Abstract
Background
We sought to characterize the clinical and molecular epidemiologic characteristics of Staphylococcus aureus colonization (especially extranasal colonization) and to determine the extent to which community-associated methicillin-resistant S. aureus (MRSA) has emerged in community nursing homes.
Methods
The study enrolled a total of 213 residents, with or without an indwelling device, from 14 nursing homes in southeastern Michigan. Samples were obtained from the nares, oropharynx, groin, perianal area, wounds, and enteral feeding tube site. Standard microbiologic methods were used to identify methicillin-susceptible S. aureus and MRSA. Molecular epidemiologic methods included pulsed-field gel electrophoresis, PCR detection of Panton-Valentine leukocidin, and SCCmec and agr typing.
Results
One hundred thirty-one residents (62%) were colonized with S. aureus (MRSA colonization in 86). S. aureus colonization occurred in 80 (76%) of 105 residents with indwelling devices and in 51 (47%) of 108 residents without indwelling devices (P < .001). Of the 86 residents who were colonized with MRSA, nares culture results were positive for only 56 (65%). Residents with devices in place were more likely to be colonized at multiple sites. Eleven different strains of MRSA were identified by pulsed-field gel electrophoresis. Seventy-three residents (85%) were colonized with hospital-associated SCCmec II strains, and 8 (9%) were colonized with community-associated SCCmec IV strains, 2 of which carried Panton-Valentine leukocidin.
Conclusions
Extranasal colonization with MRSA is common among nursing home residents—particularly among residents with an indwelling device. We documented the emergence of community-associated SCCmec IV MRSA strains in the community nursing home setting in southeastern Michigan.
The anterior nares are presumed to be the primary reservoir for Staphylococcus aureus [1]. The epidemiology of nasal colonization with S. aureus is well defined, but extranasal colonization has been less well studied. Early studies of S. aureus colonization patterns in healthy populations revealed variations in carriage patterns at extranasal sites; for example, the rate of oropharyngeal colonization among healthy adults varied from 4% to 60% [2–4]. A recent study found that 30% of members of a healthy outpatient military population carried S. aureus in the oropharynx but not in the nares [5], and another recent study from Switzerland noted that 380 (12.8%) of 2966 screened persons carried S. aureus only in the oropharynx and not in the nares [6].
Not surprisingly, the rates of nasal and extranasal colonization with methicillin-resistant S. aureus (MRSA) appear similar to those noted for S. aureus in general [7–9]. In a study of hospitalized adults for whom samples were obtained from the nares, rectum, and axilla, it was shown that 23% of patients colonized with MRSA would have been missed if rectal sampling had not been performed [7]. Also, in a study of intensive care unit patients, 54% of colonized patients would have remained undetected had comprehensive multisite screening for MRSA not been performed at the time of admission [9]. Observations among patients with community-associated MRSA (CA-MRSA) infection— an emerging problem in the United States and Europe—suggest that extranasal colonization may be more common than was previously noted with hospital strains of MRSA [10, 11].
A paucity of data exist on the prevalence of S. aureus colonization and on the risk factors for colonization, either with methicillin-susceptible S. aureus (MSSA) or MRSA, among residents living in community nursing homes. Prior studies from our group and others have shown that indwelling devices, such as urinary catheters and feeding tubes, and functional dependence are important risk factors for nasal S. aureus colonization among nursing home residents [12–15]. Our study evaluates the clinical epidemiology of S. aureus colonization in this population, with a focus on molecular epidemiology of MRSA. We hypothesized that residents with indwelling devices would have a heavier bacterial burden and would be colonized with MSSA or MRSA at multiple extranasal sites, compared with residents who did not have such devices. We characterized the molecular epidemiology of MRSA in community nursing homes, noting both typical hospital-associated and CA-MRSA strains in this setting [16, 17].
METHODS
Study design and population
We conducted a cross-sectional epidemiological study involving 14 community nursing homes in southeastern Michigan during the period from March 2003 to November 2004. At each nursing home, all culture specimens were obtained within a 2-week period. This project was approved by the University of Michigan and Veterans Affairs Ann Arbor Healthcare System Institutional Review Boards. Written informed consent was obtained from all eligible residents. If a resident was unable to provide informed consent because of documented cognitive difficulties, written informed consent was obtained from their durable power of attorney. Residents with an indwelling urinary catheter, a percutaneously placed gastrostomy tube, or a central intravenous catheter were enrolled. An equal number of control residents without devices were randomly selected from the same nursing homes using a random numbers table and were asked for consent to participate. Demographic data were obtained by chart review.
Microbiological methods
To assess colonization with S. aureus, samples were obtained from nares, oropharynx, feeding tube insertion site, groin, perianal area, and wounds (if present) using dacron-tipped swabs for both the study and control groups. Swabs were inoculated onto mannitol salt agar and incubated at 35°C for 48 h. Bright yellow colonies suggestive of S. aureus were grown on trypticase soy agar with 5% sheep blood and were confirmed to be S. aureus by Gram stain, catalase positivity, and agglutination with a rapid test for protein A (Fisher Healthcare). All isolates were tested for methicillin resistance by growth on Mueller-Hinton agar containing oxacillin, 6 μg/mL, and 4% NaCl. A resident colonized with both MRSA and MSSA was categorized as an MRSA carrier. One MRSA isolate from each resident colonized with MRSA was further typed using methods described below.
Molecular epidemiology methods
PFGE was performed to characterize the relatedness of MRSA isolates. Genomic DNA was prepared and digested with SmaI (New England BioLabs) [18]. SmaI fragments were separated using a CHEF DR III apparatus (BioRad) and compared using BioNumerics software (Applied Maths). All MRSA isolates were compared with MRSA strains USA100–1100 [18, 19]. Isolates were placed in the same PFGE strain group if their SmaI restriction patterns were ≥80% similar. PCR was performed to detect the Panton-Valentine leukocidin (PVL) gene [20]. Multiplex PCR was performed to characterize staphylococcal cassette chromosome mec (SCCmec) types I–V [21, 22]. Nontypeable strains were further analyzed by ccr typing using PCR with primers to identify ccr types 1–4. Multiplex PCR was also used to determine accessory gene regulator (agr) types I–IV [23, 24].
Statistical analysis
Our main outcome of interest was the frequency of extranasal colonization with MRSA and MSSA in community nursing home residents. We were also interested in the number of sites of colonization with these organisms and the impact of indwelling devices on colonization. All data were entered into an Excel database (Microsoft) and analyzed using Stata software, version 8.2 (Stata). Univariate analyses were performed to examine the spread of data. Categorical variables were compared using χ2 test. P values <.05 were considered to be statistically significant.
RESULTS
A total of 125 residents who had an indwelling urinary catheter, a percutaneously placed gastrostomy tube, or a central intravenous catheter and 125 randomly selected control residents were eligible for the study. Residents were from 14 nursing home facilities that contained a total of 1669 beds. Of these 250 residents, 37 refused consent. Therefore, 213 residents (105 residents with ≥1 indwelling device and 108 residents without an indwelling device) were enrolled in the study. Forty-six residents in the study had a urinary catheter only, 48 had an enteral feeding tube only, 6 had both, and 5 residents had a central intravenous catheter only.
Overall, 131 (62%) of 213 residents were colonized with S. aureus at ≥1 site; this included 80 (76%) of 105 residents in the device group and 51 (47%) of 108 residents in the control group (OR, 3.6; 95% CI, 1.9–6.7; P < .001). Eighty-six residents were colonized with MRSA (75 with MRSA only and 11 with both MRSA and MSSA), and 45 were colonized with MSSA only (table 1). Nares cultures were positive for MRSA in 56 (65%) of the 86 residents who were colonized with MRSA. There were no differences in nasal MSSA colonization rates between the device and control groups, although the rate of MRSA colonization was significantly greater among residents with indwelling devices (55 [52%] of 105 vs. 31 [29%] of 108; OR, 2.7; 95% CI, 1.5–5.0; P < .001). Nasal colonization with MRSA was found in 31% of residents with devices and in 21% of those without devices; nasal colonization with MSSA was found in 16% of residents in each group (P=not significant). Residents in the device group were significantly more likely to be colonized with either MSSA or MRSA in their groin and perianal area than were residents in the control group (table 1).
Table 1.
Colonization with methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) in community nursing home residents with and without indwelling devices.
| No (%) of residents |
||||
|---|---|---|---|---|
| Characteristic | Device group (n = 105) | Control group (n = 108) | OR (95% CI) | P |
| Colonization with MSSA (n = 45) | ||||
| Nares | 17 (16) | 18 (16) | 1.2 (0.5–2.6) | .7 |
| Oropharynx | 14 (13) | 10 (9) | 1.9 (0.7–4.9) | .2 |
| Groin | 15 (14) | 5 (5) | 4.6 (1.5–15.3) | .003 |
| Perianal | 14 (13) | 5 (5) | 3.2 (1.0–10.5) | .02 |
| PEG tube site | 13 (12) | … | … | |
| Wound | 6 (6) | 2 (2) | … | |
| Any site | 25 (24) | 20 (19) | 1.4 (0.7–2.1) | .3 |
| Colonization with MRSA (n = 86) | ||||
| Nares | 33 (31) | 23 (21) | 1.8 (0.9–3.5) | .09 |
| Oropharynx | 27 (26) | 12 (11) | 1.6 (0.6–3.9) | .3 |
| Groin | 26 (25) | 5 (5) | 7.0 (2.7–25) | <.001 |
| Perianal | 28 (27) | 7 (7) | 8.7 (2.7–25) | <.001 |
| PEG tube site | 22 (21) | … | … | |
| Wound | 3 (3) | 1 (1) | … | |
| Any site | 55 (52) | 31 (29) | 4.1 (2.02–8.13) | <.001 |
NOTE. Many residents had >1 site colonized. PEG, percutaneous gastrostomy tube.
Among the 49 residents who were found to be colonized at 1 site only, the nares alone was colonized in 15 (38%) of the 39 residents colonized with MRSA and in 4 (40%) of the 10 colonized with MSSA. Among the 39 residents who had MRSA found only at a single site, 8 (21%) were colonized in the oropharynx, 9 (23%) were colonized in the groin or perianal area, and 7 (18%) were colonized at the percutaneously placed gastrostomy tube site.
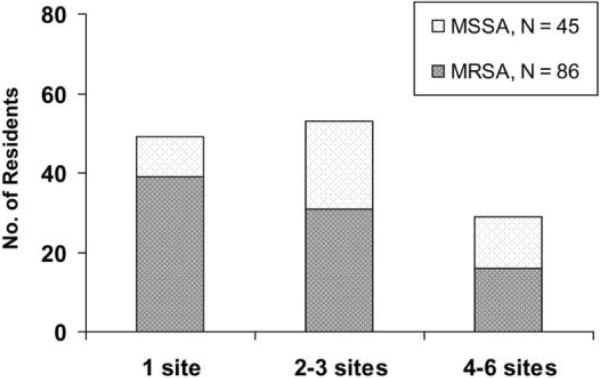
Overall, 33 (73%) of 45 nursing home residents colonized with MSSA alone were colonized at ≥2 sites, and 47 (55%) of the 86 colonized with MRSA were colonized at ≥2 sites (figure 1). Residents in the device group were more likely to be colonized at multiple sites than were those in the control group. For example, all 16 residents with MRSA colonization at ≥4 sites had an indwelling device (table 2).
Figure 1.
Frequency of colonization at multiple sites with methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) among community nursing home residents.
Table 2.
Methicillin-resistant Staphylococcus aureus (MRSA) colonization patterns in nursing home residents.
| No. of sites with MRSA carriage | No. of residents | No. (%) of residents with a device in place | Site |
|||||
|---|---|---|---|---|---|---|---|---|
| Nares | Oropharynx | Groin | Perianal site | Device | Wound | |||
| 0 | 127 | 50 (39) | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 39 | 22 (56) | 15 | 8 | 4 | 5 | 7 | 0 |
| 2 | 18 | 9 (50) | 14 | 11 | 3 | 5 | 2 | 1 |
| 3 | 13 | 8 (62) | 11 | 6 | 9 | 10 | 3 | 0 |
| 4 | 7 | 7 (100) | 7 | 6 | 6 | 6 | 2 | 1 |
| 5 | 8 | 8 (100) | 8 | 7 | 8 | 8 | 8 | 1 |
| 6 | 1 | 1 (100) | 1 | 1 | 1 | 1 | 1 | 1 |
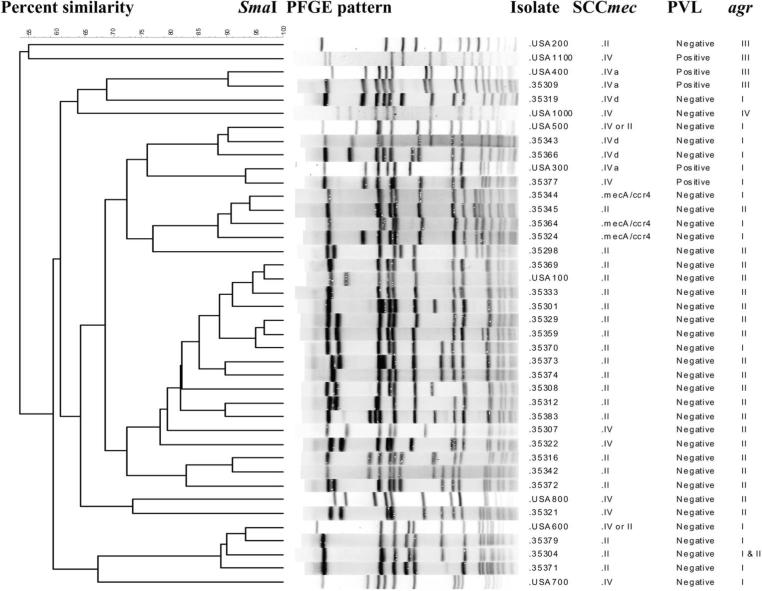
PFGE was performed on 1 isolate recovered from each of the 86 residents who had MRSA. Eleven different strains of MRSA were identified by PFGE in these residents. Representative strains are shown in figure 2. Each nursing home facility had 1–6 different circulating PFGE types. Seventy-three (85%) of the residents were colonized with SCCmec II, which typically is hospital associated; these included 61 USA100 isolates, 3 USA600 isolates, and 9 isolates belonging to PFGE groups other than USA100–1100. All SCCmec II isolates were negative for PVL, and most were agr II.
Figure 2.
PFGE-based dendrogram showing genetic relatedness among representative methicillin-resistant Staphylococcus aureus (MRSA) strains recovered from 40 community nursing home residents. Of 86 residents colonized with MRSA, 73 were colonized with SCCmec II, 8 were colonized with SCCmec IV, and 5 were colonized with mecA, ccr 4. PVL, Panton-Valentine leukocidin.
Eight (9%) of the residents (5 in the device group and 3 in the control group) were colonized with MRSA containing SCCmec IV, which is now increasingly described as a CA-MRSA. These strains were isolated from nares in 2 residents, the perianal area in 3, the oropharynx in 2, and a percutaneously placed gastrostomy tube site in 1. Two of these strains contained PVL: one was USA 300, SCC mec IV, agr I; and the other was USA 400, SCC mec IVa, agr III. Among the remaining 6 SCC mec IV isolates, 2 were USA 500, 1 was USA 100, and 3 were unique strains. Five residents were colonized with mecA ccr 4 strains (figure 2).
DISCUSSION
Ours is among the first studies to conduct an in-depth analysis of multiple sites of MSSA and MRSA colonization in the community nursing home setting. Our data show that extranasal colonization with both MSSA and MRSA is common in nursing home residents. One-third of community nursing home residents who were colonized with MRSA would have been missed if swab sample collection had been confined to nares only. The oropharynx was often the only site colonized, as noted by others who have studied hospitalized patients and outpatients [5, 6]. These results are consistent with previous studies from acute care settings that have found that 25%–50% of colonized patients would not be detected unless multiple sites were sampled at the time of hospital admission [7, 9]. A prospective study of intensive care unit patients who had undergone twice-weekly screening for MRSA carriage found that over 30% of those colonized with MRSA did not have nasal carriage [7], and another study of elderly hospitalized patients noted that 13% of MRSA carriers would have been missed if only the nares had been sampled [8].
Identifying a group of persons at high risk of extranasal colonization could help target laboratory and clinical resources. However, data on what constitutes a high-risk population for extranasal colonization with MRSA are lacking. Our study helps define a group that appears to be at risk of extranasal colonization; 23% of residents in the device group were colonized with MRSA only in their groin and perianal regions, and 21% were colonized only in the oropharynx. Patients with indwelling devices also were more likely to be colonized at multiple sites. Whether this might lead to increased risk of transmission of MRSA to others in the nursing home or might lead to a greater risk of infection in these residents is not known.
Emergence of CA-MRSA has become a major problem, often presenting as severe and recurrent skin and soft-tissue infec tions. In general, nursing home residents are colonized with hospital-acquired MRSA, typically SCC mec II. However, reports of CA-MRSA in nursing homes are slowly emerging [25–29]. In San Francisco, California, 12 (3.8%) of 318 CA-MRSA clinical isolates originated from nursing homes [25]. A cluster of infections due to SCCmec V MRSA was noted in 5 of 76 residents in a nursing home in Finland [29], and another nursing home in Germany reported that 7.6% of 197 residents were found to be colonized with PVL-containing MRSA strains [28]. A study of 949 residents in residential and continuum care facilities in Hong Kong noted that 23 (2.4%) were colonized with SCC mec IV or V MRSA strains [27]. In our study, 8 MRSA-colonized residents (9%) were colonized with SCCmec IV strains, including 2 residents who were colonized with PVL-positive strains, suggesting spread of CA-MRSA into southeastern Michigan nursing homes. The emergence of these strains in this setting will likely alter the selection of empirical antibiotic therapies for skin and soft-tissue infections and could portend increasing problems with the severity of staphylococcal infections in these often debilitated patients.
The major strength of this study—one that makes it generalizable—is that it focuses on residents from multiple community nursing homes. Most previous studies of colonization with MRSA and other drug-resistant organisms have been conducted in Veterans Affairs facilities; thus, it was unclear how applicable the findings were to community nursing homes, which care for the majority of older adults who require long-term care [13, 30, 31]. Our study has a few limitations. The cross-sectional study design does not allow one to determine a temporal relationship between extranasal MRSA colonization and the presence of indwelling devices and does not allow evaluation of the duration of colonization at various body sites. In addition, this was not a point prevalence survey of all nursing home residents. Therefore, we cannot comment on the extent to which CA-MRSA colonization occurs in community nursing home residents in southeastern Michigan but can only raise the emergence of this organism as a concern in the long-term care setting.
Acknowledgments
Financial support. National Institute of Aging Patient-Oriented Career Development Award, ASP/AGS T. Franklin Williams Research Scholarship, and Veterans Affairs Merit Review Program
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Kluytmans JA, Wertheim HF. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection. 2005;33:3–8. doi: 10.1007/s15010-005-4012-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith AJ, Robertson D, Tang MK, et al. Staphylococcus aureus in the oral cavity: a three-year retrospective analysis of clinical laboratory data. Br Dent J. 2003;195:701–3. doi: 10.1038/sj.bdj.4810832. [DOI] [PubMed] [Google Scholar]
- 3.Smith AJ, Jackson MS, Bagg J. The ecology of staphylococci in the oral cavity: a review. J Med Microbiol. 2001;50:940–6. doi: 10.1099/0022-1317-50-11-940. [DOI] [PubMed] [Google Scholar]
- 4.Williams REO. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenner J, O'Connor T, Piantanida N, et al. Rates of carriage of methicillin-resistant Staphylococcus aureus in an outpatient population. Infect Control Hosp Epidemiol. 2003;24:439–44. doi: 10.1086/502229. [DOI] [PubMed] [Google Scholar]
- 6.Mertz D, Frei R, Jaussi B, et al. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis. 2007;45:475–7. doi: 10.1086/520016. [DOI] [PubMed] [Google Scholar]
- 7.Eveillard M, Lassence AD, Lancien E, et al. Evaluation of a strategy of screening multiple anatomical sites for methicillin-resistant Staphylococcus aureus at admission to a teaching hospital. Infect Control Hosp Epidemiol. 2006;27:181–4. doi: 10.1086/500627. [DOI] [PubMed] [Google Scholar]
- 8.Simor AE, Ofner-Agostini M, Paton S, et al. Clinical and epidemiologic features of methicillin-resistant Staphylococcus aureus in elderly hospitalized patients. Infect Control Hosp Epidemiol. 2005;26:838–41. doi: 10.1086/502503. [DOI] [PubMed] [Google Scholar]
- 9.Lucet JC, Chevert S, Durand-Zaleski I, et al. Prevalence and risk factors for carriage of methicillin-resistant S. aureus at admission to the intensive care unit: results of a multicenter study. Arch Intern Med. 2003;163:181–8. doi: 10.1001/archinte.163.2.181. [DOI] [PubMed] [Google Scholar]
- 10.Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446–53. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 11.Cook HA, Furuya EY, Larson E, et al. Heterosexual transmission of community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2007;44:410–3. doi: 10.1086/510681. [DOI] [PubMed] [Google Scholar]
- 12.Terpenning MS, Bradley SF, Wan JY, et al. Colonization and infection with antibiotic-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 1994;42:1062–9. doi: 10.1111/j.1532-5415.1994.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 13.Bradley SF, Terpenning MS, Ramsey MA, et al. Methicillin-resistant Staphylococcus aureus: colonization and infection in a long-term care facility. Ann Intern Med. 1991;115:417–22. doi: 10.7326/0003-4819-115-6-417. [DOI] [PubMed] [Google Scholar]
- 14.Tada A, Watanabe T, Yokoe H, et al. Oral bacteria influenced by the functional status of elderly people and the type and quality of facilities for the bedridden. J Appl Microbiol. 2002;93:487–91. doi: 10.1046/j.1365-2672.2002.01702.x. [DOI] [PubMed] [Google Scholar]
- 15.Mody L, Maheshwari S, Galecki A, et al. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc. 2007;55:1921–6. doi: 10.1111/j.1532-5415.2007.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehnert MJ, Kruszon-Moran D, Hill HA, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193:172–9. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 17.Hidron AI, Kourbatova EV, Halvosa S, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus in patients admitted to an urban hospital: emergence of community-associated MRSA carriage. Clin Infect Dis. 2005;41:159–66. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 18.McDougal LK, Steward CD, Killgore GE, et al. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenover FC, McDougal LK, Goering RV, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44:108–11. doi: 10.1128/JCM.44.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin–producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 21.Okuma K, Iwakawa K, Turnidge JD, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo Y, Ito T, Ma XX, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007;51:264–74. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witte W, Enright M, Schmitz FJ, et al. Characteristics of a new epidemic MRSA in Germany ancestral to United Kingdom EMRSA 15. Int J Med Microbiol. 2001;290:677–82. doi: 10.1016/S1438-4221(01)80006-0. [DOI] [PubMed] [Google Scholar]
- 24.Strommenger B, Cuny C, Werner G, Witte W. Obvious lack of association between dynamics of epidemic methicillin-resistant Staphylococcus aureus in Central Europe and agr specificity groups. Eur J Clin Microbiol Infect Dis. 2004;23:15–9. doi: 10.1007/s10096-003-1046-8. [DOI] [PubMed] [Google Scholar]
- 25.Carleton HA, Diep BA, Charlebois ED, et al. Community-adapted methicillin-resistant Staphylococcus aureus: population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190:1730–8. doi: 10.1086/425019. [DOI] [PubMed] [Google Scholar]
- 26.Brady JM, Stemper ME, Weigel A, et al. Sporadic “transitional” community-associated methicillin-resistant Staphylococcus aureus strains from healthcare facilities in the United States. J Clin Microbiol. 2007;45:2654–61. doi: 10.1128/JCM.02579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho P, Wang TK, Ching P, et al. Epidemiology and genetic diversity of methicillin-resistant Staphylococcus aureus strains in residential care homes for elderly persons in Hong Kong. Infect Control Hosp Epidemiol. 2007;28:671–8. doi: 10.1086/517951. [DOI] [PubMed] [Google Scholar]
- 28.Raab U, Kahlau D, Wagenlehner F, et al. Prevalence of and risk factors for carriage of Panton-Valentine leucocidin-positive methicillin-resistant Staphylococcus aureus among residents and staff of a German nursing home. Infect Control Hosp Epidemiol. 2006;27:208–11. doi: 10.1086/500629. [DOI] [PubMed] [Google Scholar]
- 29.Kerttula AM, Lyytikainen O, Vuipio-Varkila J, et al. Molecular epidemiology of an outbreak caused by methicillin-resistant Staphylococcus aureus in a healthcare ward and associated nursing home. J Clin Microbiol. 2005;43:6161–3. doi: 10.1128/JCM.43.12.6161-6163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strausbaugh LS, Jacobson C, Sewell DL, et al. Methicillin-resistant Staphylococcus aureus in extended care facilities: experiences in a Veterans' Affairs nursing home and a review of literature. Infect Control Hosp Epidemiol. 1991;12:36–45. doi: 10.1086/646236. [DOI] [PubMed] [Google Scholar]
- 31.Muder RR, Brennen C, Wagener MM, et al. Methicillin resistant staphylococcal colonization and infection in a long-term care facility. Ann Intern Med. 1991;114:107–12. doi: 10.7326/0003-4819-114-2-1-107. [DOI] [PubMed] [Google Scholar]