Abstract
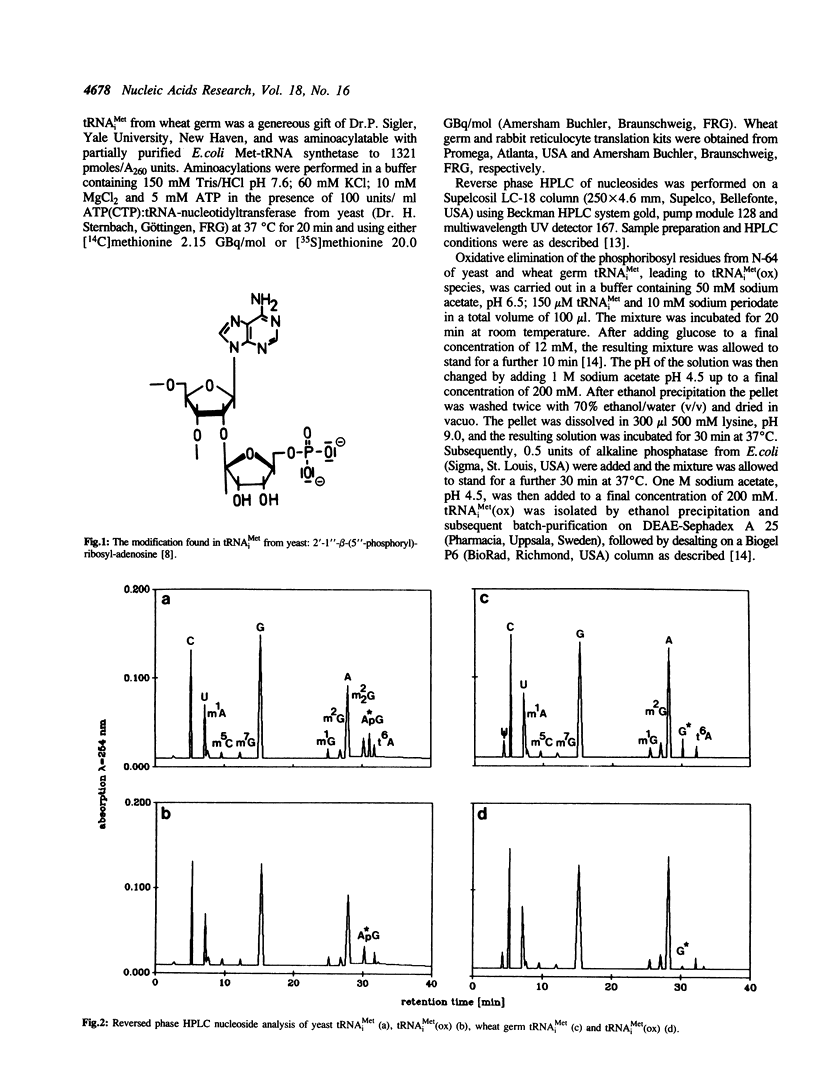
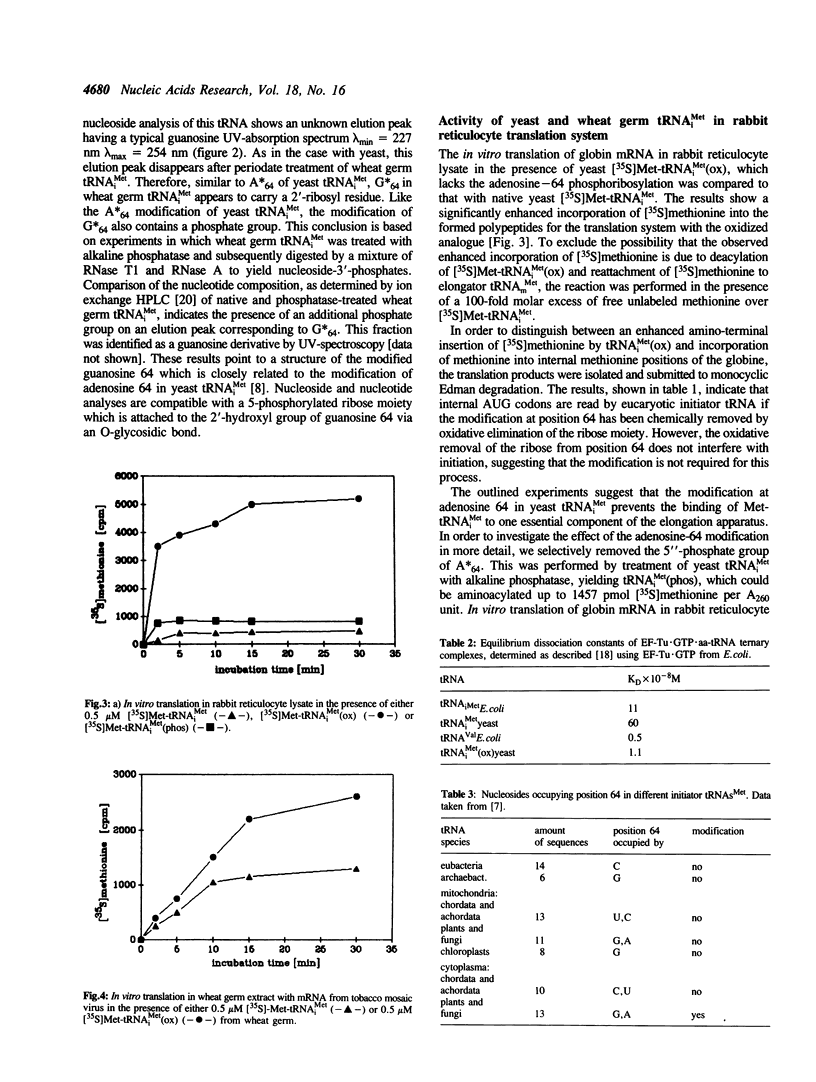
The role of 2'-ribosylated adenosine 64 in tRNA(iMet) from yeast in initiation/elongation discrimination was investigated. As measured by in vitro translation in rabbit reticulocyte lysate, the specific removal of the 2'-ribosylphosphate at adenosine 64 via periodate oxidation allows tRNA(iMet) to read internal AUG codons of the globine messenger RNA. Yeast Met-tRNA(iMet) lacking the modification of nucleoside 64 forms ternary complexes with GTP and elongation factor Tu from Escherichia coli. The lack of modification at position 64 does not prevent tRNA(iMet) from participating in the initiation process of in vitro protein synthesis. Wheat germ tRNA(iMet) has a 2'-ribosylated guanosine at position 64. Removal of this modification from the wheat germ tRNA(iMet) enables it to read internal AUG codons of globine and tobacco mosaic virus messenger RNA in reticulocyte and wheat germ translation systems, respectively.
Full text
PDF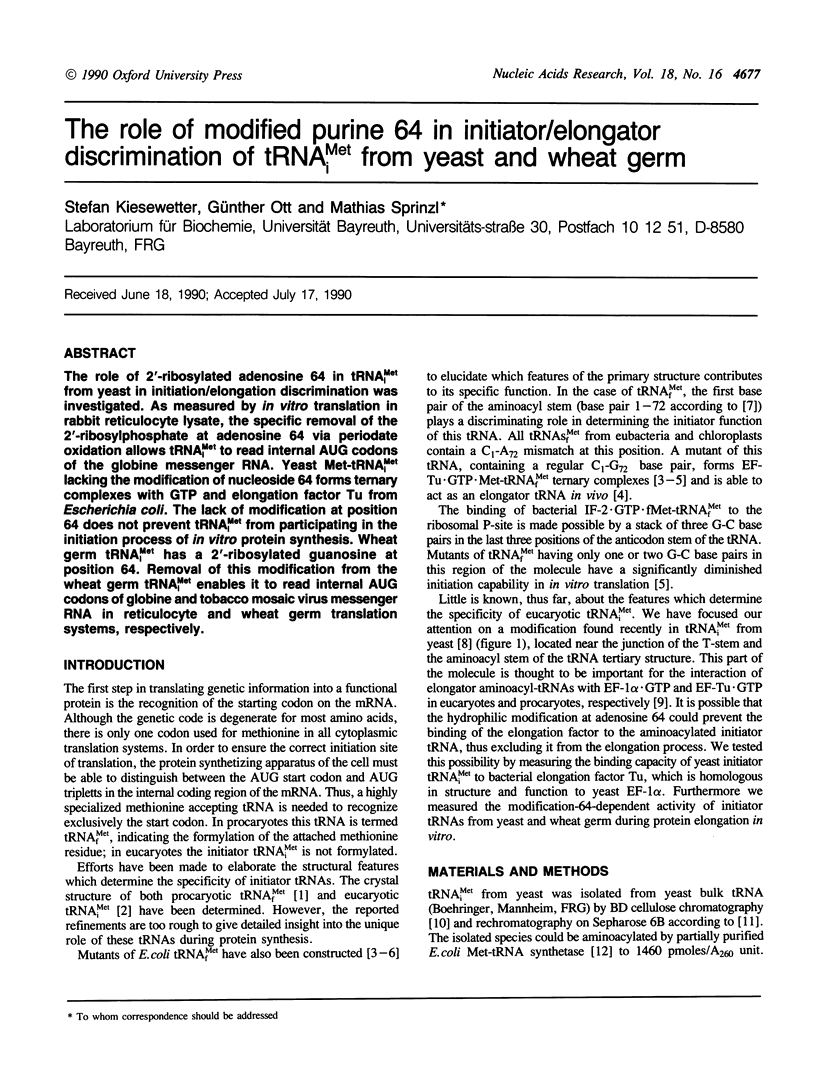



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dasmahapatra B., Chakraburtty K. Protein synthesis in yeast. I. Purification and properties of elongation factor 3 from Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):9999–10004. [PubMed] [Google Scholar]
- Desgrès J., Keith G., Kuo K. C., Gehrke C. W. Presence of phosphorylated O-ribosyl-adenosine in T-psi-stem of yeast methionine initiator tRNA. Nucleic Acids Res. 1989 Feb 11;17(3):865–882. doi: 10.1093/nar/17.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin H. J., RajBhandary U. L. Expression in vivo of a mutant human initiator tRNA gene in mammalian cells using a simian virus 40 vector. J Biol Chem. 1985 May 10;260(9):5588–5595. [PubMed] [Google Scholar]
- Fischer W., Doi T., Ikehara M., Ohtsuka E., Sprinzl M. Interaction of methionine-specific tRNAs from Escherichia coli with immobilized elongation factor Tu. FEBS Lett. 1985 Nov 11;192(1):151–154. doi: 10.1016/0014-5793(85)80062-4. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Schimmel P. Aminoacylation of RNA minihelices with alanine. Nature. 1989 Feb 2;337(6206):478–481. doi: 10.1038/337478a0. [DOI] [PubMed] [Google Scholar]
- Gross M. Control of protein synthesis by hemin. Isolation and characterization of a supernatant factor from rabbit reticulocyte lysate. Biochim Biophys Acta. 1976 Nov 1;447(4):445–459. doi: 10.1016/0005-2787(76)90082-4. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R. L., Faulhammer H., Chapeville F., Sprinzl M., Haenni A. L. Aminoacyl RNA domain of turnip yellow mosaic virus Val-RNA interacting with elongation factor Tu. Nucleic Acids Res. 1984 Oct 11;12(19):7467–7478. doi: 10.1093/nar/12.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie A., Ribeiro N. S., Reid B. R., Jurnak F. Relative affinities of all Escherichia coli aminoacyl-tRNAs for elongation factor Tu-GTP. J Biol Chem. 1984 Apr 25;259(8):5010–5016. [PubMed] [Google Scholar]
- McClain W. H., Guerrier-Takada C., Altman S. Model substrates for an RNA enzyme. Science. 1987 Oct 23;238(4826):527–530. doi: 10.1126/science.2443980. [DOI] [PubMed] [Google Scholar]
- McLaughlin L. W., Cramer F., Sprinzl M. Rapid analysis of modified tRNAphe from yeast by high-performance liquid chromatography: chromatography of oligonucleotides after RNase T1 digestion on aminopropylsilica and assignment of the fragments based on nucleoside analysis by chromatography on C18-silica. Anal Biochem. 1981 Mar 15;112(1):60–69. doi: 10.1016/0003-2697(81)90260-8. [DOI] [PubMed] [Google Scholar]
- Ott G., Faulhammer H. G., Sprinzl M. Interaction of elongation factor Tu from Escherichia coli with aminoacyl-tRNA carrying a fluorescent reporter group on the 3' terminus. Eur J Biochem. 1989 Sep 15;184(2):345–352. doi: 10.1111/j.1432-1033.1989.tb15025.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevitz R. W., Podjarny A. D., Krishnamachari N., Hughes J. J., Sigler P. B., Sussman J. L. Crystal structure of a eukaryotic initiator tRNA. Nature. 1979 Mar 8;278(5700):188–190. doi: 10.1038/278188a0. [DOI] [PubMed] [Google Scholar]
- Schneider D., Solfert R., von der Haar F. Large scale purification of tRNA ser , tRNA tyr and tRNA phe from Baker's yeast. Hoppe Seylers Z Physiol Chem. 1972 Aug;353(8):1330–1336. doi: 10.1515/bchm2.1972.353.2.1330. [DOI] [PubMed] [Google Scholar]
- Seong B. L., Lee C. P., RajBhandary U. L. Suppression of amber codons in vivo as evidence that mutants derived from Escherichia coli initiator tRNA can act at the step of elongation in protein synthesis. J Biol Chem. 1989 Apr 15;264(11):6504–6508. [PubMed] [Google Scholar]
- Seong B. L., RajBhandary U. L. Escherichia coli formylmethionine tRNA: mutations in GGGCCC sequence conserved in anticodon stem of initiator tRNAs affect initiation of protein synthesis and conformation of anticodon loop. Proc Natl Acad Sci U S A. 1987 Jan;84(2):334–338. doi: 10.1073/pnas.84.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Sternbach H., von der Haar F., Cramer F. Enzymatic incorporation of ATP and CTP analogues into the 3' end of tRNA. Eur J Biochem. 1977 Dec;81(3):579–589. doi: 10.1111/j.1432-1033.1977.tb11985.x. [DOI] [PubMed] [Google Scholar]
- Tanada S., Kawakami M., Nishio K., Takemura S. Interaction of aminoacyl-tRNA with bacterial elongation factor Tu: GTP complex: effects of the amino group of amino acid esterified to tRNA, the amino acid side chain, and tRNA structure. J Biochem. 1982 Jan;91(1):291–299. doi: 10.1093/oxfordjournals.jbchem.a133687. [DOI] [PubMed] [Google Scholar]
- Wagner T., Gross M., Sigler P. B. Isoleucyl initiator tRNA does not initiate eucaryotic protein synthesis. J Biol Chem. 1984 Apr 25;259(8):4706–4709. [PubMed] [Google Scholar]
- Wagner T., Sprinzl M. Enzymic binding of aminoacyl-tRNA to Escherichia coli ribosomes using modified tRNA species and tRNA fragments. Methods Enzymol. 1979;60:615–628. doi: 10.1016/s0076-6879(79)60058-7. [DOI] [PubMed] [Google Scholar]
- Woo N. H., Roe B. A., Rich A. Three-dimensional structure of Escherichia coli initiator tRNAfMet. Nature. 1980 Jul 24;286(5771):346–351. doi: 10.1038/286346a0. [DOI] [PubMed] [Google Scholar]


