transcriptionally active and silent domains. Here we have analyzed the transition between these domains at the structural and nucleosomal level. This study was conducted with the end of chromosome 2 that is associated with cytoadherence. At this end of the chromosome, the first set of erythrocytic genes has been mapped 85 kb from the telomere. These genes are monocistronically transcribed as revealed by nuclear run-on analysis. Two of these genes, PfEMP3 and KAHRP, are deleted in cytoadherent negative mutants, whereas the third gene, GLARP, is expressed in all the strains investigated. The data indicate that the polymorphic domain at this end of chromosome 2 extends into the transcribed region. Analysis of the chromatin structure revealed that both the transcribed domain and the subtelomeric region are organized as nucleosomes with a periodicity of 155 +/- 5 bp.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. C., Workman J. L. Nucleosome displacement in transcription. Cell. 1993 Feb 12;72(3):305–308. doi: 10.1016/0092-8674(93)90109-4. [DOI] [PubMed] [Google Scholar]
- Barnwell J. W. Cytoadherence and sequestration in falciparum malaria. Exp Parasitol. 1989 Nov;69(4):407–412. doi: 10.1016/0014-4894(89)90190-2. [DOI] [PubMed] [Google Scholar]
- Barnwell J. W., Howard R. J., Miller L. H. Influence of the spleen on the expression of surface antigens on parasitized erythrocytes. Ciba Found Symp. 1983;94:117–136. doi: 10.1002/9780470715444.ch8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark D. J., Felsenfeld G. A nucleosome core is transferred out of the path of a transcribing polymerase. Cell. 1992 Oct 2;71(1):11–22. doi: 10.1016/0092-8674(92)90262-b. [DOI] [PubMed] [Google Scholar]
- Creedon K. A., Kaslow D. C., Rathod P. K., Wellems T. E. Identification of a Plasmodium falciparum histone 2A gene. Mol Biochem Parasitol. 1992 Aug;54(1):113–115. doi: 10.1016/0166-6851(92)90102-p. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990 Nov 16;63(4):751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F., Walliker D. Genetic diversity in Plasmodium falciparum. Adv Parasitol. 1990;29:75–149. doi: 10.1016/s0065-308x(08)60105-0. [DOI] [PubMed] [Google Scholar]
- Kutner S., Breuer W. V., Ginsburg H., Aley S. B., Cabantchik Z. I. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J Cell Physiol. 1985 Dec;125(3):521–527. doi: 10.1002/jcp.1041250323. [DOI] [PubMed] [Google Scholar]
- Lanzer M., Wertheimer S. P., de Bruin D., Ravetch J. V. Chromatin structure determines the sites of chromosome breakages in Plasmodium falciparum. Nucleic Acids Res. 1994 Aug 11;22(15):3099–3103. doi: 10.1093/nar/22.15.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., de Bruin D., Ravetch J. V. A sequence element associated with the Plasmodium falciparum KAHRP gene is the site of developmentally regulated protein-DNA interactions. Nucleic Acids Res. 1992 Jun 25;20(12):3051–3056. doi: 10.1093/nar/20.12.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., de Bruin D., Ravetch J. V. Transcription mapping of a 100 kb locus of Plasmodium falciparum identifies an intergenic region in which transcription terminates and reinitiates. EMBO J. 1992 May;11(5):1949–1955. doi: 10.1002/j.1460-2075.1992.tb05248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., de Bruin D., Ravetch J. V. Transcriptional differences in polymorphic and conserved domains of a complete cloned P. falciparum chromosome. Nature. 1993 Feb 18;361(6413):654–657. doi: 10.1038/361654a0. [DOI] [PubMed] [Google Scholar]
- Lanzer M., de Bruin D., Wertheimer S. P., Ravetch J. V. Organization of chromosomes in Plasmodium falciparum: a model for generating karyotypic diversity. Parasitol Today. 1994 Mar;10(3):114–117. doi: 10.1016/0169-4758(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Chien S., Usami S. Decreased deformability of Plasmodium coatneyi-infected red cells and its possible relation to cerebral malaria. Am J Trop Med Hyg. 1972 Mar;21(2):133–137. doi: 10.4269/ajtmh.1972.21.133. [DOI] [PubMed] [Google Scholar]
- Pasloske B. L., Baruch D. I., van Schravendijk M. R., Handunnetti S. M., Aikawa M., Fujioka H., Taraschi T. F., Gormley J. A., Howard R. J. Cloning and characterization of a Plasmodium falciparum gene encoding a novel high-molecular weight host membrane-associated protein, PfEMP3. Mol Biochem Parasitol. 1993 May;59(1):59–72. doi: 10.1016/0166-6851(93)90007-k. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Pavlovec A., Shio H., Ravetch J. V. Primary structure and subcellular localization of the knob-associated histidine-rich protein of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7139–7143. doi: 10.1073/pnas.84.20.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Raventos-Suarez C., Kaul D. K., Macaluso F., Nagel R. L. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3829–3833. doi: 10.1073/pnas.82.11.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Triglia T., Stahl H. D., Crewther P. E., Scanlon D., Brown G. V., Anders R. F., Kemp D. J. The complete sequence of the gene for the knob-associated histidine-rich protein from Plasmodium falciparum. EMBO J. 1987 May;6(5):1413–1419. doi: 10.1002/j.1460-2075.1987.tb02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Aikawa M., Berzins K., Wahlgren M., Perlmann P. Cytoadherence of knobless Plasmodium falciparum-infected erythrocytes and its inhibition by a human monoclonal antibody. Nature. 1989 Apr 27;338(6218):763–765. doi: 10.1038/338763a0. [DOI] [PubMed] [Google Scholar]
- Wright J. H., Gottschling D. E., Zakian V. A. Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes Dev. 1992 Feb;6(2):197–210. doi: 10.1101/gad.6.2.197. [DOI] [PubMed] [Google Scholar]
- de Bruin D., Lanzer M., Ravetch J. V. Characterization of yeast artificial chromosomes from Plasmodium falciparum: construction of a stable, representative library and cloning of telomeric DNA fragments. Genomics. 1992 Oct;14(2):332–339. doi: 10.1016/s0888-7543(05)80223-x. [DOI] [PubMed] [Google Scholar]
- de Bruin D., Lanzer M., Ravetch J. V. The polymorphic subtelomeric regions of Plasmodium falciparum chromosomes contain arrays of repetitive sequence elements. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):619–623. doi: 10.1073/pnas.91.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]