Abstract
Synaptic transmission is a finely regulated mechanism of neuronal communication. The release of neurotransmitter at the synapse is not only the reflection of membrane depolarization events, but rather, is the summation of interactions between ion channels, G protein coupled receptors, second messengers, and the exocytotic machinery itself which exposes the components within a synaptic vesicle to the synaptic cleft. The focus of this review is to explore the role of G protein signaling as it relates to neurotransmission, as well as to discuss the recently determined inhibitory mechanism of Gβγ dimers acting directly on the exocytotic machinery proteins to inhibit neurotransmitter release.
Keywords: presynaptic regulation, exocytosis, SNARE proteins, heterotrimeric G proteins
1 Introduction
Efficient communication between neurons in the brain is crucial to the normal functioning of the nervous system as it ensures integration of both external and internal sensory input, and permits the generation of appropriate behaviors to meet the demands of an individual’s environment (Goodman et al., 2001). As a result, understanding the process of synaptic transmission is essential to understanding how normal processes are coordinated, but also how they may be disrupted by injury and disease. In its most elementary form, synaptic transmission is simply the communication between one presynaptic neuron and a single postsynaptic cell as well as the processing by the postsynaptic cell of the signal that it receives (Kandel et al., 2000). At chemical synapses, signal transduction is achieved by the rapid conversion of an arriving electrical signal into a chemical one that diffuses between the cells (Schoch and Gundelfinger, 2006; Zhai and Bellen, 2004). Structurally, these complex, asymmetrical cell-cell contact sites are formed from the axon terminal membrane of the presynaptic neuron, juxtaposed with the postsynaptic density on the postsynaptic cell (Dresbach et al., 2001; Schoch and Gundelfinger, 2006).
Membrane depolarization caused by the arrival of presynaptic action potentials induces the opening of voltage-dependent calcium channels (VDCC), with the resulting calcium transient stimulating synaptic vesicle exocytosis from the active zone of the presynaptic terminal (Sudhof, 2004). Such regulated exocytosis releases neurotransmitter into the synaptic cleft whereupon it activates receptors or channels on the postsynaptic membrane to ensure continued propagation of the signal (Dresbach et al., 2001; Sudhof, 2004). When these chemical synapses are examined, two distinct types of vesicles are evident: small, clear synaptic vesicles which are filled with classical neurotransmitters such as acetylcholine, glutamate, and the monoamines, and large dense-core vesicles filled with neuropeptides and neurohormones. While these vesicles differ in their morphology, release kinetics, and distribution, they maintain conserved machinery for fusion events, and both exhibit calcium-dependence for exocytosis (Park and Kim, 2009).
Released neurotransmitters bind to ligand-gated ion channels on postsynaptic neurons to mediate voltage changes in the postsynaptic cell. Major modulators of neurotransmitter action are G protein coupled receptors (GPCRs). These seven membrane-spanning α-helical proteins bind specifically to their respective neurotransmitters, causing a change in the structure of the receptor, and resulting in activation of heterotrimeric guanine-nucleotide binding proteins. Gi/o-coupled autoreceptors on both pre-and postsynaptic cells guard against overstimulation by release of G protein βγ subunits which postsynaptically activate G protein-coupled inward rectifier K+ (GIRK) channels and presynaptically inhibit voltage activated calcium channels (Kajikawa et al., 2001; Takahashi et al., 1996). Gβγ subunits can also directly interact with the vesicle exocytotic machinery, soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins (Blackmer et al., 2001; Delaney et al., 2007; Gerachshenko et al., 2005). Gi/o-coupled heteroreceptors mediate circuit-level modulation of neurotransmitter responses.
The goal of this review is to discuss the regulation of exocytosis at the synapse by G protein coupled receptor signaling pathways with a focus on the role and mechanism of Gβγ signaling and the novel direct modulation on the exocytotic machinery.
2 The Exocytotic Machinery
2.1 Regulatory Components Controlling Vesicle Docking, Priming, and Exocytosis at the Presynaptic Membrane
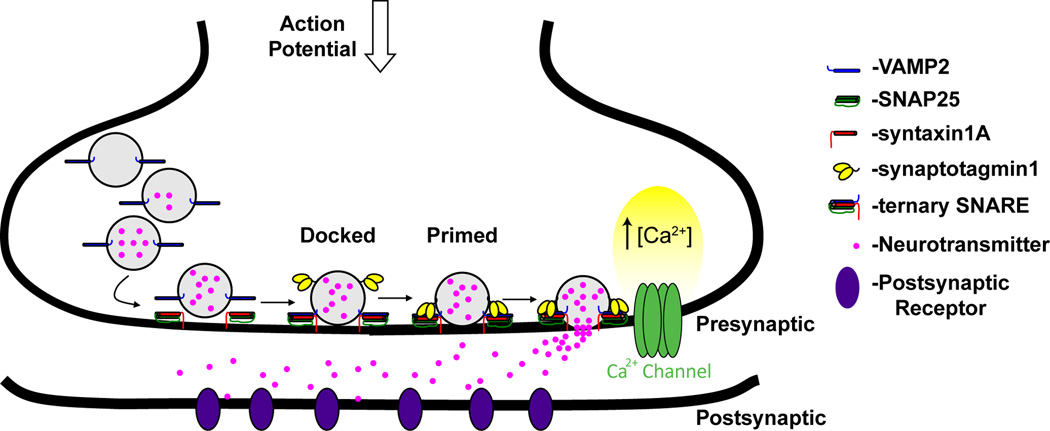
The short latency between calcium influx at a presynaptic terminal and the release of neurotransmitter suggests that a population of vesicles sits poised to fuse with the plasma membrane immediately following calcium entry (Katz, 1969; Weimer and Richmond, 2005). To achieve such temporal precision, vesicles undergo a series of maturation steps at the presynaptic membrane known as docking and priming, in order to become fusion competent (see Figure 1). Docked vesicles were traditionally defined morphologically based on electron micrographs as those without measurable distance between vesicle and plasma membrane (Verhage and Sorensen, 2008). Based on this definition, docked vesicles were often reported to cluster around electron-dense pyramidal densities at the presynaptic terminal, and were suggested to make direct contact with the active zone (Fernandez-Busnadiego et al., 2010; Weimer and Richmond, 2005). Unfortunately, while ultrastructure studies are unmatched in resolution, they cannot easily be used to study vesicle dynamics and preclude identification of different docked states, while potentially overestimating the number of docked vesicles through inclusion of those which are primed and/or being endocytosed (Toonen et al., 2006; Weimer and Richmond, 2005). Further, chemical fixation required for electron microscopy is known to introduce crosslinking artifacts which can alter the distribution of synaptic vesicles at a presynaptic terminal (Fernandez-Busnadiego et al., 2010). More recent studies observing single synaptic vesicles using tools such as total internal reflection fluorescence microscopy show evidence for three distinct docking states by which vesicles approach the plasma membrane for variable lengths of time and contact it only during fusion events (Fernandez-Busnadiego et al., 2010; Weimer and Richmond, 2005). Such studies suggest that docking may be an easily reversible event prior to priming where stable interactions with the plasma membrane would be established (Fernandez-Busnadiego et al., 2010; Weimer and Richmond, 2005). Despite increasing numbers of perturbation and biophysical studies, however, relatively few docking phenotypes have been associated with genetic mutations compared to secretion phenotypes (Verhage and Sorensen, 2008). Given the spatial fidelity by which synaptic vesicles cluster at an active zone, however, it is likely that specific molecular recognition mechanisms mediate attachment of the vesicles to the target membrane during the docking process.
Figure 1. Overview of neurotransmission across a synapse.
In the presynaptic neuron, synaptic vesicles expressing VAMP2, among other proteins embedded in their membrane, are loaded with neurotransmitters. Simplistically, docking of the vesicle occurs via interaction of VAMP2 with t-SNARE (SNAP-25/syntaxin 1A), a process that is guided and regulated by numerous proteins, while priming has been described as further zippering of the SNARE complex with interaction of other major components such as synaptotagmin, MUNC13, and MUNC18. Upon the arrival of an action potential, VDCC facilitate a large increase in intracellular calcium concentration. This rise is sensed by proteins within the synaptic complex of the vesicle at the membrane such that fusion of the vesicle membrane with the presynaptic membrane occurs, resulting in release of neurotransmitter into the synaptic cleft to activate its respective receptor on the post-synaptic neuron ensuring propagation of action potentials from one neuron to another.
Comparatively more is known about priming, a vesicle maturation process wherein vesicles transition from a docked but unprimed pool to a releasable pool which can fuse with the plasma membrane immediately upon calcium entry (Becherer and Rettig, 2006; Malsam et al., 2008; Weimer and Richmond, 2005). While the fidelity of vesicle priming and subsequent exocytosis has been shown to depend on the coordinated actions of a large number of proteins, the core machinery underlying this process revolves around members of the SNARE complex. Further regulation is provided by a series of ancillary proteins such as the Sec-1/Munc-18 (SM) proteins Munc-18 and Munc-13, and the calcium sensor, synaptotagmin (Parpura and Mohideen, 2008). The SM proteins have been shown to have important interactions with syntaxin1A that regulate the docking of vesicles to the synaptic membrane by influencing the open and closed state of syntaxin1A (de Wit, 2010; Gerber et al., 2008; Misura et al., 2000; Rizo et al., 2006; Rizo and Rosenmund, 2008; Sudhof, 2004; Sudhof and Rothman, 2009; Toonen et al., 2006; Wojcik and Brose, 2007). In contrast, synaptotagmin1 plays a role in both priming and docking by its ability to interact with t-SNARE, the complex of SNAP25 and syntaxin1A at the synaptic membrane, to stabilize it for further interaction with VAMP2 on the synaptic vesicle (Weninger et al., 2008).
2.2 Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins
The protein machinery that drives and regulates exocytosis has been shown to be remarkably conserved, with SNARE proteins ubiquitously used for membrane fusion in organisms ranging from yeast to humans (Jahn and Scheller, 2006; Parpura and Mohideen, 2008). A mechanistic model has subsequently emerged termed the ‘SNARE Hypothesis’ whereby membrane fusion is thought to occur through formation of a four-helix bundle which draws opposing membranes into close proximity and releases enough free energy to drive fusion events (Jahn and Scheller, 2006; Sollner et al., 1993a; Sollner et al., 1993b; Weber et al., 1998). Central to this hypothesis are the three proteins which make up the SNARE complex. The representative major isoforms in brain of t-SNAREs are syntaxin 1A and synaptosome-associated protein of 25 kDa (SNAP-25), and of the v-SNARE is synaptobrevin/VAMP (vesicle-associated membrane protein)-2. When the structure of syntaxin 1A is examined, three conserved domains are apparent: a carboxy-terminal transmembrane domain anchored to the presynaptic membrane, and two conserved α-helical structures - a SNARE domain single α–helix and an amino-terminal triple α–helix Habc domain - which play a role in regulating synaptic transmission. The SNARE domain mediates interaction of syntaxin with other members of the SNARE complex to form the exocytotic machinery. By folding back on itself to cover the SNARE domain, the Habc domain places syntaxin in a ‘closed’ conformation and prevents the formation of the core fusion complex through further interaction with chaperone proteins such as Munc-18 (Sudhof, 2004; Teng et al., 2001).
Similarly, synaptobrevin is also a transmembrane protein whose cytoplasmic domain forms an α-helical SNARE domain. While in vitro studies have suggested synaptobrevin may interact with other synaptic proteins such as voltage-dependent calcium and sodium channels (Sampo et al., 2000), the primary role for these proteins at this time appears to be mediating vesicle endo- and exocytosis (Hong, 2005; Seagar et al., 1999). The final member of the SNARE complex, SNAP-25, is unique in that while it is found localized to the presynaptic membrane, it lacks a transmembrane domain. Rather, it is anchored via post-translational palmitoylation of four cysteine residues located near the central region of the molecule between two α-helical SNARE domains (Matteoli et al., 2009). Two other members of this family, SNAP-23 and SNAP-29 have also been shown to be important for regulating exocytotic events, but despite these isoforms co-localizing to regions such as the cerebellar cortex, SNAP-25 appears to be the major homolog mediating regulated exocytosis in the brain (Matteoli et al., 2009).
The predominant view of SNARE complex formation is that initial assembly of the intermediate binary complex between syntaxin and SNAP-25 (t-SNARE) is followed by formation of the ternary complex through interactions with synaptobrevin on the secretory vesicle as the vesicle is drawn to the presynaptic membrane during priming. As vesicles approach the presynaptic membrane, the SNARE motifs interact in a process referred to as “zippering”, forming a stable, four-helical bundle which brings the vesicle progressively closer to the presynaptic membrane to allow fusion to occur.
2.3 Sec-1/Munc-18 (SM) Proteins
While formation of the SNARE complex has been shown to be sufficient to drive exocytosis, a number of studies examining fusion kinetics have suggested that additional proteins may be required. One group of such proteins includes the cytosolic SM proteins, Munc-18 and Munc-13 (Sudhof, 2004). All members of the SM family are composed of a conserved ≈600 amino acid sequence which folds into an arch-shaped structure important for protein-protein interactions (Sudhof and Rothman, 2009). Munc-18 is thought to serve multiple roles in exocytosis, the first being as an inhibitory protein which prevents assembly of the SNARE complex at inopportune times and which plays an important role in mediating vesicle docking (de Wit, 2010; Dulubova et al., 1999; Toonen et al., 2006; Voets et al., 2001; Wojcik and Brose, 2007; Yang et al., 2000). Evidence for a second, post-docking role for this SM protein, however, comes from analysis of Munc-18-1 knockout mice which exhibit a total block of neurotransmitter release (Burgoyne and Morgan, 2011; Verhage et al., 2000). Recent evidence suggests that rather than dissociate completely, Munc-18 actually remains anchored to syntaxin by its N-terminal lobe, allowing it to take on an additional regulatory role. As ternary SNARE formation proceeds, Munc-18 is believed to fold back over the four helix SNARE bundle and in doing so, restrict the diffusion of the SNARE proteins in the membrane such that they are maintained in the correct orientation for fusion to occur, as well as allow energy transduction to the membranes (Rizo and Rosenmund, 2008; Rizo, 2009; Sudhof and Rothman, 2009).
A second SM protein, Munc-13, has also been shown to play a prominent role in vesicle exocytosis, although as opposed to Munc-18, Munc-13 primarily regulates the priming process to increase the number of primed vesicles. Munc-13 null mice demonstrate total abolishment of both evoked and spontaneous neurotransmitter release while docking remains unaffected (Augustin et al., 1999), and this phenotype is also found in knockouts of homologs in both C. elegans (Richmond et al., 1999) and Drosophila (Aravamudan et al., 1999). Its actions appear to be downstream of Munc-18 as overexpression of Munc-13 does not affect the number of docked vesicles, while open forms of syntaxin 1A are able to at least partially rescue the unc-13 null phenotype in worms (Weimer et al., 2006) but not that of unc-18 (Hammarlund et al., 2007; Verhage and Sorensen, 2008). This role in priming is thought to be mediated through both its C-terminal C2 domain (C2C) as well as the Munc homology domains (MHD) located in the C-terminal third of the protein. These MHDs allow binding to syntaxin 1A after it forms a heterodimer with SNAP-25 or the fully formed SNARE complex (Becherer and Rettig, 2006; Rizo and Rosenmund, 2008; Stevens et al., 2005). In doing so, Munc-13 may help transform syntaxin to an open conformation to facilitate SNARE complex formation, or stabilize the exocytotic machinery to promote vesicle fusion once the complex is fully formed.
2.4 Synaptotagmins
In addition to SM proteins, synaptotagmin has also been shown to be essential for vesicle fusion to occur (Brose et al., 1992). Synaptotagmins are a family of calcium binding proteins (Fukuda, 2003a; b; Schiavo et al., 1998; Südhof, 2002). Genetic ablation of synaptotagmin-1 results in abrogation of fast, synchronous neurotransmitter release without affecting asynchronous events, leading to the suggestion that this protein acts as a calcium sensor for calcium-dependent vesicle fusion (Geppert et al., 1994; Maximov and Sudhof, 2005; Rizo et al., 2006; Tang et al., 2006). Gene mutation in mice of the calcium-binding site of synaptotagmin, that disrupts calcium binding but no other structural features of synaptotagmin, results in a decrease in calcium sensitivity of neurotransmitter release, but has no effect on the size or spontaneous release of the ready releasable pool of synaptic vesicles (Fernandez-Chacon et al., 2001). Synaptotagmin-1 is localized to both synaptic and large dense core vesicle membranes and is characterized by two tandem, cytoplasmic, PKC-like C2 domains: C2A and C2B respectively. Two of the most important binding partners for this protein are SNARE proteins and the lipid membrane, and it’s believed that through cooperative interactions with each of these, the C2 domains play an important role in exocytosis. Specifically, calcium influx has been shown to induce binding of synaptotagmin-1 to both syntaxin and the C-terminal of SNAP-25, as well as t-SNARE heterodimers and fully formed SNARE complexes (Dai et al., 2007; Gerona et al., 2000; Rizo et al., 2006; Zhang et al., 2002), a process which may be mediated at least in part through interactions between acidic residues on SNAP-25 and basic residues in the second loop of the C2A domain (Lynch et al., 2007), or perhaps with the polybasic region of the C2B domain (Rizo and Rosenmund, 2008).
Anionic phospholipids are a second important effector for synaptotagmin-1(Chapman and Jahn, 1994; Chapman and Davis, 1998; Fernandez et al., 2001) and mutations which reduce calcium-dependent synaptotagmin binding to the lipid membrane exhibit a decrease in synaptic release probability (Zhang et al., 2002) suggesting that this function is essential for its role in exocytosis. While calcium-dependent binding of synaptotagmin to both SNARE proteins and lipid membranes is essential for fast exocytosis, debate still exists as to how exactly this occurs. One possibility is that synaptotagmin interacts with SNARE complexes prior to calcium influx, bringing the vesicle and plasma membranes into close apposition. This would be expected to promote vesicle fusion as it would enable tighter coiling of the SNARE complex, while subsequent interactions with the lipid bilayer upon calcium entry may accelerate membrane fusion through electrostatic interactions acting to perturb the lipid bilayer and overcome the energy barrier to fusion (Rizo and Rosenmund, 2008). Another unanswered question is how synaptotagmin interacts on a molecular level with the SNARE complex. Fluorescence resonance energy transfer–based studies detected an interaction of the SNARE complex on the opposing surfaces of the C2 domains of synaptotagmin (Choi et al., 2010). Further, NMR data demonstrated a primary interaction interface with SNARE may be through a polybasic region on the C2B domain (Dai et al., 2007). Most recently, electron paramagnetic resonance spectroscopy data reveal a structurally heterogeneous interaction maintaining the polybasic and C2B domain interactions, but also a site near loop 2 of the calcium binding site in C2A; an interaction not seen in the other two studies mentioned (Lai et al., 2011). These authors suggest that this new binding mode allows for simultaneous interaction with the SNARE complex as well as two membrane surfaces in apposition such as the cell membrane and the docked synaptic vesicle. Regardless of how exactly this process functions, however, synaptotagmin clearly plays a major role in regulating synchronous release with both SNARE interactions and phospholipid binding playing a crucial part. Further, synaptotagmins or other calcium sensors may play an important role in regulating asynchronous release events which are unrelated to action potentials. Studies examining synaptotagmin 1 and 2 knockout mice both demonstrate increases in the frequency of spontaneous release, suggesting an important role for these proteins in regulating this process (Kochubey and Schneggenburger, 2011; Sun et al., 2007). Similarly, doc2, a protein that is related to synaptotagmins in that it also has two homologous calcium-binding domains, has been shown to act as the calcium sensor for asynchronous release in cerebellar slices (Groffen et al., 2010) and cultured hippocampal neurons (Groffen et al., 2010; Yao et al., 2011), highlighting the importance of these sensors in many aspects of vesicle exocytosis.
2.5 Complexin, tomosyn, and heterotrimeric G proteins
Taken together, the SNARE proteins, as well as ancillary players such as SM proteins and synaptotagmin, function as the classical exocytotic machinery required for fusion events to occur. Studies in which individual components have been removed or inhibited, display severe disruption of synaptic transmission. Further exploration of processes which modulate their function has provided useful information about how exocytosis is regulated. Several additional proteins such as complexin, tomosyn, and heterotrimeric G proteins have also been shown to interact with either the proteins individually, the t-SNARE dimer, or the full ternary SNARE. Complexin is a regulatory protein thought to play a role in calcium-sensitive fusion (Chicka and Chapman, 2009; Tang et al., 2006) as well as vesicle priming (Hobson et al., 2011; McMahon et al., 1995; Tang et al., 2006). X-ray crystallography studies demonstrate that it interacts with the ternary SNARE complex by binding as a fifth α-helix to the SNARE bundle of ternary SNARE (Bracher et al., 2002; Chen et al., 2002). More recent x-ray crystallography and FRET data suggest that complexins can simultaneously bind to two neighboring incompletely “zippered” SNARE complexes stabilizing a structural state that is incompatible with fusion. After full incorporation of VAMP2 into the ternary SNARE bundle, complexin remains bound to the SNARE complex, but loses its interaction with the neighboring SNARE complex (Kümmel et al., 2011). Tomosyn is a protein that binds to the t-SNARE complex (Fujita et al., 1998) and prevents the association of VAMP2 (Hatsuzawa et al., 2003). Tomosyn has two domains, an N-terminal domain of two sets of 7 WD 40 repeats that form two tandem 7-bladed β-propellers, and a C-terminal domain with a SNARE binding motif. Both domains are needed to regulate exocytosis in vivo(Burdina et al., 2011). The C-terminal SNARE motif binds to t-SNARE in a way similar to that of VAMP2 binding to t-SNARE (Pobbati et al., 2004). Finally, the G-protein dimer, Gβγ, interacts with ternary SNARE as well as the individual SNARE proteins to mediate inhibition of exocytosis by GPCRs (Blackmer et al., 2001; Blackmer et al., 2005; Gerachshenko et al., 2005; Photowala et al., 2006; Yoon et al., 2007; Yoon et al., 2008). This novel interaction and its role in the context of GPCR regulation of synaptic transmission is the subject of the remainder of this manuscript.
3 GPCR Mediated Regulation of Synaptic Transmission
3.1 G protein Coupled Receptors
GPCRs are a large superfamily of proteins which convey the majority of signal transduction across cell membranes and mediate a vast array of cellular responses necessary for the normal physiology of the body (Eglen and Reisine, 2009; Millar and Newton, 2010; Oldham and Hamm, 2008). Encoded by nearly 800 different genes in humans, these proteins are activated by a wide variety of ligands ranging from single photons, odorants, and amino acids to hormones, neurotransmitters, and proteolytic enzymes as well as many others (Millar and Newton, 2010; Oldham and Hamm, 2008). As depicted in Figure 2A, while all GPCRs share a common architecture consisting of seven transmembrane-spanning α-helices, an extracellular N-terminus, an intracellular C-terminus, and three interhelical loops on each side of the membrane (Oldham and Hamm, 2007; 2008), they exhibit unique combinations of signal transduction pathways as well as complex regulatory processes controlling their activity and expression (Rosenbaum et al., 2009). At present, these receptors are phylogenetically divided into five main families: Glutamate, Rhodopsin, Adhesion, Frizzled-Taste-2, and Secretin, based on sequence and structural similarities (Schiöth and Fredriksson, 2005) with each family exhibiting unique ligand binding properties.
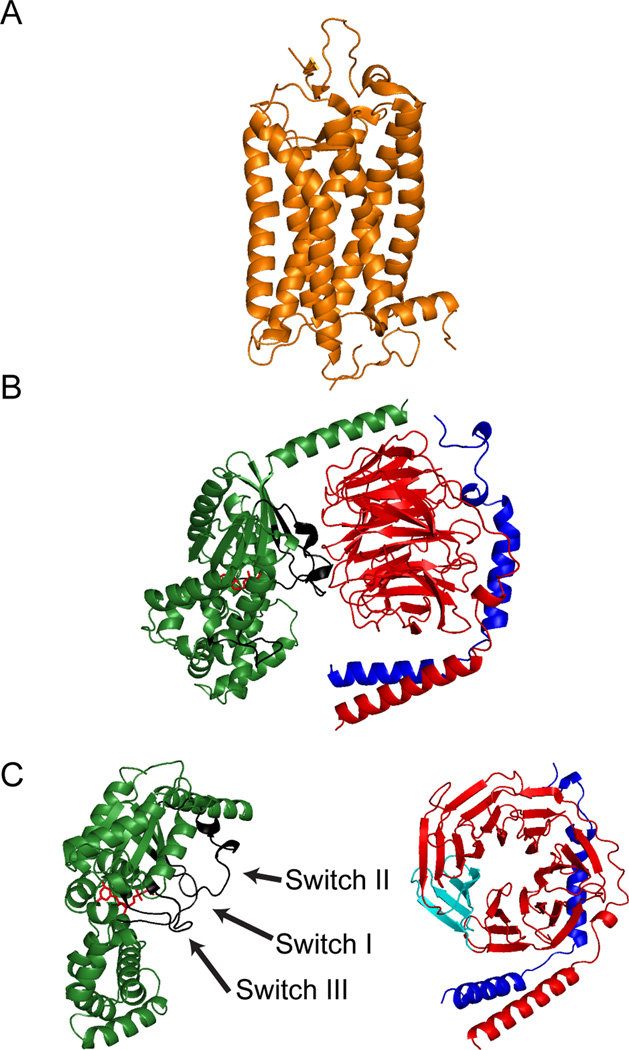
Figure 2. Structure of G protein Coupled Receptors and Heterotrimeric G proteins.
Shown in ribbon diagrams are representative examples of GPCRs and heterotrimeric G proteins. A) The structure of rhodopsin (PDB ID: 1F88) (Palczewski et al., 2000) is shown in orange, and it depicts the seven transmembrane α-helices and the respective extracellular and intracellular surfaces that interact with agonists and heterotrimeric G proteins, respectively. B) The structure of the heterotrimer transducin (PDB ID: 1GOT) (Lambright et al., 1996) is shown. The Gα subunit (green) has two domains, a GTPase domain and an α-helical domain. Within the GTPase domain, there are three regions termed Switch I, II, and III, (C), that have different orientations depending on which guanine nucleotide is present. In the GDP-bound form, Switches I–III form the major interface that interacts with the Gβγ dimer. The structure of the Gβ subunit (red) is an N-terminal α-helix followed by a series of β sheets that make a seven-bladed propeller or toroid. A single blade (light blue) is based on the amino acid motif termed WD-40 repeat. The structure of the Gγ subunit (dark blue) is two tandem α-helices that form interactions with the N-terminal α-helix of Gβ and a surface of the Gβ toroid on the opposite face from where Gα interacts with Gβ.
GPCRs are known to mediate their effects through coupling to heterotrimeric G proteins in order to modulate downstream effectors, but it is only recently that combinations of biochemical, crystallographic, and biophysical studies have begun to elucidate the structural basis by which this is accomplished (Rosenbaum et al., 2009). These studies demonstrate a complex picture by which specific ligands stabilize unique active state receptor conformations for interaction with particular effector molecules (Rosenbaum et al., 2009). The results of these conformational changes is an increase the receptor’s affinity for the G protein heterotrimer, resulting in the formation of a transient ligand-GPCR-G protein ternary complex necessary for G protein activation (Buranda et al., 2007).
3.2 Heterotrimeric G proteins
Despite the diversity of the GPCR superfamily, interestingly enough, they mediate their effects through relatively few heterotrimeric G proteins. G proteins consist of α, β, and γ subunits, and in humans, 16 genes and 11 splice variants account for 27 Gα isoforms (Wilkie et al., 1992; Yokoyama and Starmer, 1992), while 5 and 12 genes encode 5β and 13γ protein subunits, respectively (Downes and Gautam, 1999; Hildebrandt, 1997). Heterotrimers are generally classified into four families, Gs, Gi/o, Gq, and G12/13, based on the functional similarity of the Gα subunit (Simon et al., 1991), but all Gα subunits share a similar tertiary structure composed of two domains: a GTPase domain and a helical domain (Oldham and Hamm, 2007). Crystal structures demonstrate that the GTPase domain is conserved among all members of the G protein superfamily, including small G proteins and elongation factors (Oldham and Hamm, 2008). This domain contains the site of GTP hydrolysis as well as the sites for binding to the Gβγ dimer, receptors, and a wide variety of effectors. In contrast, the helical domain is unique to the heterotrimeric Gα subunit and functions as a lid to cover the nucleotide-binding pocket such that it is retained deep within the core of the protein (see Figure 2B). Further, post-translational modifications at the N-terminus of all Gα subunits help regulate membrane localization and protein-protein interactions (Oldham and Hamm, 2008).
G protein β subunits are made up of two structurally distinct regions, an amino terminal segment which is an α helix of approximately 20 amino acids, and a propeller-like structure comprising seven blades of four anti-parallel β strands known as a WD repeat (Clapham and Neer, 1997; Gautam et al., 1998; Smrcka, 2008) (see Figure 2B). In contrast, the Gγ subunit is much smaller, composed of only two α-helices, a carboxy-terminal helix which extends across the surface of blade 5 on Gβ as well as a small section of the N-terminal region, and an amino terminus helix which forms a coiled-coil with the amino terminal non-WD repeat region of the β subunit (Clapham and Neer, 1997) (see Figure 2B). All Gγ subunits are post-translationally modified at the C-terminus with either a farnesyl or geranylgeranyl moiety which aids in membrane localization and may play a role in receptor interactions (Oldham and Hamm, 2007; Smrcka, 2008). Together, Gβ and Gγ form a functional monomer as subunits cannot be dissociated except with denaturants (Smrcka, 2008) and neither subunit can signal on its own.
3.3 Specificity of Gβγ Interactions
Sequence comparisons show that Gβ1–4 share up to 90% sequence identity compared to 50% identity for Gβ5 while Gγ subunits are much more divergent, with isoforms sharing only 30–70% sequence identity (Betty et al., 1998; Smrcka, 2008). Given the high sequence identity shared among the isoforms, it might be expected that functional dimerization of all isoforms would be possible. While most Gβ and Gγ subunits have been shown to form dimer pairs in vitro, studies have shown that subtypes do not randomly associate, but rather show distinct affinities for one another, and even, that certain combinations are unable to pair. As evidence has shown with Gβγ dimerization, it is increasingly becoming evident that unique Gβγ combinations play specific roles in mediating interactions with receptors and effectors (Albert and Robillard, 2002; Betty et al., 1998; Lim et al., 2001; Lindorfer et al., 1998). Indeed, while early biochemical studies found few clear functional differences in the ability of isoform combinations to regulate effectors in vitro, subsequent studies using anti-sense oligonucleotides, site directed ribozymes, and genetic deletion have suggested very specific roles for Gβ and Gγ isoforms in signaling in intact cells (Smrcka, 2008). For example, Gγ7 knockout mice exhibit reduced expression of Gαolf and reduced adenylyl cyclase activity (Schwindinger et al., 2003), while Gγ3 knockout mice show increase susceptibility to seizures, reduced body weight, and decreased adiposity (Schwindinger et al., 2004). It is possible even, that isoforms may show tissue-dependent specificity for an individual effector (Robishaw and Berlot, 2004). Given that most cell types express multiple Gα, Gβ, and Gγ subtypes, a greater understanding of Gβγ-effector regulation is necessary to fully elucidate how specificity is achieved.
3.4 Gβγ Interaction with Effectors
Initially, Gβγ was thought to play a passive, inhibitory role by facilitating the completion of intracellular information transfer by merely returning Gα to a heterotrimeric state while also preventing Gα-mediated signaling in the absence of receptor activation (Cabrera-Vera et al., 2003). An independent role in cellular signaling was initially discovered, however, in 1987 when Logothetis et al. showed that Gβγ subunits could activate potassium-selective ion channels in cardiac atrial cells (Logothetis et al., 1987). Today, Gβγ subunits are known to regulate a wide variety of effectors including phospholipase Cβ, adenylyl cyclase, calmodulin, and components of the mitogen-activated protein kinase cascade (among others), as well as many effectors which are involved in mediating synaptic transmission such as calcium and potassium channels and members of the exocytotic machinery (Blackmer et al., 2005; Cabrera-Vera et al., 2003; Gerachshenko et al., 2005; Smrcka, 2008) (see Figure 3).
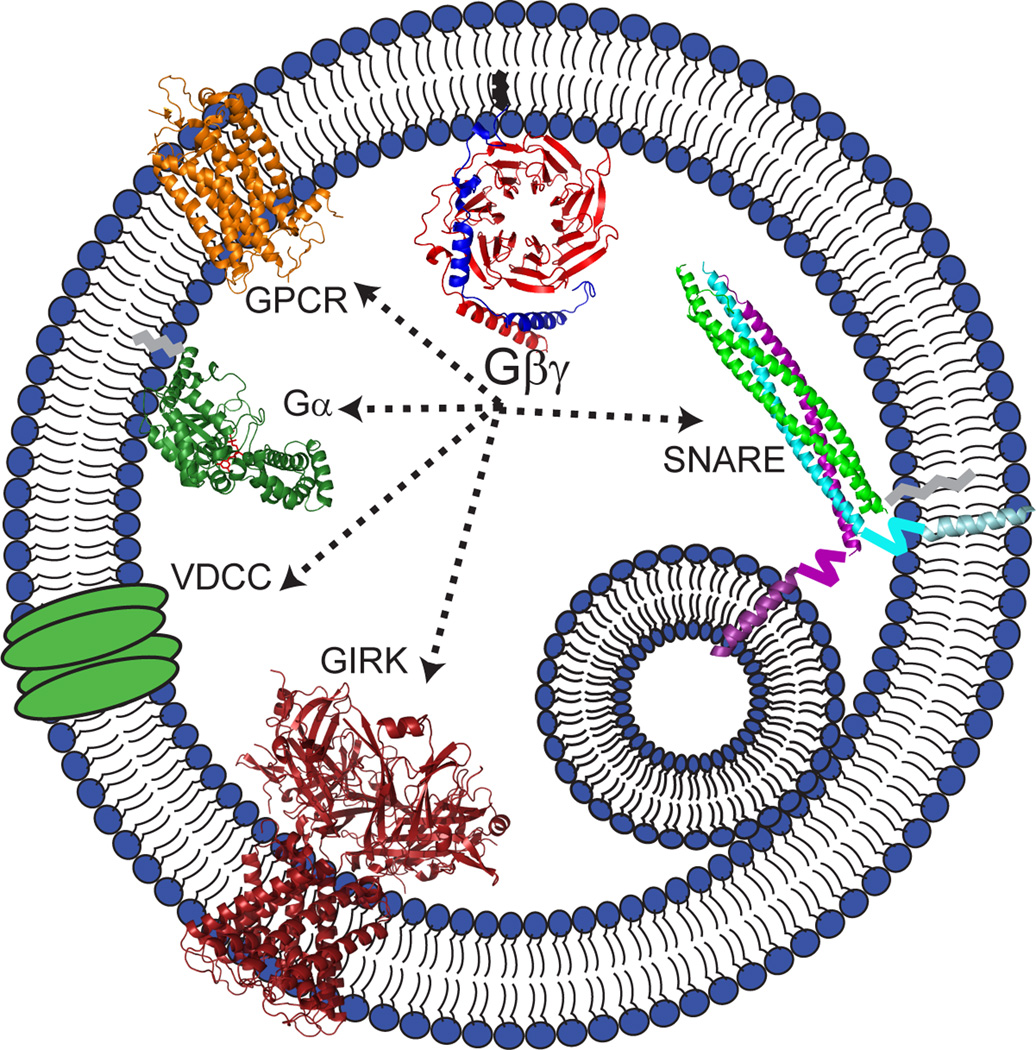
Figure 3. The interactions with Gβγ during exocytosis.
Gβγ subunits are known to have a variety of interactions during exocytosis - GPCRs and Gα subunits during its activation, as well as a number of effectors. Shown are the structures, if known, of those proteins that interact with Gβγ and play a role in exocytosis. THE GPCRs and Gα are discussed in Figure 2. The SNARE proteins (SNAP-25/Syntaxin 1A/VAMP2) form a trimeric complex through interaction of their α-helical SNARE motifs, SNAP-25 having 2 two of them (PDB ID: 1SFC)(Sutton et al., 1998). VAMP2 (purple) and syntaxin 1A (cyan) have transmembrane domains through which they interact with membranes. SNAP-25 (green) has a palmitoylation site near the C-terminal portion of its first SNARE motif by which it is tethered to the membrane. Not shown is the N-terminal helical domain of syntaxin 1A, Habc. The crystal structure of syntaxin 1A without the transmembrane domain confirms the triple helix Habc domain at its N-terminus. The GIRK channel is represented by the crystal structure for the structurally similar ATP-sensitive Kir channel 10 which is a tetramer with both transmembrane and intracellular domains (PDB ID: 2X6A) (Clarke et al., 2010). There is no structure determined yet for VDCC; it is shown as a simple transmembrane cartoon.
Although Gβγ-effector interaction sites were initially hypothesized to be localized to the Gα interface, this is not the only site important for effector regulation. Mutagenesis studies have since identified binding regions within the blades of the Gβ propeller and along its N-terminal coiled-coil, as well as areas outside of the Gα subunit interface, and regions on Gγ subunits (Clapham and Neer, 1997; Myung et al., 2006; Panchenko et al., 1998; Peng et al., 2003; Smrcka, 2008). An example of this is the interaction between Gβγ and the GIRK channels. Zhao et al. (2003) demonstrated that mutation of residues Thr-86, Thr-87, and Gly-131 on Gβ1, which are outside of the Gα binding site, resulted in decreased GIRK activity (Zhao et al., 2003). As such, while the Gα interface likely represents a core site for effector binding being that it is primarily exposed when a GPCR has activated the Gα subunit, other regions may also play important roles in facilitating downstream signaling for Gβγ subunits.
3.5 Mechanisms of Presynaptic Regulation By Heterotrimeric G proteins
GPCRs have been shown to play an important role in controlling synaptic transmission, as activation of presynaptic Gi/o-coupled auto- and heteroreceptors such as 5HT1 serotonin receptors, D2 dopamine receptors, M4 muscarinic receptors, α2A adrenergic receptors, opioid receptors, as well as many others is known to inhibit evoked transmitter release (Stephens and Mochida, 2005; Stephens, 2009). Such regulation is attributed to the actions of both the α and βγ subunits of heterotrimeric G proteins. Activation of Gα subunits leads to diffusible second messengers that can have effects at a distance from the site of activation. The Gα subunits of Gs and Gq have facilitatory roles on exocytosis through activation of adenylyl cyclase and phospholipase C leading to second messengers that activate downstream kinases (protein kinase A, by increasing cyclic adenosine monophosphate levels by Gαs activation of adenylyl cyclase, and protein kinase C, by Gαq activation of phospholipase Cβ). The phosphorylation of proteins involved in vesicle recruitment, docking, and fusion can cause both inhibition or facilitation of synaptic transmission (Brown and Sihra, 2008). Activation of presynaptic Gαq has been described to play a role in slow voltage-independent inhibition (Delmas et al., 1998; Haley et al., 2000) through activation of phospholipase C and reduction of the second messenger phosphatidylinositol-4, 5-bisphosphate (PIP2) (Gamper et al., 2004). Other regulatory pathways downstream of GPCRs include arrestin signaling, which can also affect second messenger systems (DeFea, 2011). Arrestin signaling processes can affect, for example, scaffolding of phosphodiesterase close to Gs-coupled receptors (Lynch et al., 2005).
The activation of presynaptic GPCRs also leads to dissociation of Gβγ subunits whose actions remain local as they are membrane-associated via Gγ C-terminal prenylation. Autoreceptors sensing neurotransmitter released from the activated synapse, or heteroreceptors sensing neurotransmitters from neighboring nerve terminals, activate Gi/o receptors, and release Gβγ. Obviously, the effects of neurotransmitters depend not only on the pharmacology of the receptors, but also on the spatial arrangement in relation to the sites of transmitter release. Some presynaptic GPCRs are found distant from the active zone (Shigemoto et al., 1997), and thus are not likely to affect active zone processes, but most probably work by traditional mechanisms of second messenger generation. Those presynaptic Gi/o-coupled GPCRs that are localized to the active zone or peri-active zone region (Corti et al., 2002) are likely to generate Gβγ subunits that can diffuse to the exocytotic machinery and mediate fast regulation of exocytosis (Blackmer et al., 2005; Gerachshenko et al., 2005). The detailed localization of GPCRs must be done by electron microscopic immunocytochemistry, and though there are perhaps hundreds of presynaptic receptors, fine localization is limited to a very few subtypes, (see Table I). Further work will be needed to determine subcellular localization of other Gi/o-coupled presynaptic receptors. On the other hand, there is much more information on the ability of presynaptic GPCRs to influence postsynaptic responses in paired recordings. Whether they work by modifying voltage-gated Ca2+ channels, or downstream of Ca2+ entry directly on the exocytotic machinery is known in some cases via studies of the effects of blocking various voltage-dependent calcium channels or inhibition of “minis” (see Table 1).
Table 1. Inhibitory GPCR function at synapses and cell bodies in the central nervous system.
Listed are examples of Gi/o-coupled GPCRs known to inhibit synaptic transmission via actions on voltage-dependent calcium channels or exocytotic machinery downstream of calcium entry. Receptors are separated depending on localization to the synapse or soma of neurons in the central nervous system.
| Synapse | Soma | ||
|---|---|---|---|
| VDCC | Exocytotic fusion apparatus | VDCC | |
| M2/M4 Ach receptor | (Higley et al., 2009) | (Bellingham and Berger, 1996; Scanziani et al., 1995) | (Allen and Brown, 1993; Delmas et al., 1998) |
| Group II mGlu receptor | (Capogna, 2004; Glitsch, 2006; Scanziani et al., 1995) | ||
| Group III mGlu receptor | (Giustizieri et al., 2005) | (Gereau and Conn, 1995) | |
| GABAB receptor | (Dittman and Regehr, 1996; Isaacson, 1998; Wu and Saggau, 1995) | (Dittman and Regehr, 1996; Harvey and Stephens, 2004; Wu and Saggau, 1995) | |
| μ opioid receptor | (Heinke et al., 2011) | (Heinke et al., 2011) | (Wilding et al., 1995) |
| CB1 cannabinoid receptor | (Brown et al., 2004) | ||
| A1 adenosine receptor | (Dittman and Regehr, 1996; Scholz and Miller, 1992; Umemiya and Berger, 1994) | (Scholz and Miller, 1992) | (Umemiya and Berger, 1994) |
| D2 dopamine receptor | (Hamilton and Smith, 1991; Higley and Sabatini, 2010) | (Nicola and Malenka, 1997) | (Cabrera-Vera et al., 2004) |
| α2 adrenergic receptor | (Boehm, 1999) | (Delaney et al., 2007) | (Boehm, 1999) |
Presynaptic Gβγ modulation of synaptic transmission occurs mainly through three mechanisms: inhibition of voltage-dependent calcium channels at presynaptic nerve terminals, regulation downstream of calcium entry via direct interaction with of one or more of the components of the exocytotic machinery (Miller, 1998), or regulation of GIRK channels that affect the shape of the invading action potential (Fernandez-Alacid et al., 2009). Such diversity highlights the physiological importance of G proteins in regulating synaptic transmission but also raises numerous questions about which mechanism functions at particular synapses, the relevance of each mechanism in vivo, and the contribution of each mechanism to modulation. While we attempt to highlight some of the key findings for each mechanism below, further work is needed to clarify these questions and assess the importance of each at a systems level.
3.5.1 Gβγ Regulation of Presynaptic Voltage-Dependent Calcium Channels
As mentioned, GPCR mediated regulation of synaptic transmission has been shown to occur through multiple mechanisms, the best characterized of which is through direct inhibition of VDCC. These channels play an essential role in regulating vesicle exocytosis as they control calcium influx into the cell. As the presynaptic membrane is depolarized, VDCC become activated resulting in an influx of calcium into the cell which can then bind to synaptotagmin to trigger exocytotic events. At present, five classes of VDCC have been identified in mammals based on their pharmacological and electrophysiological properties, with further delineation of subtypes into low-voltage (T-type) and high-voltage (N-, P/Q-, and R-type) activated channels based on the strength of depolarization necessary to trigger them (Catterall et al., 2005). The core of the calcium channel complex which forms the channel is the α1 subunit. This subunit has 4 transmembrane subdomains and is believed to organize and form the pore. Other subunits that have been determined to associate with and modulate the calcium channel are an intracellular β subunit and a α2δ subunit complex. Lastly, there is a γ subunit with variable tissue expression that also is part of some calcium channels (Catterall et al., 2005). Gβγ can directly bind to N-and P/Q-type calcium channels (Currie, 2010; Herlitze et al., 1996; Ikeda, 1996; Tedford et al., 2006; Tedford and Zamponi, 2006). Examination of the structural determinants which underlie this modulation have identified regions within the N-terminal loop (Canti et al., 1999), the I–II loop (Herlitze et al., 1997; Zamponi et al., 1997), and the C-terminal loop (Furukawa et al., 1998; Li et al., 2004; Qin et al., 1997) of the pore-forming α1-subunit of the calcium channels which are important for Gβγ modulation. Within the I–II loop, there are two regions that have been implicated in binding to the Gβγ dimer. The first overlaps with the α-interaction domain (AID) and contains the consensus sequence QXXER (De Waard et al., 1997; Herlitze et al., 1997; Zamponi et al., 1997). A second domain within the I–II is located C-terminal to the AID, and is termed the G protein interaction domain (Zamponi et al., 1997). Although still subject to a great deal of study, it’s believed that Gβγ binding actually stabilizes the channel in a conformational state which permits only reluctant opening, thus preventing vesicle exocytosis (Bean, 1989; Boland and Bean, 1993; Elmslie, 1992; Golard and Siegelbaum, 1993; Jarvis et al., 2000; Kasai and Aosaki, 1989).
G protein inhibition of inward calcium currents was initially demonstrated by Dunlap and Fischbach (1978) when they were able to show that the contribution of calcium channels to action potentials in chick dorsal root ganglion neurons was reduced following activation of gamma-aminobutyric acid (GABA)B, serotonin, or adrenergic receptors (Dunlap and Fischbach, 1978). Subsequent studies over the next 20 years have implicated primarily Gi/o-coupled GPCRs in this regulation as muscarinic, opioid, dopamine, and many other Gi/o-coupled receptors all have the propensity to exert negative control over calcium currents and exhibit sensitivity to pertussis toxin (Bean, 1989; Boland and Bean, 1993; Elmslie, 1992; Golard and Siegelbaum, 1993; Kasai and Aosaki, 1989). However, it should be noted that modulation of VDCC may not occur solely through the actions of Gi/o-coupled GPCRs as activation of Gs and Gz-coupled G proteins has also been shown to mediate voltage-dependent inhibition of N-type calcium channels (Zhu and Ikeda, 1994). Whether this reflects a ubiquitous mechanism of regulation remains unclear, however, it is apparent that inhibition of calcium channels by GPCRs is widespread and an important regulatory factor mediating control of neurotransmitter release.
In addition to Gβγ regulation of VDCC, there is evidence of syntaxin1A regulation of the channels themselves, as well as an intersection between VDCC, syntaxin, and Gβγ. Syntaxin1A was initially found to be associated with N-type calcium channels in a pre-fusion complex (Bennett et al., 1992; Leveque et al., 1994; O'Connor et al., 1993), however, later studies confirmed the molecular and functional interactions between these proteins (Bezprozvanny et al., 2000; Sheng et al., 1994). Further, in addition to the previously mentioned studies of Gβγ regulation of VDCC above, there is evidence of syntaxin1A promoting inhibition of VDCC by Gβγ (Jarvis et al., 2000; Jarvis and Zamponi, 2001) and that this may occur through Gβγ and VDCC binding to separate domains of syntaxin1A (Jarvis et al., 2002).
3.5.2 Gβγ Regulation of G protein-Activated Inward Rectifying (GIRK) Potassium Channels
GIRK channels are largely believed to be expressed somatodendritically (Fernandez-Alacid et al., 2009; Kulik et al., 2006; Luscher et al., 1997) where they act to hyperpolarize the cell membrane, thereby making it harder to reach the threshold for initiation of an action potential (Luscher et al., 1997; Luscher and Slesinger, 2010). They are members of the inwardly rectifying potassium channel (Kir) superfamily of proteins and are abundantly distributed in atrial cells and neurons where they function to regulate heart rate and neuronal excitability (Clapham and Neer, 1997; Ikeda et al., 2003; Yokogawa et al., 2011). Upon activation, they facilitate efflux of potassium ions out of the cell, hyperpolarizing the cell membrane and dampening electrical activity by making it more difficult to elicit an action potential (Finley et al., 2004). To date, four mammalian GIRK subunits (GIRK 1–4) have been identified which assemble to form functional channels although studies have shown that generally only heterotetramers composed of GIRK 1/2 and to a lesser extent GIRK 2/3 as well as GIRK 2 homotetramers are expressed in the brain (Dascal, 1997; Finley et al., 2004; Peng et al., 2003; Sadja and Reuveny, 2009).
Unique to the Kir family, however, the activity of postsynaptic GIRK channels is regulated through activation of Gi/o-coupled GPCRs such as opioid, M2 muscarinic, α2 adrenergic, 5-HT1a serotonin, and GABAB receptors (Ikeda et al., 2003; Ponce et al., 1996; Sadja et al., 2003), with activation occurring as a result of direct interactions of Gβγ subunits with the N and C-termini (Huang et al., 1995; Logothetis et al., 1987; Sadja and Reuveny, 2009). As with many Gβγ effectors, regulation of GIRK channels was initially proposed to occur at sites along the Gα-Gβγ interface. Studies by Ford et al. (Ford et al., 1998) and Albsoul-Younes et al. (Albsoul-Younes et al., 2001) demonstrated that eight residues falling within this region (Leu55, Lys78, Ile80, Lys89, Trp99, Asp228, Asp246 and Trp332) were important for channel activation. Subsequent work went on to identify additional sites of interaction with Absoul-Younes et al. first showing that mutation of residues along or near the loops connecting blades 1 and 2 of the Gβ torus was sufficient to disrupt GIRK channel activation. Subsequent work by Mirshahi et al. (Mirshahi et al., 2002) and Zhao et al. (Zhao et al., 2003) narrowed this to five specific residues within these loops (Ser67, Thr86, Thr87, Thr128, and Gly131) as playing a crucial role in GIRK activation, while Li et al. (Li et al., 2005) recently reported the ability of the C-terminal region of Gβ2 (residues 271–305) to induce GIRK channel activation. It should be noted, however, that in addition to Gβ, Gγ has been shown to be essential for G protein activation of GIRK channels. A recent study by Peng et al. demonstrated that residues 35–71 of Gγ2 are required for full stimulation of GIRK4 currents by Gβ1γ2 (Peng et al., 2003). Further, within this region, ten residues form an intricate network of interactions with Gβ to determine the stimulatory effects on the channel while Asp36 and Asp48 maintain the conformation of the Gβ1γ2 subunits to allow full stimulation of GIRK4. Together, these results highlight the fact that Gβγ regulation of GIRK channels may be much more complicated than originally believed and illustrate the need for further research in this area.
Studies examining specificity of GIRK channel regulation have further shown that only Gβγ dimers containing Gβ1–4 could enhance GIRK channel activity while those containing Gβ5 subunits actually suppress it (Lei et al., 2000; Lei et al., 2001; Reuveny et al., 1994; Takao et al., 1994; Wickman et al., 1994; Yamada et al., 1994). While the exact reason for this discrepancy is still being elucidated, a plausible explanation presented by Lei et al. is that Gβ5 containing dimers actually displace activating Gβγ pairs by competitively binding to the same regions on the cytoplasmic domains of the GIRK channels (Lei et al., 2003). Although no hypothesis was put forth to explain how this would prevent channel opening, given that Gβ5 is the most divergent of the Gβ subunits, it is possible that while sufficient sequence identity exists between the isoforms to permit binding to cytoplasmic domains, critical residues necessary for channel activation may be missing. As such, by competitively displacing activating Gβγ dimers, Gβ5 pairs could occupy binding sites and prevent channel activation. Conversely, however, Xie et al. (2010) recently showed that Gβ5 subunits in complex with members of the Regulator of G protein Signaling (RGS) 7 family bound to the C-terminal cytoplasmic domains of GIRK channels in order to facilitate their coupling to GABAB receptors (Xie et al., 2010). In doing so, they found that the Gβ5/RGS complex acted to influence the sensitivity of the inhibitory signaling pathway in hippocampus and that further, the absence of the Gβ5 subunits disrupted the temporal fidelity of the GIRK response. Such discrepancies highlight that the role of Gβ5 subunits in regulating potassium currents merits further investigation.
While a postsynaptic role for GIRK channels has been recognized in many studies, consistent presynaptic labeling of these channels via immunostaining and electron microscopy in the proximity of the active zone also hints for a role in modulating exocytotic release (Fernandez-Alacid et al., 2009; Ladera et al., 2008; Lujan et al., 2009; Michaeli and Yaka, 2010; Ponce et al., 1996). At least in the case of GABA signaling, there is evidence to support this assumption as recent reports from Ladera et al. (2008) as well as Michaeli and Yaka (2010) suggest new mechanisms in which presynaptic GIRK channels play a significant role. For example, Ladera et al. (2008) demonstrated via electron microscopy that GIRK channels and GABAB receptors were co-expressed in a restricted subset of cerebrocortical nerve terminals and that further, these GIRK channels mediated the inhibition of glutamate release. Similarly, Michaeli and Yaka (2010) recently proposed a new mechanism for dopamine-induced inhibition of GABAA neurotransmission whereby activation of D2-like receptors is thought to lead to activation of presynaptic GIRK channels in ventral tegmental neurons (Michaeli and Yaka, 2010). As opposed to postsynaptic GIRK regulation, however, such effects may not be mediated through Gβγ subunits. In case of the GABAB receptor-GIRK pathway in cerebrocortical nerve terminals, responses exhibited resistance to pertussis toxin, whereas in the case of GABAA currents in the ventral tegmental area, the authors propose regulation through protein kinase A substrates given attenuation of effects with protein kinase A inhibitors. It remains to be elucidated whether this represents regulation unique to presynaptic GIRK channels in addition to the well characterized regulation by Gβγ subunits.
Although a great deal of work has been done to decipher the mechanism by which Gβγ subunits regulate activation of GIRK channels and thus synaptic transmission, many questions remain unanswered. Given the diversity of binding sites on both the channel and the G protein, it is possible that multiple dimers bind to the channel at the same time to elicit the rotation and expansion of the N- and C-termini required for GIRK channel activation (Sadja and Reuveny, 2009). How this may occur and how it would affect synaptic regulation remains to be seen but such a possibility certainly highlights that a great deal of work still remains.
3.6 Modulation of Synaptic Transmission Downstream of Ion Channels: Gβγ Modulation of SNARE Proteins
While G protein modulation of calcium and potassium entry is well established, more recently, modulation of synaptic transmission distal to calcium entry has been demonstrated. G protein modulation of vesicle release in a manner independent of calcium entry via calcium channels was first noted by Silinsky in 1984, who observed that evoked acetylcholine release at the frog neuromuscular junction could be reduced following activation of presynaptic adenosine A1 receptors (Silinsky, 1984). He suggested that this inhibition was exerted at an intracellular site associated with the exocytotic process, such as the synaptic vesicles or the active zone, as miniature end plate potentials could still be reduced when the normal route of calcium entry was bypassed (Miller, 1998; Silinsky, 1984). These observations have been corroborated over the years as studies investigating receptor function in the hippocampus and other brain regions have indicated that activation of Gi/o-coupled presynaptic receptors such as such as GABAB, muscarinic, metabotropic glutamate (mGluR), serotonergic, and opioid receptors all result in a reduction in the frequency of miniature excitatory post-synaptic currents (Miller, 1998). Similarly, the use of agents such as ionomycin and α-latrotoxin which enhanced secretion while bypassing calcium channels lent further support to this conclusion as GPCR-mediated inhibition was maintained in permeabilised cells (Blackmer et al., 2005; Meldolesi et al., 1984). Taken together, these results suggested the presence of a novel mechanism controlling vesicle release which needed to be characterized.
Given that receptor-mediated inhibition can be mimicked by treatment with non-hydrolyzable GTP analogues (Ahnert-Hilger et al., 1987; Knight and Baker, 1985; Luini and De Matteis, 1990), a great deal of work went into identifying the G protein responsible for mediating the effect. This was initially challenging due to an inability of exogenous peptides to access presynaptic terminals but was overcome by direct injection of Gβγ subunits into the Calyx of Held (Kajikawa et al., 2001) and lamprey reticulospinal-motor neuron synapses (Blackmer et al., 2001). In the latter case, Gβγ subunits not only were able to inhibit evoked transmitter release, but did so without an effect on presynaptic calcium entry (Blackmer et al., 2001) suggesting that these subunits were responsible for the observed inhibition. Subsequent injection of a Gβγ scavenger, G protein-coupled receptor kinase 2 (GRK2) (Blackmer et al., 2001) or phosducin (Blackmer et al., 2005) supported this, as the inhibitory actions of Gβγ were completely alleviated. Investigations into the mechanism by which Gβγ inhibited vesicle exocytosis initially focused on known effectors in Gβγ signaling cascades. However, as blockade of classical effectors did little to attenuate Gβγ-mediated inhibition (Blackmer et al., 2001) and the onset of inhibition due to laser uncaging of 5HT was too rapid to affect docking and priming mechanisms (Gerachshenko et al., 2005), it was proposed that Gβγ may act directly upon the already-primed exocytotic machinery. In fact, inhibition was already initiated by 20 ms after uncaging 5HT, suggesting that both the GPCR and the Gβγ were close to the active zone. Interestingly, the ability of Gβγ to modulate the inhibitory effect on exocytosis was abolished by treatment with botulinum toxin A, which cleaves 9 amino acids from the C-terminus of SNAP-25 (Binz et al., 1994; Gerachshenko et al., 2005; Schiavo et al., 1993). The relevance of this C-terminal region of SNAP-25 was additionally demonstrated as the C-terminal 14 amino acid peptide from SNAP-25 itself eliminated the Gβγ-mediated inhibition (Blackmer et al., 2005; Gerachshenko et al., 2005). In addition, removal of the C-terminal residues from SNAP-25 decreased binding to Gβγ in vitro (Yoon et al., 2007; Zhao et al., 2010). These findings provided a “fingerprint” for studies of the mechanism of inhibition of exocytosis downstream from modulation of voltage-dependent Ca2+ channels directly on the exocytotic fusion apparatus that later studies have taken advantage of (Delaney et al., 2007; Zhang et al., 2011; Zhao et al., 2010).
In vitro binding studies also demonstrated direct interaction between Gβγ subunits and syntaxin 1A, SNAP-25B and VAMP/synaptobrevin that make up the SNARE complex (Blackmer et al., 2001; Blackmer et al., 2005; Yoon et al., 2007) while subsequent electrophysiological studies, fluorescence-based assays, and PC12 “cracked cell”-based amperometry studies provided evidence of Gβγ binding to the H3 SNARE domain of syntaxin and confirmed the importance of the carboxy-terminal residues of SNAP-25 (Yoon et al., 2007; Zhao et al., 2010). Additional sites of interaction may be important including the Habc domain (Yoon et al., 2007) or even the C-terminal transmembrane domain of syntaxin which has been suggested to potentially form a binding pocket for anesthetic molecules (Herring et al., 2011). Similarly, mutagenesis studies suggest SNARE binding sites on Gβγ subunits may reside outside the Gα binding face, the site of interaction with many Gβγ effectors (Ford et al., 1998). Blackmer et al. (2005) demonstrated that mutation of residues that contact Gα subunits, as well as numerous other effectors such as adenylyl cyclase, GRK2, and phospholipase C β2, did not induce a loss of function in the ability of the Gβγ subunit to inhibit calcium-triggered exocytosis. Rather, several mutations demonstrated increased potency for inhibiting secretion, suggesting further studies are necessary to characterize the binding surface required for Gβγ-SNARE interactions.
Regardless of the site at which Gβγ binds to SNARE proteins, in doing so, it is thought to prevent the tight zippering of the SNARE complex necessary to drive membrane fusion, and thus can inhibit vesicle exocytosis. However, the H3 domain and the C-terminus of SNAP-25 are also known to constitute functionally important calcium-dependent synaptotagmin binding sites, and detailed examination has revealed that Gβγ subunits and synaptotagmin actually compete with one another for binding to the SNARE proteins as calcium levels increase; at low intracellular calcium, Gβγ binding predominates, while after increasing intracellular calcium, synaptotagmin binding actually competes with Gβγ (Yoon et al., 2007). Although the significance of such competition is as yet unknown, the current working hypothesis is that Gβγ inhibits exocytosis by competing with synaptotagmin for binding to the SNARE proteins. Increasing intracellular calcium due to repetitive stimulation of neurons may serve to attenuate this by increasing synaptotagmin’s affinity for the SNARE proteins and allowing it to displace Gβγ subunits from the exocytotic machinery. Thus, depending on presynaptic Gi/o-coupled GPCR activation and calcium influx in response to synaptic activity, it is possible that Gβγ-mediated regulation of synaptic transmission could occur independently through more than one mechanism, or potentially even be additive or synergistic at a particular synapse (see Figure 4). It should be noted, however, that regardless of whether G protein modulation occurs via calcium currents or not, intact SNARE proteins have been shown in each instance to be required for the effect to be seen (Searl and Silinsky, 2005; Silinsky, 2008; Stanley and Mirotznik, 1997). Furthermore, synaptotagmin or another calcium sensor in addition to intact SNARE was important for G protein modulation of calcium dependent miniature endplate potentials, but not calcium independent miniature endplate potentials (Searl and Silinsky, 2005).
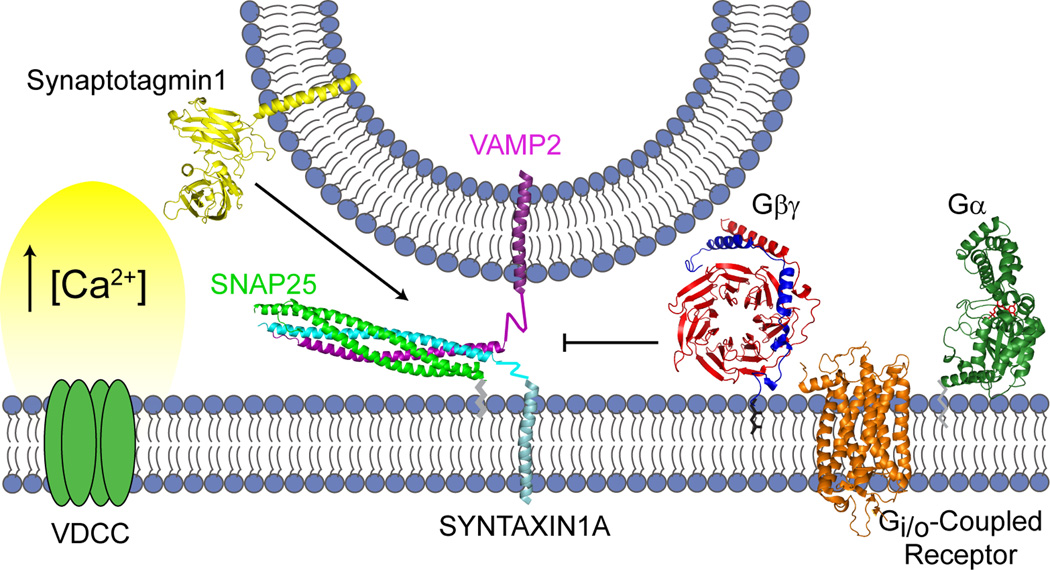
Figure 4. Hypothesis of Gβγ regulation of presynaptic vesicle release.
Synaptic vesicles are primed by a tethering interaction between VAMP2 on the vesicle and the SNAP-25/syntaxin 1A dimer at the plasma membrane. At low intracellular concentrations of calcium, activation of Gi/o-coupled receptors results in release of Gβγ that will bind to the SNARE proteins and prevent binding of synaptotagmin. However, high enough intracellular calcium concentrations, such as with repetitive neuronal stimulation, synaptotagmin is able to compete with Gβγ for binding to SNARE, and thereby promote fusion of the vesicles with the plasma membrane. Figure adapted from Wells et al. (Wells et al., 2011).
While the implications for such a graded physiological mechanism are profound, currently little is known about the occurrence or relevance of this mechanism in vivo. Studies examining rhythmic motor activities in vertebrate species, have suggested a role for Gβγ interactions in modulating fictive locomotion, which drives the coordinated contraction of muscles necessary for behaviors such as swimming. In their 2005 paper, Schwartz et al., demonstrated that inhibition of glutamate release via activation of 5HT1D receptors prolonged the frequency of ventral root bursting in lamprey reticulospinal neurons by presynaptically inhibiting synaptic transmission (Schwartz et al., 2005). In doing so, 5-HT was shown to slow the rhythm of fictive locomotion (Gabriel et al., 2009; Gerachshenko et al., 2009; Photowala et al., 2006; Schwartz et al., 2005). As extensive evidence previously demonstrated a role for Gβγ/SNARE interactions in the lamprey during serotonergic modulation, the authors suggest that 5-HT mediates its effects through activation of presynaptic receptors to inhibit transmitter release and decrease glutamatergic synaptic drive (Schwartz et al., 2005). This mechanism was elucidated further in 2009, when Gerachshenko et al. demonstrated that 5-HT1D receptor-mediated reductions in glutamate release acted to modulate locomotor rhythms (Gerachshenko et al., 2009). During physiologically relevant trains of activity similar to those that evoke locomotion, the authors were able to show that 5-HT/Gβγ-mediated inhibition is relieved over time, consistent with the idea that rising presynaptic calcium concentrations permit synaptotagmin competition with Gβγ (Gerachshenko et al., 2009). As a result, frequency-dependent increases in synaptic concentrations of glutamate are observed which serve to reestablish synaptic vesicle release (Gerachshenko et al., 2009). Modulation of locomotor patterns and motor outputs by presynaptic GPCRs has further been explored in other vertebrate species including Xenopus (Sillar et al., 1992), neonatal rats (Liu and Jordan, 2005), and zebrafish (Gabriel et al., 2009). For example, Gabriel et al. (2009), show that 5-HT is endogenously released during fictive locomotion in juvenile and adult zebrafish and acts to potentiate inhibitory synaptic transmission resulting in a decrease in the locomotor frequency, similar to that seen in lamprey (Gabriel et al., 2009). While the exact mechanism is not elucidated in their papers, it is conceivable that much like what is seen in lamprey, release of Gβγ subunits modulates SNARE function to limit frequency and duration of synaptic vesicle release and thus alter locomotor behaviors.
While such effects in lower vertebrate species is interesting, extrapolating these results to mammals is difficult, particularly as very few of the mechanisms by which Gi/o-coupled GPCRs modulate synaptic transmission in humans have been fully explored; further, those that have primarily point to activated Gβγ modifying channel properties. Some recent studies have begun to counteract this, giving evidence for the relevance of the Gβγ/SNARE mechanism in a variety of systems. For example, insulin secretion from pancreatic β cells has been shown to be reduced following activation of GPCRs in a number of ways, the most powerful of which is a distal inhibitory effect occurring downstream of increased intracellular calcium (Komatsu et al., 1995). Zhao et al., demonstrated in 2010 that the ability of noradrenaline to mediate this inhibition was due to a reduction in the number of exocytotic events without changes in vesicle size or fusion pore properties (Zhao et al., 2010). Further efforts attributed these effects to Gβγ subunits as the βγ-activating peptide, mSIRK, inhibited exocytosis to a similar extent as noradrenaline, while anti-Gβ antibodies were able to eliminate it (Zhao et al., 2010). Further, the authors went on to suggest that such modulation occurs via Gβγ binding directly to SNARE proteins, as pretreatment with botulinum toxin A, which cleaves the C-terminal 9 amino acids of SNAP-25, a functionally important Gβγ binding site (Yoon et al., 2007), was able to prevent noradrenaline from inhibiting exocytosis. Finally when the duration of calcium influx was extended via longer depolarizing pulses, the authors were able to show that both noradrenaline-induced and mSIRK-mediated inhibition was abolished, consistent with the idea that synaptotagmin is able to successfully compete with Gβγ for binding to SNAP-25 (Blackmer et al., 2005; Gerachshenko et al., 2005; Zhao et al., 2010). While the relative importance of this mechanism in vivo has yet to be fully elucidated, it is conceivable that it may play an important modulatory role which is disrupted in diabetic patients. As such, further efforts into understanding its role may provide insight into disease progression and highlight new avenues of treatment.
Such actions also appear to exist in the central nervous system as Delaney et al. (2007) recently provided evidence to support the idea that the nociceptive actions of noradrenaline may be mediated through Gβγ/SNARE interactions in the central nucleus of the amygdala (Delaney et al., 2007). This region receives dense ascending noradrenergic projections and exhibits high levels of α2a adrenergic receptors, stimulation of which modulates nociceptive information into the amygdala via a presynaptic mechanism (Delaney et al., 2007). Using whole-cell recording techniques, the authors were able to show that both noradrenaline and clonidine dose-dependently inhibited parabranchial excitatory input into the central amygdala, an effect which was reversed through application of the selective α2-adrenoceptor antagonist, yohimbine (Delaney et al., 2007). Using strontium, they went on to demonstrate that the effect occurred via a presynaptic mechanism while application of pertussis toxin or the sulphydryl alkylating agent N-ethylmaleimide both abolished inhibition, confirming a Gi/o-mediated effect (Delaney et al., 2007). Such actions were further attributed to Gβγ subunits as application of a Gβγ binding peptide blocked the effect of noradrenaline, while examination of changes in the action potential-driven rise in terminal free calcium levels confirmed the effect was downstream of calcium entry (Delaney et al., 2007). Finally, direct interactions with SNARE proteins were confirmed through incubation with botulinum toxin A, yielding similar results to previously published reports (Blackmer et al., 2005; Gerachshenko et al., 2005; Yoon et al., 2007) whereby noradrenergic inhibition was reduced with increasing incubation times (Delaney et al., 2007). Similarly, Iremonger and Bains (2009) demonstrated that dynorphin released from dendritic vesicles in response to postsynaptic activity acted in a retrograde manner to inhibit excitatory neurotransmission through a presynaptic mechanism downstream of calcium entry in the hypothalamus. Using whole-cell patch recordings from magnocellular neurosecretory cells in the paraventricular nuclei of the hypothalamus, the authors showed that dynorphin produced a robust inhibition of glutamate release onto vasopressin-expressing magnocellular neurosecretory cells via activation of presynaptic κ-opioid receptors. Further, they showed that this effect did not require inhibition of the adenylyl cyclase/protein kinase A pathway, and that it persisted despite inhibition of N- or P/Q-type calcium channels. Rather, the authors suggest that activation of presynaptic κ-opioid receptors directly modulates glutamate release through direct interaction of the Gβγ subunits with the exocytotic machinery, given that such inhibition could be alleviated through rising intracellular calcium, consistent with competition for binding to SNARE proteins by synaptotagmin (Iremonger and Bains, 2009).
While these examples support the idea that Gβγ/SNARE interactions play important roles in transient presynaptic inhibition, the question remains as to whether they play a role in long-term alterations of presynaptic signals associated with long-term potentiation (LTP) or long-term depression (LTD). Activity dependent, long-term changes in synaptic strength associated with LTP and LTD play important roles in CNS functions such as learning and memory, development of neural networks, and fine-tuning of synaptic connections (Zhang et al., 2011). GPCRs have been shown to be central to induction of LTD, but until recently it was believed that this process was primarily mediated by Gαi subunits. This was recently questioned as Zhang et al. sought to investigate whether Gβγ released along with Gαi may also be necessary for the transition from transient inhibition to presynaptic LTD in the hippocampus (Zhang et al., 2011). Using botulinum toxin-A, the authors showed that the C-terminus of SNAP-25 is critical for LTD but not LTP at Schaffer collateral-CA1 synapses. Cleavage of residues 198–206 on SNAP-25 completely prevented the induction of stimulus-evoked LTD, but alterations in extracellular calcium concentrations demonstrated that this reduction by botulinum toxin-A was not simply due to a reduction in vesicle release probabilities (Zhang et al., 2011). To examine the possibility that binding of Gβγ to SNAP-25 was a necessary step in mediating the presynaptic component of LTD, the authors then went on to electroporate a 14 amino acid peptide of the C-terminus of SNAP-25 into presynaptic CA3 pyramidal neurons as this peptide had previously been shown to scavenge free Gβγ (Gerachshenko et al., 2005). Compared to a scrambled peptide, infusion of the SNAP-25 peptide significantly reduced mGluR-mediated presynaptic depression and stimulus-evoked LTD. Further, infusion of a Gβγ binding peptide, mSIRK, which similarly blocked induction of LTD, suggested that scavenging of Gβγ at Schaffer collateral release sites could reverse GPCR mediated reductions in transmitter release (Zhang et al., 2011). Finally, imaging of calcium concentrations showed that presynaptic calcium influx was not significantly altered following cleavage of SNAP-25, suggesting that presynaptic LTD is not a result of long-term reductions in calcium concentrations (Zhang et al., 2011). Taken together, such data led the authors to conclude that Gβγ released along with Gαi may play an important role in mediating long term changes at presynaptic release sites and postulate that a greater understanding of such molecular mechanisms will be critical to evaluating the role that presynaptic long-term plasticity plays in persistent changes associated with learning and memory (Zhang et al., 2011).
4 Implications of G protein Modulation of Synaptic Transmission for Disease
As many Gi/o-coupled GPCRs act as feedback regulators for transmitter release from presynaptic terminals, a greater understanding of the mechanisms by which they operate will be important for understanding normal neural processing, but also because dysregulation of this process can have serious health consequences. For example, mutations in the promoter of the 5HT1a serotonin receptor results in abnormally high autoreceptor expression, decreased serotonin release, and increased susceptibility to depression (Le François et al., 2008). Patients known to carry a risk-associated α2a-adrenergic receptor (ADRA2A) polymorphism have been shown to express increased levels of presynaptic α2A-adrenergic receptors and reduced glucose-stimulated insulin release, resulting in higher incidences of diabetes (Rosengren et al., 2010). This effect was also seen in mice with restricted overexpression of ADRA2A in pancreatic islet cells (Devedjian et al., 2000). In Alzheimer’s patients, activation of M2 autoreceptors during anticholinesterase treatment has been suggested to actually contribute to disease progression by reducing acetylcholine release (Albrecht et al., 1999) while continuous D2 autoreceptor–mediated attenuation of dopamine release during dopamine agonist treatment is thought to contribute to the deterioration of cognitive function in Parkinson’s patients (Arnsten et al., 1994; Breitenstein et al., 2006). As such, these receptors represent major targets in drug development, with agonists such as risperidone (Janssen et al., 1988), buspirone (Taylor et al., 1985), and guanfacine (Hunt et al., 1995) targeting the D2 dopamine, 5HT1a serotonin, and α2a adrenergic receptors for the treatment of schizophrenia, anxiety disorders, and attention deficit hyperactivity disorder, respectively. In addition, a number of drugs at various stages in the development pipeline target this class of receptors, such as M4 muscarinic receptors for schizophrenia, Parkinson’s disease, psychosis and dystonia, H3 histamine receptors for cognitive deficits, sleep-wake disorders, and attention-deficit disorder, mGluR2 and 3 metabotropic glutamate receptors for schizophrenia and anxiety, and mGluR4 receptors for Parkinson’s disease and dystonia. Unfortunately, many current therapies exhibit significant side effects which limit their efficacy. For instance, risperidone is often associated with severe weight gain while guanfacine can induce orthostatic hypotension in patients. In some cases, these side effects may be due to off-target effects such as seen with risperidone where weight gain is thought to be mainly associated with an affinity of the molecule for serotonin 5HT2a receptors; in others, the effects may be because these receptors modulate differing functions in various brain regions. Regardless, these examples highlight the fact that targeting the GPCRs directly may mediate both the therapeutically desirable effect but also untoward side effects. As such, a better understanding of the downstream mechanisms by which presynaptic inhibitory GPCRs modulate exocytosis, such as through Gβγ/SNARE interactions, may reveal more disease-selective targets for therapeutic intervention.
5 Open Questions
A number of open questions remain regarding the role Gβγ-SNARE interactions play in regulating exocytosis. For example, what is the role of individual Gβγ isoforms in this process? Despite the 5 Gβ and 13 Gγ subunits sharing high levels of sequence identity (Downes and Gautam, 1999; Hildebrandt, 1997), it is known that pairs can exhibit functional selectivity in their interactions with both receptors and effectors (Dingus et al., 2005; Kleuss et al., 1993; Richardson and Robishaw, 1999). Unfortunately, little is known about the subcellular localization of particular isoforms to specific presynaptic terminals, nor which isoforms are activated by a particular receptor. Given that most cell types express multiple Gα, Gβ, and Gγ subtypes, how is specificity for one mechanism over another achieved? Does modulation of ion channels or SNARE proteins at a given synapse occur through activation of distinct Gβγ subunits or is regulation specified by a particular ligand or receptor? Further, do conditions exist which would allow switching between mechanisms for a particular synapse, and what will the functional consequences of such a situation be? In any case, a greater understanding of cellular distributions as well as Gβγ-receptor and Gβγ-effector interactions will be necessary to fully elucidate this process.
Similarly, the question arises as to how applicable Gβγ regulation of release is in other cell types which don’t involve the classical neuronal SNAREs of syntaxin 1a, SNAP-25, and synaptobrevin. Multiple isoforms of all three proteins exist, with SNAP-23 and SNAP-29 both known to be expressed in the brain and be important for exocytosis. Further, secretion is a common physiological event in many other cell types besides neurons. Some such as insulin secretion from pancreatic β cells is through the same SNARE proteins as neurons, so the extension is straightforward from neurons to β cells. Other systems such as platelets (Chen et al., 2000; Graham et al., 2009; Jardin et al., 2007; Polgar et al., 2002), endothelial cells (Predescu et al., 2005; Pulido et al., 2011; Yamakuchi et al., 2008), and immune cells such as neutrophils, eosinophils, macrophages, and mast cells (Stow et al., 2006) primarily use SNAP-23 instead of SNAP-25, as well as a variety of different syntaxin and VAMP isoforms as well. While there is a high degree of sequence similarity between the SNARE isoforms, and a preponderance of GPCR signaling in their regulation and activation, little is known at this point about whether Gβγ or activation of Gi/o-coupled receptors have a similar role in these systems as is seen in the brain.
Another unanswered question is what the full extent of Gβγ regulation on vesicle release is beyond competition with synaptotagmin 1. As reviewed above, there are other critical members of the vesicle docking/priming/fusion process that interact with the exocytotic machinery which could be affected by Gβγ interaction with SNARE proteins. Gβγ interaction with syntaxin 1A is not limited to the extreme C-terminus where synaptotagmin1 binds (Chapman et al., 1995; Yoon et al., 2007). An interaction with the N-terminal Habc domain may have implications with Munc18-syntaxin 1A interactions for example and thereby influence vesicle docking and/or priming. The ability of Gβγ to bind directly to other members of the exocytotic process has not been reported. Another facet of this question is a stoichiometric one. Does the interaction of one Gβγ dimer with a docked vesicle inhibit its fusion, or does each SNARE complex require a bound Gβγ? Furthermore, is there a gradation depending on the stoichiometry of SNARE complexes surrounding a given fusion pore of a vesicle? Based on the three-dimensional structure of a toroid shape of the Gβγ dimer and the rod like coiled-coil of four α-helices of the ternary SNARE complex, does a Gβγ dimer interact simultaneously with two juxtaposed SNARE complexes at a fusion pore? The interaction of Gβγ with the SNARE complexes may provide a unique ability to address these questions and to answer fundamental unknown mechanisms of exocytosis.
There are many proteins that have varied roles in exocytosis, and the implications of Gβγ regulation in their function is not fully understood. Dynamin, for example, is a GTPase protein that is important in both endocytosis, playing a role in forming synaptic endosomes, as well as exocytosis by its ability to regulate quantal size and to have a dual function in both kiss-and-run and full fusion modes in chromaffin cells (Chan et al., 2010). Further, dynamin has been shown to interact with Gβγ in vitro (Lin and Gilman, 1996) and in cultured neuroendocrine cells (Liu et al., 1997), and this interaction can inhibit it’s GTPase activity. Would Gβγ control quantal size by directly modulating the activity of dynamin at the exocytotic pore in addition to competing with synaptotagmin for binding to SNARE proteins? Another interesting protein is the cysteine string protein (CSP), a member of the heat shock protein 40 family that has implications in preventing presynaptic neurodegeneration (Schmitz and Fernández-Chacón, 2009), and recently has been shown to be a chaperone for SNAP-25 (Sharma et al., 2011). CSP can bind both Gβγ and Gα, and it acts as a nucleotide exchange factor for Gα exchanging GDP for GTP (Natochin et al., 2005). The binding of CSP by Gβγ has been shown to facilitate the ability of Gβγ to inhibit calcium channels (Miller et al., 2003). Do Gβγ-SNARE and Gβγ-CSP interactions intersect at the synapse to provide cross-regulation of exocytosis and its preservation through chaperoning SNAP-25? Additionally, two proteins already mentioned in this review that bind SNARE proteins are tomosyn and complexin. What role does Gβγ binding of SNARE proteins play in their function, or is there competition or synergy of their interactions? Lastly, this manuscript focused on the competition between synaptotagmin1 and Gβγ for SNARE protein binding, but could Gβγ interfere with other isoforms of synaptotagmins, or perhaps with doc2’s role in asynchronous release of synaptic vesicles?
Finally, the question remains as to the physiological relevance of Gβγ regulation to synaptic function. While it is known for example, that Gβγ can modulate both SNARE proteins and VDCC, it is as yet unclear what the relative contribution of either mechanism is to normal synaptic functions. The differential calcium requirement for these mechanisms provides for differential calcium-sensitivity which may complete a feedback loop, because Gβγ may control both Ca2+ influx through VDCC and access of the Ca2+ effector synaptotagmin to its site on the SNARE. Thus, depending on presynaptic Gi/o-coupled GPCR activation and Ca2+ influx in response to synaptic activity, a range of efficiencies of exocytosis potentially exists. Does such dynamic regulation exist, and if so, what role does it play in presynaptic integration and plasticity? Further, what are the implications for human health and disease if this process is disrupted?
6 Conclusion
GPCR mediated regulation of synaptic transmission is complex and transcends many different pathways. While a great deal has been learned to date, numerous questions still remain as to the mechanisms underlying this process in the brain as well as other systems. While interactions between Gβγ subunits and classical effectors is well established, newer mechanisms such as Gβγ-SNARE interactions highlight levels of intricacy as yet unknown. Further research into this novel protein-protein interaction will be essential for elucidating its role in regulating synaptic transmission, as well as its integration with other effectors. Only in doing so will it be possible to accurately describe normal synaptic functioning as well as develop strategies to correct situations where it has gone awry.
Highlights.
> This review focuses on the presynaptic regulation of neurotransmission.
> Heterotrimeric G protein signaling is an important part of that regulation.
> Gβγ has a novel means of regulating synaptic neurotransmission.
> This regulation may have a large impact on disease pathology and treatment.
> There are many open questions relating to G protein signaling and neurotransmission.
Acknowledgements
Research in the authors’ laboratory was supported by a grant from the National Institute of Health, R01 EY010291.
Abbreviations
- 5-HT
serotonin
- ADRA2A
α2a adrenergic receptor gene
- ATP
adenosine triphosphate
- CSP
cysteine string protein
- GABA
gamma-aminobutyric acid
- GIRK
G protein-coupled inward rectifier K+ channels
- GPCR
G protein coupled receptors
- GRK2
G protein-coupled receptor kinase 2
- Kir
inwardly rectifying potassium channel
- LTD
long-term depression
- LTP
long-term potentiation
- mGluR
metabotropic glutamate receptor
- MHD
Munc homology domain
- RGS
regulator of G protein signaling
- SM
Sec-1/Munc-18
- SNAP
synaptosome-associated protein
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- VAMP
vesicle-associated membrane protein
- VDCC
voltage-dependent calcium channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katherine M. Betke, Email: katherine.m.betke@Vanderbilt.Edu.
Christopher A. Wells, Email: christopher.wells@Vanderbilt.Edu.
References
- Ahnert-Hilger G, Brautigam M, Gratzl M. Ca2+-stimulated catecholamine release from alpha-toxin-permeabilized PC12 cells: biochemical evidence for exocytosis and its modulation by protein kinase C and G proteins. Biochemistry. 1987;26(24):7842–7848. doi: 10.1021/bi00398a046. [DOI] [PubMed] [Google Scholar]
- Albert PR, Robillard L. G protein specificity: traffic direction required. Cell. Signal. 2002;14(5):407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- Albrecht C, Bloss HG, Jackisch R, Feuerstein TJ. Evaluation of autoreceptor-mediated control of [3H]acetylcholine release in rat and human neocortex. Exp. Brain Res. 1999;128(3):383–389. doi: 10.1007/s002210050858. [DOI] [PubMed] [Google Scholar]
- Albsoul-Younes AM, Sternweis PM, Zhao P, Nakata H, Nakajima S, Nakajima Y, Kozasa T. Interaction sites of the G protein beta subunit with brain G protein-coupled inward rectifier K+ channel. J. Biol. Chem. 2001;276(16):12712–12717. doi: 10.1074/jbc.M011231200. [DOI] [PubMed] [Google Scholar]
- Allen TG, Brown DA. M2 muscarinic receptor-mediated inhibition of the Ca2+ current in rat magnocellular cholinergic basal forebrain neurones. J. Physiol. 1993;466:173–189. [PMC free article] [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila Unc-13 is essential for synaptic transmission. Nat. Neurosci. 1999;2(11):965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- Arnsten A, Cai J, Murphy B, Goldman-Rakic P. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116(2):143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400(6743):457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Becherer U, Rettig J. Vesicle pools, docking, priming, and release. Cell Tissue Res. 2006;326(2):393–407. doi: 10.1007/s00441-006-0243-z. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J. Neurophysiol. 1996;76(6):3758–3770. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Betty M, Harnish SW, Rhodes KJ, Cockett MI. Distribution of heterotrimeric G-protein beta and gamma subunits in the rat brain. Neuroscience. 1998;85(2):475–486. doi: 10.1016/s0306-4522(97)00623-4. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Zhong P, Scheller RH, Tsien RW. Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating. Proc. Natl. Acad. Sci. USA. 2000;97(25):13943–13948. doi: 10.1073/pnas.220389697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J. Biol. Chem. 1994;269(3):1617–1620. [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292(5515):293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon EJ, Preininger AM, Alford S, Hamm HE, Martin TF. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat. Neurosci. 2005;8(4):421–425. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- Boehm S. Presynaptic alpha2-adrenoceptors control excitatory, but not inhibitory, transmission at rat hippocampal synapses. J. Physiol. 1999;519(Pt 2):439–449. doi: 10.1111/j.1469-7793.1999.0439m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland L, Bean B. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J. Neurosci. 1993;13(2):516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher A, Kadlec J, Betz H, Weissenhorn W. X-ray Structure of a Neuronal Complexin-SNARE Complex from Squid. J. Biol. Chem. 2002;277(29):26517–26523. doi: 10.1074/jbc.M203460200. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Korsukewitz C, Floel A, Kretzschmar T, Diederich K, Knecht S. Tonic Dopaminergic Stimulation Impairs Associative Learning in Healthy Subjects. Neuropsychopharmacology. 2006;31(11):2552–2564. doi: 10.1038/sj.npp.1301167. [DOI] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin - A Calcium Sensor On The Synaptic Vesicle Surface. Science. 1992;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Brown DA, Sihra TS. Presynaptic signaling by heterotrimeric G-proteins. Handb. Exp. Pharmacol. 2008;(184):207–260. doi: 10.1007/978-3-540-74805-2_8. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J. Neurosci. 2004;24(24):5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buranda T, Waller A, Wu Y, Simons PC, Biggs S, Prossnitz ER, Sklar LA. Some Mechanistic Insights into GPCR Activation from Detergent-Solubilized Ternary Complexes on Beads. In: Stephen RS, editor. Adv. Protein Chem. Academic Press; 2007. pp. 95–135. [DOI] [PubMed] [Google Scholar]
- Burdina AO, Klosterman SM, Shtessel L, Ahmed S, Richmond JE. In Vivo Analysis of Conserved C. elegans Tomosyn Domains. PLoS One. 2011;6(10):e26185. doi: 10.1371/journal.pone.0026185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Chaperoning the SNAREs: a role in preventing neurodegeneration? Nat. Cell. Biol. 2011;13(1):8–9. doi: 10.1038/ncb0111-8. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G Protein Structure, Function, and Regulation. Endocr. Rev. 2003;24(6):765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Hernandez S, Earls LR, Medkova M, Sundgren-Andersson AK, Surmeier DJ, Hamm HE. RGS9-2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc. Natl. Acad. Sci. USA. 2004;101(46):16339–16344. doi: 10.1073/pnas.0407416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C, Page KM, Stephens GJ, Dolphin AC. Identification of Residues in the N Terminus of α1B Critical for Inhibition of the Voltage-Dependent Calcium Channel by Gβγ. J. Neurosci. 1999;19(16):6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogna M. Distinct properties of presynaptic group II and III metabotropic glutamate receptor-mediated inhibition of perforant pathway-CA1 EPSCs. Eur. J. Neurosci. 2004;19(10):2847–2858. doi: 10.1111/j.1460-9568.2004.03378.x. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and Structure-Function Relationships of Voltage-Gated Calcium Channels. Pharmacol. Rev. 2005;57(4):411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chan S-A, Doreian B, Smith C. Dynamin and Myosin Regulate Differential Exocytosis from Mouse Adrenal Chromaffin Cells. Cell. Mol. Neurobiol. 2010;30(8):1351–1357. doi: 10.1007/s10571-010-9591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER, Jahn R. Calcium-Dependent Interaction Of The Cytoplasmic Region Of Synaptotagmin With Membranes - Autonomous Function Of A Single C-2-Homologous Domain. J. Biol. Chem. 1994;269(8):5735–5741. [PubMed] [Google Scholar]
- Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 1995;270(40):23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 1998;273(22):13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95(3):921–929. [PubMed] [Google Scholar]
- Chen X, Tomchick DR, Kovrigin E, Araç D, Machius M, Südhof TC, Rizo J. Three-Dimensional Structure of the Complexin/SNARE Complex. Neuron. 2002;33(3):397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- Chicka MC, Chapman ER. Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes. Biochemistry. 2009;48(4):657–659. doi: 10.1021/bi801962d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi UB, Strop P, Vrljic M, Chu S, Brunger AT, Weninger KR. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat. Struct. Mol. Biol. 2010;17(3):318–324. doi: 10.1038/nsmb.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Clarke OB, Caputo AT, Hill AP, Vandenberg JI, Smith BJ, Gulbis JM. Domain Reorientation and Rotation of an Intracellular Assembly Regulate Conduction in Kir Potassium Channels. Cell. 2010;141(6):1018–1029. doi: 10.1016/j.cell.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110(3):403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Currie KP. Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors. Cell. Mol. Neurobiol. 2010;30(8):1201–1208. doi: 10.1007/s10571-010-9596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Shen N, Arac D, Rizo J. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 2007;367(3):848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. Signalling via the G protein-activated K+ channels. Cell. Signal. 1997;9(8):551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- De Waard M, Liu H, Walker D, Scott VE, Gurnett CA, Campbell KP. Direct binding of G-protein betagamma complex to voltage-dependent calcium channels [see comments] Nature. 1997;385(6615):446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- de Wit H. Molecular mechanism of secretory vesicle docking. Biochem. Soc. Trans. 2010;38:192–198. doi: 10.1042/BST0380192. [DOI] [PubMed] [Google Scholar]
- DeFea KA. Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell. Signal. 2011;23(4):621–629. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW, Sah P. Noradrenaline Modulates Transmission at a Central Synapse by a Presynaptic Mechanism. Neuron. 2007;56(5):880–892. doi: 10.1016/j.neuron.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Delmas P, Abogadie FC, Dayrell M, Haley JE, Milligan G, Caulfield MP, Brown DA, Buckley NJ. G-proteins and G-protein subunits mediating cholinergic inhibition of N-type calcium currents in sympathetic neurons. Eur. J. Neurosci. 1998;10(5):1654–1666. doi: 10.1046/j.1460-9568.1998.00170.x. [DOI] [PubMed] [Google Scholar]
- Devedjian JC, Pujol A, Cayla C, George M, Casellas A, Paris H, Bosch F. Transgenic mice overexpressing alpha2A-adrenoceptors in pancreatic beta-cells show altered regulation of glucose homeostasis. Diabetologia. 2000;43(7):899–906. doi: 10.1007/s001250051467. [DOI] [PubMed] [Google Scholar]
- Dingus J, Wells CA, Campbell L, Cleator JH, Robinson K, Hildebrandt JD. G Protein betagamma dimer formation: Gbeta and Ggamma differentially determine efficiency of in vitro dimer formation. Biochemistry. 2005;44(35):11882–11890. doi: 10.1021/bi0504254. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J. Neurosci. 1996;16(5):1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62(3):544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED. The presynaptic cytomatrix of brain synapses. Cell. Mol. Life Sci. 2001;58(1):94–116. doi: 10.1007/PL00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO. J. 1999;18(16):4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium component of sensory neurone action potentials. Nature. 1978;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Reisine T. New insights into GPCR function: implications for HTS. Methods Mol. Biol. 2009;552:1–13. doi: 10.1007/978-1-60327-317-6_1. [DOI] [PubMed] [Google Scholar]
- Elmslie KS. Calcium current modulation in frog sympathetic neurones: multiple neurotransmitters and G proteins. J. Physiol. 1992;451(1):229–246. doi: 10.1113/jphysiol.1992.sp019162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alacid L, Aguado C, Ciruela F, Martin R, Colon J, Cabanero MJ, Gassmann M, Watanabe M, Shigemoto R, Wickman K, Bettler B, Sanchez-Prieto J, Lujan R. Subcellular compartment-specific molecular diversity of pre- and post-synaptic GABA-activated GIRK channels in Purkinje cells. J. Neurochem. 2009;110(4):1363–1376. doi: 10.1111/j.1471-4159.2009.06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Busnadiego R, Zuber B, Maurer UE, Cyrklaff M, Baumeister W, Lucic V. Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J. Cell Biol. 2010;188(1):145–156. doi: 10.1083/jcb.200908082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410(6824):41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fernandez I, Arac D, Ubach J, Gerber SH, Shin OH, Gao Y, Anderson RGW, Sudhof TC, Rizo J. Three-dimensional structure of the synaptotagmin 1 C2B-domain: Synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32(6):1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- Finley M, Arrabit C, Fowler C, Suen KF, Slesinger PA. betaL-betaM loop in the C-terminal domain of G protein-activated inwardly rectifying K(+) channels is important for G(betagamma) subunit activation. J. Physiol. 2004;555(Pt 3):643–657. doi: 10.1113/jphysiol.2003.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang C-S, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular Basis for Interactions of G Protein β Subunits with Effectors. Science. 1998;280(5367):1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, Scheller RH, Takai Y. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20(5):905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Molecular Cloning, Expression, and Characterization of a Novel Class of Synaptotagmin (Syt XIV) Conserved from Drosophila to Humans. J. Biochem. 2003a;133(5):641–649. doi: 10.1093/jb/mvg082. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Molecular cloning and characterization of human, rat, and mouse synaptotagmin XV. Biochem. Biophys. Res. Commun. 2003b;306(1):64–71. doi: 10.1016/s0006-291x(03)00911-2. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Miura R, Mori Y, Strobeck M, Suzuki K, Ogihara Y, Asano T, Morishita R, Hashii M, Higashida H, Yoshii M, Nukada T. Differential Interactions of the C terminus and the Cytoplasmic I–II Loop of Neuronal Ca2+ Channels with G-protein α and β Subunits. J. Biol. Chem. 1998;273(28):17595–17603. doi: 10.1074/jbc.273.28.17595. [DOI] [PubMed] [Google Scholar]
- Gabriel JP, Mahmood R, Kyriakatos A, Soll I, Hauptmann G, Calabrese RL, El Manira A. Serotonergic modulation of locomotion in zebrafish: endogenous release and synaptic mechanisms. J. Neurosci. 2009;29(33):10387–10395. doi: 10.1523/JNEUROSCI.1978-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol [correction] 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. 2004;24(48):10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N, Downes GB, Yan K, Kisselev O. The G-protein betagamma complex. Cell. Signal. 1998;10(7):447–455. doi: 10.1016/s0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79(4):717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat. Neurosci. 2005;8(5):597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- Gerachshenko T, Schwartz E, Bleckert A, Photowala H, Seymour A, Alford S. Presynaptic G-protein-coupled receptors dynamically modify vesicle fusion, synaptic cleft glutamate concentrations, and motor behavior. J. Neurosci. 2009;29(33):10221–10233. doi: 10.1523/JNEUROSCI.1404-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber SH, Rah J-C, Min S-W, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, Verhage M, Rosenmund C, Südhof TC. Conformational Switch of Syntaxin-1 Controls Synaptic Vesicle Fusion. Science. 2008;321(5895):1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RWT, Conn PJ. Multiple presynatic metabotropic glutamate recptors modulate excitatory and inhibitory synaptic transmission in hippocampal area C1. J. Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerona RRL, Larsen EC, Kowalchyk JA, Martin TFJ. The C Terminus of SNAP25 Is Essential for Ca2+-dependent Binding of Synaptotagmin to SNARE Complexes. J. Biol. Chem. 2000;275(9):6328–6336. doi: 10.1074/jbc.275.9.6328. [DOI] [PubMed] [Google Scholar]
- Giustizieri M, Bernardi G, Mercuri NB, Berretta N. Distinct mechanisms of presynaptic inhibition at GABAergic synapses of the rat substantia nigra pars compacta. J. Neurophysiol. 2005;94(3):1992–2003. doi: 10.1152/jn.00171.2005. [DOI] [PubMed] [Google Scholar]
- Glitsch M. Selective inhibition of spontaneous but not Ca2+ -dependent release machinery by presynaptic group II mGluRs in rat cerebellar slices. J. Neurophysiol. 2006;96(1):86–96. doi: 10.1152/jn.01282.2005. [DOI] [PubMed] [Google Scholar]
- Golard A, Siegelbaum S. Kinetic basis for the voltage-dependent inhibition of N-type calcium current by somatostatin and norepinephrine in chick sympathetic neurons. J. Neurosci. 1993;13(9):3884–3894. doi: 10.1523/JNEUROSCI.13-09-03884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LS, Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill; 2001. [Google Scholar]
- Graham GJ, Ren Q, Dilks JR, Blair P, Whiteheart SW, Flaumenhaft R. Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo. Blood. 2009;114(5):1083–1090. doi: 10.1182/blood-2009-03-210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen AJ, Martens S, Arazola RD, Cornelisse LN, Lozovaya N, de Jong APH, Goriounova NA, Habets RLP, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M. Doc2b Is a High-Affinity Ca2+ Sensor for Spontaneous Neurotransmitter Release. Science. 2010;327(5973):1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley JE, Delmas P, Offermanns S, Abogadie FC, Simon MI, Buckley NJ, Brown DA. Muscarinic inhibition of calcium current and M current in Galpha q-deficient mice. J. Neurosci. 2000;20(11):3973–3979. doi: 10.1523/JNEUROSCI.20-11-03973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BR, Smith DO. Autoreceptor-mediated purinergic and cholinergic inhibition of motor nerve terminal calcium currents in the rat. J. Physiol. 1991;432:327–341. doi: 10.1113/jphysiol.1991.sp018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Palfreyman MT, Watanabe S, Olsen S, Jorgensen EM. Open syntaxin docks synaptic vesicles. PLoS Biol. 2007;5(8):e198. doi: 10.1371/journal.pbio.0050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey VL, Stephens GJ. Mechanism of GABA receptor-mediated inhibition of spontaneous GABA release onto cerebellar Purkinje cells. Eur. J. Neurosci. 2004;20(3):684–700. doi: 10.1111/j.1460-9568.2004.03505.x. [DOI] [PubMed] [Google Scholar]
- Hatsuzawa K, Lang T, Fasshauer D, Bruns D, Jahn R. The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis. J. Biol. Chem. 2003;278(33):31159–31166. doi: 10.1074/jbc.M305500200. [DOI] [PubMed] [Google Scholar]
- Heinke B, Gingl E, Sandkuhler J. Multiple targets of mu-opioid receptor-mediated presynaptic inhibition at primary afferent Adelta- and C-fibers. J. Neurosci. 2011;31(4):1313–1322. doi: 10.1523/JNEUROSCI.4060-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380(6571):258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Hockerman GH, Scheuer T, Catterall WA. Molecular determinants of inactivation and G protein modulation in the intracellular loop connecting domains I and II of the calcium channel a1A subunit. Proc. Natl. Acad. Sci. USA. 1997;94(4):1512–1516. doi: 10.1073/pnas.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, McMillan K, Pike CM, Marks J, Fox AP, Xie Z. Etomidate and propofol inhibit the neurotransmitter release machinery at different sites. J. Physiol. 2011;589(5):1103–1115. doi: 10.1113/jphysiol.2010.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat. Neurosci. 2009;12(9):1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat. Neurosci. 2010;13(8):958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt JD. Role of subunit diversity in signaling by heterotrimeric G proteins. Biochem. Pharmacol. 1997;54(3):325–339. doi: 10.1016/s0006-2952(97)00269-4. [DOI] [PubMed] [Google Scholar]
- Hobson RJ, Liu Q, Watanabe S, Jorgensen EM. Complexin Maintains Vesicles in the Primed State in C. elegans. Current Biology. 2011;21(2):106–113. doi: 10.1016/j.cub.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim. Biophys. Acta. 2005;1744(2):120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Huang C-L, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15(5):1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Hunt RD, Arnsten AFT, Asbell MD. An Open Trial of Guanfacine in the Treatment of Attention-Deficit Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34(1):50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Yoshii M, Sora I, Kobayashi T. Opioid receptor coupling to GIRK channels. In vitro studies using a Xenopus oocyte expression system and in vivo studies on weaver mutant mice. Methods Mol. Med. 2003;84:53–64. doi: 10.1385/1-59259-379-8:53. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380(6571):255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J. Neurosci. 2009;29(22):7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. GABAB receptor-mediated modulation of presynaptic currents and excitatory transmission at a fast central synapse. J. Neurophysiol. 1998;80(3):1571–1576. doi: 10.1152/jn.1998.80.3.1571. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Janssen PA, Niemegeers CJ, Awouters F, Schellekens KH, Megens AA, Meert TF. Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin-S2 and dopamine-D2 antagonistic properties. J. Pharmacol. Exp. Ther. 1988;244(2):685–693. [PubMed] [Google Scholar]
- Jardin I, Ben Amor N, Hernández-Cruz JM, Salido GM, Rosado JA. Involvement of SNARE proteins in thrombin-induced platelet aggregation: Evidence for the relevance of Ca2+ entry. Arch. Biochem. Biophys. 2007;465(1):16–25. doi: 10.1016/j.abb.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Magga JM, Beedle AM, Braun JEA, Zamponi GW. G Protein Modulation of Ntype Calcium Channels Is Facilitated by Physical Interactions between Syntaxin 1A and Gβγ. J. Biol. Chem. 2000;275(9):6388–6394. doi: 10.1074/jbc.275.9.6388. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. Distinct molecular determinants govern syntaxin 1A-mediated inactivation and G-protein inhibition of N-type calcium channels. J. Neurosci. 2001;21(9):2939–2948. doi: 10.1523/JNEUROSCI.21-09-02939.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis SE, Barr W, Feng ZP, Hamid J, Zamponi GW. Molecular determinants of syntaxin 1 modulation of N-type calcium channels. J. Biol. Chem. 2002;277(46):44399–44407. doi: 10.1074/jbc.M206902200. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, Saitoh N, Takahashi T. GTP-binding protein beta gamma subunits mediate presynaptic calcium current inhibition by GABA(B) receptor. Proc. Natl. Acad. Sci. U S A. 2001;98(14):8054–8058. doi: 10.1073/pnas.141031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4th ed. New York: McGraw-Hill, Health Professions Division; 2000. [Google Scholar]
- Kasai H, Aosaki T. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflugers Arch. 1989;414(2):145–149. doi: 10.1007/BF00580956. [DOI] [PubMed] [Google Scholar]
- Katz B. The release of Neural Transmitter Substances (The Sherrington Lectures X) Springfield, IL: Charles C. Thomas; 1969. [Google Scholar]
- Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993;259(5096):832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- Knight DE, Baker PF. Guanine nucleotides and Ca-dependent exocytosis. Studies on two adrenal cell preparations. FEBS Lett. 1985;189(2):345–349. doi: 10.1016/0014-5793(85)81053-x. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Schneggenburger R. Synaptotagmin Increases the Dynamic Range of Synapses by Driving Ca2+-Evoked Release and by Clamping a Near-Linear Remaining Ca2+ Sensor. Neuron. 2011;69(4):736–748. doi: 10.1016/j.neuron.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Schermerhorn T, Aizawa T, Sharp GW. Glucose stimulation of insulin release in the absence of extracellular Ca2+ and in the absence of any increase in intracellular Ca2+ in rat pancreatic islets. Proc. Natl. Acad. Sci. USA. 1995;92(23):10728–10732. doi: 10.1073/pnas.92.23.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J. Neurosci. 2006;26(16):4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel D, Krishnakumar SS, Radoff DT, Li F, Giraudo CG, Pincet F, Rothman JE, Reinisch KM. Complexin cross-links prefusion SNAREs into a zigzag array. Nat. Struct. Mol. Biol. 2011;18(8):927–933. doi: 10.1038/nsmb.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladera C, del Carmen Godino M, Jose Cabanero M, Torres M, Watanabe M, Lujan R, Sanchez-Prieto J. Pre-synaptic GABA receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J. Neurochem. 2008;107(6):1506–1517. doi: 10.1111/j.1471-4159.2008.05712.x. [DOI] [PubMed] [Google Scholar]
- Lai AL, Huang H, Herrick DZ, Epp N, Cafiso DS. Synaptotagmin 1 and SNAREs Form a Complex That Is Structurally Heterogeneous. J. Mol. Biol. 2011;405(3):696–706. doi: 10.1016/j.jmb.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379(6563):311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- Le François B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55(6):977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, Bayliss DA. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein beta gamma subunits. Proc. Natl. Acad. Sci. USA. 2000;97(17):9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves Gαq family subunits, phospholipase C, and a readily diffusible messenger. J. Biol. Chem. 2001;276(20):16720–16730. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- Lei Q, Jones MB, Talley EM, Garrison JC, Bayliss DA. Molecular mechanisms mediating inhibition of G protein-coupled inwardly-rectifying K+ channels. Mol. Cells. 2003;15(1):1–9. [PubMed] [Google Scholar]
- Leveque C, el Far O, Martin-Moutot N, Sato K, Kato R, Takahashi M, Seagar MJ. Purification of the N-type calcium channel associated with syntaxin and synaptotagmin. A complex implicated in synaptic vesicle exocytosis. J. Biol. Chem. 1994;269(9):6306–6312. [PubMed] [Google Scholar]
- Li B, Zhong H, Scheuer T, Catterall WA. Functional Role of a C-Terminal Gβγ-Binding Domain of Cav2.2 Channels. Mol. Pharmacol. 2004;66(3):761–769. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Li X, Hummer A, Han J, Xie M, Melnik-Martinez K, Moreno RL, Buck M, Mark MD, Herlitze S. G protein beta2 subunit-derived peptides for inhibition and induction of G protein pathways. Examination of voltage-gated Ca2+ and G protein inwardly rectifying K+ channels. J. Biol. Chem. 2005;280(25):23945–23959. doi: 10.1074/jbc.M414078200. [DOI] [PubMed] [Google Scholar]
- Lim WK, Myung CS, Garrison JC, Neubig RR. Receptor-G protein gamma specificity: gamma11 shows unique potency for A(1) adenosine and 5-HT(1A) receptors. Biochemistry. 2001;40(35):10532–10541. doi: 10.1021/bi010950c. [DOI] [PubMed] [Google Scholar]
- Lin HC, Gilman AG. Regulation of Dynamin I GTPase Activity by G Protein βγ Subunits and Phosphatidylinositol 4,5-Bisphosphate. J. Biol. Chem. 1996;271(45):27979–27982. doi: 10.1074/jbc.271.45.27979. [DOI] [PubMed] [Google Scholar]
- Lindorfer MA, Myung CS, Savino Y, Yasuda H, Khazan R, Garrison JC. Differential activity of the G protein beta5 gamma2 subunit at receptors and effectors. J. Biol. Chem. 1998;273(51):34429–34436. doi: 10.1074/jbc.273.51.34429. [DOI] [PubMed] [Google Scholar]
- Liu J-P, Yajima Y, Li H, Ackland S, Akita Y, Stewart J, Kawashima S. Molecular interactions between dynamin and G-protein βγ-subunits in neuroendocrine cells. Mol. Cell. Endocrinol. 1997;132(1–2):61–71. doi: 10.1016/s0303-7207(97)00120-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the Parapyramidal Region of the Neonatal Rat Brain Stem Produces Locomotor-Like Activity Involving Spinal 5-HT7 and 5-HT2A Receptors. J. Neurophysiol. 2005;94(2):1392–1404. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Luini A, De Matteis MA. Evidence that the receptor-linked G protein inhibits exocytosis by a post-second messenger mechanism in AtT-20 cells. J. Neurochem. 1990;54:30–38. doi: 10.1111/j.1471-4159.1990.tb13279.x. [DOI] [PubMed] [Google Scholar]
- Lujan R, Maylie J, Adelman JP. New sites of action for GIRK and SK channels. Nat. Rev. Neurosci. 2009;10(7):475–480. doi: 10.1038/nrn2668. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19(3):687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010;11(5):301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KL, Gerona RR, Larsen EC, Marcia RF, Mitchell JC, Martin TF. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol. Biol. Cell. 2007;18(12):4957–4968. doi: 10.1091/mbc.E07-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MJ, Baillie GS, Mohamed A, Li X, Maisonneuve C, Klussmann E, van Heeke G, Houslay MD. RNA silencing identifies PDE4D5 as the functionally relevant cAMP phosphodiesterase interacting with beta arrestin to control the protein kinase A/AKAP79-mediated switching of the beta2-adrenergic receptor to activation of ERK in HEK293B2 cells. J. Biol. Chem. 2005;280(39):33178–33189. doi: 10.1074/jbc.M414316200. [DOI] [PubMed] [Google Scholar]
- Malsam J, Kreye S, Sollner TH. Membrane fusion: SNAREs and regulation. Cell. Mol. Life Sci. 2008;65(18):2814–2832. doi: 10.1007/s00018-008-8352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M, Pozzi D, Grumelli C, Condliffe SB, Frassoni C, Harkany T, Verderio C. The synaptic split of SNAP-25: Different roles in glutamatergic and GABAergic neurons? Neuroscience. 2009;158(1):223–230. doi: 10.1016/j.neuroscience.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Maximov A, Sudhof TC. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 2005;48(4):547–554. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Südhof TC. Complexins: Cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83(1):111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Huttner WB, Tsien RY, Pozzan T. Free cytoplasmic Ca2+ and neurotransmitter release: studies on PC12 cells and synaptosomes exposed to alpha-latrotoxin. Proc. Natl. Acad. Sci. USA. 1984;81(2):620–624. doi: 10.1073/pnas.81.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli A, Yaka R. Dopamine inhibits GABA(A) currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience. 2010;165(4):1159–1169. doi: 10.1016/j.neuroscience.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol. Endocrinol. 2010;24(1):261–274. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LC, Swayne LA, Kay JG, Feng Z-P, Jarvis SE, Zamponi GW, Braun JEA. Molecular determinants of cysteine string protein modulation of N-type calcium channels. Journal of Cell Science. 2003;116(14):2967–2974. doi: 10.1242/jcs.00595. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annu. Rev. Pharmacol. Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Mittal V, Zhang H, Linder ME, Logothetis DE. Distinct sites on G protein beta gamma subunits regulate different effector functions. J. Biol. Chem. 2002;277(39):36345–36350. doi: 10.1074/jbc.M205359200. [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404(6776):355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Myung CS, Lim WK, DeFilippo JM, Yasuda H, Neubig RR, Garrison JC. Regions in the G protein gamma subunit important for interaction with receptors and effectors. Mol. Pharmacol. 2006;69(3):877–887. doi: 10.1124/mol.105.018994. [DOI] [PubMed] [Google Scholar]
- Natochin M, Campbell TN, Barren B, Miller LC, Hameed S, Artemyev NO, Braun JE. Characterization of the G alpha(s) regulator cysteine string protein. J. Biol. Chem. 2005;280(34):30236–30241. doi: 10.1074/jbc.M500722200. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J. Neurosci. 1997;17(15):5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor VM, Shamotienko O, Grishin E, Betz H. On the structure of the 'synaptosecretosome'. Evidence for a neurexin/synaptotagmin/syntaxin/Ca2+ channel complex. FEBS Lett. 1993;326(1–3):255–260. doi: 10.1016/0014-5793(93)81802-7. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. How do receptors activate G proteins? Adv. Protein Chem. 2007;74:67–93. doi: 10.1016/S0065-3233(07)74002-0. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2008;9(1):60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Trong IL, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal Structure of Rhodopsin: A G Protein-Coupled Receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Panchenko MP, Saxena K, Li Y, Charnecki S, Sternweis PM, Smith TF, Gilman AG, Kozasa T, Neer EJ. Sites important for PLCbeta2 activation by the G protein betagamma subunit map to the sides of the beta propeller structure. J. Biol. Chem. 1998;273(43):28298–28304. doi: 10.1074/jbc.273.43.28298. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim K-T. Short-term plasticity of small synaptic vesicle (SSV) and large dense-core vesicle (LDCV) exocytosis. Cell. Signal. 2009;21(10):1465–1470. doi: 10.1016/j.cellsig.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Parpura V, Mohideen U. Molecular form follows function: (un)snaring the SNAREs. Trends Neurosci. 2008;31(9):435–443. doi: 10.1016/j.tins.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Peng L, Mirshahi T, Zhang H, Hirsch JP, Logothetis DE. Critical determinants of the G protein gamma subunits in the Gbetagamma stimulation of G protein-activated inwardly rectifying potassium (GIRK) channel activity. J. Biol. Chem. 2003;278(50):50203–50211. doi: 10.1074/jbc.M308299200. [DOI] [PubMed] [Google Scholar]
- Photowala H, Blackmer T, Schwartz E, Hamm HE, Alford S. G protein betagamma-subunits activated by serotonin mediate presynaptic inhibition by regulating vesicle fusion properties. Proc. Natl. Acad. Sci. U S A. 2006;103(11):4281–4286. doi: 10.1073/pnas.0600509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati AV, Razeto A, Böddener M, Becker S, Fasshauer D. Structural Basis for the Inhibitory Role of Tomosyn in Exocytosis. J. Biol. Chem. 2004;279(45):47192–47200. doi: 10.1074/jbc.M408767200. [DOI] [PubMed] [Google Scholar]
- Polgar J, Chung SH, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100(3):1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- Ponce A, Bueno E, Kentros C, Vega-Saenz de Miera E, Chow A, Hillman D, Chen S, Zhu L, Wu MB, Wu X, Rudy B, Thornhill WB. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J. Neurosci. 1996;16(6):1990–2001. doi: 10.1523/JNEUROSCI.16-06-01990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu SA, Predescu DN, Shimizu K, Klein IK, Malik AB. Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J. Biol. Chem. 2005;280(44):37130–37138. doi: 10.1074/jbc.M505659200. [DOI] [PubMed] [Google Scholar]
- Pulido IR, Jahn R, Gerke V. VAMP3 is associated with endothelial Weibel-Palade bodies and participates in their Ca(2+)-dependent exocytosis. Biochim. Biophys. Acta. 2011;1813(5):1038–1044. doi: 10.1016/j.bbamcr.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of Gβγ with a C-terminal Gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 1997;94(16):8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994;370(6485):143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Richardson M, Robishaw JD. The alpha2A-adrenergic receptor discriminates between Gi heterotrimers of different betagamma subunit composition in Sf9 insect cell membranes. J. Biol. Chem. 1999;274(19):13525–13533. doi: 10.1074/jbc.274.19.13525. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C-elegans. Nat. Neurosci. 1999;2(11):959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16(7):339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 2008;15(7):665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J. SNAREs. In: Larry RS, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 11–19. [Google Scholar]
- Robishaw JD, Berlot CH. Translating G protein subunit diversity into functional specificity. Curr. Opin. Cell Biol. 2004;16(2):206–219. doi: 10.1016/j.ceb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, Nagaraj V, Reinbothe TM, Tuncel J, Eliasson L, Groop L, Rorsman P, Salehi A, Lyssenko V, Luthman H, Renstrom E. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327(5962):217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39(1):9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- Sadja R, Reuveny E. Activation gating kinetics of GIRK channels are mediated by cytoplasmic residues adjacent to transmembrane domains. Channels (Austin) 2009;3(3):205–214. doi: 10.4161/chan.3.3.9136. [DOI] [PubMed] [Google Scholar]
- Sampo B, Tricaud N, Leveque C, Seagar M, Couraud F, Dargent B. Direct interaction between synaptotagmin and the intracellular loop I–II of neuronal voltage-sensitive sodium channels. Proc. Natl. Acad. Sci. USA. 2000;97(7):3666–3671. doi: 10.1073/pnas.97.7.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Thompsom SM. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippcampus: are Ca2+ channels involved? Neuropharmacology. 1995;34:1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Osborne SL, Sgouros JG. Synaptotagmins: More Isoforms Than Functions? Biochem. Biophys. Res. Commun. 1998;248(1):1–8. doi: 10.1006/bbrc.1998.8527. [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen. Comp. Endocrinol. 2005;142(1–2):94–101. doi: 10.1016/j.ygcen.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Fernández-Chacón R. Cysteine-String Proteins (CSPs) In: Larry RS, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 285–292. [Google Scholar]
- Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326(2):379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- Scholz KP, Miller RJ. Inhibition of quantal transmitter release in the absence of calcium influx by a G protein linked adenosine receptor at hippocampal synapses. Neuron. 1992;8:1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- Schwartz EJ, Gerachshenko T, Alford S. 5-HT prolongs ventral root bursting via presynaptic inhibition of synaptic activity during fictive locomotion in lamprey. J. Neurophysiol. 2005;93(2):980–988. doi: 10.1152/jn.00669.2004. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD. Loss of G protein gamma 7 alters behavior and reduces striatal alpha(olf) level and cAMP production. J. Biol. Chem. 2003;278(8):6575–6579. doi: 10.1074/jbc.M211132200. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Giger KE, Betz KS, Stauffer AM, Sunderlin EM, Sim-Selley LJ, Selley DE, Bronson SK, Robishaw JD. Mice with Deficiency of G Protein γ3 Are Lean and Have Seizures. Mol. Cell. Biol. 2004;24(17):7758–7768. doi: 10.1128/MCB.24.17.7758-7768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagar M, Leveque C, Charvin N, Marqueze B, Martin-Moutot N, Boudier JA, Boudier JL, Shoji-Kasai Y, Sato K, Takahashi M. Interactions between proteins implicated in exocytosis and voltage-gated calcium channels. Philos. Transr. Soc. Lond. B Biol. Sci. 1999;354(1381):289–297. doi: 10.1098/rstb.1999.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searl TJ, Silinsky EM. Modulation of Ca(2+)-dependent and Ca(2+)-independent miniature endplate potentials by phorbol ester and adenosine in frog. British journal of pharmacology. 2005;145(7):954–962. doi: 10.1038/sj.bjp.0706248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Burre J, Sudhof TC. CSP[alpha] promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat. Cell. Biol. 2011;13(1):30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-Type calcium channels. Neuron. 1994;13(6):1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17(19):7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J. Physiol. 1984;346(1):243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM. Selective disruption of the mammalian secretory apparatus enhances or eliminates calcium current modulation in nerve endings. Proc. Natl. Acad. Sci. USA. 2008;105(17):6427–6432. doi: 10.1073/pnas.0708814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillar KT, Wedderburn JF, Simmers AJ. Modulation of swimming rhythmicity by 5-hydroxytryptamine during post-embryonic development in Xenopus laevis. Proc. Biol. Sci. 1992;250(1328):107–114. doi: 10.1098/rspb.1992.0137. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Smrcka AV. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell. Mol. Life Sci. 2008;65(14):2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whitehart SW, Brunner M, Erdjumentbromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Stanley EF, Mirotznik RR. Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels. Nature. 1997;385(6614):340–343. doi: 10.1038/385340a0. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Mochida S. G protein βγ subunits mediate presynaptic inhibition of transmitter release from rat superior cervical ganglion neurones in culture. J. Physiol. 2005;563(Pt 3):765–776. doi: 10.1113/jphysiol.2004.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ. G-protein-coupled-receptor-mediated presynaptic inhibition in the cerebellum. Trends Pharmacol. Sci. 2009;30(8):421–430. doi: 10.1016/j.tips.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Wu ZX, Matti U, Junge HJ, Schirra C, Becherer U, Wojcik SM, Brose N, Rettig J. Identification of the minimal protein domain required for priming activity of Munc13-1. Curr. Biol. 2005;15(24):2243–2248. doi: 10.1016/j.cub.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nature reviews. Immunology. 2006;6(12):919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Synaptotagmins: Why So Many? J. Biol. Chem. 2002;277(10):7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450(7170):676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4A resolution. Nature. 1998;395(6700):347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274(5287):594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Takao K, Yoshii M, Kanda A, Kokubun S, Nukada T. A region of the muscarinic-gated atrial K+ channel critical for activation by G protein beta gamma subunits. Neuron. 1994;13(3):747–755. doi: 10.1016/0896-6273(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126(6):1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Taylor DP, Eison MS, Riblet LA, Vandermaelen CP. Pharmacological and clinical effects of buspirone. Pharmacol. Biochem. Behav. 1985;23(4):687–694. doi: 10.1016/0091-3057(85)90438-1. [DOI] [PubMed] [Google Scholar]
- Tedford HW, Kisilevsky AE, Peloquin JB, Zamponi GW. Scanning Mutagenesis Reveals a Role for Serine 189 of the Heterotrimeric G-Protein Beta 1 Subunit in the Inhibition of N-Type Calcium Channels. J. Neurophysiol. 2006;96(1):465–470. doi: 10.1152/jn.00216.2006. [DOI] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G Protein Modulation of Cav2 Calcium Channels. Pharmacol. Rev. 2006;58(4):837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- Teng FY, Wang Y, Tang BL. The syntaxins. Genome Biol. 2001;2(11) doi: 10.1186/gb-2001-2-11-reviews3012. reviews3012.1–3012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen RF, Kochubey O, de Wit H, Gulyas-Kovacs A, Konijnenburg B, Sorensen JB, Klingauf J, Verhage M. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 2006;25(16):3725–3737. doi: 10.1038/sj.emboj.7601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994;13(6):1439–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, Berg TKvd, Missler M, Geuze HJ, Südhof TC. Synaptic Assembly of the Brain in the Absence of Neurotransmitter Secretion. Science. 2000;287(5454):864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Verhage M, Sorensen JB. Vesicle docking in regulated exocytosis. Traffic. 2008;9(9):1414–1424. doi: 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Sudhof TC, Neher E, Verhage M. Munc18-1 promotes large dense-core vesicle docking. Neuron. 2001;31(4):581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: Minimal Machinery for Membrane Fusion. Cell. 1998;92(6):759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Weimer RM, Richmond JE. Synaptic vesicle docking: a putative role for the Munc18/Sec1 protein family. Curr. Top. Dev. Biol. 2005;65:83–113. doi: 10.1016/S0070-2153(04)65003-4. [DOI] [PubMed] [Google Scholar]
- Weimer RM, Gracheva EO, Meyrignac O, Miller KG, Richmond JE, Bessereau JL. UNC-13 and UNC-10/rim localize synaptic vesicles to specific membrane domains. J. Neurosci. 2006;26(31):8040–8047. doi: 10.1523/JNEUROSCI.2350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CA, Betke KM, Lindsley CW, Hamm HE. Label-Free Detection of G Protein–SNARE Interactions and Screening for Small Molecule Modulators. ACS Chemical Neuroscience. 2011 doi: 10.1021/cn200102d. [serial on the Internet] Available from: http://dx.doi.org/10.1021/cn200102d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT. Accessory proteins stabilize the acceptor complex for synaptobrevin, the:1:1 syntaxin/SNAP-25 complex. Structure. 2008;16(2):308–320. doi: 10.1016/j.str.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368(6468):255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Womack MD, McCleskey EW. Fast, local signal transduction between the mu opioid receptor and Ca2+ channels. J. Neurosci. 1995;15(5 Pt 2):4124–4132. doi: 10.1523/JNEUROSCI.15-05-04124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie TM, Gilbert DJ, Olsen AS, Chen XN, Amatruda TT, Korenberg JR, Trask BJ, de Jong P, Reed RR, Simon MI, et al. Evolution of the mammalian G protein alpha subunit multigene family. Nat. Genet. 1992;1(2):85–91. doi: 10.1038/ng0592-85. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron. 2007;55(1):11–24. doi: 10.1016/j.neuron.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. J. Physiol. 1995;485(Pt 3):649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Allen KL, Kourrich S, Colon-Saez J, Thomas MJ, Wickman K, Martemyanov KA. Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat. Neurosci. 2010;13(6):661–663. doi: 10.1038/nn.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ho YK, Lee RH, Kontanill K, Takahashill K, Katadall T, Kurachi Y. Muscarinic K+ channels are activated by beta gamma subunits and inhibited by the GDP-bound form of alpha subunit of transducin. Biochem. Biophys. Res. Commun. 1994;200(3):1484–1490. doi: 10.1006/bbrc.1994.1618. [DOI] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Morrell CN, Matsushita K, Fletcher CA, Cao W, Lowenstein CJ. Exocytosis of endothelial cells is regulated by N-ethylmaleimide-sensitive factor. Methods Mol. Biol. 2008;440:203–215. doi: 10.1007/978-1-59745-178-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez LC, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 2000;148(2):247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Gaffaney J, Kwon S, Chapman E. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 2011;147(3):666–743. doi: 10.1016/j.cell.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa M, Osawa M, Takeuchi K, Mase Y, Shimada I. NMR analyses of the Gbetagamma binding and conformational rearrangements of the cytoplasmic pore of G protein-activated inwardly rectifying potassium channel 1 (GIRK1) J. Biol. Chem. 2011;286(3):2215–2223. doi: 10.1074/jbc.M110.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Starmer WT. Phylogeny and evolutionary rates of G protein alpha subunit genes. J. Mol. Evol. 1992;35(3):230–238. doi: 10.1007/BF00178599. [DOI] [PubMed] [Google Scholar]
- Yoon EJ, Gerachshenko T, Spiegelberg BD, Alford S, Hamm HE. Gbetagamma interferes with Ca2+-dependent binding of synaptotagmin to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. Mol. Pharmacol. 2007;72(5):1210–1219. doi: 10.1124/mol.107.039446. [DOI] [PubMed] [Google Scholar]
- Yoon EJ, Hamm HE, Currie KP. G protein betagamma subunits modulate the number and nature of exocytotic fusion events in adrenal chromaffin cells independent of calcium entry. J Neurophysiol. 2008;100(5):2929–2939. doi: 10.1152/jn.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385(6615):442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology. 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- Zhang X-l, Upreti C, Stanton PK. Gβγ and the C Terminus of SNAP-25 Are Necessary for Long-Term Depression of Transmitter Release. PLoS One. 2011;6(5):e20500. doi: 10.1371/journal.pone.0020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TFJ. Ca2+-Dependent Synaptotagmin Binding to SNAP-25 Is Essential for Ca2+-Triggered Exocytosis. Neuron. 2002;34(4):599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Kawano T, Nakata H, Nakajima Y, Nakajima S, Kozasa T. Interaction of G protein beta subunit with inward rectifier K(+) channel Kir3. Mol. Pharmacol. 2003;64(5):1085–1091. doi: 10.1124/mol.64.5.1085. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fang Q, Straub SG, Lindau M, Sharp GW. Noradrenaline inhibits exocytosis via the G protein betagamma subunit and refilling of the readily releasable granule pool via the alpha(i1/2) subunit. J Physiol. 2010;588(Pt 18):3485–3498. doi: 10.1113/jphysiol.2010.190090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. VIP inhibits N-type Ca2+ channels of sympathetic neurons via a pertussis toxininsensitive but cholera toxin-sensitive pathway. Neuron. 1994;13(3):657–669. doi: 10.1016/0896-6273(94)90033-7. [DOI] [PubMed] [Google Scholar]