Abstract
Aging is associated with suboptimal CD8 T cell responses to viral infections. It is not clear if these poor responses are due to environmental influences or quantitative and qualitative changes in the pool of responding CD8 T cells. Our studies demonstrated several deleterious age-related changes in the pool of antigen-specific CD8 T cells that respond to infection. The majority of CD8 T cells from uninfected aged mice were CD44Hi and had increased expression of inhibitory receptors including PD1, LAG3, 2B4 and CD160. These aged CD44Hi CD8 T cells were transcriptionally similar to exhausted CD8 T cells found during chronic infections. In addition, the number of virus-specific precursors in aged mice prior to infection was decreased up to 10 fold and many of these antigen-specific precursors had high expression of CD44 and PD1. Finally, TCR transgenic studies demonstrated that the CD44Hi antigen-specific CD8 T cells from unimmunized aged and young mice were qualitatively inferior compared to CD44Lo CD8 T cells from aged or young donors. Thus, a decrease in precursor frequency as well as qualitative changes of CD8 T cells during aging are directly related to impaired immunity.
Introduction
Aging is associated with a decline in T cell function and an increase in susceptibility to infections. Some of the immunological changes associated with aging include reduced thymic output, a decrease in naïve T cells, expansion of memory phenotype CD8 T cells, cellular senescence, changes in telomerase expression or telomere length, reduced proliferation, altered signaling, and reduced IL-2 production (1–5). Two prominent age-associated changes, the involution of thymus and a global shift of T cell phenotype from naïve to memory, lead to a decrease in the number of naïve T cells (CD44Lo) and an increase in the proportion of T cells with memory phenotype (CD44Hi) (6, 7). Several studies showed that the transfer of CD44Lo CD8 T cells into RAG-1−/−recipients (lymphopenic environment) results in the transition to a CD44Hi memory phenotype within 3 weeks. By transferring different numbers of naïve (CD44Lo) cells to syngeneic RAG-1−/− recipients, the transition to a memory (CD44Hi) phenotype was demonstrated to be directly proportional to the extent of homeostatic proliferation (8) suggesting that the gradual decline in new naïve T cell production during aging could lead to a gradual increase in homeostatic proliferation of the existing T cell pool. At least in young mice, T cells generated during lymphopenia-induced homeostatic proliferation can have increased functionality after in vivo antigen stimulation compared to naïve CD44Lo T cells (9) and these homeostatically induced CD44Hi T cells provided protective immunity to bacterial infections (10). However, two subpopulations of proliferating T cells exist under lymphopenic conditions, a slow proliferative subset and a subset that rapidly proliferates to CFSE negative (11). Interestingly, the rapidly proliferative subset is thought to be driven by environmental and/or self antigens and upregulates the inhibitory receptor PD-1 (12). It is unclear, however, how changes in the T cell compartment prior to infection, whether driven by lymphopenia or other signals, impact antiviral immunity.
A diverse repertoire of naive T cells is essential for a vigorous response to infection (13, 14). A loss of naïve T cells can result in a decline in naïve antigen-specific precursors and this decline can impact the generation of an effective immune response (15, 16). Moreover, clonal expansions of memory-phenotype T cells have been observed in mice, primates and humans (17). These expansions are believed to be driven by chronic infections, altered cytokine milieu of the host, or both and could further restrict the T cell repertoire and/or compete with other T cell responses (18). Thus, changes in the global T cell pool and/or changes in T cell competition might account for some changes in T cell immunity observed with age.
Although less well documented, cell-intrinsic changes of naïve T cells could also contribute to impaired immunity with aging. Recently, several studies observed phenotypic alterations in the CD4 T cell pool in aged mice including changes in the expression of several molecules such as PD1, ICOS, CTLA4 and KLRG1, though the consequences of these phenotypic changes remain poorly understood (19–21). Recent studies also demonstrated that decreased thymic output with age leads to an accumulation of long-lived naïve CD4 T cells that express low amounts of Bim and have functional defects (22). Less information is available regarding the impact of aging on the naïve CD8 T cell pool.
Here we demonstrated that aging in mice was associated with an increase in the expression of several inhibitory receptors including PD1, LAG3, 2B4 and CD160 on CD8 T cells. This was especially pronounced on the CD44Hi population of CD8 T cells from uninfected aged mice with these changes first becoming apparent sometime between 8 to 12 months of age concurrent with a shift from CD44Lo to predominantly CD44Hi T cells. We also demonstrated that the precursor frequency of virus-specific CD8 T cells from uninfected aged mice declines by 10-fold or more. Moreover, aging resulted not just in a decreased precursor frequency but also in changes in the quality of these precursors. Many antigen-specific CD8 T cell precursors were CD44Hi and PD1 positive. Transcriptional profiling of total CD44Hi CD8 T cells from naïve aged mice revealed strong similarities to exhausted CD8 T cells found during chronic infections. Studies with aged and young TCR transgenic T cells indicated substantial proliferative defects of CD44Hi T cells from uninfected mice. These studies suggest both quantitative and qualitative defects in the pool of antigen-specific CD8 T cells prior to immunization or infection in the elderly and point to possible opportunities for reversing such age-related defects.
Materials and Methods
Mice
Female mice were purchased from the Taconic at either 3–5 months or 8-months of age and then aged to 12–14-months- and 17–22-months-old (aged mice) in the Wistar Institute aging colony. Additional female mice were purchased from Taconic at 2–3 months of age. These mice were housed in the same colony but used when 3–5-months old (young mice). All animals in the Wistar aging colony were housed in micro-isolator cages at the AAALAC-approved Animal Care Facility at the Wistar Institute and screened by an independent commercial service (Charles River) every 6 months for a panel of 17 rodent pathogens including LCMV and mouse norovirus (MNV). All screens for rodent pathogens over the 5 years of existence of this aging colony were negative. In an independent set of studies, aged mice (17–22 months old) were obtained from the aging colony at the National Institutes on Aging (NIA) and examined directly upon receipt. Aged animals with obvious cancer or lymphoma were excluded from the studies. For all adoptive transfer experiments, congenic mice (obtained from National Cancer Institute) differing in Ly5 (Ly5.1 versus Ly5.2) or Thy (Thy1.1 versus Thy1.2) were used. P14 TCR transgenic mice were bred at the Wistar Institute. OT1 Rag2−/− female mice were obtained from Taconic at 2 months of age. All mice were used in accordance with Institutional Animal Care and Use Committee guidelines.
Adoptive transfer, virus and infection
CD8 T cells from young and aged P14 Ly5.1 or P14 Thy1.1 mice were purified (>90% purity) using magnetic beads (CD8+ T cell isolation kit, MACS beads; Miltenyi Biotec) and then sorted based on CD44 expression. After sort, 5×103 P14 (DbGP33-specific) CD8 T cells from each group (young CD44Lo, young CD44Hi, aged CD44Lo and aged CD44Hi) were transferred i.v. into wild type recipient mice (Ly5.2+Thy1.2+) which were then infected with LCMV Armstrong (LCMV Arm, 2×105 pfu) i.p. Donor populations were monitored in the peripheral blood by retro-orbital blood collection as described (23). Similarly, CD8 T cells from young OT1 Rag2−/− mice were purified using magnetic beads (CD8+ T cell isolation kit, MACS beads; Miltenyi Biotec) and then sorted based on CD44 expression. After sort, 15×103 OT1 (KbOVA-specific) CD8 T cells from each group (young CD44Lo and young CD44Hi) were transferred i.v. into wild type recipient mice (Ly5.2+) which were then infected with recombinant vesicular stomatitis virus expressing OVA (VSV-OVA, 2×106 pfu) i.v. Donor populations were monitored in the peripheral blood by retro-orbital blood collection as described (23).
Isolation of lymphocytes from tissues
Lymphocytes were isolated from peripheral blood and spleens as described (24).
Tetramer staining and magnetic bead sorting
Spleens were isolated from uninfected mice at 4, 7, 12, 14, 17 and 19 months of age; tetramer staining (DbGP33 or KbOVA) and magnetic bead sorting was done as previously described (9, 25). CD8 and CD3 were used as positive gates. B220, CD19, CD4, CD11b, CD11c and MHC II were used as dump gates. In addition to tetramer staining, samples were stained for CD44 and PD1.
Flow cytometry, intracellular cytokine staining and BrdU staining
Lymphocytes were stained using standard techniques and analyzed by flow cytometry. Virus-specific CD8 T cells were quantified using MHC class I peptide tetramer staining. MHC class I peptide tetramers were made and used as described (23). Antibodies to CD8, CD44, PD1 (RPMI-30), CD3ε (145-2C11) and CD28 (37.51) were purchased from Biolegend (San Diego, CA). Antibodies to LAG3, 2B4, CD160 and IFNγ were purchased from eBioscience (San Diego, CA). Antibodies to CD4, Mip1α, KLRG1 and Tbet were purchased from Invitrogen (Carlsbad, CA), R&D Systems (Minneapolis, MN), Beckman Coulter (Fullerton, CA) or Santa Cruz Biotechnology (Santa Cruz, CA), respectively. All other antibodies were purchased from BD Biosciences (San Diego, CA). Staining and analysis were performed as previously described (23).
Function was investigated by intracellular cytokine staining following stimulation. Briefly, 1×106 splenocytes were cultured in the absence or presence of the anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) for 5 h at 37°C. Following staining for surface antigens as described above, cells were stained for intracellular cytokines (IFNγ, IL-2, Mip1α) using the Cytofix/Cytoperm kit (BD Biosciences).
For BrdU analysis, mice received a single intraperitoneal injection (1 mg/mouse) of BrdU 12 h before sacrifice. BrdU staining was carried out using a BrdU Flow Kit (BD Pharmingen) in accordance with the manufacturer’s instructions. BrdU was detected using FITC-conjugated anti-BrdU antibody.
Samples were collected using a LSR II flow cytometer (Becton Dickinson, San Jose, CA).
Cell Sorting and Microarray Analysis
CD8 T cells from uninfected young and aged mice were purified (>90% purity) using magnetic beads (CD8+ T cell isolation kit, MACS beads; Miltenyi Biotec) and then sorted directly into TriZol (GIBCO/BRL Life Technologies, Rockville, MD) based on CD44 expression. Purity was assessed by FACS analysis, and was greater than 95–98% for all samples. Total RNA was isolated from sorted CD44Hi CD8 T cells from uninfected young and aged mice using TriZol according to the manufacturer’s instructions. cDNA was fragmented, labeled and amplified as previously described (26). Samples were hybridized to the Affymetrix GeneChip Mouse Exon 1.0 ST arrays (Affymetrix, Santa Clara, CA) at the University of Pennsylvania Microarray Core Facility. Transcript levels were summarized from the Affymetrix-CEL files using the RMA (Robust Multichip Average) algorithm (27), as implemented by the Affymetrix Power Tool apt-probeset-summarize. There were four technical replicates for each young and aged CD44Hi population. Differentially expressed genes were identified using the Class Neighbors module of GenePattern (28), and the Significance Analysis of Microarrays (SAM) algorithm (29). GSEA was performed as described previously (26).
Statistical analysis
Data were analyzed using a two-tailed Student’s t-test and a p value of 0.05 was considered significant. For microarray data analysis we used the Class Neighbors and SAM algorithms as described previously (28, 29).
Results
Phenotypic changes of T cells from uninfected mice during aging
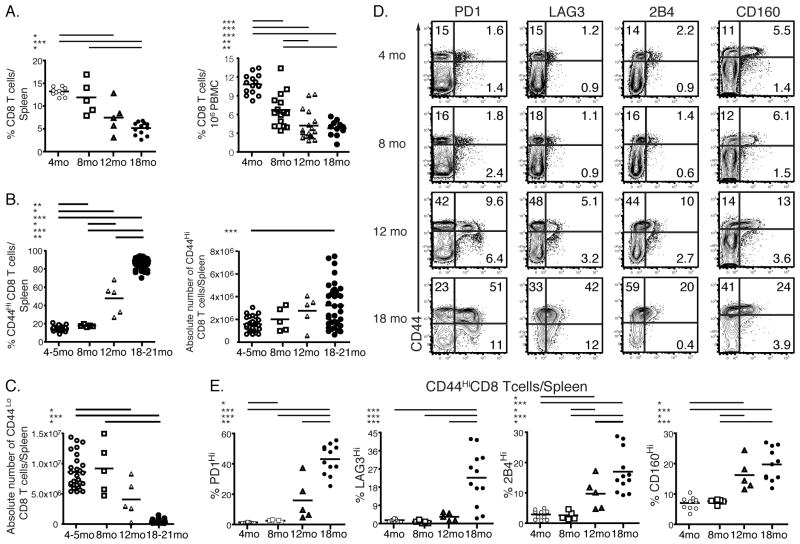
To begin to understand age-related changes in antiviral immunity, we analyzed the percentages and numbers of T cells in mice at different times in their lifespan. The analysis of mice at 4, 8, 12 and 18 months of age demonstrated that the percentages and numbers of CD8 T cells were diminished with age. This was true in both spleen and blood (Fig. 1A and Supplemental Fig. 1A) consistent with previous studies (30–33). Moreover, we demonstrated that a previously observed global shift from naïve to memory phenotype T cells (13, 34, 35) that results in an increase in the frequency and number of CD44Hi T cells and a concomitant decrease in the number of CD44Lo naïve T cells started as early as 8–12 months of age (Fig. 1A, B and C).
FIGURE 1.
Age-associated changes in CD8 T cells. Frequencies of CD8 T cells were analyzed in the spleens and blood of uninfected mice at 4, 8, 12 and 18 months of age (A). The percentages and numbers of CD44Hi and CD44Lo CD8 T cells in the spleens of uninfected mice at age of 4, 8, 12 and 18 months are shown in B and C, respectively. CD8 T cells were isolated from uninfected young and aged spleens at 4, 8, 12 and 18 months of age and stained for the expression of inhibitory receptors PD1, LAG3, 2B4 and CD160. The representative graphs of individual stains of CD8 T cells from spleen are shown in D. The percentages of different inhibitory receptors on CD44Hi CD8 T cells from spleens are shown in E. 1.48 ± 0.09% (Mean ± SEM) of CD44Hi CD8 T cells in the spleens of 4 months old mice are PD1 positive compared to 2.58 ± 0.33%, 15.93 ± 6.12%, 43.18 ± 2.81% of PD1 positive CD44Hi CD8 T cells from the spleens of 8, 12 and 18 months old mice, respectively. Data are representative of 2 independent experiments including 12–20 mice/experiment. Some data are representative of several pooled experiments * = 0.05 > P > 0.01, ** = 0.01 > P > 0.001, *** = P < 0.001 by unpaired two-tailed t test.
We next examined the phenotype of T cells from the spleen and blood of uninfected aged and young mice and found an increase in the expression of KLRG1 and Tbet for CD8 T cells from uninfected aged mice (Supplemental Fig. 1B and C) which could reflect senescence or terminal differentiation as previously reported (36, 37). Expression of other markers such as CD44, CD62L and CD49d (α4 integrin) was also altered (data not shown), but one of the most striking observations was the difference in expression of inhibitory receptors between CD8 T cells from uninfected aged and young mice. In particular, CD44Hi CD8 T cells from aged mice had higher expression of PD1, LAG3, 2B4 and CD160 compared to CD8 T cells from young mice (Fig. 1D). The upregulation of inhibitory receptor expression started between 8–12 months of age and became more prominent between 12 and 18 months (Fig. 1E and Supplemental Fig. 1D). Mean fluorescence intensity (MFI) was also increased for both PD1 and LAG3 on CD44Hi CD8 T cells from 12 and 18 months old mice (Supplemental Fig. 1E and F). While the MFI was unchanged (for 2B4) or decreased (for CD160) (Supplemental Fig. 1E and F), the percentages of CD44Hi cells that were 2B4 and CD160 positive increased at 12 and 18 months of age (Fig. 1D and E and Supplemental Fig. 1D). The change in inhibitory receptors expression with age was also evident in the total population of CD8 T cells, though most of the increased expression of PD1, LAG3, 2B4 and CD160 was restricted to the CD44Hi subset (Fig. 1D and data not shown). While many of these inhibitory receptors are involved in self-tolerance and are upregulated by self-reactive T cells (38), these molecules also can be induced during chronic infections (39). Mice in our aging colony were routinely screened for common rodent pathogens and were always negative for all agents tested including LCMV and MNV (data not shown). Moreover, we observed a similar upregulation of inhibitory receptors on T cells from an independent source of aged mice by examining mice from the aging colony operated by the National Institutes on Aging (Supplemental Fig. 1G-H).
The increase in the inhibitory receptors on CD8 T cells from aged mice is not due exclusively to oligoclonal expansions
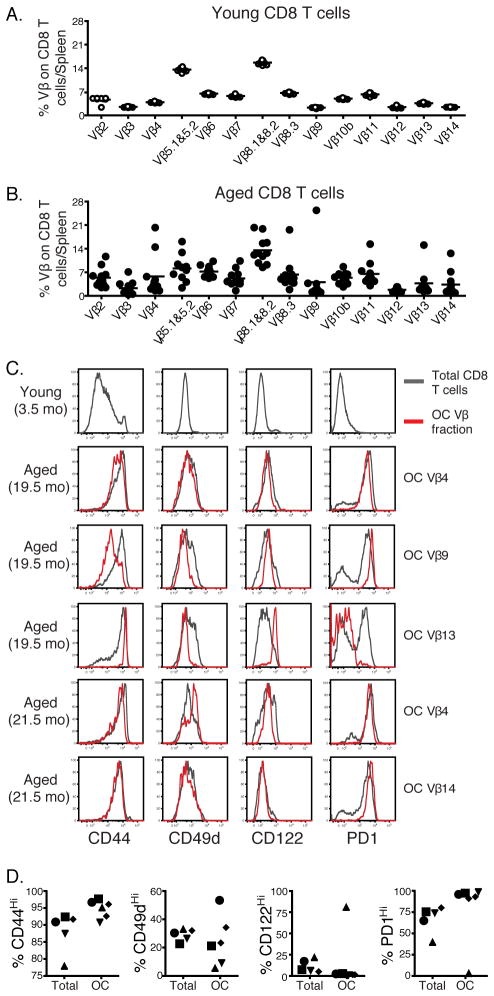
To investigate if the increase in inhibitory receptors on CD8 T cells from aged mice was due to oligoclonal expansions, which have been associated with aging (17), we examined T cell receptor (TCR) Vβ expression on CD8 T cells from young (4 months old) and aged (20–22 months old) uninfected mice. Aged mice had higher variability in the expression of Vβ receptors compared to young animals in agreement with prior work (Fig. 2A and B) (40) and in some mice the clonal expansions of certain Vβ receptors (e.g. Vβ 4, Vβ 8.3, Vβ 9, Vβ 13 and Vβ 14) were detected. While modest clonal expansions were occasionally present, they represented at most a quarter and usually < 10% of the total CD8 T cell pool (Fig. 2B). Oligoclonally expanded populations were examined for the expression of different markers including CD44, CD49d (α4 integrin), CD122 and PD1. The expression profile of these markers within each Vβ fraction was then compared with the expression profiles on the remaining CD8 T cells within the same mouse (Fig. 2C and D). The expression patterns of these markers on oligoclonally expanded populations were variable and ranged from indistinguishable to slightly or very different from the total CD8 T cell pool, depending on the specific oligoclonal expansion and marker tested. Thus, these data suggested that the increase in the inhibitory receptors on CD8 T cells from aged mice was not due exclusively to oligoclonal expansions.
FIGURE 2.
The increase in the inhibitory receptors on CD8 T cells from aged mice is not due exclusively to oligoclonal expansions. CD8 T cells were isolated from spleens of uninfected young (4 months old, white circles) and aged (20–21 months old, black circles) mice and were stained for panel of different Vβ receptors. The percentages of different Vβ populations on young and aged naïve CD8 T cells are shown in panels A and B, respectively. A clonal expansion was defined as at least 3 standard deviations above the mean Vβ use in young mice. Panel C shows histograms of different markers on total or oligoclonally expanded population of CD8 T cells from one young and several aged mice. Panel D shows the percentages of expression of the same markers as shown in C on total and oligoclonally expanded cells. Data are representative of 2 independent experiments including 10 mice/experiment. * = 0.05 > P > 0.01, ** = 0.01 > P > 0.001, *** = P < 0.001 by unpaired two-tailed t test.
Age-related changes in antigen-specific CD8 T cell precursor numbers and phenotype in uninfected mice
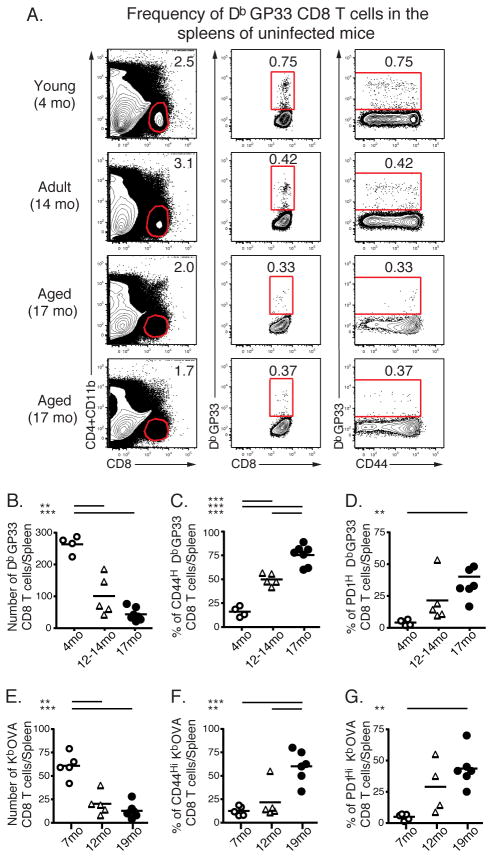
In young animals the majority of antigen-specific but inexperienced CD8 T cells are CD44Lo. Given the loss in CD44Lo CD8 T cells in aged mice, we next investigated the number and phenotype of virus-specific CD8 T cells in aged mice prior to infection. Using MHC class I/peptide tetramer enrichment staining as previously described (9, 25), we compared the numbers of DbGP33-specific and KbOVA-specific CD8 T cell precursors in the spleens of young and aged uninfected mice. Young mice (4 months old) contained ~260 DbGP33-specific precursors/spleen consistent with previous estimates using this (41) and other techniques (42) (Fig. 3A and B). This number declined in mice 12–14 months old and was further reduced by 17 months. Aged mice (17 months) exhibited at least a 10-fold reduction in the number of DbGP33-specific CD8 T cell precursors per spleen (~20–30 DbGP33-specific precursors/spleen). Similarly, the number of KbOVA-specific CD8 T cell precursors in the spleens of aged mice was significantly reduced compared to the number of these precursors in the spleens of young mice. Young mice (7 months old) contained ~61 KbOVA-specific precursors/spleen, while in aged mice (19 months) the number of KbOVA-specific CD8 T cell precursors per spleen was ~13 (Fig. 3E). These observations suggest that to reach the same numbers of antigen-specific T cells at the peak of the response, virus-specific CD8 T cells in aged mice would have to undergo at least 3 extra rounds of division, assuming antigen-specific precursors in aged mice are qualitatively similar to those in young mice. To investigate this second issue, we examined differentiation markers expressed by virus-specific precursors in uninfected aged versus young mice. In aged mice a higher proportion of DbGP33- or KbOVA-specific precursors were CD44Hi (Fig. 3C and 3F), while the vast majority of these DbGP33- or KbOVA-specific precursors in young mice were CD44Lo. In addition, many of the DbGP33- or KbOVA-specific aged CD8 T cells expressed increased levels of PD1 (Fig. 3D and 3G). Thus, not only were the numbers of antigen-specific precursors declining with age, but the remaining antigen-specific precursors were phenotypically altered.
FIGURE 3.
Age-related changes in antigen-specific CD8 T cell precursor numbers and phenotype in naïve mice. Spleens of uninfected mice at 4, 7, 12, 14, 17 and 19 months of age were stained with DbGP33- or KbOVA- tetramers. Representative staining for DbGP33-specific precursors are shown in A. The absolute numbers of naïve DbGP33-specific precursors at different ages are shown in B. C and D show the percent of expression of CD44 and PD1 on DbGP33-specific precursors, respectively. The absolute numbers of naïve KbOVA-specific precursors at different ages are shown in E. F and G show the percent of expression of CD44 and PD1 on KbOVA-specific precursors, respectively. Data are from two independent experiments and are displayed as the pooled results from both. * = 0.05 > P > 0.01, ** = 0.01 > P > 0.001, *** = P < 0.001 by unpaired two-tailed t test.
Functional changes of T cell populations from naïve mice during aging
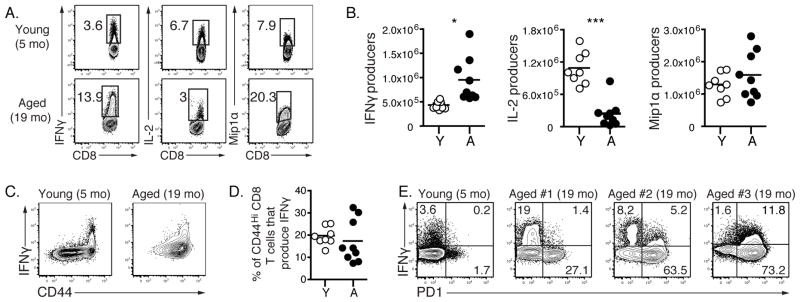
We next investigated if the changes in the phenotype and precursor frequency of the CD8 T cell pool from aged uninfected mice were accompanied by changes in the functionality of these cells. T cells from young and aged naïve mice were stimulated in vitro using anti-CD3 and anti-CD28 antibodies and cytokine production examined. During polyclonal stimulation there were numerically more IFNγ producers in aged mice (Fig. 4A and B). Young and aged CD8 T cells produced similar amounts of Mip1α, but CD8 T cells from aged mice were poor producers of IL-2 compared to CD8 T cells from young mice in agreement with previous data examining CD4 T cells from aged mice (43). We failed to detect IL-17 in the in vitro CD8 T cell assay, but we observed that aged CD8 T cells had slightly higher IL-10 production than young CD8 T cells upon activation (data not shown). While both aged and young CD44Hi CD8 T cells produced IFNγ, there were some aged mice in which this function was well preserved and another subset of animals in which the CD8 T cells appeared less functional per cell (Fig. 4C and D). IFNγ production was also compared to PD1 expression. Very little IFNγ was found in the small PD1+ subset of CD8 T cells from young naïve mice (Fig. 4E). While there were some aged mice in which IFNγ was produced by PD1+ CD8 T cells, in general the PD1+ CD8 T cells from aged mice made less IFNγ /cell compared to the PD1lo cells. Overall, these results indicate that aging alters some components of CD8 CD44Hi T cell cytokine responses, though there was clearly heterogeneity with preserved functionality in some mice and perhaps moderately reduced IFNγ production/per cell in other mice.
FIGURE 4.
Functional changes of naïve T cells during aging. CD8 T cells were isolated from the spleens of uninfected young (4–5 months old) and aged (19–20 months old) mice. Splenocytes were stimulated in vitro using anti-CD3 and anti-CD28 antibodies. 5 hours later cells were stained for the production of different cytokines. The representative graphs for IFNγ, IL-2 and Mip1α staining are shown in A. Graphs in B show absolute numbers of young and aged naïve CD8 T cells that produce IFNγ, IL-2 and Mip1α. Representative plots of CD8 T cells show IFNγ production versus CD44 expression (C). The percentages of CD44Hi CD8 T cells that produce IFNγ are shown in D. E is gated on CD8 T cells from young and aged mice and shows IFNγ versus PD1 expression. Data are representative of 2 independent experiments including 8–9 mice/experiment. * = 0.05 > P > 0.01, ** = 0.01 > P > 0.001, *** = P < 0.001 by unpaired two-tailed t test.
The transcriptional profiles of CD44Hi CD8 T cells from uninfected aged mice indicate a signature similar to that of exhausted CD8 T cells
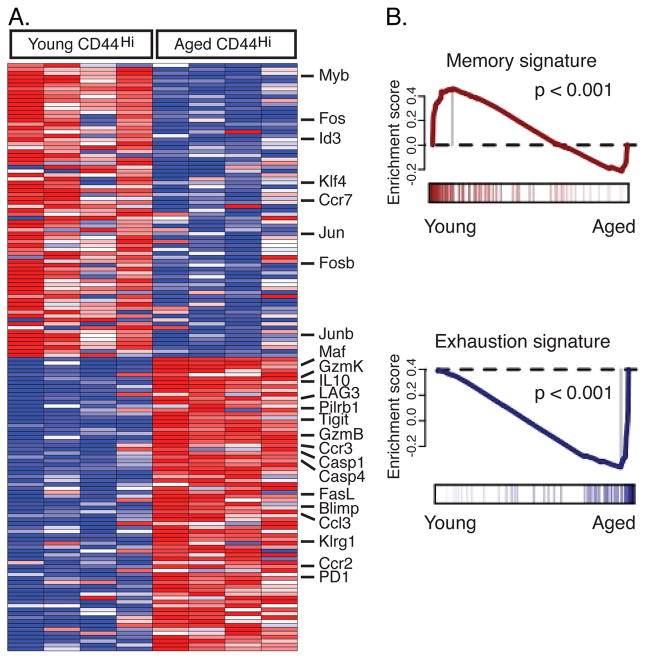
We next examined the global gene-expression profiles of CD44Hi CD8 T cells from young and aged mice. CD44Hi CD8 T cells from young and aged uninfected mice showed differential expression of a number of transcripts known to play important roles in CD8 T cells (Fig 5A and Supplemental Table 1 and 2). Using both the Class Neighbors and the Significance Analysis of Microarrays algorithms we identified many transcripts that have also been implicated in modulating CD8 T cell exhaustion or dysfunction during chronic infections (e.g. PD1, LAG3, IL-10, Blimp-1; Table S1 and S2) (26). To further probe the similarities between aging and exhaustion, we used Gene Set Enrichment Analysis (GSEA) to evaluate the global similarity between our samples and a previously defined signature of exhausted CD8 T cells (26). There was a clear enrichment of the transcriptional signature of CD8 T cell exhaustion in the aged CD44Hi CD8 T cells, with a p < 0.001 (Fig. 5B). Moreover, there is a corresponding bias of a similarly defined memory signature toward the young CD44Hi CD8 T cell samples, p < 0.001. Together, these gene expression data indicate that aging is associated with a transcriptional program in CD8 T cells that has similarities to that found in exhausted T cells. One notable set of differences, however, was the increase in KLRG1 and Tbet expression by CD8 T cells from aged mice. In contrast, expression of these molecules is reduced in exhausted CD8 T cells during chronic LCMV infection (26, 44). Nonetheless, inhibitory receptors, cell death pathways and transcription factors were identified as specific genes and pathways and may provide important targets for future analysis.
FIGURE 5.
The transcriptional profiles of CD44Hi CD8 T cells from uninfected aged mice indicate a signature similar to that of exhausted CD8 T cells. CD8 T cells were isolated from spleens of uninfected young (4 months old) and aged (20–21 months old) mice and sorted based on CD44 expression. RNA was isolated and hybridized to the Affymetrix GeneChip Mouse Exon 1.0 ST arrays. ClassNeighbors analysis was performed to identify genes that differentiate CD44Hi CD8 T cells from uninfected young and aged mice (n=4 for each set, A). To evaluate the global similarity to previously defined signatures of CD8 T cell memory and exhaustion we performed Gene Set Enrichment Analysis (GSEA) of CD44Hi CD8 T cells from uninfected young and aged mice (B). There is a clear enrichment of the exhaustion signature in the aged CD44Hi CD8 T cell samples, P < 0.001. There is a corresponding bias of a similarly defined memory signature toward the young CD44Hi CD8 T cell samples, P < 0.001.
Poor responsiveness of antigen-specific CD44Hi CD8 T cells from uninfected aged and young mice
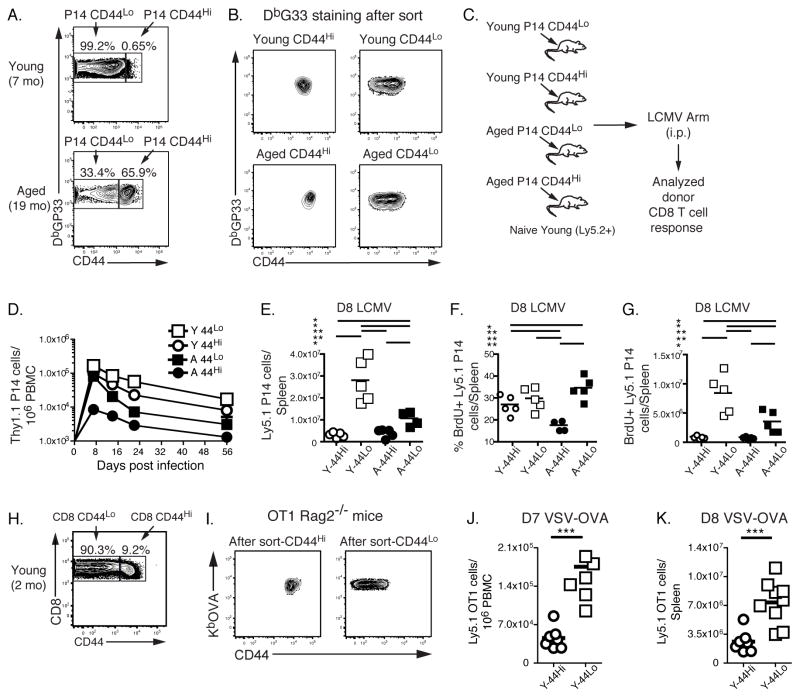
We next examined the impact of qualitative changes in the pool of antigen-specific CD8 T cells from uninfected aged mice in vivo during viral infection. Hence, we isolated DbGP33-specific CD8 T cells from uninfected young and aged P14 TCR transgenic mice that are reactive to the GP33 epitope of LCMV. Note that, while aged P14 mice had a clear population of CD44Hi DbGP33 tetramer positive CD8 T cells, all PD1 expressing CD8 T cells in these mice were tetramer negative (Supplemental Fig. 2). This observation suggests the use of a non-transgenic TCR and perhaps a role for TCR stimulation in age-related increases in PD1 expression. We sorted these P14 CD8 T cells into two groups based on CD44: CD44Lo (naïve CD8 T cells) and CD44Hi (memory-like CD8 T cells, Fig. 6A). To test the impact of a shift from CD44Lo to CD44Hi CD8 T cells with age, equal numbers of either young or aged CD44Lo and CD44Hi P14 cells were purified and adoptively transferred to young congenic recipient mice that were subsequently challenged with LCMV (Fig. 6C). While there was clearly a hierarchy in the responsiveness of these different donor populations (Fig. 6D and E), the CD44Hi P14 cells from aged mice were 10–20 fold less efficient in the initial expansion and accumulated poorly in the blood compared to the other populations. Also, a lower percentage of these CD44Hi aged donor cells incorporated BrdU compared to young P14 cells (17.3 ± 1.2% vs. 26.7 ± 1.9%, P = 0.0056, Figure 6F and G). Despite perhaps a slight disadvantage of CD44Lo CD8 T cells from aged mice in expansion (Fig 6E), we found similar BrdU incorporation between young CD44Lo and aged CD44Lo cells (~ 29.6 ± 2.3% and 34.4 ± 2.3%, respectively). We also observed that, in the spleen, CD44Hi P14 cells from young donors had moderately diminished responses compared to CD44Lo cells from same donors though CD44Hi P14 cells from aged mice clearly had the most defective proliferative expansion of the populations examined (Fig. 6E and G). Previous studies indicated that CD44Hi CD8 T cells generated by homeostatic proliferation in lymphopenic hosts responded more vigorously in response to bacterial challenge compared to CD44Lo CD8 T cells (10). We therefore extended our studies of naturally arising CD44Hi CD8 T cells from young mice to another setting. KbOVA-specific CD8 T cells were isolated from uninfected young OT1 TCR transgenic mice on Rag2−/−background (OT1 Rag2−/− mice). In OT1 Rag2−/− mice all CD8 T cells are OVA-specific. We sorted these CD8 T cells into CD44Lo (naïve CD8 T cells) and CD44Hi (memory-like CD8 T cells, Fig. 6H). Equal numbers of purified CD44Lo and CD44Hi OVA-specific CD8 T cells from young mice were adoptively transferred to young congenic recipient mice and those recipients were infected with vesicular stomatitis virus expressing OVA. Once again naturally arising CD44Hi cells from young mice were 3–4 fold less efficient in the initial expansion and accumulated to lower frequency and absolute number in the blood and spleen compared to the CD44Lo populations (Fig. 6J and K). Thus, using two different TCR transgenic models and two different types of infections we demonstrated that although CD44Hi CD8 T cells from young mice can serve as precursors, their response to infection is reduced compared to responses that arise from CD44Lo precursors. These data suggest that at least part of the reason for poor antiviral CD8 T cell responses in old mice is the due to the non-lymphopenia induced natural shift of some antigen-specific precursors to a CD44Hi phenotype.
FIGURE 6.
Poor responsiveness of antigen-specific CD44Hi CD8 T cells from uninfected aged and young mice. P14 (DbGP33-specific) CD8 T cells were purified from uninfected young and aged P14 mice (Ly5.1+) and sorted based on the expression of CD44 (A). B shows DbGP33 tetramer staining on sorted P14 populations. After sort, equal numbers of young and aged CD44Hi and CD44Lo P14 cells were transferred into recipient mice (Ly5.2+). Recipient mice were then challenged i.p. with LCMV (2×105 pfu/mouse, C). The responses of the donor P14 CD8 T cells were monitored in the blood (D) and spleens (day 8 p.i, E-G) of recipient mice. Lower frequencies of CD44Hi P14 CD8 T cells derived from aged and young donors were observed compared to their CD44Lo counterparts. Panels E and F, show the absolute number and frequency of BrdU+ donor P14 CD8 T cells derived from young and aged CD44Hi and CD44Lo cells in the spleens of recipient mice at day 8 p.i. In these experiments, twelve hours preanalysis, mice were injected with BrdU i.p. Data are representative of 2 independent experiments including 4–5 mice/experiment. Similarly OT1 (KbOVA-specific) CD8 T cells were purified from uninfected young OT1 Rag2−/− mice (Ly5.1+) and sorted based on the expression of CD44 (H). I shows KbOVA tetramer staining on sorted OT1 populations. After sort, equal numbers of young CD44Hi and CD44Lo OT1 cells were transferred into recipient mice (Ly5.2+). Recipient mice were then challenged i.v. with VSV-OVA (2×106 pfu/mouse). The responses of the donor OT1 CD8 T cells were monitored in the blood (day 7 p.i., J) and spleens (day 8 p.i, K) of recipient mice. Lower numbers of CD44Hi OT1 CD8 T cells (white circles) were observed compared to CD44Lo OT1 CD8 T (white squares). * = 0.05 > P > 0.01, ** = 0.01 > P > 0.001, *** = P < 0.001 by unpaired two-tailed t test.
Discussion
Aging-related susceptibility to infectious diseases and cancer is associated with diminished immune function including reduced T cell responses. While some aspects of declining T cell responses with age reflect systemic changes (e.g. an increase in regulatory T cells, etc.), others are due to cell-intrinsic defects (45). In the current study we demonstrated both quantitative and qualitative age-related changes in T cells and found that these changes were linked to defective responses upon viral infection. The decrease in peripheral T cell numbers likely reflects thymic involution that starts early (perhaps as early as 6 weeks in mice and age 1 in humans) and continues through life (3, 46). Although some functional thymic tissue can be found at old age (47), T cell numbers in the periphery are thought to be retained increasingly by homeostatic proliferation as thymic output declines. Both homeostatic proliferation and exposure to environmental antigens are believed to be responsible for the phenotypic changes in T cells that occur with age. Indeed, T cells that undergo a homeostasis driven-proliferation under lymphopenic conditions have been shown to upregulate CD44 (8, 48). Interestingly, CD44Hi T cells can be also found in germ-free mice (9, 49) suggesting that these cells can arise during physiological lymphopenia in the process of aging in the absence of commensal microbes.
CD44Hi CD8 T cells from uninfected aged mice express high levels of several inhibitory receptors including PD1, LAG3, 2B4 and CD160. Upregulated expression of inhibitory receptors, such as PD1, is typically found on T cells responding to persisting antigen stimulation and chronic infection in both mice and humans, but PD1 and related pathways also have a central role in regulating peripheral self-tolerance and autoimmunity (50, 51). Recent studies showed that PD1 expression correlates with impaired function of LCMV-, HIV- or HCV-specific CD8 T cells and that the blockade of PD1 and/or other inhibitory receptors results in improved CD8 T cell functionality (39). Furthermore, CD4 T cells from aged mice can also express increased PD1 (19) and PD1 positive CD4 T cells from aged mice exhibit proliferative hyporesponsiveness in vitro (20) and in vivo (52). Blockade of PD1/PDL1 pathway in vitro moderately improved cytokine production, but did not restore proliferation of PD1 positive T cells from aged mice (21). The impact of high expression of PD1 on CD8 T cells during aging remains less well defined. In addition, PD1 is clearly not the only inhibitory receptor expressed by these cells and, as occurs during chronic infections, co-expression of inhibitory receptors might influence the function of these aged cells. In combination with microarray studies, these observations suggest a global age-associated change in gene expression in CD8 T cells from uninfected mice that has similarities to what occurs for exhausted CD8 T cells. In addition to inhibitory receptors, these gene expression profiles also identified a number of other immunoregulatory and/or transcriptional pathways that differed between CD8 T cells from young and aged mice. These differences included components of AP-1 (e.g. Fos, Fosb, Jun, JunB), other transcription factors (Myb, Klf4, Maf, Prdm1) and IL10 and FasL. It will be interesting to investigate the importance of these transcriptional changes in the altered responses of T cells during aging.
An interesting question that arises is what is the cause for accumulating inhibitory receptor expression with age. Despite the similarities to T cells found during chronic infections, it is important to note that our aged mice were consistently negative for all common rodent pathogens including viruses such as LCMV and MNV. This information suggests that, while signatures of T cell exhaustion might reflect exposure to unknown infectious agents, other age-related events (such as increased self-reactivity) might underlie the inhibitory receptor expression and other transcriptional changes. Indeed, T cells undergoing recognition of self antigen in vivo express PD-1 during the induction of tolerance (38). While most of these tolerized T cells are typically physically deleted in young mice, we propose that during aging some of these tolerized T cells escape deletion, persist and accumulate over time. Oligoclonally expanded T cells have also been previously shown to express PD1 (40). Although we observed some oligoclonal expansion of the CD8 T cells in uninfected aged mice, these expansions could not explain all of the PD1 expression, since the percent of oligoclonally expanded cells was typically < 10–15% while 20–60% of CD8 T cells expressed PD1. It is interesting that PD1 positive cells were found predominantly in the polyclonal pool of CD44Hi CD8 T cell from naïve mice. While tetramer positive CD44Hi cells from aged P14 transgenic mice were PD1 negative, these mice did contain a population of tetramer negative CD8 T cells that were PD1 positive (Supplemental Fig. 2). It is possible that tetramer negative CD8 PD1Hi T cells in these P14 mice crossreact with self- or environmental antigens. Future studies are necessary to test this idea, but if true, it could suggest that the pool of antigen-specific CD8 T cells in aged mice prior to infection (i.e. CD44HiPD-1+ CD8 T cells) would be more crossreactive compared to the CD8 T cell pool in young animals.
It is interesting to note, however, that even for the CD44Hi PD-1Lo CD8 T cells from naïve aged animals, responses were qualitatively inferior compared to CD44Lo CD8 T cells. We also observed diminished proliferative responses of naturally arising CD44Hi cells from young transgenic animals compared to CD44Lo cells. These observations were somewhat surprising given the work by others demonstrating enhanced responsiveness of homeostatically generated CD44Hi CD8 T cells (9, 10). Together, these data suggest that naturally arising CD44Hi CD8 T cells that accumulate with age might be functionally distinct from those generated by strong homeostatic emptiness following adoptive transfer of CD44Lo T cells into lymphopenic hosts (e.g. over ~3–4 weeks in irradiated or Rag−/− mice). One possible explanation for some of these differences could be that transient bacterial release after sublethal irradiation influences the functional quality of CD44Hi memory cells; if these CD44Hi cells are generated in antibiotic-treated hosts they show dramatic impairment in protective immunity against bacterial infections (53). In addition, the decreased ability of CD44Hi precursors to respond to infection is consistent with the changes in global gene expression in these cells, including the prominent increase in multiple cell intrinsic (e.g. PD-1) and potentially cell extrinsic (e.g. IL-10) negative regulatory pathways. The global shift to CD44Hi CD8 T cells with age is obviously associated with the accumulation of functional, antigen-induced memory T cells generated by infection or vaccination. However, our data now suggest that the shift of some naïve precursors into the CD44Hi pool in the absence of overt antigen stimulation or severe lymphopenia is associated with reduced proliferative capacity and potentially other functional defects. These observations suggest the possibility that generating high quality memory T cells prior to this shift to defective precursors (i.e. when young) might enhance immunity into old age. Studies to directly test this notion are underway.
Another key finding of the current study was that the precursor frequency of antigen-specific CD8 T cells present prior to infection was 10 fold lower in aged compared to young mice. Previous studies demonstrated that age-associated changes in immunodominance could be due to TCR repertoire changes and/or low precursor frequency leading to holes in the repertoire (15). We have quantified this loss of virus-specific precursors for two different antigen specificities and further demonstrated that many antigen-specific precursors from uninfected aged mice are CD44Hi and express PD1. The decrease in both the number and quality of antigen-specific precursors in aged mice could mean that these cells must undergo greater numbers of divisions upon infection to achieve the same clonal expansion causing a delay in the early antiviral effector CD8 T cell response in aged mice consistent with previous observations (24). This delay in clonal expansion during an acute viral infection could result in a substantial increase in viral replication and dissemination. Moreover, the increased number of divisions could also result in defects in effector and memory T cell responses, drive terminal differentiation or even skew the responding T cells towards functional exhaustion. The expression of PD1 prior to infection could mean even further difficulties in proliferation, expansion and /or functionality of these cells and enhanced PD-1 signaling could alter CD8 T cell differentiation through induction of transcriptional pathways such as BATF (54).
Thus, we demonstrate here that the ability of CD8 T cells from young and aged mice to respond to infection is at least partially influenced by intrinsic and population-based changes in CD8 T cells. Precursor frequency plays a significant role in T cell memory quality (55) and our data now demonstrate major changes in antigen-specific CD8 T cell precursor frequency are associated with age-dependent defects in antiviral immunity. Understanding these aging-associated changes could help define the molecular mechanisms underlying defective CD8 T cell responses. Strategies that result in vigorous production of new CD44Lo truly naïve T cells from the thymus, prevention of T cell senescence and modulation/manipulation of immunoregulatory pathways are some of the attractive approaches that could enhance immunity and responses to vaccines in the elderly.
Supplementary Material
Acknowledgments
This work was supported by grants from the American Heart Association, NIH (HHSN266200500030C), R01AG028082 and the Ellison Medical Foundation. The authors have no competing financial interests to declare.
We thank Mohammed-Alkhatim A. Ali and Kathleen Mansfield for technical assistance and the members of the Wherry lab for helpful discussions. We also thank Dr. Ross M. Kedl at the University of Colorado Health Sciences Center for help with tetramer staining and bead sorting methodology.
References
- 1.Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp Gerontol. 2007;42:416–420. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 4.Dicarlo AL, Fuldner R, Kaminski J, Hodes R. Aging in the context of immunological architecture, function and disease outcomes. Trends Immunol. 2009;30:293–294. doi: 10.1016/j.it.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 7.Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–307. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho BK, V, Rao P, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 11.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204:2321–2333. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Lewinsohn DA, Nikolich-Zugich J. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clambey ET, Kappler JW, Marrack P. CD8 T cell clonal expansions & aging: a heterogeneous phenomenon with a common outcome. Exp Gerontol. 2007;42:407–411. doi: 10.1016/j.exger.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008 doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mechanisms of ageing and development. 2009;130:709–712. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M. Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp Gerontol. 2009;44:517–522. doi: 10.1016/j.exger.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/ PD-L1 pathway. Aging Cell. 2010;9:785–798. doi: 10.1111/j.1474-9726.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of virology. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decman V, Laidlaw BJ, Dimenna LJ, Abdulla S, Mozdzanowska K, Erikson J, Ertl HC, Wherry EJ. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol. 2010;184:5151–5159. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- 25.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Irizarry RA, Ooi SL, Wu Z, Boeke JD. Use of mixture models in a microarray-based screening procedure for detecting differentially represented yeast mutants. Stat Appl Genet Mol Biol. 2003;2:Article1. doi: 10.2202/1544-6115.1002. [DOI] [PubMed] [Google Scholar]
- 28.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 29.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, Marcato A, Passeri G, Ortolani C, Forti E, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–2773. [PubMed] [Google Scholar]
- 32.Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 33.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 34.Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Opin Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pawelec G. Immunity and ageing in man. Exp Gerontol. 2006;41:1239–1242. doi: 10.1016/j.exger.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 40.Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci U S A. 2008;105:12997–13002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 47.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 49.Huang T, Wei B, Velazquez P, Borneman J, Braun J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clinical immunology (Orlando, Fla. 2005;117:221–230. doi: 10.1016/j.clim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 52.Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N. PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc Natl Acad Sci U S A. 2009;106:15807–15812. doi: 10.1073/pnas.0908805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton SE, Jameson SC. The nature of the lymphopenic environment dictates protective function of homeostatic-memory CD8+ T cells. Proc Natl Acad Sci U S A. 2008;105:18484–18489. doi: 10.1073/pnas.0806487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann DE, Walker BD, Ebert B, Haining WN. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.