Abstract
Objective
Thrombosis and restenosis remain problematic for many intravascular procedures. Previously, it has been demonstrated that modifying an injured vascular surface with a protein-reactive polymer could block undesirable platelet deposition. As an added benefit, it would be advantageous if one could target therapeutics to the injured site. This study investigates a site-specific delivery system to target microspheres to vascular surfaces modified with a reactive polyethylene glycol tagged with biotin.
Methods
Rabbit femoral arteries were injured with a 2F embolectomy catheter. Modification of the vascular surface was achieved using a channeled balloon catheter or small diameter tube. Microspheres were injected intravenously through catheterization of the ear vein. Polymer modification on the injured surface and delivery of microspheres was quantified using epi-fluorescence microscopy at 0, 24, 48, and 72 h.
Results
Polymer modification of the vascular surface could be achieved using a channeled drug delivery catheter or small diameter tube with similar results. Maximum polymer coverage occurred at 0 h and decreased to 85% maximal at 24 h, 72% at 48 h, and 67% at 72 h. The initial number of microspheres per mm2 binding to modified, injured arteries was 304 versus 141 for the unmodified, damaged control (P < .01). At subsequent times, the number of adherent microspheres to modified, injured arteries decreased by 50%, 70%, and 84% at 24, 48, and 72 h, respectively; while non-specific binding to unmodified, injured arteries quickly decreased by 93%. Initial microsphere binding to modified, healthy arteries was 153 microspheres/mm2 as opposed to 26 for the unmodified, healthy controls (P < .01).
Conclusions
Chemical modification of injured vessels following intravascular procedures can be readily accomplished in vivo to create a substrate for targeted delivery systems. As a proof of concept, targeted microspheres preferentially adhered to polymer-modified surfaces as opposed to injured, unmodified or healthy vascular surfaces.
INTRODUCTION
Disruption of the endothelial layer during intravascular procedures such as balloon angioplasty often leads to restenosis. Exposure of a thrombogenic surface leads to platelet and leukocyte activation which contribute to thrombus formation and inflammation 1 In addition, balloon inflation can lead to dissections within the arterial wall, causing smooth muscle cell injury. Factors released by platelets and leukocytes as well as direct injury cause smooth muscle cells to proliferate, migrate, and deposit more extracellular matrix, resulting in neointimal hyperplasia.2, 3 Stents have reduced but not eliminated the problem of restenosis, and have introduced new risks of stent thrombosis related to delayed re-endothelialization, often requiring long term anti-platelet therapy. Thus, strategies to mitigate the cascade of pathophysiologic events that lead to restenosis and contribute to stent thrombosis would have significant clinical value.
In the current study, a protein-reactive polymer, N-hydroxysuccinimide-polyethylene glycol (NHS-PEG), was employed in an in vivo rabbit femoral artery model of vascular injury to directly modify injured vascular surfaces. The goal of the study was do demonstrate the potential for polymer modification of the vascular surface to block acute thrombosis and provide a site for the targeted delivery of therapeutics. The NHS reactive group covalently links with primary amines, the most accessible being the epsilon amine found on the amino acid lysine.4 A stable amide bond is formed covalently linking the protein-reactive polymer with a primary amine of a protein on a vascular surface.5 Previous reports have demonstrated that modification of vascular surfaces with a protein-reactive PEG forms a molecular barrier preventing acute platelet and leukocyte adhesion.6, 7
In addition to blocking thrombosis at sites of vascular injury, it would be advantageous to have a means of targeting intravenously injected agents to labeled vascular segments for further therapeutic benefit. This could be accomplished by the presence of a signaling molecule, such as biotin, on the non-reactive terminus of the PEG molecule. NHS-PEG-biotin might thus modify vascular tissue and provide a site for the targeted delivery of agents relying upon the high affinity between biotin and avidin (Ka = 1015 L/mol).4 Targeting systems exploiting the strong interaction between biotin and avidin have previously been explored as drug delivery vehicles for tumors and whole organs.8, 9 Our objective here was to evaluate this concept (Figure 1) in a rabbit model using avidin-coated microspheres to simulate particulate drugs, liposomes, or vesicles targeted to PEG-biotin modified arterial segments. The dependence of PEG-biotin coverage on the delivery method and time after modification were examined, as was the ability to target microspheres to the modified vascular segment over a 72 hour period.
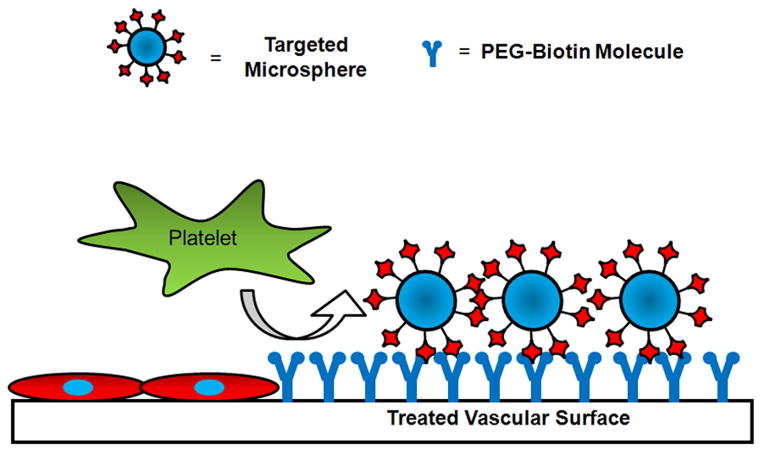
Figure 1.
Schematic of the proposed targeted delivery system. The reactive polymer is covalently attached to the vascular surface forming a molecular barrier, inhibiting platelet and leukocyte adhesion. Targeted microspheres or cells will specifically adhere to vascular surfaces labeled with the polymer.
MATERIALS AND METHODS
Surgical Procedure
The animal studies were completed following approval by the University of Pittsburgh Institutional Animal Care & Use Committee. This study employed a rabbit femoral artery model to evaluate the proposed modification and targeting system.6 Female New Zealand White rabbits (Myrtle’s Rabbitry, Inc., Thompson Station, TN) with an average weight of 4.25 kg were anesthetized by intramuscular injection of 40 mg/kg ketamine and 5 mg/kg xylazine and maintained on inhaled 1.5–2.5% isoflurane (Webster Veterinary, Sterling, MA). An ear vein was cannulated with a 22 G × 1 in JELCO IV catheter (Webster Veterinary), and a slow drip of lactated Ringers buffer (Webster Veterinary) was administered through a Venoset Microdrip IV set (Webster Veterinary). The common femoral, saphenous, and popliteal arteries were exposed by dissection, and minor side branches off the common femoral artery were ligated and removed. SUNDT Slim-Line aneurysm clips (Codman, Raynham, MA) were placed on the common femoral, deep femoral branch, and on the popliteal arteries. An arteriotomy was made in the saphenous artery to allow access to the lumen of the common femoral artery. Only one leg was used in survival studies to minimize trauma to the animal and allow for adequate mobility for water and food intake. In acute studies, the same procedure was repeated on the other leg of the animal to minimize the number of animals required.
Balloon Injury
A 2F Fogarty embolectomy catheter (Edwards Lifesciences, Irvine, CA) was inserted through the arteriotomy in the saphenous artery until it reached the clamp on the common femoral artery. The balloon was inflated with air using a 1 mL slip-tip disposable tuberculin syringe (Becton Dickinson, Franklin Lakes, NJ) until the vessel distended slightly. The inflated balloon was withdrawn from the vessel until it reached the branch where the popliteal and saphenous emerge from the common femoral artery. The balloon was deflated and the procedure repeated three times to ensure vascular injury.10
Denudation of the endothelial layer and vascular injury was confirmed in a small cohort of animals using 10 mg/kg Evans blue dye given intravenously 2 h prior to euthanasia. Evans blue is an azo dye that binds with circulating albumin. An intact endothelium would serve as a barrier to this protein-dye complex, but the complex adsorbs onto denuded arterial segments staining the surface a royal blue color.11 Both the injured common femoral artery and the uninjured common femoral artery in the contralateral leg were harvested and inspected. Only femoral arteries injured with the embolectomy catheter appeared blue (data not shown).
Arterial Modification with PEG
Arterial lumen surface modification was accomplished using either a 0.63 mm outer diameter Micro-Renathane (MR) tubing (Braintree Scientific, Braintree, MA) or 2.5 mm channeled drug delivery balloon catheter (Remedy, Boston Scientific, Natick, MA), which is a dual lumen balloon and drug-delivery catheter with 24 distinct channels on the outer balloon surface leading to a series of 100 μm holes for delivery of the therapeutic agent directly to the arterial wall and independent of the inflation of the balloon. The channeled catheter was used to evaluate a more clinical approach, while the MR tubing was more practical for animal and proof of concept studies. The MR tubing or channeled catheter was introduced into the common femoral artery through the saphenous arteriotomy and threaded until the tip reached the clamp. Phosphate buffered saline (PBS, Cambrex, Walkersville, MD) was added to NHS-PEG-biotin (MW 3,400) or NHS-PEG-fluorescein (MW 5,000, Nektar Therapeutics, San Carlos, CA) to make 1 mL of a 10 mM solution, and injected through the port on the channeled catheter or using a 27G × ½ in needle (BD PrecisionGlide™) inserted into the end of the MR tubing to allow for perfusion of the polymer into the vessel. The polymer solution was continuously flushed through the vessel lumen for 45 s, after which, the MR tubing or balloon catheter was withdrawn and a previously placed ligature was tightened at the saphenous artery branch point. When the total incubation time for the polymer in the artery reached 1 min, the aneurysm clips were removed from the popliteal and common femoral arteries to restore blood flow. The incubation time and concentration of the infused solution were based upon previous in vitro studies12. Control arteries were treated in an identical manner except that the vehicle, PBS, was perfused instead of the reactive polymer solution.
For control experiments in undamaged arterial segments, the tip of a 30G × ½ in needle (BD PrecisionGlide) was bent to a 90° angle and inserted into the common femoral artery lumen near the clamp. The polymer was continuously flushed through the lumen of the vessel and out the site of the arteriotomy for a period of 45 s. After addition of the polymer, the needle was withdrawn, and a previously placed ligature was tightened at the saphenous artery branch point. A small amount of Avitene® (Bard Davol, Inc., Warwick, RI) was placed at site of access to control bleeding. When the total incubation time for the polymer in the artery reached 1 min, the aneurysm clips were removed from the popliteal and common femoral arteries to restore blood flow through the femoral artery and remove any un-reacted polymer. A subset of arteries were treated in an identical manner except that the vehicle, PBS, was added instead of the reactive polymer solution.
Modified and control vessels from injured and uninjured animals were harvested at 0, 24, 48, and 72 h post operatively (n=4–6 animals for each time point). For non-acute studies, surgical sites were closed with 2-0 Vicryl sutures (Ethicon, Somerville, NJ), and the animals recovered. Intramuscular injections of 1 mg/kg ketoprofen and 100 mg cefazolin (Webster Veterinary) were administered twice daily until the animals were euthanized.
Targeting of Microspheres
A deglycosylated form of avidin, NeutrAvidin, which has similar biotin binding characteristics and lower non-specific binding was utilized in these studies as a model of targeted drug delivery vehicles. All microspheres were delivered to the peripheral circulation through a cannulated ear vein. In the case of the 0 h time point, intravenous access was already established at the beginning of the surgical procedure, which permitted administration of the microspheres. In the case of the 0 h time point, intravenous access was already established at the beginning of the surgical procedure, which permitted administration of the microspheres. For the extended time points, the animals were anesthetized and an ear vein catheterized as described previously in the Surgical Procedure section. A bolus of 4.80 ×108 particles of 1-μm yellow-green fluorescent NeutrAvidin-labeled microspheres (Fluospheres, Molecular Probes, Eugene, OR) in 1 mL of PBS was injected over a period of 30 s via an 18 G × 1 ½ in needle (BD PrecisionGlide) inserted into an access port on the Venoset Microdrip IV set and then flushed with Lactated Ringers solution. The microspheres were allowed to circulate in the bloodstream of the rabbit for 1 h before the vessels were harvested as described above.
Specimen Collection
Animals were maintained on 2.5% isoflurane. A midline incision below the rib cage to the pelvic region was used to expose and isolate the vena cava and aorta from the surrounding tissue. The isoflurane was increased to 5% and the animals euthanized with a supersaturated potassium chloride (KCl) solution (Sigma, St. Louis, MO). The aorta was cannulated with an 18 G × 1 in IV catheter (JELCO, Webster Veterinary) and the hind limb portion of the animals was flushed with 100 mL of Lactated Ringers through a Venoset IV set (Webster Veterinary). The vena cava was severed to allow the excess blood and fluid to drain from the hind limb region, and 100 mL of Shandon Glyo-Fixx™ (Thermo Electron Corporation, Waltham, MA) was administered to pressure fix the arteries for examination. Experimental and control femoral arteries were explanted from the rabbit after fixation along with the unmodified carotid arteries, which served as the control for acute experiments where both femoral arteries were modified. Excess tissue was removed from the exterior of the arteries which were then filleted lengthwise to expose the lumen. Minutien pins (Fine Science Tools, Foster City, CA) were used to secure the edges of the vessel to pink dental wax (Electron Microscopy Sciences, Hatfield, PA) with the lumen facing upwards for evaluation using epi-fluorescence microscopy.
Fluorescence Microscopy of Samples
Fluorescence intensity of the surface deposited polymer in the modification experiments and quantification of microsphere binding in targeting experiments were performed using an inverted fluorescence microscope (Axiovert, Carl Zeiss, Inc., Thornwood, NY). Micrographs of the explanted vessels were taken at the same exposure at 1 mm increments from the saphenous artery branch to the site of the clamp on the common femoral artery. The average fluorescence intensity of the polymer (490 nm emission, 514 nm excitation) was measured for each image with IP Lab software (Scanalytics Inc., Billerica, MA) to determine the persistence of the polymer modification over time. Quantification of the number of adherent microspheres was performed on images collected at excitation/emission spectra of 505 nm and 515 nm, respectively.
Statistical Analysis
Statistical analysis of the data acquired from the vessel images was performed in SPSS (version 13.0, SPSS Inc.). Data points are represented as mean values + standard deviation (st dev). Differences between control and treated groups for PEG duration and microsphere targeting to balloon injured rabbit femoral arteries, at all time points, were evaluated using a two-way ANOVA to determine statistical significance. For microsphere targeting experiments to healthy endothelium, controls versus treated group comparisons were made using a Student’s t-test with P < .05 being considered statistically significant.
RESULTS
Arterial Modification Technique
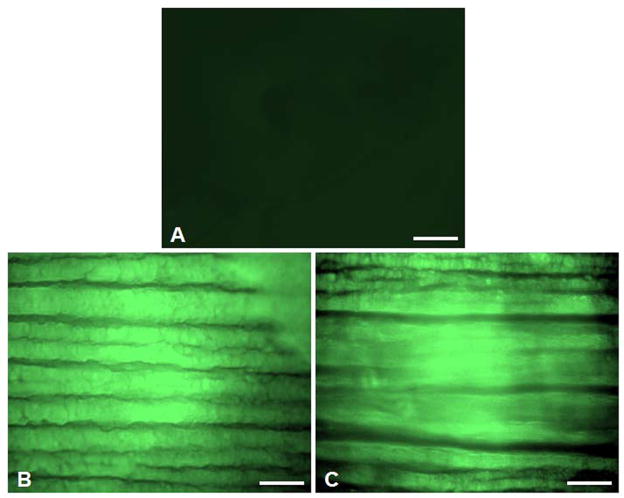
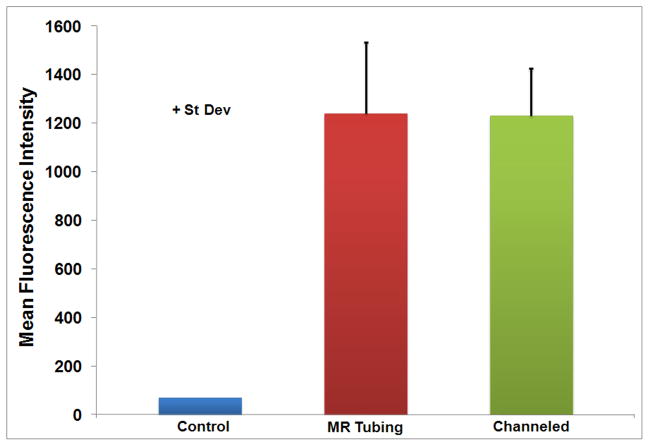
Visual inspection of the arterial specimens showed limited background fluorescence for control vessels and similar patterns of staining across treated arteries (Figure 2). No statistically significant differences were found in the mean fluorescence intensity (FU) of NHS-PEG-fluorescein delivered to balloon injured arteries using the MR tubing (1,241 FU) relative to the channeled drug delivery catheter (1,230 FU) (Figure 3). Control vessels showed little to no autofluorescence with a mean fluorescence intensity of 69 FU (Figure 3). Since there was no difference in the ability to deliver the polymer with either technique, subsequent experiments evaluating the duration of the polymer in the vessel or ability to target microspheres to the polymer were completed using the MR tubing. The MR tubing was selected because of its smaller size in comparison to the channeled drug delivery balloon catheter, which allowed for easier introduction into the rabbit saphenous artery with fewer complications.
Figure 2.
Fluorescent micrographs of different techniques to modify balloon injured vessels with reactive PEG-fluorescein: (A) Vehicle (PBS) control, (B) Micro-Renathane tubing, (C) Remedy channeled drug delivery balloon catheter. Scale bar is 100 μm.
Figure 3.
Mean fluorescence intensity comparing the vehicle, Micro-Renathane tubing, and Remedy channeled drug delivery balloon catheter delivery methods. Data are shown as FU + st dev.
Duration of PEG coverage
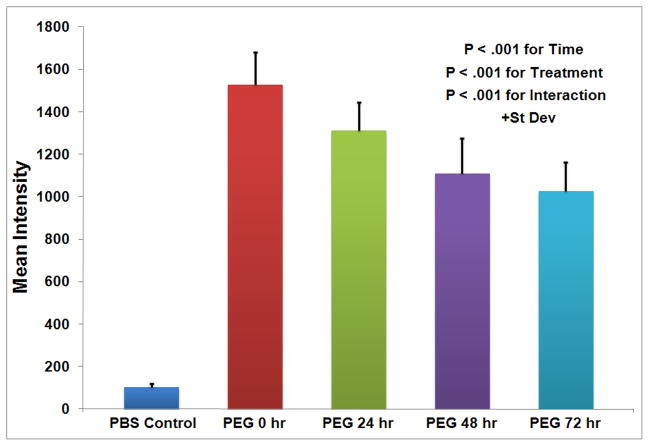
In order to assess the ability of the polymeric barrier to inhibit thrombosis and serve as a site for the targeted delivery of therapeutics, it was important to establish the duration of the polymer on the vascular surface post-modification. There was little to no auto-fluorescence associated with denuded control vessels at any time point with a mean fluorescence intensity of only 101 FU (Figure 4). The maximum fluorescence for the polymer-modified vessels occurred at 0 h with a mean intensity of 1,529 FU, which then decreased to 1,028 FU over the next 72 h (Figure 4). There was a statistically significant increase in fluorescent intensity over controls P < .001), which decreased with time (P < .05) in a linear manner (FU = 1500 -7.1*time, P < .0001). Even though the polymer remaining on the balloon injured vessel declined over time, greater than 67% of the original fluorescence was maintained at 72 h (Figure 4).
Figure 4.
Duration of PEG-fluorescein on balloon injured rabbit femoral arteries after modification at 0, 24, 48, and 72 hrs. Data are shown as FU + st dev with N = 4 at all times and treatments and the results are significant with P < .001 for the time, treatment, and interaction.
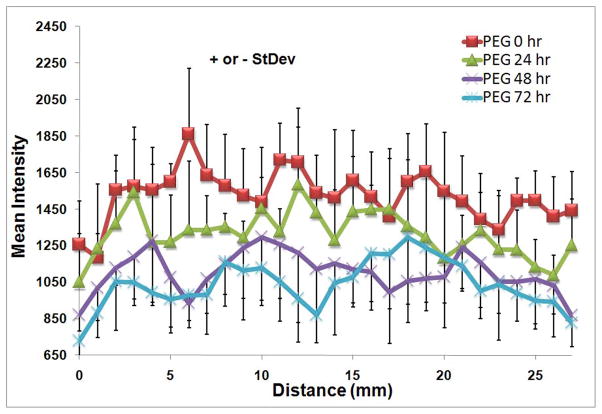
In addition to evaluating the temporal maintenance of the polymer, fluorescence intensity data from the images along the entire length of the balloon injured vessels modified with the fluorescent PEG were used to determine the consistency of the polymer coverage for each time point. There was some fluctuation of the mean intensity of the fluorescent polymer over the length of the vessel, but there was no significant effect with axial or circumferential vessel position as determined by the 2-factor ANOVA (Figure 5).
Figure 5.
Distribution of PEG-fluorescein modified, balloon injured rabbit femoral arteries along the vessel length at 0, 24, 48, and 72 hrs as measured from the saphenous artery branch to the site of the clamp on the common femoral artery Data are shown as FU +/− st dev. N=4.
Microsphere Targeting
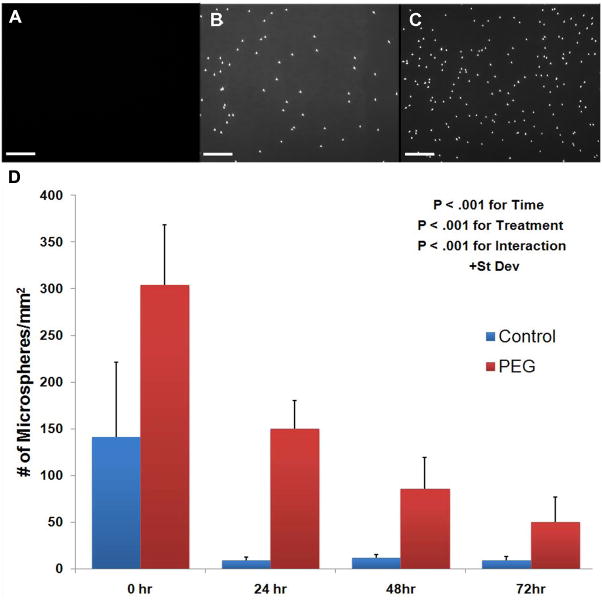
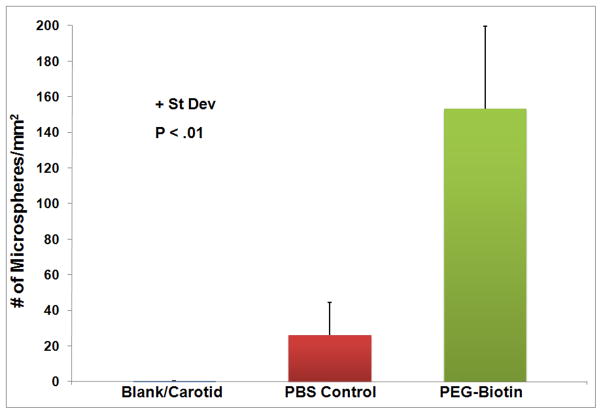
NeutrAvidin-coated microspheres preferentially adhered to balloon injured arteries modified with PEG-biotin as opposed to balloon injured, unmodified vascular surfaces at all time points evaluated (Figure 6). A total of 304 + 64 microspheres per mm2 bound to PEG-biotin modified balloon injured femoral arteries as compared to 141 + 80 microspheres per mm2 for the injured, unmodified control at 0 h (P < .001). The number of adherent microspheres per mm2 for untreated, balloon injured rabbit femoral arteries decreased sharply from 0 to 24 h and then remained constant out to 72 h, while the total number of adherent microspheres to PEG-biotin modified, balloon injured arteries gradually decreased as the time post-modification increased, with 50 + 27 microspheres/mm2 remaining at 72 h (P < .001). The ability of the same targeting strategy to target agents to healthy vascular tissue by addition of the reactive polymer or vehicle control to uninjured rabbit femoral arteries was also assessed. NeutrAvidin-coated microspheres were preferentially targeted to healthy vascular tissue modified with the polymer as opposed to the unmodified arteries (Figure 7). At 0 h, a total of 153 + 46 microspheres per mm2 bound to PEG-biotin modified healthy femoral arteries compared to 26 + 18 microspheres per mm2 for the unmodified control at 0 h (P < 0.01) (Figure 7). The total number of microspheres adhering to PEG-biotin modified healthy tissue at 0 h (153 + 46 microspheres/mm2) was less than that seen with the PEG-biotin modified, balloon injured arteries (303 + 65 microspheres/mm2).
Figure 6.
Targeted microsphere adherence to rabbit femoral arteries. A) Unmanipulated carotid, (B) balloon injured control femoral artery, and (C) PEG-biotin modified balloon injured femoral arteries at 0 hr. Scale bar is 100 μm. D) Quantification of targeted microspheres adhering to control and PEG-biotin treated, balloon injured rabbit femoral arteries in vivo at 0, 24, 48, and 72 hrs post-modification. Data are shown + st dev. P < .001 for delivery time, treatment, and interaction.
Figure 7.
Number of targeted microspheres adhering to control and PEG-biotin treated healthy rabbit endothelium in vivo at 0 hrs. Data are shown + st dev and the results are significant with P < .01.
DISCUSSION
Modifying vascular surfaces with a reactive PEG provides multiple options for preventing thrombosis or restenosis by serving as a molecular barrier as well as providing a site for targeted drug delivery. Reactive PEG has previously been shown to form a molecular barrier on damaged vascular surfaces7, 13 as well as biomaterials6, 14, 15 to inhibit platelet deposition, and we demonstrate that suitable levels are maintained for greater than 72 h. Nanocoatings of hyaluron and chitosan deposited onto damaged vascular surfaces have also been used to inhibit platelet deposition, but typically require multiple administrations of the polyelectrolytes.16 Likewise, concealment of extracellular matrix ligands with a PEGylated fibronectin, which interacts with fibrillar collagens, to reduce platelet recognition requires 30 min to cover the injured vascular surface.17 Both the hyaluron and chitosan nanocoatings and PEGylated fibronectin systems for inhibiting intravascular thrombosis require extended application periods or repeated administrations, which may be detrimental in time-sensitive procedures. Hydrogel barriers formed by photopolymerization have also demonstrated an ability to inhibit thrombosis and reduce intimal thickening on injured vascular surfaces. However, formation of the hydrogel barriers using photopolymerization requires additional steps such as adsorption of a photoinitiator on the injured vessel surface, flushing of excess initiator, administration of the hydrogel precursors, and adequate illumination (external or internal) to form the gel.18, 19
A major advantage of the reactive PEG employed in the current system from the standpoint of clinical implementation is that a single application could rapidly modify vascular surfaces (less than 1 min), potentially while an intervention is being performed at the site using a channeled drug delivery catheter. Establishing the consistency of the polymeric coverage along the entire length of the modified vessel was also important in evaluating the effectiveness of the barrier to inhibit thrombosis or the signal to deliver targeted therapeutics. The molecular barrier thickness could theoretically be manipulated by changing the molecular weight or branching of the polymer molecule and thus reduce some of these effects. Further, the PEG modification was sustained for at least 72 h, and if one were to assume that the linear trend continued, 25% of the polymer would remain after 160 h in vivo. The process by which the PEG is removed from the surface is likely due to a combination of the host-response to the foreign material that cleaves the modified protein, or the covalent PEG-protein bond itself. Because the microsphere binding capacity was only 20% of the acute capacity by 72 h, we hypothesize that the further reduction in polymer availability may be due to the non-linearity of these types of binding events, which have been modeled previously20. A loss of the microspheres through dissociation between streptavidin and biotin is unlikely because of lengthy dissociation time that contributes to the high affinity of the bond.21 Although the use of PEG is associated with a reduction in protein and platelet deposition 13, 22, masking of the binding target for NeutrAvidin by fouling processes cannot be dismissed. PEG hydrogels have also been synthesized to release nitric oxide (NO) or YC-1, a benzyl indazole derivative, as therapeutic agents to reduce platelet adhesion and smooth muscle cell proliferation after vascular injury.23, 24 Therapeutics such as these and others might be delivered to PEG-modified vascular sites using the targeting strategy developed in this paper.
Another method of inhibiting thrombosis and restenosis at sites of arterial injury involves local administration of drugs or gene therapy vectors to the injured vessels. Local delivery of these agents can be accomplished using specialized catheters that employ passive, pressure-driven, electrically, or mechanically enhanced diffusion for delivery.25, 26 Using these catheters for direct delivery of the agents only allows for one application or requires an indwelling line that is prone to thrombosis. A single application of reactive PEG that subsequently serves as a specific target for the drug or therapeutic vector to be systemically injected over time may prove to be a more suitable system for long-term delivery.
Local delivery of therapeutics is also possible by targeting the molecules or using targeted carriers to direct the therapy to specific vascular segments. While some studies rely on local delivery 27, 28, others incorporate additional homing capabilities. One such method targets heparin or low molecular weight heparin conjugated to antibodies against cross-linked heparin, which is deposited at the site of arterial injury, to reduce neointimal formation.29 Other methods target microspheres or particles to selected vascular sites using antibodies to exposed surface markers. Hyaluron microspheres conjugated to antibodies against E- and P-selectin preferentially adhered to inflammatory vascular sites and successfully delivered plasmid DNA.30 Similarly, biodegradable particles conjugated to antibodies for E- and P-selectin, ICAM-1, and VCAM-1 also showed targeting to inflamed endothelium.31 In this balloon injury model, the attachment of NHS-PEG is intended here not to modify cells, but to modify the exposed basement membrane, which is the source of acute thrombosis, and PEG has previously been shown to interrupt platelet deposition and acute thrombosis on this surface6. Previous in vitro studies indicate that it is possible to attain high surface densities of the PEG-biotin signaling molecule 12 In addition, therapeutic agents can be targeted to specific vascular segments using a tag on the end of the polymer molecule.12 Although there is a reduction in the available polymer over time, repeated delivery of therapeutic vehicles, such as microspheres, is still possible since not all of the sites will be occupied after the initial dose due to the limited concentration of the microspheres in the boundary layer that occurs with systemic administration. Because the protein-reactive PEG permits modification of both healthy endothelium and exposed matrix following vascular injury, treatment would not be dependent upon the expression and availability of specific surface markers that are up-regulated in certain disease states. Systemic administration of the reactive molecule itself, however, would not be feasible because of the promiscuous nature of the reaction between the PEG-NHS and surface amines, which would result in PEG modification of blood proteins encountered and any other molecules with free amine groups.
Drug-eluting stents, which release anti-mitotic agents such as sirolimus (CYPHER, Cordis J&J) and paclitaxel (TAXUS, Boston-Scientific), have demonstrated significant efficacy in reducing restenosis rates. Randomized-controlled trials have demonstrated a reduction in the need for repeat revascularization after coronary interventions by 60–80% when using drug-eluting stents.32 However, long-term results of the effects of drug-eluting stents are not well-defined. As drug-eluting stents are applied to more complex lesions, there have been increasing occurrences of late stent thrombosis.33–35 The polymer coating that serves as a reservoir for drug delivery has also come under scrutiny. There are numerous reports suggesting that a hypersensitivity reaction to the polymer coating on the stents results in extensive inflammation of the vessel wall.33, 34, 36, 37 Modification of injured vascular surfaces and stents with a PEG-reactive polymer provides another strategy that might be employed in conjunction with drug-eluting stents to further improve outcomes of coronary interventions.38, 39
There were several limitations in this technique which should be addressed in future studies. First and foremost, this report represents a demonstration of the potential for the technique and drug release or the therapeutic effects of a particular drug was not studied. The use of 1-μm spheres as a model delivery vehicle, while having a reduced footprint for drag forces, also have a reduced binding density which can limit their ability to withstand shear forces following the initial binding event and may pose an issue for long-term drug releasing systems.40 Nanoparticles might provide a better platform since they appear to be unaffected by shear forces but would potentially have a smaller drug payload.41 The use of alternative ligand-receptor chemistry also might provide a means to improve upon the density of particulate adherence. While the biotin-streptavidin bond is extraordinarily stable and well characterized21, its use with microparticle delivery systems is limited in low-shear envronments.42 Other particulate-surface interactions could also be designed using lock and key mechanisms that have more attractive kinetics, including aptamer technology.43
In summary, this study demonstrates that a protein-reactive PEG can be used to modify healthy and injured vascular segments. Furthermore, this polymer attachment approach can be used as a target for the site-specific delivery of microspheres to the labeled vascular tissue. Microspheres are representative of a particulate drug or carrier that could be loaded with various therapeutics designed to treat various ailments. In cases of vascular injury, such as with balloon angioplasties, carotid endarterectomies, or anastamoses, delivery of anti-thrombotics or anti-mitotics might assist in inhibiting intimal hyperplasia.
Acknowledgments
Funding for parts of this research was provided by the National Institutes of Health (# HL58617), The National Tissue Engineering Center (# 02059001) and a fellowship from The Hartwell Foundation. In addition, we would like to thank the animal surgical staff and monitors at the McGowan Institute for Regenerative Medicine that assisted with the in vivo studies. In particular, we would like to acknowledge Lisa Gordon and Aaron Dean who assisted with the surgical procedures and monitored the anesthesia for the animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conde ID, Kleiman NS. Arterial thrombosis for the interventional cardiologist: from adhesion molecules and coagulation factors to clinical therapeutics. Catheter Cardiovasc Interv. 2003;60(2):236–46. doi: 10.1002/ccd.10635. [DOI] [PubMed] [Google Scholar]
- 2.Garas SM, Huber P, Scott NA. Overview of therapies for prevention of restenosis after coronary interventions. Pharmacol Ther. 2001;92(2–3):165–78. doi: 10.1016/s0163-7258(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 3.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22(11):1769–76. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 4.Savage MD, Mattson G, Desai S, Nielander GW, Morgensen S, Conklin EJ. Avidin-Biotin Chemistry: A Handbook. 2. Rockford: Pierce Chemical Company; 1994. [Google Scholar]
- 5.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54(4):459–76. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 6.Burchenal JE, Deible CR, Deglau TE, Russell AJ, Beckman EJ, Wagner WR. Polyethylene glycol diisocyanate decreases platelet deposition after balloon injury of rabbit femoral arteries. J Thromb Thrombolysis. 2002;13(1):27–33. doi: 10.1023/a:1015364024487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deible CR, Beckman EJ, Russell AJ, Wagner WR. Creating molecular barriers to acute platelet deposition on damaged arteries with reactive polyethylene glycol. J Biomed Mater Res. 1998;41(2):251–6. doi: 10.1002/(sici)1097-4636(199808)41:2<251::aid-jbm10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Huhtinen P, Soukka T, Lovgren T, Harma H. Immunoassay of total prostate-specific antigen using europium(III) nanoparticle labels and streptavidin-biotin technology. J Immunol Methods. 2004;294(1–2):111–22. doi: 10.1016/j.jim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Hoya K, Guterman LR, Miskolczi L, Hopkins LN. A novel intravascular drug delivery method using endothelial biotinylation and avidin-biotin binding. Drug Deliv. 2001;8(4):215–22. doi: 10.1080/107175401317245895. [DOI] [PubMed] [Google Scholar]
- 10.Zou J, Huang Y, Cao K, Yang G, Yin H, Len J, et al. Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci. 2000;68(2):153–63. doi: 10.1016/s0024-3205(00)00925-5. [DOI] [PubMed] [Google Scholar]
- 11.Barone GW, Farley PC, Conerly JM, Flanagan TL, Kron IL. Morphological and functional techniques for assessing endothelial integrity: the use of Evans blue dye, silver stains, and endothelial derived relaxing factor. J Card Surg. 1989;4(2):140–8. doi: 10.1111/j.1540-8191.1989.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 12.Deglau TE, Johnson JD, Villanueva FS, Wagner WR. Targeting microspheres and cells to polyethylene glycol-modified biological surfaces. J Biomed Mater Res A. 2007;81(3):578–85. doi: 10.1002/jbm.a.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deible CR, Petrosko P, Johnson PC, Beckman EJ, Russell AJ, Wagner WR. Molecular barriers to biomaterial thrombosis by modification of surface proteins with polyethylene glycol. Biomaterials. 1998;19(20):1885–93. doi: 10.1016/s0142-9612(98)00098-2. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Kaar JL, Russell AJ, Wagner WR. Characterizing the modification of surface proteins with poly(ethylene glycol) to interrupt platelet adhesion. Biomaterials. 2006;27(16):3125–35. doi: 10.1016/j.biomaterials.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann J, Groll J, Heuts J, Rong H, Klee D, Ziemer G, et al. Blood cell and plasma protein repellent properties of star-PEG-modified surfaces. J Biomater Sci Polym Ed. 2006;17(9):985–96. doi: 10.1163/156856206778366059. [DOI] [PubMed] [Google Scholar]
- 16.Thierry B, Winnik FM, Merhi Y, Tabrizian M. Nanocoatings onto arteries via layer-by-layer deposition: toward the in vivo repair of damaged blood vessels. J Am Chem Soc. 2003;125(25):7494–5. doi: 10.1021/ja034321x. [DOI] [PubMed] [Google Scholar]
- 17.Geho DH, Smith WI, Jr, Liotta LA, Roberts DD. Fibronectin-based masking molecule blocks platelet adhesion. Bioconjug Chem. 2003;14(4):703–6. doi: 10.1021/bc034037v. [DOI] [PubMed] [Google Scholar]
- 18.West JL, Hubbell JA. Separation of the arterial wall from blood contact using hydrogel barriers reduces intimal thickening after balloon injury in the rat: the roles of medial and luminal factors in arterial healing. Proc Natl Acad Sci U S A. 1996;93(23):13188–93. doi: 10.1073/pnas.93.23.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill-West JL, Chowdhury SM, Slepian MJ, Hubbell JA. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc Natl Acad Sci U S A. 1994;91(13):5967–71. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maul TM, Dudgeon DD, Beste MT, Hammer DA, Lazo JS, Villanueva FS, et al. Optimization of ultrasound contrast agents with computational models to improve selection of ligands and binding strength. Biotechnol Bioeng. 2010;107(5):854–64. doi: 10.1002/bit.22857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swift JL, Heuff R, Cramb DT. A Two-Photon Excitation Fluorescence Cross-Correlation Assay for a Model Ligand-Receptor Binding System Using Quantum Dots. Biophysical Journal. 2006;90(4):1396–410. doi: 10.1529/biophysj.105.069526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombotz WR, Wang GH, Horbett TA, Hoffman AS. Protein adsorption to poly(ethylene oxide) surfaces. J Biomed Mater Res. 1991;25(12):1547–62. doi: 10.1002/jbm.820251211. [DOI] [PubMed] [Google Scholar]
- 23.Tulis DA, Bohl Masters KS, Lipke EA, Schiesser RL, Evans AJ, Peyton KJ, et al. YC-1-mediated vascular protection through inhibition of smooth muscle cell proliferation and platelet function. Biochem Biophys Res Commun. 2002;291(4):1014–21. doi: 10.1006/bbrc.2002.6552. [DOI] [PubMed] [Google Scholar]
- 24.Bohl KS, West JL. Nitric oxide-generating polymers reduce platelet adhesion and smooth muscle cell proliferation. Biomaterials. 2000;21(22):2273–8. doi: 10.1016/s0142-9612(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 25.Alfke H, Wagner HJ, Calmer C, Klose KJ. Local intravascular drug delivery: in vitro comparison of three catheter systems. Cardiovasc Intervent Radiol. 1998;21(1):50–6. doi: 10.1007/s002709900211. [DOI] [PubMed] [Google Scholar]
- 26.Du X, Yang Y, Le Visage C, Chen HH, DeJong R, Qiu B, et al. In vivo US monitoring of catheter-based vascular delivery of gene microspheres in pigs: feasibility. Radiology. 2003;228(2):555–9. doi: 10.1148/radiol.2282020539. [DOI] [PubMed] [Google Scholar]
- 27.Westedt U, Kalinowski M, Wittmar M, Merdan T, Unger F, Fuchs J, et al. Poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) nanoparticles for local delivery of paclitaxel for restenosis treatment. J Control Release. 2007;119(1):41–51. doi: 10.1016/j.jconrel.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Kuji T, Masaki T, Goteti K, Li L, Zhuplatov S, Terry CM, et al. Efficacy of local dipyridamole therapy in a porcine model of arteriovenous graft stenosis. Kidney Int. 2006;69(12):2179–85. doi: 10.1038/sj.ki.5000383. [DOI] [PubMed] [Google Scholar]
- 29.Thomas AC, Campbell JH. Targeted delivery of heparin and LMWH using a fibrin antibody prevents restenosis. Atherosclerosis. 2004;176(1):73–81. doi: 10.1016/j.atherosclerosis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Yun YH, Goetz DJ, Yellen P, Chen W. Hyaluronan microspheres for sustained gene delivery and site-specific targeting. Biomaterials. 2004;25(1):147–57. doi: 10.1016/s0142-9612(03)00467-8. [DOI] [PubMed] [Google Scholar]
- 31.Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, et al. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2003;100(26):15895–900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodgson JM, Bottner RK, Klein LW, Walpole HT, Jr, Cohen DJ, Cutlip DE, et al. Drug-eluting stent task force: final report and recommendations of the working committees on cost-effectiveness/economics, access to care, and medicolegal issues. Catheter Cardiovasc Interv. 2004;62(1):1–17. doi: 10.1002/ccd.20025. [DOI] [PubMed] [Google Scholar]
- 33.Virmani R, Farb A, Guagliumi G, Kolodgie FD. Drug-eluting stents: caution and concerns for long-term outcome. Coron Artery Dis. 2004;15(6):313–8. doi: 10.1097/00019501-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia V, Bhatia R, Dhindsa M. Drug-eluting stents: new era and new concerns. Postgrad Med J. 2004;80(939):13–8. doi: 10.1136/pmj.2003.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibbing D, Laugwitz KL, Bott-Flugel L, Pache J. Very late stent thrombosis 42 months after implantation of sirolimus-eluting stent and discontinuation of antiplatelet therapy. Case Report Med. 2009;2009:713292. doi: 10.1155/2009/713292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120(5):391–9. doi: 10.1161/CIRCULATIONAHA.109.854398. [DOI] [PubMed] [Google Scholar]
- 37.Young JJ. Neointimal formation following drug-eluting stents: physiology, timeline, and the influence of drug delivery systems. Rev Cardiovasc Med. 2007;8 (Suppl 1):S3–10. [PubMed] [Google Scholar]
- 38.Billinger M, Buddeberg F, Hubbell JA, Elbert DL, Schaffner T, Mettler D, et al. Polymer stent coating for prevention of neointimal hyperplasia. J Invasive Cardiol. 2006;18(9):423–6. discussion 7. [PubMed] [Google Scholar]
- 39.Scott EA, Nichols MD, Cordova LH, George BJ, Jun YS, Elbert DL. Protein adsorption and cell adhesion on nanoscale bioactive coatings formed from poly(ethylene glycol) and albumin microgels. Biomaterials. 2008;29(34):4481–93. doi: 10.1016/j.biomaterials.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maul TM, Dudgeon DD, Beste MT, Hammer DA, Lazo JS, Villanueva FS, et al. Optimization of Ultrasound Contrast Agents with Computational Models to Improve Selection of Ligands and Binding Strength. Biotechnology and Bioengineering. 2010;107(5):854–64. doi: 10.1002/bit.22857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haun JB, Hammer DA. Quantifying Nanoparticle Adhesion Mediated by Specific Molecular Interactions. Langmuir. 2008;24(16):8821–32. doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- 42.Pierres A, Touchard D, Benoliel A-M, Bongrand P. Dissecting Streptavidin-Biotin Interaction with a Laminar Flow Chamber. Biophysical Journal. 2002;82(6):3214–23. doi: 10.1016/S0006-3495(02)75664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy-Nissenbaum E, Radovic-Moreno AF, Wang AZ, Langer R, Farokhzad OC. Nanotechnology and aptamers: applications in drug delivery. Trends Biotechnol. 2008;26(8):442–9. doi: 10.1016/j.tibtech.2008.04.006. [DOI] [PubMed] [Google Scholar]