Abstract
Much of our dietary uptake of heavy metals is through the consumption of plants. A long-sought strategy to reduce chronic exposure to heavy metals is to develop plant varieties with reduced accumulation in edible tissues. Here, we describe that the fission yeast (Schizosaccharomyces pombe) phytochelatin (PC)-cadmium (Cd) transporter SpHMT1 produced in Arabidopsis (Arabidopsis thaliana) was localized to tonoplast, and enhanced tolerance to and accumulation of Cd2+, copper, arsenic, and zinc. The action of SpHMT1 requires PC substrates, and failed to confer Cd2+ tolerance and accumulation when glutathione and PC synthesis was blocked by l-buthionine sulfoximine, or only PC synthesis is blocked in the cad1-3 mutant, which is deficient in PC synthase. SpHMT1 expression enhanced vacuolar Cd2+ accumulation in wild-type Columbia-0, but not in cad1-3, where only approximately 35% of the Cd2+ in protoplasts was localized in vacuoles, in contrast to the near 100% found in wild-type vacuoles and approximately 25% in those of cad2-1 that synthesizes very low amounts of glutathione and PCs. Interestingly, constitutive SpHMT1 expression delayed root-to-shoot metal transport, and root-targeted expression confirmed that roots can serve as a sink to reduce metal contents in shoots and seeds. These findings suggest that SpHMT1 function requires PCs in Arabidopsis, and it is feasible to promote food safety by engineering plants using SpHMT1 to decrease metal accumulation in edible tissues.
Nonessential heavy metal(loid)s including cadmium (Cd2+), arsenic (As), and lead (Pb2+) are widespread in the biosphere due largely to industrial pollution. The adverse effects on agriculture and human health are well noted (Mohammed et al., 2011). In China alone, nearly one-fifth of the cultivated land, approximately 20 million hectares, are contaminated with Cd2+, As, and/or Pb2+, leading to approximately 12 million tons of contaminated grains that translate to approximately 20 billion Yuan of economic loss per year (Chen, 1996). In some areas, Cd2+ concentration in rice (Oryza sativa) was found as high as 1 to 2 mg/kg (Cheng, 2003), far beyond the highest level allowed by China National Standard (GB2762–2005). Heavy metal contamination is also widespread in Eastern Europe and is increasingly recognized in many parts of the developing world (Krämer, 2005). The past two decades have seen much discussion on the prospects of phytoremediation—the use of plants to remediate polluted soils (Cunningham and Ow, 1996; Salt et al., 1998; Singh et al., 2003). Likewise, much interest has been raised on the genetic improvement of crop varieties that could reduce dietary intake of these pollutants, as might be possible if toxic metals accumulate less in edible tissues (Ueno et al., 2010). Toward these aims, a better understanding of heavy metal allocation and detoxification in plants is of great importance.
To date, chelation and subcellular sequestration appear to represent essential metal detoxification mechanisms in yeasts (Saccharomyces cerevisiae) and plants (Hall, 2002; Verbruggen et al., 2009). The best-characterized heavy-metal-binding chemicals are small Cys-rich molecules, including metallothioneins, glutathione (GSH), and phytochelatins (PCs; Cobbett and Goldsbrough, 2002; Verbruggen et al., 2009). Metallothioneins are diverse proteins encoded by a superfamily in both animals and plants (Grennan, 2011). PCs are found in plants, algae, some fungi, and surprisingly in Canenorhabditis elegans (Grill et al., 1985; Vatamaniuk et al., 2001; Pal and Rai, 2010). PCs are serial oligopeptides with a general structure of (γ-Glu-Cys)n-Gly (n = 2–11), and are synthesized from GSH by PC synthase (PCS; Grill et al., 1987, 1989; Clemens et al., 1999; Ha et al., 1999; Vatamaniuk et al., 1999). PCs chelate Cd2+ with higher affinity than do GSH molecules (Kneer and Zenk, 1992; Howden et al., 1995a), and both types of complex can be sequestered into vacuoles (Ortiz et al., 1992; Li et al., 1996). In fission yeast (Schziosaccharomyces pombe), the inability of a Cd2+-sensitive mutant to accumulate vacuolar PC-Cd led to the discovery of HMT1, a half-molecule ATP-binding cassette (ABC) transporter that recognizes PCs and PC-Cd (Ortiz et al., 1992, 1995). Likewise, a Cd2+-sensitive mutant of yeast led to YCF1, a full-molecule ABC transporter that mediates vacuolar sequestration of a GS2-Cd complex [bis(glutathionato) Cd] (Li et al., 1996, 1997).
Subsequent studies have suggested that SpHMT1 might also transport GS2-Cd conjugates when overexpressed in fission yeast mutants that lack PCs for substrate (Prévéral et al., 2009). Whether GS2-Cd or glutathione S-conjugate (GS-X) are physiologically relevant substrates remains to be determined, as during Cd2+ stress, much more of the Cd ions are bound to PCs rather than to GSH, and GS2-Cd as well as GS-X transport activities are detected in vacuoles even in the absence of HMT1 (Ortiz et al., 1995). Nevertheless, this fact does raise the possibility that in fission yeast and C. elegans, HMT1 might have evolved from an ancient but more universal role in GS-X transport.
Despite the subsequent identification of CeHMT1, a C. elegans PC transporter, and more recently a Drosophila melanogaster HMT1 homolog that likely transports GSH conjugates, a HMT1 ortholog has not been identified in higher plants (Vatamaniuk et al., 2005; Sooksa-Nguan et al., 2009). The likelihood that higher plants have evolved a different PC transport system has received recent support from the discovery of two ABCC subfamily members of ABC transporters in Arabidopsis (Arabidopsis thaliana), for which vacuolar PC-As(III) transport was shown (Song et al., 2010). A most recent study further reported substantial decrease of vacuolar Cd2+ in the atabcc1 atabcc2 mutant, indicating that AtABCC1 and AtABCC2 play a significant role in vacuolar Cd2+ sequestration. However, the relative contribution by PCs and GSH to this process remains to be determined, as GSH might possibly be a substrate of AtABCC1 and AtABCC2 under certain conditions (Park et al., 2012).
In this study, we found that PCs play a primary role in vacuolar Cd2+ sequestration in Arabidopsis, and ectopic expression of SpHMT1 enhanced this process. SpHMT1 function requires PCs, as its effects are not seen in the PC-deficient mutant cad1-3. We further demonstrate the possibility of engineering plants using SpHMT1 to improve food safety by reducing heavy metal translocation into plant edible parts.
RESULTS
Expression of SpHMT1 in Arabidopsis
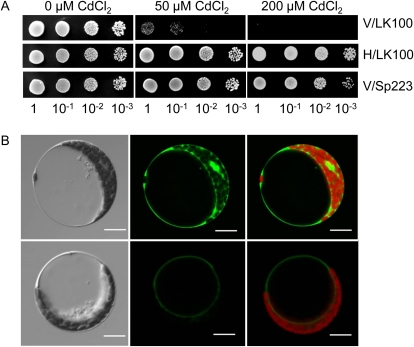
A SpHMT1 expression construct pART1-SpHMT1 was generated containing the native 5′-untranslated region and a c-myc tag fused to the 3′ terminus of the SpHMT1 gene. The construct complements the Cd2+-sensitive phenotype in the hmt1- strain LK100, showing that the c-myc tag did not substantially affect SpHMT1 activity (Fig. 1A; Supplemental Fig. S1). The c-myc-tagged SpHMT1 fragment was placed under the control of the cauliflower mosaic virus 35S promoter and transferred to an Agrobacterium tumefaciens vector to generate pBI121-35S/SpHMT1:cmyc for transformation of Arabidopsis. Reverse transcription-PCR analyses of transgenic plants derived from wild-type Columbia-0 (Col-0) and the PC-deficient mutant cad1-3 showed that SpHMT1 was expressed in both genetic backgrounds (Supplemental Fig. S2, A and B). Further subcellular localization analyses using Arabidopsis protoplasts showed that SpHMT1 is tonoplast localized (Fig. 1B), consistent with the results in fission yeast (Ortiz et al., 1992, 1995).
Figure 1.
Rescue of Cd2+ sensitivity in hmt1- mutant LK100 and subcellular localization analysis. A, LK100 transformed with empty vector pART1 (V/LK100) or c-myc-tagged SpHMT1 (H/LK100). Wild-type strain Sp223 transformed with pART1 (V/Sp223) was used as positive control. B, Subcellular localization of SpHMT1. Left, bright field; middle, fluorescence; and right, merged images of Enhanced Yellow Fluorescent Protein (EYFP; top) and EYFP:SpHMT1 (bottom). Red color represents autofluorescence of chloroplasts. Bars = 20 μm.
SpHMT1 Enhanced Metal Tolerance and Accumulation in Arabidopsis
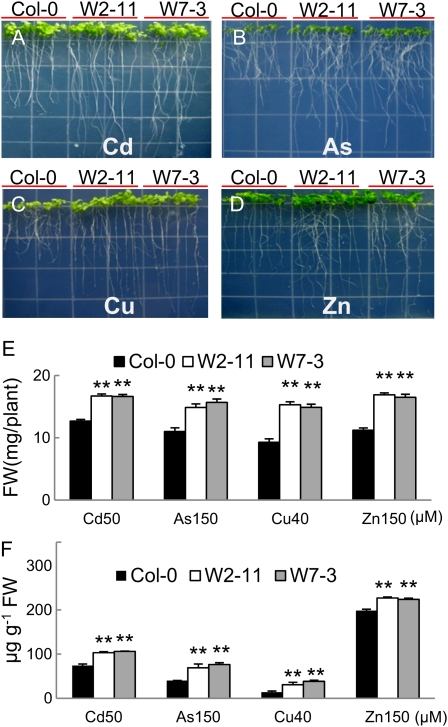
Representative transgenic lines expressing SpHMT1 were tested for tolerance to heavy metals. When germinated without heavy metals, transgenic lines W2-11 and W7-3 showed similar growth and biomass as the wild-type Col-0 (Supplemental Fig. S3; P > 0.05). However, when germinated on 50 μm Cd2+ (Fig. 2A), 150 μm As (Fig. 2B), 40 μm copper (Cu; Fig. 2C), or 150 μm zinc (Zn; Fig. 2D), lines W2-11 and W7-3 grew longer roots than did the wild-type control. Total biomass and metal content were also significantly higher than those of the control plants (Fig. 2, E and F; P < 0.01). For Cd2+, 39% to 42% more of the metal were found in the transgenic lines. Adjusting for biomass differences, this translates to 1.7- to 1.9-fold more Cd2+ per fresh weight of tissue. Similar increases in accumulation of As, Cu2+, or Zn2+ were also found (Fig. 2F; P < 0.01).
Figure 2.
Enhanced metal tolerance and accumulation in SpHMT1 plants. Surface sterile seeds plated onto one-fourth Murashige and Skoog medium supplemented with 50 μm CdCl2 (A), 150 μm KH2AsO4 (B), 40 μm CuSO4 (C), and 150 μm ZnSO4 (D), and grown vertically for 4 weeks. W2-11 and W7-3 are independent lines transgenic for pBI121-35S/SpHMT1:cmyc in wild-type Col-0 background. Fresh weight (FW; E) and metal content (F) were determined in plants grown under conditions in A to D. Values are mean ± sd, n = 12 for E, or n = 4 for F, each containing greater than five plants. Asterisks indicate significant difference between wild type and the transgenic lines at P < 0.05 (*) and 0.01 (**) by t tests.
Cd Tolerance and Accumulation in Transgenic Plants Aborted by BSO
PCs and PC-Cd were described as substrates of SpHMT1 (Ortiz et al., 1992, 1995; Mendoza-Cózatl et al., 2010). Subsequent studies from overexpression of SpHMT1 also implicated GS2-Cd as a likely substrate when PCs were absent (Prévéral et al., 2009; Sooksa-Nguan et al., 2009). The production of both PCs and GSH can be abolished by BSO that inhibits a key enzyme in GSH biosynthesis (Howden et al., 1995a). Should the enhanced metal tolerance and accumulation phenotype in W2-11 and W7-3 be due to SpHMT1 recognition of PC-Cd and GS2-Cd, then BSO should indirectly block SpHMT1 function. When germinated with 0.5 mm BSO, both transgenic and wild-type plants showed similar phenotype and biomass (Supplemental Fig. S4, A and C). When 10 μm CdCl2 was included along with 0.5 mm BSO, reduced growth and chlorotic leaves were observed, and to the same degree between transgenic and wild-type plants (Supplemental Fig. S4, B and C). Cd2+ content in roots, rosette leaves, or shoots also failed to show a significant difference among transgenic and wild-type plants exposed to Cd2+ and BSO (Supplemental Fig. S4D; P > 0.05). We conclude that BSO neutralized the effect of SpHMT1 for metal tolerance and accumulation.
SpHMT1 Failed to Increase Cd Tolerance and Accumulation in cad1-3
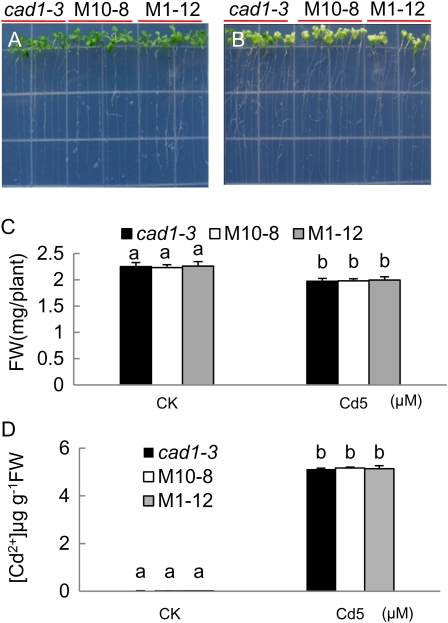
The mutant cad1-3 harbors a defective PCS gene. While PC synthesis is impaired, GSH production is unaffected (Howden et al., 1995a). If GSH were necessary for SpHMT1 function, an expectation would be that overexpression of SpHMT1 in the cad1-3 mutant would still enhance Cd2+ tolerance and accumulation through recognition of GSH. However, this was not the case, as representative lines of transgenic SpHMT1 in the cad1-3 mutant background exhibited similar growth as the cad1-3 mutant, either in the absence of metal stress (Fig. 3, A and C), or when germinated with 5 μm Cd2+ where both cad1-3 and the transgenic derivatives M10-8 and M1-12 showed similar chlorosis and reduced growth (Fig. 3, B and C). Given that a relatively low Cd2+ concentration had to be used due to the metal hypersensitivity of cad1-3 mutation, a root-bending assay that allows treatment with increased dosage was also conducted. When exposed to 20 μm Cd2+ (Supplemental Fig. S5A), growth hooks could be observed in both cad1-3 and the transgenic plant roots (M10-8, M1-12, and M1-4), but not at 30 μm Cd2+ (Supplemental Fig. S5B). In contrast, significant growth hooks were observed in transgenic plants W2-11, W3-13, and W7-3, but not in the wild-type Col-0 when exposed to 150 μm Cd2+ (Supplemental Fig. S6). In sum, a difference between cad1-3 and its SpHMT1 derivatives was not observed with respect to Cd2+ tolerance and accumulation (Fig. 3D). As the cad1-3 mutant is proficient in GSH production, the simplest interpretation is that SpHMT1 requires PCs rather than GSH to enhance tolerance and accumulation of Cd2+ in Arabidopsis.
Figure 3.
Cd2+ sensitivity and accumulation in cad1-3 expressing SpHMT1. Plants were germinated and grown vertically on one-fourth Murashige and Skoog plates under control condition (A) or with 5 μm CdCl2 (B). M10-8 and M1-12 represent cad1-3 plants transformed with pBI121-35S/SpHMT1:cmyc. Photographs taken at day 7 (A) and day 20 (B) post germination. C, Fresh weights of plants grown under control condition (CK) in A or with 5 μm CdCl2 (Cd5) in B. D, Cd2+ concentration in seedlings grown under conditions in A and B. Values are mean ± sd, n = 12 for C, or n = 4 for D, each containing greater than five plants. Lowercase letters indicate whether averages were statistically different at P < 0.05 by t tests. [See online article for color version of this figure.]
PC-Assisted Cd2+ Distribution between Vacuoles and Cytosol
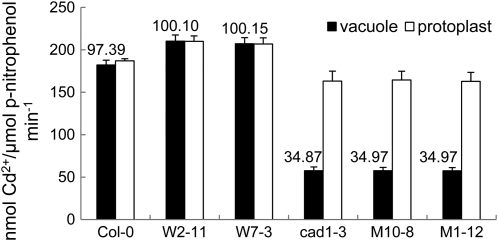
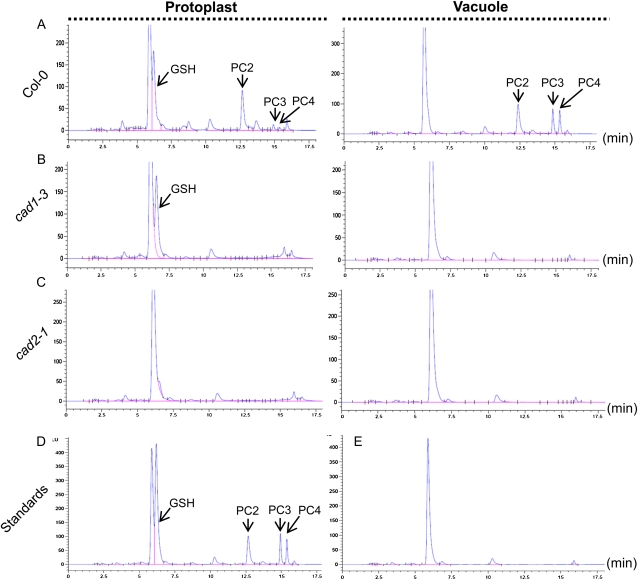
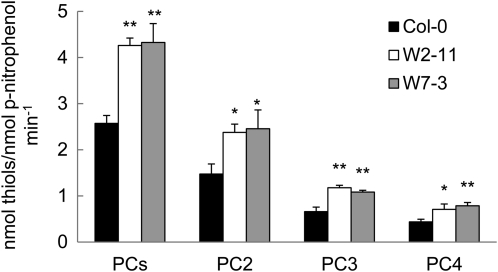
To test whether SpHMT1 promotes vacuolar sequestration of Cd2+ in Arabidopsis, the distribution of Cd2+ was examined in isolated protoplasts and vacuoles (Supplemental Table S1). The SpHMT1 transgenic lines W2-11 and W7-3 showed higher protoplast and vacuolar Cd2+ content than the wild-type control, but a difference was not observed among the PC-deficient lines cad1-3, M10-8, and M1-12 (Fig. 4). More Cd2+ accumulated in Col-0, W2-11, and W7-3 than in cad1-3, M10-8, and M1-12, especially in the vacuoles, and nearly 100% of the cellular Cd2+ in Col-0, W2-11, and W7-3 was vacuolar (Fig. 4). However, the lack of PCs did not abolish vacuolar sequestration entirely in cad1-3, M10-8, and M1-12, as approximately 35% of the cellular Cd2+ was in vacuoles of these PC-deficient lines. To assess whether this approximately 35% vacuolar accumulation of Cd2+ might be GSH mediated, the same analysis was extended to mutant cad2-1 that produces substantially decreased amounts of GSH and PCs (Howden et al., 1995b). Even less Cd2+ accumulated in protoplasts and vacuoles of cad2-1 compared to cad1-3, with only 24% of the protoplast Cd2+ localized in vacuoles (Supplemental Fig. S7). HPLC analyses showed that in Col-0, both GSH and PCs (PC2, PC3, and PC4) were detected in protoplasts, while only PCs were detected in vacuoles (Fig. 5A). In cad1-3, PCs were not detected in protoplasts and vacuoles, but consistently, GSH was detected only in protoplasts (Fig. 5B). Although it appeared that a small amount of GSH might accumulate in cad2-1 protoplasts, detectable GSH or PCs were not observed in vacuoles (Fig. 5C). Interestingly, in W2-11 and W7-3, more PCs, approximately 165% of the wild-type level, were detected in vacuoles (Fig. 6; P < 0.05). Taken together, the data indicate that PCs play a predominant role in vacuolar Cd2+ sequestration. GSH also contributes, but other Cd2+ cross-tonoplast transport mechanisms other than PC or GSH assisted are also involved. Additionally, the significantly higher Cd2+ and PC contents found in W2-11 and W7-3 vacuoles over those of the control are consistent with an interpretation that SpHMT1 promotes PC-Cd sequestration into the plant vacuoles.
Figure 4.
Subcellular localization of Cd2+. Protoplasts and vacuoles isolated from leaves of soil-grown plants (Col-0, cad1-3, and transgenic W2-11, W7-3, M1-12, and M10-8) were measured for Cd2+ content. Values above the bars represent the percentage of vacuolar Cd2+ relative to the total in protoplasts. Acid phosphatase (ACP) activity specific to vacuole was determined and used to normalize Cd2+ accumulation; data are mean ± se from three independent experiments.
Figure 5.
Distribution of GSH and PCs. Protoplasts and vacuoles were extracted from soil-grown plant leaves of Col-0 (A), cad1-3 (B), and cad2-1 (C). GSH and PCs measured by fluorescence HPLC. D, Synthesized GSH, PC2, PC3, and PC4 used as standards. E, Blank control. Peaks are indicated by arrows. [See online article for color version of this figure.]
Figure 6.
Increased vacuolar PCs in transgenic SpHMT1 plants. Soil-grown plants were used for protoplasts and vacuoles isolation. PC contents in vacuoles from Col-0 and the transgenic plants W2-11 and W7-3 were determined as described in “Materials and Methods.” Acid phosphatase (ACP) activity specific to vacuoles was determined and used to normalize PC accumulation. Data are mean ± se from three independent experiments. Asterisks indicate significant difference between wild type and the transgenic lines at P < 0.05 (*) and 0.01 (**) by t tests.
Long-Distance Root-to-Shoot Transport of Cd2+ Was Delayed by SpHMT1
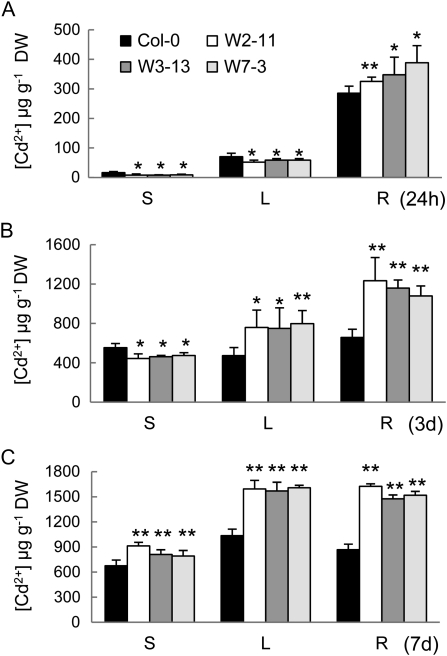
To examine if SpHMT1 affects Cd2+ long-distance transport, Cd2+ contents in roots and shoots were measured. When exposed to 10 μm CdCl2 for 24 h, more Cd2+ accumulated in the roots while less Cd2+ was detected in rosette leaves and stems of transgenic plants compared to the wild-type control (Fig. 7A; P < 0.05). By day 3, more Cd2+ accumulated in leaves as well as in the roots, but less in the stems of the transgenic plants (Fig. 7B; P < 0.05). After a week, the transgenic plants accumulated higher Cd2+ content in all three tissue types (Fig. 7C; P < 0.01). Consistently, delayed Cd2+ entry into xylem sap by SpHMT1 was also observed (Supplemental Fig. S8), where sap Cd2+ increase in transgenic plants was slower than in Col-0. Note that at day 3, Cd2+ concentration in wild-type sap began to decline, which possibly resulted from a more severely inhibited transpiration rate. Taken together, these data show that SpHMT1 plants delayed long-distance transport of Cd2+ from roots to shoots.
Figure 7.
Long-distance Cd2+ transport from roots to shoots. Plants were grown hydroponically for 4 weeks before treatment with 10 μm CdCl2 for 24 h (A), 3 d (B), and 7 d (C). Cd2+ concentrations in stems (S), rosette leaves (L), and roots (R) of wild-type Col-0 and transgenic lines (W2-11, W7-3, and W3-13) were determined. Values are mean ± sd, n = 4, each containing greater than eight plants. Asterisks indicate significant difference between wild type and the transgenic lines at P < 0.05 (*) and 0.01 (**) by t tests.
Root-Specific Expression of SpHMT1 Reduced Heavy Metal Accumulation in Plant Aerial Parts
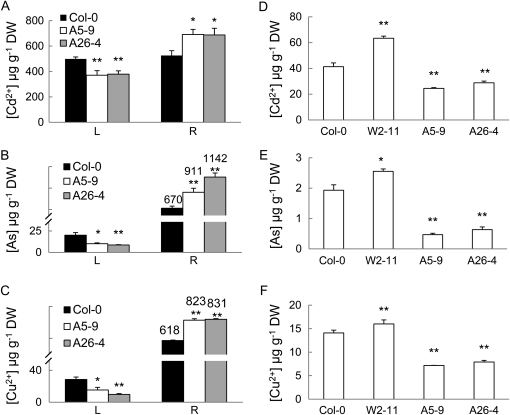
Given that constitutive SpHMT1 expression delayed Cd2+ transport to aerial parts (Fig. 7), we sought to test if root-specific expression of SpHMT1 could further reduce Cd2+ accumulation in leaves and seeds, the edible parts of leafy vegetables, or grain crops. For this purpose, the SpHMT1 fragment was placed under the control of the Arabidopsis alcohol dehydrogenase (Adh) promoter (Gong et al., 2003) in the construct pBI121-Adh/SpHMT1:cmyc, and root-specific expression of SpHMT1 was found in transgenic lines A5-9 and A26-4 (Supplemental Fig. S2, C and D). When treated with 10 μm CdCl2, 100 μm KH2AsO4, or 20 μm CuSO4 for 3 d, more metals accumulated in the roots of these plants compared to the wild-type Col-0 (Fig. 8, A–C; P < 0.05). Conversely, significantly less toxic metals were detected in leaves (Fig. 8, A–C; P < 0.01). Furthermore, seeds derived from plants grown with 5 μm CdCl2, KH2AsO4, or CuSO4 accumulated less of these toxic metals. Compared to the wild-type control, approximately 40% less of Cd2+ (Fig. 8D), approximately 70% less of As (Fig. 8E), and approximately 45% less of Cu2+ (Fig. 8F), were found in A5-9 and A26-4 seeds. These results suggest that root-specific expression of SpHMT1 substantially decreases heavy metal accumulation in aerial tissues.
Figure 8.
Targeted SpHMT1 expression in roots decreased metal accumulation in leaves and seeds. Metal content in rosette leaves (L), roots (R), and seeds (D–F) measured in plants grown hydroponically to 4 weeks of age and exposed to 10 μm CdCl2 (A), 100 μm KH2AsO4 (B), or 20 μm CuSO4 (C) for 3 d before sampling, or in 2-week-old hydroponically grown plants supplemented with 5 μm CdCl2 (D), KH2AsO4 (E), or CuSO4 (F) until maturation of seeds. A5-9 and A26-4 represent independent pBI121-Adh/SpHMT1:cmyc transformed lines, while W2-11 represents a pBI121-35S/SpHMT1:cmyc transgenic line. Values are mean ± sd, n = 4, each containing greater than eight plants. Asterisks indicate significant difference between wild type and the transgenic lines at P < 0.05 (*) and 0.01 (**) by t tests.
DISCUSSION
SpHMT1-Mediated Sequestration of Cd2+ Requires PCs
SpHMT1 was originally identified as a principal vacuolar PC-Cd transporter in fission yeast (Ortiz et al., 1992, 1995). In this study, ectopic expression of SpHMT1 in Arabidopsis wild type enhanced both metal tolerance and accumulation (Fig. 2). However, application of BSO that inhibits GSH biosynthesis and thus inhibits PC synthesis, aborted both the increased metal tolerance and accumulation in transgenic plants (Supplemental Fig. S4), while in transgenic cad1-3 plants, which are deficient in PCs but proficient for GSH production (Howden et al., 1995a; Ha et al., 1999), significant increase in either metal tolerance or accumulation was not observed (Fig. 3; Supplemental Fig. S5). Furthermore, transgenic SpHMT1 significantly increased vacuolar Cd2+ and PC levels in Col-0 (Figs. 4 and 6). These data suggest that PCs are required for SpHMT1-mediated Cd2+ sequestration in Arabidopsis.
However, previous studies suggested that GSH instead of PCs is required for SpHMT1 function in yeast (Prévéral et al., 2009; Sooksa-Nguan et al., 2009), in contrast to the current result in Arabidopsis. The inconsistency possibly resulted from the unusual conditions used in yeast, where high abundance of SpHMT1 protein might recognize GSH that is devoid of competition from PCs. Alternatively, plants may have developed a mechanism that predominantly relies on PCs, thus limiting substrate availability to ectopically expressed proteins. This latter hypothesis was supported by isolation and characterization of the Arabidopsis native PC transporters AtABCC1 and AtABCC2 (Song et al., 2010). In any case, the substrates recognized by SpHMT1 might be the reason that SpHMT1 enhanced As tolerance in plants but not in yeast, as PCs are known to be more efficient than GSH in metal(loid)s detoxification (Howden et al., 1995a; Gong et al., 2003; Song et al., 2010). Further support for this model came from the result that SpHMT1 did effect As sensitivity in a dose-dependent manner in yeast (Prévéral et al., 2009; Sooksa-Nguan et al., 2009).
PCs Play a Primary Role in Metal Sequestration into Arabidopsis Vacuoles
This study also calls into question the physiological relevance of GSH for Cd sequestration by plant tonoplast transporters. Subcellular localization analyses showed that in wild-type Col-0, nearly 100% of the protoplast Cd2+ was localized in vacuoles, but in protoplasts of the PC-deficient mutant cad1-3, only 35% is present in vacuoles (Fig. 4), comparable to the result in atabcc1 atabcc2 mutant in which PC and possibly GSH transport was disrupted (Park et al., 2012). These data consistently suggest that PCs play a primary role in vacuolar Cd2+ sequestration in Arabidopsis. In cad2-1, approximately 24% of the protoplast Cd2+ was localized in vacuoles (Supplemental Fig. S7), in contrast to approximately 35% in cad1-3, which further suggest that GSH contributes about 13% to 18% of the vacuolar sequestration of Cd2+, given that a general 20% to 40% GSH of wild-type levels is present in cad2-1 (Howden et al., 1995b; Cobbett et al., 1998). Note that the contribution by GSH might be indirect due to the lack of detectable GSH in vacuoles (Fig. 5).
Engineering of SpHMT1 Is a Feasible and Efficient Way to Tackle Heavy-Metal-Related Food Safety Problems
Plant heavy metal uptake not only reduces crop yields, but also transits the pollutants to the human diet, resulting in serious diseases including neurodegenerative conditions, dysfunction of vital organs, and cancers (Lee and White, 1980). Phytoextraction has been proposed to tackle this problem (Salt et al., 1998), though it seemed to be less efficient in situations with a wide range of multiple heavy metal pollutions (Cheng, 2003). Considering from this aspect, it might be more feasible to reduce toxic metal accumulation in plant edible parts.
Transgenic SpHMT1 led to 30% to 40% increase of Cd2+, As, Cu, or Zn2+ accumulation in plants (Fig. 2F), which might not be sufficient for rapid cleanup of soil contamination. Even if this could translate to field applications, it would only shorten somewhat the estimated decades (century)-long process of soil cleanup by plants. For the purpose of reducing dietary intake of toxic metals, however, the root-specific expression of SpHMT1 reduced seed accumulation of Cd2+ and Cu by half, and of As to one-third of the wild-type level (Fig. 8, D–F). Decrease of metals in leafy tissue was also found though not as impressive (Fig. 8, A–C). Given that the consumption of grains such as rice represents the greatest proportion of daily calorie intake in many developing countries, especially in countries where significant toxic metal pollution has occurred, the development of grain crops with one-third to half of the normal amount of dietary toxic metals could lead to significant health benefits.
Previously, the engineering of the budding yeast YCF1 in Arabidopsis was reported to have enhanced Cd2+ and Pb2+ tolerance and accumulation (Song et al., 2003). However, the Cd2+ and Pb2+ accumulation was not in roots, but in the plant aerial parts. In this work, we found that the aerial portion could accumulate more Cd2+, but the kinetics of Cd2+ accumulation suggested that there is a delay in root-to-shoot translocation (Fig. 7; Supplemental Fig. S8), as though the roots serve as an initial sink for the toxic metals. Root-specific expression of SpHMT1 substantiated this deduction, with proportionally more metals in roots than shoots or seeds (Fig. 8), indicating that the roots can have high capacity for toxic metals storage. Given that root-targeted expression of the wheat (Triticum aestivum) PCS gene TaPCS1 in Arabidopsis failed to trap Cd2+ in roots, but instead greatly enhanced long-distance Cd2+ transport to plant aerial parts (Gong et al., 2003), our data demonstrate that engineering vacuolar PC sequestration could overcome the plant overflow protection mechanism in which PC synthesis facilitates Cd2+ translocation and hence limits the metal concentration in a specific organelle (Gong et al., 2003), thus this sequestration strategy could be applied to targeting heavy metals to specific tissues.
In summary, PCs were found to play a primary role in Cd2+ sequestration into Arabidopsis vacuoles, and ectopic expression of SpHMT1 enhanced this process. We further demonstrated that the engineering of SpHMT1 could be a feasible and efficient way to tackle food safety concerns in countries with a wide range of heavy metal pollution.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Metal Sensitivity Analyses
Arabidopsis (Arabidopsis thaliana) plants were grown in quarter-strength hydroponics as described (Arteca and Arteca, 2000; Gong et al., 2003). At 4 weeks of age, plants were exposed to CdCl2 or CdCl2 BSO as indicated. Root, rosette leaf, and stem samples were harvested as described (Gong et al., 2003). Alternatively, Arabidopsis plants were grown in soil to 2 weeks old with a 16/8-h day/night period, and bottom flooded with 0.5 L of 40 μm CdCl2 twice for 1 week prior to leaf tissue sampling. Seedling metal sensitivity analyses were performed on one-fourth Murashige and Skoog basal medium (Sigma-Aldrich) with 1 g/L MES, 1.5% Bacto agar, and heavy metals with or without BSO as indicated. Plants grew vertically for indicated time intervals prior to fresh weight and metal content measurements. Root-bending assay was performed as described (Howden and Cobbett, 1992) with minor modification: 6-d-old seedlings were transferred onto one-fourth Murashige and Skoog plates supplemented with indicated concentrations of CdCl2, and the plates were then placed upside down and incubated for another 5 d.
DNA Constructs and Transformation into Yeast and Plants
The fission yeast (Schizosaccharomyces pombe) SpHMT1 cDNA was amplified by reverse transcription-PCR, into which two restriction enzyme sites BamHI and SpeI were introduced using forward primer 5′-AGGATCCAAATTAGCAATTGAAGCAGTAACAA-3′ and reverse primer 5′-AACTAGTGAATGAGTTTCAGCAGAAGTTTTT-3′. The SpHMT1 cDNA fragment was sequenced prior to inserting into BamHI and SpeI sites of binary vector pBI121-35S/TaPCS1:cmyc (Gong et al., 2003) to generate pBI121-35S/SpHMT1:cmyc. The same fragment was subsequently transferred to binary vector pBI121-Adh/TaPCS1:cmyc (Gong et al., 2003) to yield pBI121-Adh/SpHMT1:cmyc. Both constructs were used for Arabidopsis floral-dip transformation (Clough and Bent, 1998) into wild-type Col-0 and PC-deficient mutant cad1-3 (Howden et al., 1995a) background. Transgenic lines with a segregation rate of 3:1 on kanamycin plates were used for further screening of homozygotes and strong alleles. The SpHMT1 cDNA from pBI121-35S/SpHMT1:cmyc was also transferred as a BamHI to SacI fragment to fission yeast vector pART1 (Ortiz et al., 1992) to yield pART1-SpHMT1 for transformation by the lithium acetate method into fission yeast strain Sp223 (h- ade6-216 leul-32 ura4-294) and its isogenic SpHMT1 mutant strain LK100 (h- ade6-216 leul-32 ura4-294 hmt1-; Ortiz et al., 1992). Transformants were selected on Edinburgh minimal medium (EMM) plates without Leu (Okazaki et al., 1990) and cells were grown at 30°C in EMM supplemented with appropriate amino acids. For metal sensitivity assays, yeast (Saccharomyces cerevisiae) cells were grown to saturation, then diluted to an OD600 of 0.5, grown for approximately 4 h to log phase, and further diluted as indicated and incubated with different concentrations of CdCl2. Cell density was determined after 16 to 20 h. Alternatively, the diluted cultures were spotted onto EMM plates containing CdCl2 at indicated concentrations and grown for 3 to 5 d.
Isolation of Intact Protoplasts and Vacuoles and Determination of Metal and PC Levels
Rosette leaves from soil-grown Arabidopsis plants were used to isolate intact protoplasts and vacuoles as described (Robert et al., 2007) with minor modification: The purified protoplasts were divided into two equal aliquots, and one was used for further isolation of vacuoles. The purified protoplasts and vacuoles were then subsampled and subjected to determination of Cd2+ concentrations and marker enzyme activities as described (Vögeli-Lange and Wagner, 1990; Ma et al., 2005), respectively. Cd2+ contents in protoplasts and vacuoles or other metal contents in sampled plant tissues were measured by inductively coupled plasma-mass spectrometry (ELAN DRC-e, PerkinElmer) as described (Gong et al., 2003; Li et al., 2010) with corresponding modification. Protoplasts and vacuoles from Cd2+-treated plants were analyzed by HPLC (Agilent 1200 series) using a reversed-phase C18 column (Agilent 300Extend-C18 4.6 × 150 mm, 3.5 μm particle size) as described (Minocha et al., 2008) with corresponding modification. Thiols were labeled by monobromobimane as described (Gong et al., 2003). PC standards, (γ-GluCys)2Gly (PC2), (γ-GluCys)3Gly (PC3), and (γ-GluCys)4Gly (PC4) were synthesized and purchased from AnaSpec. The activities of acid phosphatase and cytochrome c oxidase were determined using plant ACP colorimetry assay kit and COX assay kit (GenMed Sci Inc.) following the manufacturer’s instructions.
EYFP Fusion and Subcellular Localization
Two restriction sites BglII and XhoI were introduced into SpHMT1 cDNA using forward primer 5′-AAGATCTATGGTTCTACGTTACAACAGCCC-3′ and reverse primer 5′-ACTCGAGTTAATGAGTTTCAGCAGAAGTTTTT-3′, by which the confirmed SpHMT1 cDNA fragment was inserted into vector pMON530-35S/EYFP to make an EYFP:SpHMT1 fusion construct. The resulted pMON530-35S/EYFP:SpHMT1 construct together with the control pA7-35S/EYFP were transiently expressed in Arabidopsis protoplasts via polyethylene-glycol-mediated transformation as described (Yoo et al., 2007). The transformed protoplasts were treated and imaged as described (Li et al., 2010), and >100 protoplasts were analyzed from each of three replicates.
Statistical Analyses
Two-tailed Student’s t tests were used to compare fresh weights and metal contents between transgenic plants and wild type. Differences were deemed significant at P < 0.05, and extremely significant at P < 0.01.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number CAA78419/Z14055.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Rescue of Cd2+ sensitivity in hmt1- mutant LK100 by c-myc tagged SpHMT1.
Supplemental Figure S2. RT-PCR analysis of SpHMT1 expression.
Supplemental Figure S3. Transgenic lines harboring SpHMT1 showed similar growth with the wild type Col-0 under control condition.
Supplemental Figure S4. Enhanced Cd2+ tolerance and accumulation aborted by BSO.
Supplemental Figure S5. SpHMT1 fails to rescue Cd2+ sensitivity in cad1-3 mutant.
Supplemental Figure S6. SpHMT1 enhanced cadmium tolerance in Col-0.
Supplemental Figure S7.Subcellular localization of Cd2+.
Supplemental Figure S8.Cd2+ concentration in xylem sap.
Supplemental Table S1. Determination of vacuole purity.
Supplementary Material
Acknowledgments
We thank Drs. Christopher Cobbett (University of Melbourne, Australia) and Julian Schroeder (University of California, San Diego) for providing cad1-3 and cad2-1 seeds and Xiao-Shu Gao and Laisheng Ji (Core Facility, Shanghai Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences) for helping with confocal microscopy and ultracentrifugation.
References
- Arteca RN, Arteca JM. (2000) A novel method for growing Arabidopsis thaliana plants hydroponically. Physiol Plant 108: 188–193 [Google Scholar]
- Chen HM. (1996) Pollution of Heavy Metals in Soil-Plant System. Science Press, Beijing, pp 1–344 [Google Scholar]
- Cheng S. (2003) Heavy metal pollution in China: origin, pattern and control. Environ Sci Pollut Res Int 10: 192–198 [DOI] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18: 3325–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P. (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- Cunningham SD, Ow DW. (1996) Promises and prospects of phytoremediation. Plant Physiol 110: 715–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JM, Lee DA, Schroeder JI. (2003) Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci USA 100: 10118–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grennan AK. (2011) Metallothioneins, a diverse protein family. Plant Physiol 155: 1750–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Löffler S, Winnacker EL, Zenk MH. (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86: 6838–6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. (1985) Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 230: 674–676 [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. (1987) Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84: 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O’Connell MJ, Goldsbrough PB, Cobbett CS. (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL. (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53: 1–11 [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS. (1995b) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Cobbett CS. (1992) Cadmium-sensitive mutants of Arabidopsis thaliana. Plant Physiol 100: 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS. (1995a) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107: 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneer R, Zenk MH. (1992) Phytochelatins protect plant enzymes from heavy-metal poisoning. Phytochemistry 31: 2663–2667 [Google Scholar]
- Krämer U. (2005) Phytoremediation: novel approaches to cleaning up polluted soils. Curr Opin Biotechnol 16: 133–141 [DOI] [PubMed] [Google Scholar]
- Lee JS, White KL. (1980) A review of the health effects of cadmium. Am J Ind Med 1: 307–317 [DOI] [PubMed] [Google Scholar]
- Li JY, Fu YL, Pike SM, Bao J, Tian W, Zhang Y, Chen CZ, Zhang Y, Li HM, Huang J, et al. (2010) The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 22: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA. (1997) A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci USA 94: 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Szczypka M, Lu YP, Thiele DJ, Rea PA. (1996) The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem 271: 6509–6517 [DOI] [PubMed] [Google Scholar]
- Ma JF, Ueno D, Zhao FJ, McGrath SP. (2005) Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220: 731–736 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Zhai Z, Jobe TO, Akmakjian GZ, Song WY, Limbo O, Russell MR, Kozlovskyy VI, Martinoia E, Vatamaniuk OK, et al. (2010) Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. J Biol Chem 285: 40416–40426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha R, Thangavel P, Dhankher OP, Long S. (2008) Separation and quantification of monothiols and phytochelatins from a wide variety of cell cultures and tissues of trees and other plants using high performance liquid chromatography. J Chromatogr A 1207: 72–83 [DOI] [PubMed] [Google Scholar]
- Mohammed AS, Kapri A, Goel R. (2011) Heavy metal pollution: source, impact, and remedies. Environ Pollut 20: 1–28 [Google Scholar]
- Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. (1990) High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res 18: 6485–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz DF, Kreppel L, Speiser DM, Scheel G, McDonald G, Ow DW. (1992) Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J 11: 3491–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz DF, Ruscitti T, McCue KF, Ow DW. (1995) Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem 270: 4721–4728 [DOI] [PubMed] [Google Scholar]
- Pal R, Rai JPN. (2010) Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 160: 945–963 [DOI] [PubMed] [Google Scholar]
- Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y. (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69: 278–288 [DOI] [PubMed] [Google Scholar]
- Prévéral S, Gayet L, Moldes C, Hoffmann J, Mounicou S, Gruet A, Reynaud F, Lobinski R, Verbavatz JM, Vavasseur A, et al. (2009) A common highly conserved cadmium detoxification mechanism from bacteria to humans: heavy metal tolerance conferred by the ATP-binding cassette (ABC) transporter SpHMT1 requires glutathione but not metal-chelating phytochelatin peptides. J Biol Chem 284: 4936–4943 [DOI] [PubMed] [Google Scholar]
- Robert S, Zouhar J, Carter C, Raikhel N. (2007) Isolation of intact vacuoles from Arabidopsis rosette leaf-derived protoplasts. Nat Protoc 2: 259–262 [DOI] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. (1998) PHYTOREMEDIATION. Annu Rev Plant Physiol Plant Mol Biol 49: 643–668 [DOI] [PubMed] [Google Scholar]
- Singh OV, Labana S, Pandey G, Budhiraja R, Jain RK. (2003) Phytoremediation: an overview of metallic ion decontamination from soil. Appl Microbiol Biotechnol 61: 405–412 [DOI] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107: 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Sohn EJ, Martinoia E, Lee YJ, Yang YY, Jasinski M, Forestier C, Hwang I, Lee Y. (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat Biotechnol 21: 914–919 [DOI] [PubMed] [Google Scholar]
- Sooksa-Nguan T, Yakubov B, Kozlovskyy VI, Barkume CM, Howe KJ, Thannhauser TW, Rutzke MA, Hart JJ, Kochian LV, Rea PA, et al. (2009) Drosophila ABC transporter, DmHMT-1, confers tolerance to cadmium: DmHMT-1 and its yeast homolog, SpHMT-1, are not essential for vacuolar phytochelatin sequestration. J Biol Chem 284: 354–362 [DOI] [PubMed] [Google Scholar]
- Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF. (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA 107: 16500–16505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk OK, Bucher EA, Sundaram MV, Rea PA. (2005) CeHMT-1, a putative phytochelatin transporter, is required for cadmium tolerance in Caenorhabditis elegans. J Biol Chem 280: 23684–23690 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Bucher EA, Ward JT, Rea PA. (2001) A new pathway for heavy metal detoxification in animals: phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J Biol Chem 276: 20817–20820 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu YP, Rea PA. (1999) AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proc Natl Acad Sci USA 96: 7110–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H. (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12: 364–372 [DOI] [PubMed] [Google Scholar]
- Vögeli-Lange R, Wagner GJ. (1990) Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves: implication of a transport function for cadmium-binding peptides. Plant Physiol 92: 1086–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.