Abstract
Context:
Obesity has emerged as one of the leading medical challenges of the 21st century. The resistance of this disorder to effective, long-term treatment can be traced to the fact that body fat stores are subject to homeostatic regulation in obese individuals, just as in lean individuals. Because the growing obesity epidemic is linked to a substantial increase in daily energy intake, a key priority is to delineate how mechanisms governing food intake and body fat content are altered in an obesogenic environment.
Evidence Acquisition:
We considered all relevant published research and cited references that represented the highest quality evidence available. Where space permitted, primary references were cited.
Evidence Synthesis:
The increase of energy intake that has fueled the U.S. obesity epidemic is linked to greater availability of highly rewarding/palatable and energy-dense food. Obesity occurs in genetically susceptible individuals and involves the biological defense of an elevated body fat mass, which may result in part from interactions between brain reward and homeostatic circuits. Inflammatory signaling, accumulation of lipid metabolites, or other mechanisms that impair hypothalamic neurons may also contribute to the development of obesity and offer a plausible mechanism to explain the biological defense of elevated body fat mass.
Conclusions:
Despite steady research progress, mechanisms underlying the resistance to fat loss once obesity is established remain incompletely understood. Breakthroughs in this area may be required for the development of effective new obesity prevention and treatment strategies.
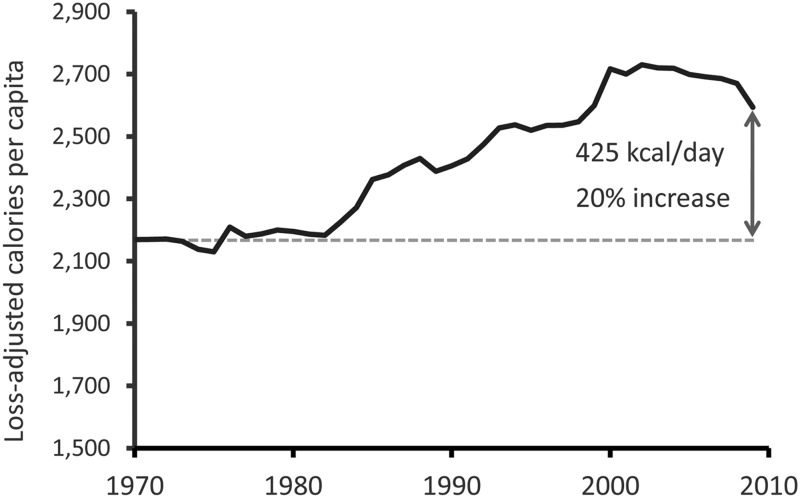
Over the course of industrialization, affluent populations have experienced an “epidemiological transition” characterized by an increased prevalence of certain disorders that are uncommon both in nonindustrial populations and in wild animals (1). Among these, obesity is perhaps the most conspicuous. Affecting approximately one third of adults in the United States (with an additional one third falling into the overweight category) (2), obesity has become a leading cause of morbidity, mortality, and reduced quality of life (3), a problem aggravated by the limited efficacy of nonsurgical treatments. Although the obesity epidemic can be traced to an increase in per capita energy intake over the past 40 yr (Fig. 1) (4, 5), which in turn is linked to changes in our collective diet, little is known about the physiological mechanisms underlying this trend. Particularly vexing is the question of why this disorder, once established, is so refractory to treatment. Here, we review the mechanisms controlling food intake in the context of energy homeostasis (the biological process that matches energy intake to energy expenditure over long time intervals), with a focus on obesity pathogenesis and the dramatic recent increase in obesity prevalence.
Fig. 1.
Per capita energy intake in the United States, 1970–2009. According to U.S. Department of Agriculture (USDA) food disappearance data (corrected for waste), per capita daily energy intake in the United States has increased 20% since 1970 (133). The increase began shortly after 1980, closely paralleling the rapid increment in obesity prevalence. Although USDA data are calculated from food disappearance measurements, this recent increase is broadly consistent with estimates calculated via other methods.
Short-Term, Meal-Related Determinants of Food Intake
Because humans, like most mammals, consume food in discrete bouts (or meals), total daily energy intake is a function of the size, frequency, and composition of meals. The perception of hunger and the decision to initiate a meal involve complex and poorly understood interactions between genetic, social, learned, environmental, circadian, and humoral cues (6, 7). As such, this process is quite variable and, although several endogenous peptides have been identified with the ability to stimulate feeding (6, 7), a unifying, physiological explanation for the experience of hunger and the decision to commence eating is still awaited.
A popular notion in the lay literature merits comment. Nearly 60 yr ago, Jean Mayer and his contemporaries hypothesized that because hypoglycemia potently stimulates appetite, hunger is normally triggered by sensing of declining plasma glucose levels or rates of glucose utilization by hypothalamic “feeding centers” (8). Decades of subsequent research have demonstrated that to the contrary, meal onset is not causally related to preprandial blood glucose (or insulin) levels within the normal physiological range (9). Yet the notion that low glucose (or elevated insulin) levels drive feeding behavior and promote fat gain remains widely popular, due largely to the marketing of commercial diet plans based on the glycemic index or reduced carbohydrate content. Among various scientific rationales that have been advanced for such diets is that excessive insulin secretion induced by rapidly digested carbohydrate foods causes a subsequent, transient fall of plasma glucose levels; this, in turn, triggers excess feeding and ultimately causes obesity. Another idea proposes that increased fasting and/or postprandial insulin levels induced by carbohydrate foods acts directly on adipocytes to increase fat storage, promoting fat gain over time. Although clinical trials have established that reduced carbohydrate diets can safely induce modest long-term weight loss (10), the mechanisms typically advanced to explain this benefit have little in the way of experimental support and are not informative with respect to the control of food intake. Moreover, reduced carbohydrate diets do not necessarily outperform diets with a higher carbohydrate content (e.g. the “Mediterranean” diet) when outcomes are measured after 1 yr or more (10).
Once feeding commences, the amount consumed is determined by factors involved in satiety perception (Fig. 2). The term “satiation” refers to the perception of fullness that leads to meal termination, whereas “satiety” describes the reduced interest in food after a meal. These responses ensure that feeding is terminated before gastric capacity is reached and that an appropriate interval passes to allow the disposition of ingested nutrients before the next meal begins (11). Unlike hunger perception, these processes are relatively well understood, involving combined effects of gastric distention and the release of peptide signals from enteroendocrine cells lining the gastrointestinal tract (12).
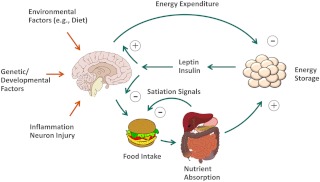
Fig. 2.
Model for CNS regulation of food intake and body fat mass. The CNS is hypothesized to regulate body fat mass via coordinated adjustments of both food intake and energy expenditure in response to afferent long-term and short-term signals of energy status. Environmental factors (e.g. the availability of highly palatable/rewarding foods, food composition, physical activity, etc.), genetic and developmental factors, hypothalamic inflammation, and neuron injury collectively determine the biologically defended level of body fat mass. Brain image: Patrick J. Lynch.
Gastric distension is sensed by mechanoreceptor neurons in the stomach and relayed to the hindbrain via vagal afferent and spinal sensory nerves (13). The majority of satiation-inducing gut peptides also mediate their effects via vagal afferent fibers, although some enter the brain from the circulation and exert their effects directly (12). Examples of satiation/satiety peptides released from intestinal enteroendocrine cells include cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), oxyntomodulin, peptide YY (PYY3–36), apolipoprotein A-IV, and enterostatin (12). Additional satiety-inducing peptides released from the endocrine pancreas include pancreatic polypeptide, glucagon, and amylin (14–18). CCK is a prototypic satiation peptide that is secreted by the duodenal and jejunal mucosa primarily in response to fat and protein ingestion, and it decreases food intake rapidly but transiently via the activation of vagal afferents (12, 19, 20). Meal size is increased by interventions that disrupt CCK signaling, establishing a physiological role for this peptide in satiation (21). GLP-1 and amylin are the only other satiation peptides shown to meet this criterion thus far (18, 22).
Ghrelin is an acylated peptide secreted from the gastric mucosa that, unlike satiety peptides, stimulates feeding and is implicated in meal initiation (6). Consistent with this concept, circulating ghrelin peaks just before meal onset and declines rapidly during and immediately after the meal (6, 12). However, because mice lacking a ghrelin signal do not exhibit altered meal patterns (23), the contribution of ghrelin to meal initiation relative to other factors remains uncertain.
Afferent signals involved in satiety are processed initially in the hindbrain. The nucleus of the solitary tract (NTS) plays a particularly important role in the processing of satiety-related input from vagal sensory fibers, and the adjacent area postrema, being located outside the blood-brain barrier, can sense input from circulating peptides directly (12). Evidence that hindbrain circuitry is itself sufficient to regulate meal size stems from experiments in “decerebrate” rats, in which the hindbrain and forebrain are surgically disconnected (24, 25). Although these animals are incapable of spontaneously initiating meals and rely on liquid food delivered directly into the mouth, they nonetheless terminate meals normally in response to gastric distention or satiation peptides such as CCK (24, 25). Because decerebrate rats fail to adjust meal size to compensate for changes in energy balance (26), however, communication between forebrain and hindbrain circuits appears necessary for adaptive changes of meal size to occur in response to changing energy needs. The neurocircuitry involved in this integration includes hypothalamic neurons that sense humoral input involved in energy homeostasis (e.g. leptin) and project to the hindbrain, where they modulate the sensitivity of NTS neurons to satiety signals in a manner that compensates for changes of body fat mass (27).
Long-Term Regulation of Food Intake and Energy Balance
The hormone leptin is secreted by adipocytes in proportion to body fat mass and plays a key role in energy homeostasis by informing the brain of changes in both energy balance and the amount of fuel stored as fat (Fig. 2) (28–30). Leptin acts in the brain as a negative feedback regulator of adiposity, constraining fat mass by limiting energy intake and supporting energy expenditure (28). Decreased leptin signaling promotes increased food intake, positive energy balance, and fat accumulation (28–30). Although plasma leptin levels reliably reflect body fat mass under weight-stable conditions, they can also change in response to short-term alterations of energy balance, well before significant changes of fat mass have occurred (31).
Leptin's effects on energy balance are mediated via leptin receptors in hypothalamic areas such as the arcuate nucleus (ARC), paraventricular nucleus, ventromedial hypothalamic nucleus, and lateral hypothalamic area (LHA) (28). Many extrahypothalamic regions are also leptin-sensitive, including the NTS and midbrain regions central to reward and motivation (32). The ARC is a major site for sensing and integrating peripheral energy balance signals, including hormones (leptin, insulin, and ghrelin) and nutrients (fatty acids, amino acids, and glucose) (28). These effects are mediated by at least two distinct leptin-sensitive neuron subpopulations in the ARC. Neurons that express proopiomelanocortin (POMC) synthesize and release melanocortin peptides such as α-MSH that are potently anorexigenic (inhibit food intake) and are stimulated by leptin and insulin (28). Melanocortin signaling appears to play a key physiological role to defend against excess fat gain, particularly during exposure to energy-dense, highly palatable foods (33, 34).
Located adjacent to POMC cells are orexigenic neurons that express neuropeptide Y (NPY) and agouti-related peptide (AgRP; an endogenous antagonist of the melanocortin-4 receptor). These cells are regulated in a manner reciprocal to POMC cells (28), and they play a key role to enable/stimulate feeding behavior (35). NPY/AgRP neurons are also GABAergic, and γ-aminobutyric acid is implicated as an important regulator of feeding released by these neurons (36, 37). NPY/AgRP neurons synapse onto and inhibit POMC neurons, and both cell types regulate energy balance via projections to brain regions that influence motivation/reward, energy expenditure, hunger, and ingestive behaviors (28).
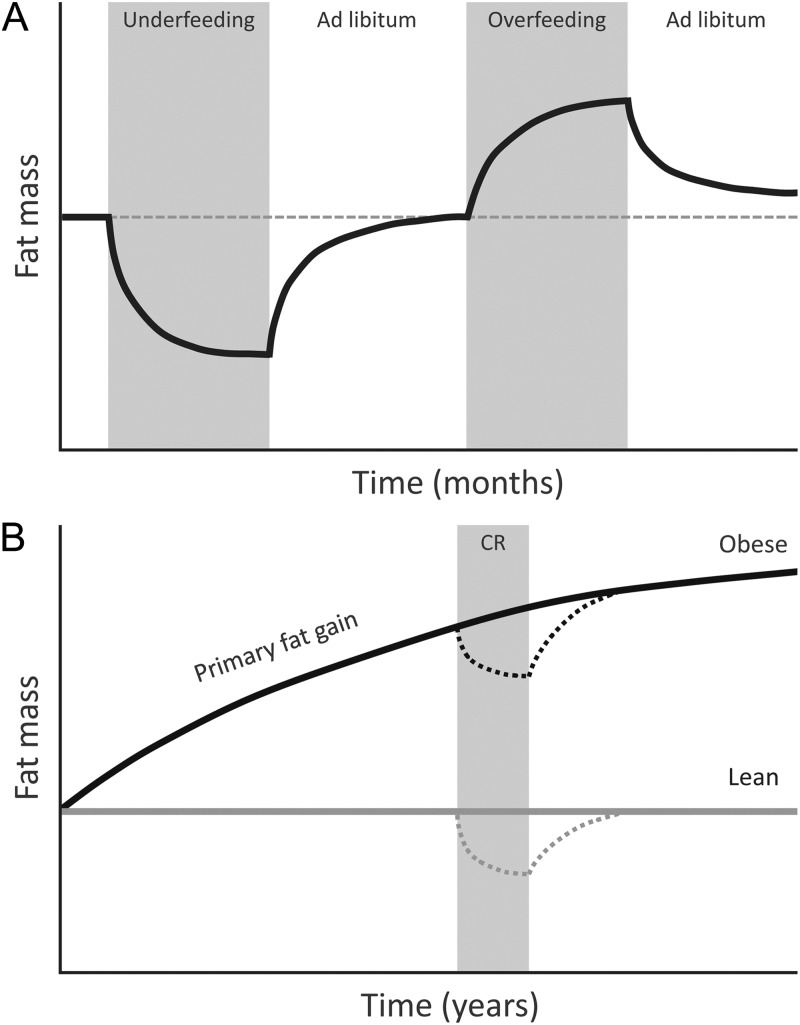
The adaptive response to fat loss induced by calorie-restricted diets illustrates how coordinate regulation of ARC neurons participates in energy homeostasis. Negative energy balance and loss of body fat lower plasma levels of adiposity negative feedback signals (e.g. leptin and insulin) while also raising ghrelin levels. In response, NPY/AgRP neurons are activated, whereas POMC cells are inhibited, a combination that potently promotes hyperphagia (increased food intake), positive energy balance, and the recovery of lost fat (28). These responses to fat loss appear to be operative in obese as well as lean individuals (Fig. 3).
Fig. 3.
Homeostatic regulation of body fat mass. A, Return of fat mass to baseline after short-term underfeeding or overfeeding. B, Gradual increase in the defended level of fat mass with age in an obese individual. The homeostatic response to fat loss induced by calorie restriction (CR) occurs in obese as well as lean individuals, resulting in the recovery of lost fat.
Food Reward and Palatability
In the United States, fast food has increased from 2 to 18% of total food spending over the last 50 yr, whereas soft drink consumption has increased 3.5-fold (38). These trends can be traced to increased food availability, systematic efforts to increase the reward value of food, and aggressive marketing that in combination have undoubtedly helped drive the obesity epidemic over the past four decades (4, 5).
Reward can be defined as the process whereby certain behaviors are reinforced in response to specific environmental stimuli. Animals and humans rapidly learn to associate olfactory, gustatory, and environmental cues with food properties that, in turn, reinforce behaviors related to the acquisition and consumption of rewarding food (39). Relevant food properties include caloric density and texture as well as the content of fat, starch, simple sugars, salt and free glutamate; under certain conditions, these factors can influence food intake and body fatness (39–47). In addition, a variety of other cues can be associated with these factors and become rewarding over time, resulting in acquired preferences (40, 41, 47). Palatability is defined as the pleasure or hedonic value associated with food, and it has been consistently shown to influence meal size in humans (48).
The “cafeteria diet” model of rodent obesity illustrates the importance of food reward and palatability in appetite regulation and obesity pathogenesis (49). In this model, rodents are provided with an assortment of human commercial “junk foods” in addition to standard unpurified chow. The animals overconsume palatable foods at the expense of less palatable yet more nutritious chow, and susceptible rat strains develop obesity rapidly (50). The impressive behavioral impact of food reward is illustrated by the observation that rats will voluntarily endure foot shocks or extreme cold to obtain a cafeteria diet, even when standard chow is freely available in unlimited amounts (51, 52).
Central nervous system (CNS) circuits involved in food reward are integrated with those governing energy homeostasis to allow food-seeking behaviors to be adjusted according to energy needs. Although the mechanisms underlying this integration are not fully understood, hormones including leptin, ghrelin, PYY, CCK, and insulin are implicated (32). The LHA is an important example of an area that participates in both reward processing (53, 54) and energy homeostasis (55), and hence may integrate inputs pertinent to food reward with those involved in body fat mass regulation.
Numerous brain regions coordinately evaluate and reinforce the rewarding value of food (56–58), including corticolimbic, hypothalamic, and midbrain circuits involved in reward processing (e.g. dopaminergic neurons of the ventral tegmental area and substantia nigra) the insula, amygdala, striatum, nucleus accumbens, orbitofrontal cortex, and the LHA (58). Signaling by dopamine and opioid peptides is particularly important in the reward and hedonic valuation of food, respectively (32, 58). Specifically, dopamine signaling is thought to contribute to the “wanting” of food (e.g. motivational aspects), whereas opioids are implicated in the “liking” of food (e.g. hedonic value or palatability) (59). Consistent with this view, opioid receptor agonists strongly increase the intake of palatable food over standard chow in rodents, whereas opioid antagonists decrease both the intake and the hedonic value of palatable food (60, 61). Although the μ-opioid receptor antagonist naltrexone was recently shown in clinical trials to lower body weight in obese subjects when combined with the norepinephrine-dopamine reuptake inhibitor bupropion (62), efforts to obtain Food and Drug Administration (FDA) approval for this combination have thus far proven unsuccessful. The mechanism of action may involve enhanced melanocortin signaling, consistent with a role for opioid and dopamine signaling to influence energy homeostasis circuits (62).
The endocannabinoid system includes the ligands anandamide and 2-arachydonlyglycerol that can act on either of two cannabinoid receptor subtypes (CB1 and CB2). This system earned its name after confirmation that marijuana exerts its psychoactive effects primarily through CB1 receptor activation (63). Endocannabinoids are small lipophilic molecules ubiquitously present in brain reward areas (64) and, whereas CB1 agonism selectively increases the consumption of palatable food (65) and is used to enhance appetite in the setting of cancer and other chronic disease states, antagonists of these receptors cause weight loss in overweight humans and selectively suppress the consumption of highly palatable foods in rodents (66, 67). The mechanism of fat loss is complex but includes reduced food intake and enhanced leptin sensitivity and is primarily mediated by CB1 receptors in the CNS (68–70). Despite unquestioned weight loss efficacy, the FDA has yet to approve CB1 antagonists for human obesity treatment, citing psychiatric side effects including an increased risk of suicide (66).
These findings collectively suggest that obesity can arise when animals or humans are confronted with foods whose palatability/reward value greatly exceeds that to which they are genetically adapted, and hence that interventions that inhibit food reward can prevent fat gain and promote fat loss. In considering these hypotheses, it is important to bear in mind that obesity in both humans and animal models involves the biological defense of an elevated level of body fat mass (Fig. 3). A key issue, therefore, is to understand how a change in the reward value of a diet impacts the energy homeostasis system.
Obesity and Energy Homeostasis
Because survival in a natural environment can be threatened by either too little or too much body fat, the energy homeostasis system is presumed to have evolved to maintain the level of adiposity appropriate for each species' ecological niche. There is general agreement that this system responds robustly to reduced fat mass by increasing both hunger and energy efficiency via a mechanism involving a low leptin signal, and that these responses are quite effective in returning fat mass to its original, preintervention level (Fig. 3A) (71, 72).
A more controversial question is the extent to which this homeostatic system defends against excess fat gain, a notion seemingly at odds with the steady increase of obesity prevalence in affluent nations (57, 73, 74). Yet numerous studies suggest that both overweight and lean individuals mount responses that resist excess fat gain and are able to lose most or all of the excess fat acquired during periods of experimental overfeeding via a mechanism involving post-overfeeding hypophagia (Fig. 3A) (75–79). This inherent resistance to fat gain in humans closely parallels what is observed in rodents that are either overfed by intragastric feeding or are exposed to refined highly palatable food and subsequently returned to an ad libitum, low-palatability chow [although the degree of fat loss can vary (80–82)]. Combined with evidence that these adaptive responses require intact leptin signaling (83), a physiological role for leptin to protect against pathological fat gain is implied.
Obesity can therefore be viewed as a state in which the defended level of body fat is increased, analogous to increased blood pressure in essential hypertension (Fig. 3B). Yet obesity development is also clearly linked to reward/hedonic drives (57, 58), raising the possibility that reward/hedonic circuits can impact homeostatic systems in a manner that favors the defense of an elevated body fat mass (Fig. 2). This concept is strengthened by the extensive and reciprocal neuroanatomical connections involved in the regulation of food intake and energy homeostasis that are shared by these two systems (84, 85).
Furthermore, interventions that alter CNS dopamine signaling can potently affect food intake and body fat mass, although both the direction and magnitude of these effects depend on the brain areas and dopamine receptor subtypes involved (86–91). As one example, variation at the dopamine D2 receptor gene locus can influence responses to palatable foods in ways that predict subsequent fat gain (91, 92), and D2 receptor availability in the striatum is reduced in obesity. These observations led to the notion that obesity arises from a “reward deficit,” in which affected individuals overeat to compensate for a diminished perception of food reward (58, 93). However, this hypothesis is inconsistent with reports of increased food reward sensitivity in obese individuals (94), and it predicts that low-reward food should cause compensatory overeating, which is not observed. To the contrary, food that is low in palatability and reward value can lower energy intake and body fat in ad libitum-fed obese rodents and humans (81, 82, 95, 96). An alternative hypothesis proposes that an inherited or acquired reduction of dopamine signaling via the D2 receptor in discrete CNS regions (perhaps accompanied by other dopaminergic alterations) influences systems governing energy homeostasis in a manner that favors the accumulation of body fat. The acquisition of this type of dopaminergic defect may involve desensitization of specific dopamine circuits caused by overexposure to highly palatable/rewarding food (97) and/or the suppressive effect of elevated leptin and insulin on reward regions in obese individuals (32).
Bariatric surgery is an interesting case study in body fat homeostasis. It is by far the most effective medical treatment for obesity, with the most commonly used procedure, Roux-en-Y gastric bypass (RYGB), yielding an average approximately 60% loss of excess body weight in morbidly obese patients (98). In direct contrast to calorie restriction, RYGB causes a reduction in hunger, reduced cravings for energy dense foods, and no alteration in circulating thyroid hormones that would suggest a homeostatic resistance to fat loss (99–101). As such, it appears to be the only well-characterized medical treatment that substantially, reliably, and durably reduces the defended level of fat mass. Although the mechanistic underpinnings of this effect remain poorly understood, plausible explanations involving changes in gut-brain communication have emerged. Studies using impermeable sleeves that cause luminal nutrients to bypass the duodenum and proximal ileum demonstrate that upper intestinal bypass per se is an important contributor to fat loss after RYGB (102). Thus, simply excluding the duodenum and/or increasing nutrient exposure of the ileum may favor the defense of a reduced level of body fat mass. After RYGB but before substantial fat loss, postprandial levels of PYY3–36 and GLP-1 increase substantially, whereas ghrelin levels decrease in most (but not all) studies (103), changes that should be anorexigenic and may therefore contribute to fat loss. Neural signals arising from gastric distention also increase due to a surgically induced reduction of stomach volume, and this may enhance the inhibitory effect of gut peptides on appetite and food intake. In addition, the duodenum, bypassed in RYGB, participates in nutrient sensing involved in reward processing (56), so removing it from contact with luminal nutrients may diminish food reward valuation. Indeed, several studies have reported alterations in reward functions after RYGB, consistent with a role for altered reward processes in the fat loss observed (101). Although the extent to which food reward is influenced by gastrointestinal or pancreatic peptides remains unclear, studies using pharmacological doses of CCK are consistent with such a possibility (104). These findings support the hypothesis that after RYGB, alterations in reward processing, in conjunction with a more anorexigenic gut peptide profile and increased gastric distension, collectively support the defense of a reduced fat mass.
Genetic Factors
Although rare, single-gene mutations can cause obesity, and much of what is known about energy homeostasis was discovered through the study of these monogenic obesity syndromes. To date, all known nondysmorphic monogenic obesity syndromes in humans arise from loss-of-function mutations in genes involved in the leptin signaling pathway (105).
Although heritable factors (reflecting a combination of genetics and epigenetics) are estimated to explain 45 to 75% of body mass index (BMI) variability in human populations (106), monogenic disorders account for only a small fraction (<5%) of human obesity. Thus, obesity susceptibility may be a polygenic trait. Consistent with this notion, genome-wide association studies (GWAS) have identified more than 40 common polymorphisms that associate with BMI variability in humans (107, 108). Their estimated collective contribution to BMI variability, however, remains quite small (<2%) (109), presumably because GWAS methodology does not detect gene-gene or gene-environment interactions. Nevertheless, GWAS, like monogenic obesity syndromes, implicate altered hypothalamic function in the pathogenesis of common obesity (107, 110).
In addition to genetic variability, developmental and epigenetic factors are implicated in obesity risk. In animal models, both in utero undernutrition and maternal overnutrition increase subsequent obesity risk in offspring, and children born to obese mothers are at an elevated risk of obesity relative to those born to lean mothers (111). Prepregnancy fat loss due to bariatric surgery attenuates subsequent obesity risk in offspring (112), suggesting that independent of genetic predisposition, developmental factors contribute meaningfully to the transgenerational transmission of obesity risk.
Growing evidence suggests that interacting genetic (113) and possibly developmental factors play an important role to determine obesity susceptibility when human populations are confronted with an obesogenic environment (Fig. 2). Relevant environmental factors include not only the types of foods available, but factors that affect food choice, such as education level, income, and related socioeconomic variables (114). From this perspective, dramatic, recent increases of obesity prevalence in Western societies can be viewed as resulting in large part from ever-increasing exposure of a genetically susceptible population to highly palatable, energy-dense foods.
Leptin Resistance: Cause or Effect of Obesity?
Obesity is characterized by increases of both fat mass and circulating leptin levels (72, 115, 116), suggesting a state of leptin resistance in which elevated leptin levels are required to overcome a defect in the leptin signaling cascade. Unlike in states of insulin resistance, in which a healthy pancreas can simply secrete more insulin as needed, increased body fat mass is required to maintain hyperleptinemia. Leptin resistance, therefore, offers a plausible mechanism to explain the defense of an elevated homeostatic “set point” for body fat mass. In support of this hypothesis, fat loss induced by caloric restriction elicits compensatory responses in obese individuals that promote the recovery of lost fat (including increased hunger and metabolic efficiency) (72, 115, 116), which can be suppressed by restoring plasma leptin concentrations to pre-fat loss levels (72).
The hypothesis that leptin resistance contributes to obesity pathogenesis is supported by evidence that rodents fed a purified, high-fat diet (HFD) acquire a striking loss of leptin sensitivity in ARC neurons (117, 118). Because targeted deletion of leptin receptors from any of several hypothalamic neuronal subpopulations is sufficient to cause obesity in mice (119, 120), the acquisition of hypothalamic leptin resistance is a plausible explanation for the defense of elevated body fatness in animal models of diet-induced obesity (DIO). Genetic interventions that prevent hypothalamic leptin resistance also protect against DIO (121–125), suggesting that the former is required for the latter to occur. Moreover, leptin resistance is detectable in ARC neurons even after relatively short periods of HFD feeding, before substantial fat gain (118). At the cellular level, inflammatory signaling has emerged as a potentially important mediator of leptin resistance (Fig. 2) (126). An induction of proinflammatory cytokines and other markers of inflammation is clearly evident in the hypothalamus of rodents with DIO (122, 126) and, just as inflammation in peripheral tissues contributes to obesity-induced insulin resistance, cellular inflammatory signaling also potently inhibits leptin signal transduction in neurons (122, 125).
Among several cellular mechanisms implicated in obesity-associated hypothalamic inflammation are activation of JNK (126) and IKK-NF-κB (122) signal transduction pathways, possibly as a consequence of endoplasmic reticulum stress, an upstream activator of these signaling pathways. Suppressor of cytokine signaling 3 and protein tyrosine phosphatase 1B are molecules downstream of NF-κB activation that inhibit leptin (and insulin) signaling and are induced in the hypothalamus of animals with DIO (127, 128). A deficiency of either of these genes confers resistance to DIO in mice (121, 123, 124), supporting a link between inflammatory signaling, CNS leptin and insulin resistance, and the development of obesity in this model. Another potential mediator of neuronal resistance to leptin (and insulin) is protein kinase C-θ, a serine-threonine kinase that is induced in the hypothalamus by intracellular accumulation of fatty acid metabolites (129, 130), analogous to its role as a mediator of free fatty acid-induced insulin resistance in skeletal muscle (131).
Very recent work reveals evidence of neuron injury occurring rapidly in the ARC of rats and mice placed on a HFD, accompanied by microglial and astroglial responses that may (at least initially) be neuroprotective in nature (132). The onset of hypothalamic injury during HFD feeding in rats and mice occurred before obesity onset, and an increased gliosis signal was detected in the hypothalamus of obese humans using magnetic resonance imaging, suggesting that a similar process may operate in humans. Although the implications of this work await further study, acquired injury of neurons in a brain area central to energy homeostasis offers a plausible mechanism for both hypothalamic inflammation and the defense of an elevated level of body fat in obese individuals (Fig. 2) (132).
Although these and related observations offer important insight into the pathogenesis of DIO, many pieces of the puzzle are still missing. Among these are cohesive explanations for the mechanisms underlying hypothalamic inflammatory signaling and injury during HFD feeding and translational studies that address the hypothesis mechanistically in humans.
Conclusion
Although substantial progress has begun to identify neurohumoral mechanisms underlying obesity, nonsurgical obesity treatment has improved little over the years. If obesity involves the biological defense of an elevated level of body fat, as current evidence suggests, advice to simply “eat less, move more” cannot be expected to remedy the problem. This is because interventions that reduce body fat stores without a corresponding decrease in the defended level of fat mass elicit compensatory responses that promote the recovery of lost fat and are difficult to consciously override. Because these responses constitute perhaps the single largest obstacle to effective obesity treatment, breakthroughs in understanding the biological defense of elevated body fat mass may be required to enable the development of effective new obesity prevention and treatment strategies.
Acknowledgments
This work was supported by a National Institutes of Health (NIH) Fellowship Training Program Award (T32DK007247), an NIH National Research Service Award (F32DK091989), the NIH-funded University of Washington Nutrition Obesity Research Center and Diabetes Endocrinology Research Center, and NIH Grants DK068384, DK083042, and DK052989 (to M.S.).
Disclosure Summary: S.G. has nothing to declare. M.S. has previously consulted for Orexigen and Pfizer.
Footnotes
- AgRP
- Agouti-related peptide
- ARC
- arcuate nucleus
- BMI
- body mass index
- CCK
- cholecystokinin
- DIO
- diet-induced obesity
- GLP-1
- glucagon-like peptide-1
- GWAS
- genome-wide association studies
- HFD
- high-fat diet
- LHA
- lateral hypothalamic area
- NPY
- neuropeptide Y
- NTS
- nucleus of the solitary tract
- POMC
- proopiomelanocortin
- PYY
- peptide YY
- RYGB
- Roux-en-Y gastric bypass.
References
- 1. Omran AR. 1971. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q 49:509–538 [PubMed] [Google Scholar]
- 2. Lee JM, Pilli S, Gebremariam A, Keirns CC, Davis MM, Vijan S, Freed GL, Herman WH, Gurney JG. 2010. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes (Lond) 34:614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flegal KM, Graubard BI, Williamson DF, Gail MH. 2007. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298:2028–2037 [DOI] [PubMed] [Google Scholar]
- 4. Duffey KJ, Popkin BM. 2011. Energy density, portion size, and eating occasions: contributions to increased energy intake in the United States, 1977–2006. PLoS Med 8:e1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swinburn BA, Sacks G, Lo SK, Westerterp KR, Rush EC, Rosenbaum M, Luke A, Schoeller DA, DeLany JP, Butte NF, Ravussin E. 2009. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr 89:1723–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. 2001. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- 7. Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. 1998. Signals that regulate food intake and energy homeostasis. Science 280:1378–1383 [DOI] [PubMed] [Google Scholar]
- 8. Mayer J. 1953. Glucostatic mechanism of regulation of food intake. N Engl J Med 249:13–16 [DOI] [PubMed] [Google Scholar]
- 9. Grossman SP. 1986. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci Biobehav Rev 10:295–315 [DOI] [PubMed] [Google Scholar]
- 10. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ. 2008. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 359:229–241 [DOI] [PubMed] [Google Scholar]
- 11. Davis JD, Smith GP. 1990. Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. Am J Physiol 259:R1228–R1235 [DOI] [PubMed] [Google Scholar]
- 12. Cummings DE, Overduin J. 2007. Gastrointestinal regulation of food intake. J Clin Invest 117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ritter RC. 2004. Gastrointestinal mechanisms of satiation for food. Physiol Behav 81:249–273 [DOI] [PubMed] [Google Scholar]
- 14. Katsuura G, Asakawa A, Inui A. 2002. Roles of pancreatic polypeptide in regulation of food intake. Peptides 23:323–329 [DOI] [PubMed] [Google Scholar]
- 15. Okada S, York DA, Bray GA, Erlanson-Albertsson C. 1991. Enterostatin (Val-Pro-Asp-Pro-Arg), the activation peptide of procolipase, selectively reduces fat intake. Physiol Behav 49:1185–1189 [DOI] [PubMed] [Google Scholar]
- 16. Lutz TA, Del Prete E, Scharrer E. 1994. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol Behav 55:891–895 [DOI] [PubMed] [Google Scholar]
- 17. Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Lush CW, Chen K, Lacerte C, Burns C, McKay R, Weyer C, Horowitz M. 2007. Low-dose pramlintide reduced food intake and meal duration in healthy, normal-weight subjects. Obesity (Silver Spring) 15:1179–1186 [DOI] [PubMed] [Google Scholar]
- 18. Geary N, Smith GP. 1982. Pancreatic glucagon and postprandial satiety in the rat. Physiol Behav 28:313–322 [DOI] [PubMed] [Google Scholar]
- 19. Gibbs J, Young RC, Smith GP. 1973. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature 245:323–325 [DOI] [PubMed] [Google Scholar]
- 20. Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. 1981. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 34:154–160 [DOI] [PubMed] [Google Scholar]
- 21. Moran TH, Ameglio PJ, Schwartz GJ, McHugh PR. 1992. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am J Physiol 262:R46–R50 [DOI] [PubMed] [Google Scholar]
- 22. Williams DL, Baskin DG, Schwartz MW. 2009. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. 2004. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 101:8227–8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grill HJ, Norgren R. 1978. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science 201:267–269 [DOI] [PubMed] [Google Scholar]
- 25. Grill HJ, Smith GP. 1988. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol 254:R853–R856 [DOI] [PubMed] [Google Scholar]
- 26. Kaplan JM, Seeley RJ, Grill HJ. 1993. Daily caloric intake in intact and chronic decerebrate rats. Behav Neurosci 107:876–881 [PubMed] [Google Scholar]
- 27. Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. 2005. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- 29. Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. 2001. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 31. Chin-Chance C, Polonsky KS, Schoeller DA. 2000. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab 85:2685–2691 [DOI] [PubMed] [Google Scholar]
- 32. Figlewicz DP, Sipols AJ. 2010. Energy regulatory signals and food reward. Pharmacol Biochem Behav 97:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. 2001. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- 34. Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. 1998. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- 35. Aponte Y, Atasoy D, Sternson SM. 2011. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14:351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. 2008. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11:998–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu Q, Boyle MP, Palmiter RD. 2009. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137:1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. 2011. Economic Research Service. U.S. Department of Agriculture. http://www.ers.usda.gov/Briefing/CPIFoodAndExpenditures/Data/Expenditures_tables/
- 39. Sclafani A. 2004. Oral and postoral determinants of food reward. Physiol Behav 81:773–779 [DOI] [PubMed] [Google Scholar]
- 40. Booth DA, Mather P, Fuller J. 1982. Starch content of ordinary foods associatively conditions human appetite and satiation, indexed by intake and eating pleasantness of starch-paired flavours. Appetite 3:163–184 [DOI] [PubMed] [Google Scholar]
- 41. Johnson SL, McPhee L, Birch LL. 1991. Conditioned preferences: young children prefer flavors associated with high dietary fat. Physiol Behav 50:1245–1251 [DOI] [PubMed] [Google Scholar]
- 42. Lucas F, Ackroff K, Sclafani A. 1989. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiol Behav 45:937–946 [DOI] [PubMed] [Google Scholar]
- 43. Morris MJ, Na ES, Johnson AK. 2008. Salt craving: the psychobiology of pathogenic sodium intake. Physiol Behav 94:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramirez I. 1991. High-fat diets stimulate transient hyperphagia whereas wet diets stimulate prolonged hyperphagia in Fischer rats. Physiol Behav 49:1223–1228 [DOI] [PubMed] [Google Scholar]
- 45. Ramirez I, Friedman MI. 1990. Dietary hyperphagia in rats: role of fat, carbohydrate, energy content. Physiol Behav 47:1157–1163 [DOI] [PubMed] [Google Scholar]
- 46. Sclafani A. 1987. Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form, taste. Neurosci Biobehav Rev 11:155–162 [DOI] [PubMed] [Google Scholar]
- 47. Yeomans MR, Gould NJ, Mobini S, Prescott J. 2008. Acquired flavor acceptance and intake facilitated by monosodium glutamate in humans. Physiol Behav 93:958–966 [DOI] [PubMed] [Google Scholar]
- 48. Sørensen LB, Møller P, Flint A, Martens M, Raben A. 2003. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes Relat Metab Disord 27:1152–1166 [DOI] [PubMed] [Google Scholar]
- 49. Sclafani A, Springer D. 1976. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav 17:461–471 [DOI] [PubMed] [Google Scholar]
- 50. Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. 2011. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 19:1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cabanac M, Johnson KG. 1983. Analysis of a conflict between palatability and cold exposure in rats. Physiol Behav 31:249–253 [DOI] [PubMed] [Google Scholar]
- 52. Oswald KD, Murdaugh DL, King VL, Boggiano MM. 2011. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord 44:203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fulton S, Woodside B, Shizgal P. 2000. Modulation of brain reward circuitry by leptin. Science 287:125–128 [DOI] [PubMed] [Google Scholar]
- 54. Hoebel BG, Teitelbaum P. 1962. Hypothalamic control of feeding and self-stimulation. Science 135:375–377 [DOI] [PubMed] [Google Scholar]
- 55. Powley TL, Keesey RE. 1970. Relationship of body weight to the lateral hypothalamic feeding syndrome. J Comp Physiol Psychol 70:25–36 [DOI] [PubMed] [Google Scholar]
- 56. Ackroff K, Yiin YM, Sclafani A. 2010. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav 99:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Berthoud HR. 2004. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav 81:781–793 [DOI] [PubMed] [Google Scholar]
- 58. Kenny PJ. 2011. Reward mechanisms in obesity: new insights and future directions. Neuron 69:664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berridge KC. 1996. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20:1–25 [DOI] [PubMed] [Google Scholar]
- 60. Olszewski PK, Levine AS. 2007. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav 91:506–512 [DOI] [PubMed] [Google Scholar]
- 61. Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS. 1993. Naloxone's anorectic effect is dependent upon the relative palatability of food. Pharmacol Biochem Behav 46:917–921 [DOI] [PubMed] [Google Scholar]
- 62. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. 2010. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376:595–605 [DOI] [PubMed] [Google Scholar]
- 63. Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. 2001. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58:322–328 [DOI] [PubMed] [Google Scholar]
- 64. Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. 1998. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411 [DOI] [PubMed] [Google Scholar]
- 65. Foltin RW, Fischman MW, Byrne MF. 1988. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 11:1–14 [DOI] [PubMed] [Google Scholar]
- 66. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. 2007. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370:1706–1713 [DOI] [PubMed] [Google Scholar]
- 67. Gessa GL, Orrù A, Lai P, Maccioni P, Lecca R, Lobina C, Carai MA, Colombo G. 2006. Lack of tolerance to the suppressing effect of rimonabant on chocolate intake in rats. Psychopharmacology (Berl) 185:248–254 [DOI] [PubMed] [Google Scholar]
- 68. Pang Z, Wu NN, Zhao W, Chain DC, Schaffer E, Zhang X, Yamdagni P, Palejwala VA, Fan C, Favara SG, Dressler HM, Economides KD, Weinstock D, Cavallo JS, Naimi S, Galzin AM, Guillot E, Pruniaux MP, Tocci MJ, Polites HG. 2011. The central cannabinoid CB1 receptor is required for diet-induced obesity and rimonabant's antiobesity effects in mice. Obesity (Silver Spring) 19:1923–1934 [DOI] [PubMed] [Google Scholar]
- 69. Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P. 2004. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648 [DOI] [PubMed] [Google Scholar]
- 70. Xie S, Furjanic MA, Ferrara JJ, McAndrew NR, Ardino EL, Ngondara A, Bernstein Y, Thomas KJ, Kim E, Walker JM, Nagar S, Ward SJ, Raffa RB. 2007. The endocannabinoid system and rimonabant: a new drug with a novel mechanism of action involving cannabinoid CB1 receptor antagonism—or inverse agonism—as potential obesity treatment and other therapeutic use. J Clin Pharm Ther 32:209–231 [DOI] [PubMed] [Google Scholar]
- 71. Keys A. 1946. Human starvation and its consequences. J Am Diet Assoc 22:582–587 [PubMed] [Google Scholar]
- 72. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. 2005. Low-dose leptin reverses skeletal muscle, autonomic, neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leibel RL. 2002. The role of leptin in the control of body weight. Nutr Rev 60:S15–S19; discussion S68–84, 85–87 [DOI] [PubMed] [Google Scholar]
- 74. Flier JS. 1998. Clinical review 94: what's in a name? In search of leptin's physiologic role. J Clin Endocrinol Metab 83:1407–1413 [DOI] [PubMed] [Google Scholar]
- 75. Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. 1992. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr 56:641–655 [DOI] [PubMed] [Google Scholar]
- 76. Pasquet P, Apfelbaum M. 1994. Recovery of initial body weight and composition after long-term massive overfeeding in men. Am J Clin Nutr 60:861–863 [DOI] [PubMed] [Google Scholar]
- 77. Roberts SB, Young VR, Fuss P, Fiatarone MA, Richard B, Rasmussen H, Wagner D, Joseph L, Holehouse E, Evans WJ. 1990. Energy expenditure and subsequent nutrient intakes in overfed young men. Am J Physiol 259:R461–R469 [DOI] [PubMed] [Google Scholar]
- 78. Sims EA, Danforth E, Jr, Horton ES, Bray GA, Glennon JA, Salans LB. 1973. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 29:457–496 [DOI] [PubMed] [Google Scholar]
- 79. Tremblay A, Després JP, Thériault G, Fournier G, Bouchard C. 1992. Overfeeding and energy expenditure in humans. Am J Clin Nutr 56:857–862 [DOI] [PubMed] [Google Scholar]
- 80. Cohn C, Joseph D. 1962. Influence of body weight and body fat on appetite of “normal” lean and obese rats. Yale J Biol Med 34:598–607 [PMC free article] [PubMed] [Google Scholar]
- 81. Guo J, Jou W, Gavrilova O, Hall KD. 2009. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS One 4:e5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shi H, Akunuru S, Bierman JC, Hodge KM, Mitchell MC, Foster MT, Seeley RJ, Reizes O. 2009. Diet-induced obese mice are leptin insufficient after weight reduction. Obesity (Silver Spring) 17:1702–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. White CL, Purpera MN, Ballard K, Morrison CD. 2010. Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol Behav 100:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stratford TR, Kelley AE. 1999. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 19:11040–11048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Barone FC, Wayner MJ, Tsai WH, Zarco de Coronado I. 1981. Effects of ventral tegmental area stimulation and microiontophoretic application of dopamine and norepinephrine on hypothalamic neurons. Brain Res Bull 7:181–193 [DOI] [PubMed] [Google Scholar]
- 86. Cincotta AH, Meier AH. 1989. Reductions of body fat stores and total plasma cholesterol and triglyceride concentrations in several species by bromocriptine treatment. Life Sci 45:2247–2254 [DOI] [PubMed] [Google Scholar]
- 87. Cincotta AH, Meier AH. 1996. Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects. Diabetes Care 19:667–670 [DOI] [PubMed] [Google Scholar]
- 88. Jones B, Basson BR, Walker DJ, Crawford AM, Kinon BJ. 2001. Weight change and atypical antipsychotic treatment in patients with schizophrenia. J Clin Psychiatry 62(Suppl 2):41–44 [PubMed] [Google Scholar]
- 89. Leibowitz SF, Rossakis C. 1979. Mapping study of brain dopamine- and epinephrine-sensitive sites which cause feeding suppression in the rat. Brain Res 172:101–113 [DOI] [PubMed] [Google Scholar]
- 90. Spitz MR, Detry MA, Pillow P, Hu Y, Amos CI, Hong WK, Wu X. 2000. Variant alleles of the D2 dopamine receptor gene and obesity. Nutr Res 20:371–380 [Google Scholar]
- 91. Stice E, Spoor S, Bohon C, Small DM. 2008. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322:449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. 2008. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 117:924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. 2001. Brain dopamine and obesity. Lancet 357:354–357 [DOI] [PubMed] [Google Scholar]
- 94. Stice E, Spoor S, Ng J, Zald DH. 2009. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav 97:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cabanac M, Rabe EF. 1976. Influence of a monotonous food on body weight regulation in humans. Physiol Behav 17:675–678 [DOI] [PubMed] [Google Scholar]
- 96. Hashim SA, Van Itallie TB. 1965. Studies in normal and obese subjects with a monitored food dispensing device. Ann NY Acad Sci 131:654–661 [DOI] [PubMed] [Google Scholar]
- 97. Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. 2001. Excessive sugar intake alters binding to dopamine and μ-opioid receptors in the brain. Neuroreport 12:3549–3552 [DOI] [PubMed] [Google Scholar]
- 98. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 99. Chikunguwo S, Brethauer S, Nirujogi V, Pitt T, Udomsawaengsup S, Chand B, Schauer P. 2007. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg Obes Relat Dis 3:631–635; discussion 635–636 [DOI] [PubMed] [Google Scholar]
- 100. Moulin de Moraes CM, Mancini MC, de Melo ME, Figueiredo DA, Villares SM, Rascovski A, Zilberstein B, Halpern A. 2005. Prevalence of subclinical hypothyroidism in a morbidly obese population and improvement after weight loss induced by Roux-en-Y gastric bypass. Obes Surg 15:1287–1291 [DOI] [PubMed] [Google Scholar]
- 101. Shin AC, Berthoud HR. 2011. Food reward functions as affected by obesity and bariatric surgery. Int J Obes (Lond) 35(Suppl 3):S40–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, Greve JW. 2010. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg 251:236–243 [DOI] [PubMed] [Google Scholar]
- 103. Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. 2011. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes Lond 35:153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pérez C, Sclafani A. 1991. Cholecystokinin conditions flavor preferences in rats. Am J Physiol 260:R179–R185 [DOI] [PubMed] [Google Scholar]
- 105. Farooqi S, O'Rahilly S. 2006. Genetics of obesity in humans. Endocr Rev 27:710–718 [DOI] [PubMed] [Google Scholar]
- 106. Farooqi IS, O'Rahilly S. 2007. Genetic factors in human obesity. Obes Rev 8(Suppl 1):37–40 [DOI] [PubMed] [Google Scholar]
- 107. O'Rahilly S. 2009. Human genetics illuminates the paths to metabolic disease. Nature 462:307–314 [DOI] [PubMed] [Google Scholar]
- 108. Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, et al. 2009. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li S, Zhao JH, Luan J, Luben RN, Rodwell SA, Khaw KT, Ong KK, Wareham NJ, Loos RJ. 2010. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. Am J Clin Nutr 91:184–190 [DOI] [PubMed] [Google Scholar]
- 110. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Mägi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segrè AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpeläinen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, et al. 2010. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Taylor PD, Poston L. 2007. Developmental programming of obesity in mammals. Exp Physiol 92:287–298 [DOI] [PubMed] [Google Scholar]
- 112. Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, Biertho L, Simard S, Kral JG, Marceau P. 2009. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab 94:4275–4283 [DOI] [PubMed] [Google Scholar]
- 113. Rokholm B, Silventoinen K, Ängquist L, Skytthe A, Kyvik KO, Sørensen TI. 2011. Increased genetic variance of BMI with a higher prevalence of obesity. PLoS One 6:e20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Drewnowski A, Darmon N. 2005. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr 82:265S–273S [DOI] [PubMed] [Google Scholar]
- 115. Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. 1995. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161 [DOI] [PubMed] [Google Scholar]
- 116. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. 1996. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- 117. Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. 2007. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5:181–194 [DOI] [PubMed] [Google Scholar]
- 118. Münzberg H, Flier JS, Bjørbaek C. 2004. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145:4880–4889 [DOI] [PubMed] [Google Scholar]
- 119. Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. 2010. The roles of leptin receptors on POMC neurons in the regulation of sex-specific energy homeostasis. Physiol Behav 100:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr 2008. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149:1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12:917–924 [DOI] [PubMed] [Google Scholar]
- 122. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. 2008. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. 2004. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10:739–743 [DOI] [PubMed] [Google Scholar]
- 124. Picardi PK, Calegari VC, Prada Pde O, Moraes JC, Araújo E, Marcondes MC, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. 2008. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology 149:3870–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Brüning JC. 2009. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 10:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. 2005. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146:4192–4199 [DOI] [PubMed] [Google Scholar]
- 127. Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. 2003. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 4:540–545 [DOI] [PubMed] [Google Scholar]
- 128. Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. 2008. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283:14230–14241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, Lefevre AL, Cruciani-Guglielmacci C, Magnan C, Yu F, Niswender K, Irani BG, Holland WL, Clegg DJ. 2009. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J Clin Invest 119:2577–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. 2009. Hypothalamic proinflammatory lipid accumulation, inflammation, insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296:E1003–E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- 132. Thaler JP, Yi C, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. 2011. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. 2011. Economic Research Service. U.S. Department of Agriculture. http://www.ers.usda.gov/data/foodconsumption/FoodAvailDoc.htm