Abstract
Thymus-derived, naturally-occurring CD4+ FoxP3+ regulatory T cells (nTreg) have suppressive activity that is important for the establishment and maintenance of immune homeostasis in the healthy state. Abundant reports have shown that they can suppress pathogenic processes in autoimmune diseases and inhibit transplant rejection and graft-versus-host disease. Far less is known about induced regulatory T cells (iTreg) that are generated from naïve T cells in the periphery or in vitro, by directing naïve T cells to acquire suppressive function under the influence of transforming growth factor-β (TGF-β) and other factors. In this review, we describe mechanisms by which naïve T cells are thought to be converted into iTreg. We also discuss the suppressive potential of iTreg, particularly in comparison to their naturally-occurring counterparts, focusing on those reports in which direct comparisons have been made. Based on current knowledge, we consider the rationale for using iTreg versus nTreg in clinical trials.
Keywords: Naturally-occurring regulatory T cells, induced regulatory T cells, immune suppression, tolerance, transplantation
1. INTRODUCTION
Numerous studies have demonstrated the importance of regulatory T cells in maintaining tolerance to auto- and allo-antigens (Ags). Naturally-occurring Treg (nTreg), that are generated in the thymus, were first described in mice by Sakaguchi et al in 1995 [1] and their suppressive function was later associated with expression of the winged helix transcription factor Forkhead Box P3 (FoxP3) [2]. Additionally, human nTreg can be identified by expression of CD4+, in combination with cell surface CD25(IL-2Rα)hi, and the absence of CD127(IL-7Rα) [3]. Adoptive transfer of nTreg has been successful in animal models of autoimmune diseases and transplantation (Tx) [4, 5]. However, as the frequency of nTreg in healthy humans is only ~5% of CD4+ T cells, it is difficult to obtain sufficient numbers of these cells for clinical testing [6, 7]. Many groups have reported the marked expansion of nTreg from a CD4+ CD25hi (in combination with CD127− or CD45RA+) starting population by T cell Ag receptor (TCR) stimulation in the presence of exogenous IL-2 [8, 9]. However, during prolonged expansion for 3–4 weeks, T effector cells (Teff) outgrow nTreg, resulting in reduction of the suppressive capacity of the cultured cells [3, 7]. A shorter expansion period would avoid this difficulty, but the limited nTreg number that can be generated under these conditions would constitute a problem when Treg are to be used in a clinical setting.
To address this issue, various groups have sought to generate Treg from a naïve T cell starting population. Beginning in 2002, the first reports were published on adaptive or induced Treg (iTreg) that are CD4+FoxP3+ cells which are generated in the periphery, and also that have suppressive capacity [10–12]. These iTreg are generated in vivo from naïve T cells in peripheral lymphoid organs during immunological responses to Ag stimulation, a phenomenon that is replicated in vitro through stimulation of mouse or human T cells under specific conditions. Various groups have observed that iTreg can be generated easily by conversion of much more abundant naïve CD4+ T cells in vitro, allowing much higher numbers of Treg to be obtained [10–12]. In the in vitro setting, iTreg can be generated in either a polyclonal or an Ag-specific fashion. The percentage of iTreg in the total population of circulating Treg in healthy mice and humans is thought to be in the range <3 to 30% [13, 14]. Here, we review current knowledge on the generation of iTreg and discuss their suppressive function in comparison to nTreg.
2. THE INDUCTION OF TREG FROM NAÏVE T CELLS
2.1 In vivo pathways of iTreg generation
Several pathways through which Treg can be induced in the periphery in vivo have been described. In general, all Ag-presenting cells (APC) have the potential to induce Treg. Thus, dendritic cells (DC) in the gut-associated lymphoid tissue (GALT) can promote iTreg conversion [15–18]. However, B cells [19]; mesenchymal stem cells [20, 21] and myeloid-derived suppressor cells [22] are also able to promote conversion.
The presentation of self- or foreign Ag is important for conversion to the Treg phenotype. In vivo generation not only occurs as a homeostatic phenomenon, but also during allo immune responses. iTreg reactive to foreign Ag are generated in response to microbes and food Ags in the intestinal mucosa [17], during the induction of tolerance to chicken ovalbumin (OVA) in the mesenteric lymph nodes [23], and by OVA-presenting DC [24]. They are also found in response to self Ag in chronically inflamed tissues [25], in response to self Ag in a mouse autoimmune diabetes model [26], and during homeostatic repopulation in lymphopenic hosts reconstituted with Treg-depleted cells [27, 28]. Moreover, generation of iTreg in response to donor tissue in transplanted organs has been studied extensively. Plasmacytoid DC play an important role in their generation under alloAg stimulation in the transplant setting [29]. Immunosuppressive therapy is also of major influence; different studies show preferential generation of iTreg with use of non-depleting anti-CD4 mAb [30], anti-CD154 mAb+rapamycin [31], or rapamycin alone [32]. By contrast, cyclosporine is detrimental for iTreg generation in vitro as well as in vivo [32].
2.2 Infectious tolerance: nTreg can generate iTreg from conventional CD4+ T cells
Although the concept of infectious tolerance has long been recognized as a phenomenon in which the T cells of a tolerant mouse or rat can transfer their suppressive activity to conventional CD4+ T cells in a naïve host [33–35], a possible mechanism underlying this phenomenon has been described much more recently. Two groups have reported the induction of Treg from CD4+CD25− T cells by nTreg [36, 37]. Both studies showed that human nTreg could induce anergic suppressor cells from a CD4+ CD25− population. Conversion occurred in a population that did not contain FoxP3+ cells; during conventional immune responses in vivo, this process is tightly regulated. Homeostatic regulation guarantees the maintenance of an appropriate balance between Treg and conventional T cells. Cell-cell contact between nTreg and naïve CD4+ T cells was necessary for the generation of iTreg, but these iTreg could, in turn, suppress proliferation of Teff in a cell contact-independent fashion. Key cytokines that have been associated with the suppressive activity of iTreg are transforming-growth factor-β (TGF-β) [37] and IL-10 [36]. The mechanisms of infectious tolerance have been further elucidated recently by Kendal et al [38], who have shown that the presence of Treg is crucial for continuous suppression of Teff cells. Peripherally-induced FoxP3+ Treg can sustain tolerance by converting naïve T cells to the next generation of FoxP3+ cells.
3. KEY COMPONENTS OF IN VITRO GENERATION OF iTREG: IL-2, TGF-β AND COSTIMULATION
IL-2 is required for the generation and expansion of nTreg, together with stimulation of the TCR (CD3) and costimulation (via CD28) [5, 9]. By contrast, the requirements for iTreg generation and expansion are still under investigation. The main factors that have been identified as crucial for induction of FoxP3 expression in CD4+CD25− cells are IL-2 and TGF-β [10, 12]. Zheng et al [12] first showed that CD4+ suppressor cells could be generated in vitro from human CD4+CD25− cells with TGF-β and stimulation by irradiated superAg-presenting B cells. The iTreg generated had a CD4+CD25hi cytotoxic T lymphocyte Ag 4 (CTLA4)+ phenotype, exhibited reduced production of interferon (IFN)-γ and IL-10, and suppressed autologous antibody (Ab) production through cell contact as well as TGF-β production. Chen et al [10] reported that TGF-β, together with anti-CD3 and APC stimulation could potently convert mouse CD4+CD25− Teff into Treg (CD4+CD25+CD45RB−) that suppressed allergic responses in a mouse asthma model. Subsequently, several groups have demonstrated that strong costimulation provided by B7 through CD28 during iTreg generation prevents FoxP3 upregulation and renders cells with poor suppressive function [11, 19, 39]. By contrast, upregulation of CTLA4, which is the negative counterpart of CD28 in B7-mediated regulation [39] is required for the suppressor function of iTreg [28, 40–43]. Fantini et al [44] observed that exogenous IL-2 is required for in vivo generation and expansion of iTreg in a murine colitis model, and that TGF-β-induced FoxP3 expression is associated with downregulation of the inhibitory signaling protein and transcription factor mothers against decapentaplegic (Smad)7, which is a TGF-β type 1 receptor antagonist. This makes iTreg susceptible to TGF-β-mediated regulatory effects. These observations were confirmed by in vitro experiments with neutralizing anti-IL-2 Abs in which IL-2 was shown to be crucial for the TGF-β-mediated conversion of naive CD4+ T cells [45]. A recent development is the use of IL-2/anti-IL-2 mAb complexes that deliver a consistent IL-2 level to the cultured cells which is not dependent on the cells’ own IL-2 secretion, and is a potent method to generate iTreg with stable FoxP3 expression [46]. Altogether, these observations suggest key roles for IL-2, TGF-β, and negative costimulation through CTLA4 in the generation of iTreg, which are summarized in Table 1.
Table 1.
Properties of nTreg and iTreg
| nTreg (references) | iTreg (references) | ||
|---|---|---|---|
| Generation | Tissue | Thymus (1, 2) | Secondary lymphoid organs, inflamed tissue, site of transplant (13, 17, 25, 30) |
| Cytokines required | IL-2 or IL-15 (6, 9) | IL-2 +TGF-β (10, 12, 45) | |
| Costimulation required | CD28 (6, 9) | CTLA4 (28, 39, 42, 43) | |
|
| |||
| Improvement of suppressive function by reagent | ATRA | No (54) | Yes (15, 17, 19, 53) |
| Rapamycin | Yes (54)/No (58) | Yes (57, 59, 63) | |
|
| |||
| Specificity | Self or alloAg (1, 2) | AlloAgs, food allergens, gut microbes, transplanted tissue, self (inflammation) (15, 17, 25, 26, 30) | |
|
| |||
| Th17 differentiation | IL-6-mediated | Yes (73, 74) | Yes (39, 71, 72) |
| Antagonized by ATRA | Yes (76) | Yes (23, 75) | |
| Antagonized by rapamycin | Has not yet been addressed | Yes (57) | |
|
| |||
| Intracellular marker | FoxP3 | Yes (1, 2) | Yes (10, 11) |
| Helios | Yes (70) | No (13) | |
4. AUGMENTATION OF iTREG GENERATION
4.1 The role of retinoic acid in iTreg conversion
Vitamin A is an important metabolite of the body that influences immune regulation. Its deficiency has been associated with infectious diseases in children [47]. DC in the GALT can produce the vitamin A derivative retinoic acid (RA), which is associated with gut-homing of T cells, as well as enhanced FoxP3 expression of CD4+ T cells in mice [19, 48] and humans [49]. RA functions synergistically with TGF-β in the mouse intestine to induce FoxP3 expression in naïve T cells upon Ag activation [15, 17]. When the active derivative of RA, all-trans retinoic acid (ATRA), is added to cultures of CD4+FoxP3− T cells, together with IL-2, anti-CD3/28 and TGF-β, a dose-dependent significant increase in FoxP3 expression is found when compared to culture without ATRA [19]. The cells express high levels of the CC chemokine receptor CCR9 and α4β7, which are homing receptors associated with the intestinal lamina propria [17, 50, 51]. The mechanism by which RA enhances FoxP3 expression and suppressive capacity in iTreg remains under investigation. Different studies demonstrate that it functions either through a cytokine-dependent mechanism, by blocking the secretion of pro-inflammatory cytokines by memory T cells [52], or through cytokine-independent mechanisms, as RA retains its inhibitory effect in the absence of cytokines [53].
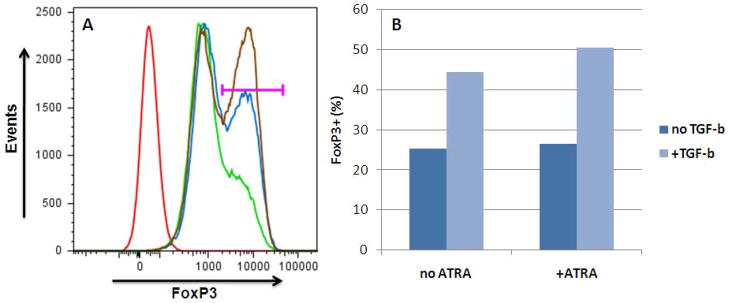
The influence of ATRA on iTreg generation has been confirmed in studies showing the generation of human iTreg with stable FoxP3 expression in vitro using ATRA+TGF-β. Combination of ATRA+TGF-β significantly enhances FoxP3 expression of iTreg, as shown by our own studies (Figure 1). Moreover, iTreg generated with ATRA+TGF-β have enhanced suppressive capacity compared to either ATRA or TGF-β alone [54].
Figure 1. Human iTreg generation with TGF-β and ATRA.
Human CD4+ T cells were obtained by magnetic bead sorting (CD4+ kit, Miltenyi) of PBMC (purity >90%; 5% FoxP3+) and stimulated with high-dose IL-2 +/− TGF-β (20 ng/ml) +/− ATRA (10 ng/ml). After 6 days, live CD4+ cells were assessed for FoxP3 expression by flow cytometry. Cells cultured in the presence of TGF-β + ATRA showed 50% FoxP3+ expression while cells cultured with only IL-2 were only 25% FoxP3+; the remaining 75% of the cells showed an intermediate level of FoxP3.
(A) Gating for FoxP3+ cells (horizontal bar). Red: isotype; green: no TGF-β, no ATRA; blue: TGF-β only; brown: TGF-β + ATRA.
(B) Quantification of FoxP3+ cells: 50% of cells cultured with TGF-β + ATRA acquired FoxP3+ phenotype.
By contrast, when human nTreg are cultured with ATRA, this is not associated with increased expansion or suppressor function, although culture of nTreg in ATRA in combination with rapamycin enhances their suppressive capacity [55]. These findings suggest an important role for ATRA in the induction, but not in the maintenance, of regulatory function in nTreg.
4.2 Rapamycin functions synergistically with TGF-β
The immunosuppressive pro-drug rapamycin is known to selectively inhibit the expansion and function of Teff by blocking the serine/threonine kinase activity of the mammalian target of rapamycin (mTOR), but still allows nTreg to proliferate [56] (for further details see Thomson et al [57]). Several groups have studied the effects of rapamycin in the context of iTreg generation. Rapamycin can enhance the de novo generation of iTreg in bulk CD4+ as well as CD4+CD25− cell populations [58]. Mechanistic studies by Haxhinasto et al [59] have revealed that the active form of the kinase AKT (AKT*) selectively suppresses the de novo expression of FoxP3 induced by TGF-β in murine CD4+CD25− cells, without affecting the already established FoxP3 levels of nTreg. This effect of AKT* is reduced by rapamycin, suggesting a protective effect of rapamycin on the induction of Treg. These findings are supported by the study of Delgoffe et al [60], who showed that mTOR is required for the development of effector T cells from bulk CD4+ T cells. In mTOR-deficient mice, T cells differentiate into iTreg even under strong cytokine-induced skewing conditions for Th1, Th2 or Th17 generation. This is associated with decreased signal transducer and activator of transcription (STAT) activation and hyperactive Smad3 phosphorylation, resulting in higher sensitivity of cells to TGF-β.
In a study of non-human primate (baboon) cells, rapamycin increased the induction of FoxP3+ cells from purified CD4+ cells by ~2-fold (11.2% to 20.6%). CD4+ CD25+ cells isolated from the expanded population potently suppressed a xenogeneic T cell proliferative response [61]. In a recent study of human cells [55]. nTreg expanded in the presence of rapamycin showed improved capacity to induce infectious tolerance. Thus, CD4+ FoxP3− cells cocultured with nTreg for 3 days showed 8% FoxP3+ expression and significant IL-2 production; however, if the nTreg were expanded in the presence of rapamycin (or rapamycin+ATRA), there was a 2-fold increase in FoxP3 induction in Teff, and IL-2 production was abrogated. Another recent study describes the potent ability of a combination of rapamycin and TGF-β to induce FoxP3 expression in human naïve T cells after expansion in culture for up to 2 weeks [62]. Moreover, the iTreg that were generated by this method suppressed GVHD in a xenogeneic model.
An important role in the conversion and function of iTreg in relation to mTOR activity has been found for the coregulatory B7 family member programmed death-ligand1 (PD-L1, = B7-H1), which is expressed on hematopoetic and parenchymal cells. Expression of PD-L1 is associated with the inhibition of autoreactive CD4+ T cell responses and peripheral T cell tolerance [63]. PD-L1 synergizes with TGF-β to inhibit the AKT-mTOR axis, thereby promoting iTreg development and function via the same pathway as rapamycin [64]. Cobbold et al [65] have linked the effect of mTOR inhibition to infectious tolerance by showing that enzymes produced by nTreg consume certain amino acids that are essential for T cell proliferation, resulting in inhibition of the mTOR pathway and induction of FoxP3 expression. These studies suggest that upregulation of PD-L1 may be used as a strategy to further optimize iTreg conversion.
Taken together, these studies show that mTOR inhibition with rapamycin can enhance induction of FoxP3+ Treg from a naïve T cell population, and function synergistically with ATRA (Table 1). This supports the concept that use of rapamycin as maintenance therapy may constitute tolerogenic immunosuppression [66, 67].
5. ALTERNATIVE APPROACH: GENETIC ENGINEERING TO INDUCE FOXP3 EXPRESSION
An alternative strategy to induce suppressor function in conventional T cells is ex vivo FoxP3 gene transfer using a viral vector. This approach was studied by Hori et al [68], who demonstrated the conversion of 30–60% of mouse CD4+CD25− cells to the Treg phenotype after forced expression of FoxP3 was induced by retroviral transduction. The iTreg generated had reduced cytokine production and a potent inhibitory effect on autologous Teff proliferation. Moreover, the FoxP3+ cells generated were capable of abrogating autoimmune disease in a mouse inflammatory bowel disease model. Lentivirus-based transduction of the FoxP3 transcription factor was explored by Allen et al [6], after it was found that overexpression of FoxP3 by regular retroviral transduction did not lead consistently to generation of suppressive Treg [69]. In contrast to conventional retroviral vectors, lentiviral vectors have the ability to replicate in non-cycling cells, rendering them very efficient in gene transduction. A lentiviral vector was used to induce FoxP3 in human FoxP3−cells, and a maximum transduction efficiency of 76–97% was achieved. The FoxP3+ cells had an nTreg phenotype and potent suppressor function. Additionally, it was shown that lentivirus-driven FoxP3 expression could be maintained for several weeks, whereas expanded nTreg lost FoxP3 expression upon prolonged culture.
These studies indicate that there are several ways to obtain high numbers of FoxP3+ suppressor cells from a naïve CD4+ T cell population, and promising results provide a strong rationale for the use of viral transduction in further studies.
6. IN VIVO PREVALENCE OF iTREG
The incidence of iTreg in vivo has been determined in different settings. When their frequency was assessed by enumeration of FoxP3+ cells after homeostatic repopulation in a FoxP3− mouse model, it was found to be 8% [28] and 4–7% [27] of total CD4+ cells, respectively. However, in a study without lymphodepletion, little to no conversion was found upon stimulation with self Ag [14]. A solution to the issue of distinguishing nTreg from iTreg would preferably be through a marker that is expressed specifically on one, but not the other cell type. The development of the T lymphocyte lineage is linked to one of the members of the Ikaros transcription factor family, Helios [70]. Expression of Helios is upregulated in nTreg by binding to the FoxP3 promoter; upon Helios elimination, FoxP3 expression and the suppressive function of nTreg is inhibited [71]. A recent study [13] used Helios expression to assess nTreg/iTreg proportions in healthy mice and humans. Helios was expressed by 100% of thymic CD4+FoxP3+ nTreg, but was not expressed when mouse or human FoxP3+ iTreg were generated in vitro. Both mouse and human CD4+FoxP3+ cells from healthy subjects are 70% Helios+[13], suggesting that 30% of circulating FoxP3+ Treg are peripherally-induced iTreg. Based on these studies, it can be concluded that there is a significant percentage of circulating iTreg in healthy individuals, although the exact proportion of iTreg within the total FoxP3+ population needs to be better defined.
7. TREG PLASTICITY: Th17 DIFFERENTIATION
Conversion of naïve T cells carries the risk that not all cells will be converted to Treg, and that remaining CD4+ T effector cells will contaminate the cultures. However, during the activation of murine naïve CD4+ T cells in the presence of TGF-β, there is a crucial role for IL-6, a common pro-inflammatory cytokine produced by Teff and macrophages. When combined with TGF-β, this acute-phase protein is associated directly with differentiation of CD4+ T cells into pro-inflammatory Th17 cells, rather than into the FoxP3+ iTreg phenotype in the mouse model, suggesting that an inflammatory milieu can drive naïve cells to convert into effector rather than suppressor cells [72, 73]. Moreover, IL-6 can convert already established FoxP3+ nTreg into Th17 cells [74, 75]. The capacity of IL-6 (or an inflammatory milieu in general) to direct T cells to an immune effector rather than regulatory phenotype is a problematic issue for expansion of a pure Treg population in vitro, and could also be of crucial importance when Treg are used in a clinical setting of autoimmune disease or transplantation, where, despite immunosuppressive therapy, some degree of inflammatory response can be expected.
In addition to the influence of IL-6, high levels of TCR/CD28 costimulation are associated with induction of nuclear factor-κB (NF-κB) activation, which potently inhibits iTreg differentiation in naïve CD4+ T cells. Indeed, when naïve CD4+ T cells are stimulated with anti-CD28 and high doses of anti-CD3, expression of the proinflammatory cytokines IL-17 and IL-9 is enhanced significantly, and the frequency of FoxP3+ cells diminished [39]. As the main goal of iTreg induction is to generate potent suppressor cells, contamination by outgrowth of effector cells is likely to have a detrimental effect on their potential application. These recent observations raise concerns with regard to the preparation of Treg for therapeutic application, and indicate that a reliable method to assess suppressive capacity might become a necessary goal.
A promising and straightforward solution to overcome the detrimental outgrowth of Th17 cells in the Treg population is the addition of ATRA or rapamycin during the expansion of naïve T cells. ATRA potently suppresses Th17 differentiation of naïve T cells and drives them to iTreg conversion with stable FoxP3+ expression, despite an IL-6-induced inflammatory environment [23, 76]. Recently, it has been shown that ATRA has similar effects on nTreg stability as on iTreg, since it prevents their conversion to Th17 cells [77]. Similar to the inhibitory effect of ATRA on TGF-β-mediated iTreg/Th17 differentiation, rapamycin inhibits the development of Th17 cells from bulk CD4+ cells, as well as CD4+CD25− populations under Th17-skewing conditions [58]. Therefore, it is likely that iTreg generated in vitro under the influence of ATRA or rapamycin will have a stable phenotype and function. It is not clear if this same effect can be obtained by in vivo administration of ATRA or rapamycin.
Importantly, in experiments involving human cells, it has been found that naïve T cells do not differentiate into Th17 cells when stimulated with TGF-β and IL-6 alone, but that this requires additional stimulation by IL-1β ([78], IL-23 [79], or DC [80]. Unfortunately, reports on human iTreg have not determined the Treg-skewing effects of ATRA or rapamycin on iTreg/Th17 differentiation. Although this knowledge is perhaps not essential for in vitro generation of these cells (since human naïve T cells need additional stimulation to differentiate into Th17 cells, and cells and cytokine levels can be strictly controlled during laboratory experiments), it will certainly be an important issue for future clinical application of iTreg.
8. iTREG VS nTREG: DIRECT COMPARISON
Induced Treg have significant suppressive function when compared to baseline levels of T cell proliferation (baseline = responder cells + stimulation only; no Treg or control T cells added), but a wide range of suppressive efficacy has been reported depending on methods of induction [10, 12, 54, 58]. To adequately compare the function of iTreg with that of nTreg, it is necessary to test both cell types in the same system and under identical conditions. Interestingly, only a few groups that have generated iTreg have undertaken this direct comparison of the function of iTreg versus nTreg. The few studies on iTreg in the mouse model that have done so all show the level of suppression of fresh nTreg compared to expanded iTreg.
Conversion of CD4+CD25− T cells by 5 days stimulation with anti-CD3, irradiated APC and IL-2 +/− TGF-β showed, when compared to fresh CD4+CD25− T cells, a significant increase in normalized FoxP3 mRNA levels which was about 50% of the nTreg level. These 5-day expanded iTreg potently suppressed baseline CD4+CD25− Teff proliferation to levels comparable to those when nTreg were assessed [11]. In another study using a similar method of induction, murine CD4+CD25− T cells were cultured for 5 days with plate-bound anti-CD3 and soluble anti-CD28 +/− IL-2 +/− TGF-β. The frequency of CD25+FoxP3+ iTreg increased significantly from 3% (no TGF-β) to 53% when IL-2+TGF-β was added to the cultures. In a suppression assay, the addition of TGF-β-expanded iTreg or fresh nTreg (1:1 to responders) resulted in similar suppression of Teff proliferation to 5–10% of baseline under both conditions. These findings were then translated to an in vivo colitis model, where iTreg infusion resulted in a marked decrease in colonic inflammation, although these results were not compared to nTreg [81].
Very promising results have also been reported by Benson et al [19], who showed almost 100% conversion to CD4+FoxP3+ iTreg by 4 days expansion of CD4+FoxP3− Tcells with anti-CD3, anti-CD28, IL-2, TGF-β and ATRA. Expanded iTreg exhibited similar suppressive activity as fresh nTreg when added to responder CD4+ cells in a 1:1 ratio. At a ratio of 1:8 (Treg:responder), iTreg retained some suppressive effect, whereas fresh nTreg had lost their suppressive capacity. These studies in the mouse model provide evidence that iTreg can exhibit suppressive function at a similar strength as nTreg.
In a study in which baboon bulk CD4+ T cells were expanded in the presence of anti-CD3/28 beads, IL-2 and rapamycin for 4 weeks, increased expression of FoxP3+ of 20% (11% when cultured without rapamycin) was found. The CD25hi cells were purified after expansion, and added to an MLR of autologous baboon CD4+CD25− responders stimulated with xenogeneic (porcine) irradiated PBMC. Compared to freshly-isolated nTreg, iTreg cultured with rapamycin showed similar, potent inhibition of the xenogeneic T cell response [61].
Interestingly, a recent study describes the induction of human Treg by bacterial superAgs, which provide potent polyclonal activation [82]. Stimulation of human CD4+CD25− T cells with Streptococcal pyrogenic exotoxin and autologous APC alone (without the addition of IL-2 or TGF-β) resulted in the induction of 20% CD25+FoxP3+ T cells after 3 days stimulation (5% without bacterial Ag stimulation). Subsequent addition of Treg in an autologous CD4+CD25− proliferation assay showed that, at 1:1 ratio, fresh nTreg could reduce proliferation to 20% of baseline, compared to 40% for iTreg; however, at 1:10, nTreg were still suppressive (55%), whereas iTreg had lost their suppressive effect (80%).
Overall, these studies provide promising evidence that, similar to the findings in the mouse model, in humans and primates iTreg generated from naïve T cells can acquire suppressive function of similar strength as their nTreg counterparts.
9. FUTURE PROSPECTS
Many questions remain unresolved regarding pathways and optimal conditions for the induction of Treg from a naïve T cell population and how the resulting population compares to nTreg. Although recent reports are promising, there are very few studies that provide a direct comparison of nTreg and iTreg. This will be of great importance when such studies are translated to clinical trials in the near future, especially in regard to the quantity of cells needed. Notably, a recent study shows that human nTreg can be expanded on a much greater scale than had been reported previously and used for the first time in clinical trials [83, 84], thus making the cell number that can be obtained of less importance when proposing the use of iTreg. To make iTreg true competitors of their naturally-occurring counterpart, the suppressive function of iTreg should be at least equal to that of nTreg.
One concern that may arise when translating studies on iTreg to clinical trials, is that not all cells from the starting population would be converted, thus leaving a remainder of contaminating Teff. However, this concern could be overcome by sorting the iTreg after expansion (e.g., based on expression of CD25hi, CD45RA+, or CCR9, depending on the method of iTreg generation).
Another critical point is the potential to drive a subset of naïve T cells to the Th17 or other effector phenotype, rather than to iTreg during stimulation - a risk that cannot be underestimated. Although it is well-demonstrated that this can be abrogated by the use of ATRA or rapamycin in in vitro culture, the potential of Th17 differentiation in vivo may remain. Interestingly, RA has also been associated recently with promotion of Teff responses, suggesting a dual function for proinflammatory as well as suppressive T cell responses [85]. A possible solution to overcome the release of pro-inflammatory under RA stimulation would be by blocking IL-15 during iTreg stimulation [86]. Moreover, most studies on iTreg have been carried out in mice, but Treg in humans have already proven different from those in the mouse. These differences can be found, for example, in the conversion of naïve T cells to Th17 effector cells (in humans this does not occur when stimulation is with TGF-β and IL-6 alone) or in the expression of FoxP3. In mice, FoxP3 expression is strictly related to suppressor function, while human activated T cells can transiently express intermediate levels of FoxP3. This however, is at a lower level than that of human nTreg, and is not associated with suppressor function [87, 88]. Moreover, activated human conventional CD4+ T cells can acquire suppressive capacity while not expressing FoxP3.
Another point of interest is that in certain diseases, e.g., in autoimmune disorders, patients may have defective nTreg, which may be the immunologic basis of their disease. Although studies have shown that e.g., in type-1 diabetes, nTreg can be expanded and exert suppressive function that is equal to that of healthy controls [89], this may not be the case in other diseases. In diseases where the nTreg of the patient are less functional, e.g., the immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, generation of iTreg may be beneficial and more effective than expansion of autologous nTreg. Moreover, these iTreg can be generated in an Ag-specific fashion, thereby making the cells a disease- (or donor-organ) specific treatment.
Remaining concerns that need to be addressed before clinical trials are initiated, include the stability of the iTreg (and nTreg) phenotype, particularly considering their interaction with inflammatory cells and immunosuppressive therapy. As described by Feuerer et al [90], various subtypes of Treg have different gene expression profiles, suggesting a different function for each type. Furthermore, the importance of appropriate migration of the cells (reviewed by Zhang et al [91]), which determines their fate after in vivo injection, and a detailed comparison of the efficacy of nTreg versus iTreg in vivo need to be studied. Many factors are likely to affect homeostasis of transferred Treg, including IL-2, TGF-β levels and those of other regulators such as mTOR inhibitors or Vitamin A. Non-human primate models can provide valuable information on the in vivo function of Treg (reviewed by Dons et al [92]); in this regard, comparison of iTreg to nTreg might provide further information on the function of both cell types, before use of these cells is translated to clinical trials.
10. CONCLUDING REMARKS
Regulatory T cells have been the subject of intense investigation for the last decade, and as the first clinical trials to ascertain the safety of ex-vivo expanded nTreg infusion in humans are being initiated [83, 84], determination of the necessary numbers of Treg has become increasingly pressing. The induction of a regulatory phenotype from naïve CD4+CD25− Teff has become a well-established approach to convert large numbers of T cells into functional Treg. Although various methods for the generation of iTreg have been published, a consensus exists in regard to the basic procedure of induction, using IL-2, TGF-β and low costimulation to generate potently suppressive FoxP3+ iTreg. Rapamycin and ATRA are proven to be valuable, as these reagents not only enhance FoxP3 induction, but also actively prevent the differentiation of naïve T cells into Th17 cells, thus providing a more stable phenotype. iTreg have proven to be stable and potent suppressor cells that seem of similar suppressive strength to nTreg. Perhaps optimal results will be achieved when both cell types are being used in concert, a concept that still needs to be explored.
Altogether, the induction of a regulatory phenotype provides a promising means to generate potently suppressive Treg with stable FoxP3 expression in significantly higher numbers than can be obtained by expansion of nTreg, thereby having the potential for use in large-scale clinical trials.
Acknowledgments
This work was supported by National Institutes of Health grants U01-AI091197 and R01-67541. Eefje M. Dons is the recipient of fellowships from the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences and the Stichting Professor Michael van Vloten Fund, The Netherlands. Giorgio Raimondi is in receipt of an American Heart Association Beginning Grant-in-Aid, an American Diabetes Association Junior Faculty Grant and the Thomas E. Starzl Transplantation Institute Joseph A. Patrick Fellowship.
ABBREVIATIONS
- Ab
antibody
- Ag
antigen
- APC
antigen-presenting cells
- ATRA
all-trans retinoic acid
- CTLA4
cytotoxic lymphocyte antigen 4
- DC
dendritic cells
- FoxP3
Forkhead box P3
- iTreg
induced regulatory T cells
- mTOR
mammalian target of rapamycin
- nTreg
naturally-occurring regulatory T cell
- RA
retinoic acid
- Teff
T effector cells
- TGF-β
transforming growth factor β
- Treg
regulatory T cells
- Tx
transplantation
Footnotes
Authors Contributions:
EMD, GR, DKCC and AWT each participated in the writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151. [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, Naldini L, Roncarolo MG, Soudeyns H, Levings MK. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16(1):194. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108(13):4260. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104(2):453. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104(3):895. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Zhang H, Ding Y, Bromberg JS. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant. 2004;4(10):1614. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169(8):4183. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 13.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med. 2007;204(9):2039. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110(13):4293. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204(8):1765. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185(1):302. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 21.Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011 doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 22.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011 doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 23.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Dawicki W, Zhang X, Town J, Gordon JR. Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25-/loFoxp3- effector T cells. J Immunol. 2010;185(9):5003. doi: 10.4049/jimmunol.0903446. [DOI] [PubMed] [Google Scholar]
- 25.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29(1):114. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Thompson LJ, Valladao AC, Ziegler SF. Cutting edge: de novo induction of functional foxp3+ regulatory CD4 T cells in response to tissue-restricted self antigen. J Immunol. 2011;186(8):4551. doi: 10.4049/jimmunol.1003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205(13):3105. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25- cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med. 2005;201(1):127. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 30.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172(10):6003. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 31.Fan Z, Spencer JA, Lu Y, Pitsillides CM, Singh G, Kim P, Yun SH, Toxavidis V, Strom TB, Lin CP, Koulmanda M. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16(6):718. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7(7):1722. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21(6):903. [PMC free article] [PubMed] [Google Scholar]
- 34.Onodera K, Lehmann M, Akalin E, Volk HD, Sayegh MH, Kupiec-Weglinski JW. Induction of “infectious” tolerance to MHC-incompatible cardiac allografts in CD4 monoclonal antibody-treated sensitized rat recipients. J Immunol. 1996;157(5):1944. [PubMed] [Google Scholar]
- 35.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. “Infectious” transplantation tolerance. Science. 1993;259(5097):974. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 36.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196(2):247. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196(2):255. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, Hori S, Waldmann H. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011;208(10):2043. doi: 10.1084/jem.20110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-{kappa}B-dependent manner. J Immunol. 2011;186(8):4609. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9(9):1202. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 41.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16(10):1391. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 43.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176(6):3321. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 44.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172(9):5149. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 45.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178(4):2018. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 46.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186(11):6329. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sommer A. Vitamin A, infectious disease, and childhood mortality: a 2 solution? J Infect Dis. 1993;167(5):1003. doi: 10.1093/infdis/167.5.1003. [DOI] [PubMed] [Google Scholar]
- 48.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179(6):3724. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 50.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74(1):185. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 51.Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M, LaRosa GJ, Yang LL, Soler D, Butcher EC, Ponath PD, Parker CM, Andrew DP. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190(9):1241. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29(5):758. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206(10):2131. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol. 2009;183(6):4119. doi: 10.4049/jimmunol.0901065. [DOI] [PubMed] [Google Scholar]
- 55.Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS One. 2011;6(1):e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 57.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7(13):1819. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205(3):565. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh AK, Horvath KA, Mohiuddin MM. Rapamycin promotes the enrichment of CD4(+)CD25(hi)FoxP3(+) T regulatory cells from naive CD4(+) T cells of baboon that suppress antiporcine xenogenic response in vitro. Transplant Proc. 2009;41(1):418. doi: 10.1016/j.transproceed.2008.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11(6):1148. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A. 2009;106(29):12055. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, Turka LA, Knechtle SJ. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant. 2008;8(4):793. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 67.Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, Roncarolo MG, Battaglia M. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes. 2008;57(9):2341. doi: 10.2337/db08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 69.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115(11):3276. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, Akashi K, Weissman IL, Fisher AG, Smale ST. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 1998;12(6):782. doi: 10.1101/gad.12.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S, Pan F, Pardoll DM, Drake CG. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47(7–8):1595. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 73.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178(11):6725. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 75.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180(11):7112. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 76.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181(4):2277. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185(5):2675. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 79.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 80.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27(4):660. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55(5):671. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor AL, Llewelyn MJ. Superantigen-induced proliferation of human CD4+CD25- T cells is followed by a switch to a functional regulatory phenotype. J Immunol. 2010;185(11):6591. doi: 10.4049/jimmunol.1002416. [DOI] [PubMed] [Google Scholar]
- 83.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential Role for Retinoic Acid in the Promotion of CD4(+) T Cell Effector Responses via Retinoic Acid Receptor Alpha. Immunity. 2011;34(3):435. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471(7337):220. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110(8):2983. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ziegler SF. FOXP3: not just for regulatory T cells anymore. Eur J Immunol. 2007;37(1):21. doi: 10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- 89.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58(3):652. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci U S A. 2010;107(13):5919. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dons EM, Raimondi G, Cooper DK, Thomson AW. Non-human primate regulatory T cells: current biology and implications for transplantation. Transplantation. 2010;90(8):811. doi: 10.1097/TP.0b013e3181ebf782. [DOI] [PMC free article] [PubMed] [Google Scholar]