Abstract
Oviductal disease is a primary cause of infertility, a problem that largely stems from excessive inflammation of this key reproductive organ. Our poor understanding of the mechanisms regulating oviductal inflammation restricts our ability to diagnose, treat, and/or prevent oviductal disease. Using mice, our objective was to determine the spatial localization, regulatory mechanism, and functional attributes of a hypothesized regulator of oviductal inflammation, the hematopoietic form of prostaglandin D synthase (HPGDS). Immunohistochemistry revealed specific localization of HPGDS to the oviduct's epithelium. In the isthmus, expression of HPGDS was consistent. In the ampulla, expression of HPGDS appeared dependent upon stage of the estrous cycle. HPGDS was expressed in the epithelium of immature and cycling mice but not in the oviducts of estrogen receptor α knockouts. Two receptor subtypes bind PGD2: PGD2 receptor and G protein-coupled receptor 44. Expression of mRNA for Ptgdr was higher in the epithelial cells (EPI) than in the stroma (P < 0.05), whereas mRNA for Gpr44 was higher in the stroma than epithelium (P < 0.05). Treatment of human oviductal EPI with HQL-79, an inhibitor of HPGDS, decreased cell viability (P < 0.05). Treatment of mice with HQL-79 increased mRNA for chemokine (C-C motif) ligands 3, 4, and 19; chemokine (C-X-C motif) ligands 11 and 12; IL-13 and IL-17B; and TNF receptor superfamily, member 1b (P < 0.02 for each mRNA). Overall, these results suggest that HPGDS may play a role in the regulation of inflammation and EPI health within the oviduct.
Tubal or oviductal disease is a major reproductive concern in women (1, 2). Tubal dysfunction is comparable in etiology with ovulatory defects or endometriosis as an indication for the treatment of female infertility (3–5), and tubal ectopic pregnancies are the primary cause of mortality for a woman in her first trimester (6, 7). Excessive or chronic inflammation of the oviduct is the primary precursor to tubal disease (8–13), yet our understanding of inflammatory mechanisms in this organ, and hence our ability to diagnose, treat, and/or manage for tubal disease, remains poor.
In this article, we provide evidence suggesting that the hematopoietic form of prostaglandin (PG) D synthase (HPGDS) plays a pivotal role in the regulation of inflammation in the oviduct. This synthase acts downstream of PG-endoperoxide synthase 2 (cyclooxygenase-2) to catalyze the conversion of PGH2 to PGD2 (14–16). PGs are known to mediate inflammation (17), maintaining homeostasis of the epithelium in the lung (18, 19), digestive tract (20, 21), urinary (22, 23) and other reproductive (24, 25) systems. In addition, the involvement of PGs in epithelial-derived cancers (26–28) is well documented. Although PGs of the E and F series and their respective receptors are well established as regulators of many aspects of reproductive biology, HPGDS-derived PGD2 has received little attention as a regulator of reproductive function and represents a potential target to improve our ability to specifically diagnose and/or treat oviductal inflammation and disease.
When originally cloned, levels of mRNA for Hpgds were found to be highly expressed in the oviduct of the rat (29). However, with the interests of those authors lying outside of reproductive biology, no comprehensive analysis of this synthase, or its end product, was reported for the oviduct. Interestingly, the spatial pattern of expression for HPGDS in the various tissues and organs of the body is unique. In their original study, Kanaoka et al. (29) conducted a broad distribution analysis by Northern blotting and observed relatively specific expression of Hpgds in samples of the spleen, followed by the oviduct. In that study, HPGDS was not detectable in the ovary and uterus, or in many of the nonreproductive tissues they evaluated.
With inflammation-induced oviductal epithelial cell (EPI) death known to precede tubal occlusion and infertility (8–13), strong and relatively tissue-specific localization of HPGDS to the oviduct and the knowledge that PG are known regulators of inflammation, we hypothesized that HPGDS-catalyzed PGD2 may be acting as a key regulator of inflammation in this organ and hence performing a vital role in the maintenance of cellular homeostasis, patency, and function. Using mice, we report that HPGDS is localized specifically to the EPI of the oviduct, expressed before puberty, temporally regulated over the course of the estrous cycle, and dependent upon functional expression of the transcription factor estrogen receptor-α (ESR1). In addition, inhibition of HPGDS was found to decrease the viability of human oviductal EPI (hOEC) in vitro and increase the level of expression of multiple genes affecting inflammation within the oviducts of mice in vivo.
Together, our results suggest that HPGDS-catalyzed PGD2 may be a regulator of oviductal inflammation and that the role of this synthase and PGD2 in oviductal biology needs to be examined in greater detail. This synthase was identified within the oviduct over 10 yr ago (29), in such quantities that the oviduct was then used as a positive control in other studies (30, 31), yet HPGDS and PGD2 had not been investigated with respect to the actual function of the oviduct itself. Identification of HPGDS as a key regulator of oviductal inflammation would have dramatic implications to the management of female reproductive health, an especially important finding when considering the lack of noninvasive treatment options for oviductal disease, as well as the financial and emotional burden posed on both women and their families by oviductal-based infertility (3–5, 32–34).
Materials and Methods
Animals
Animal procedures involved in these studies were approved by the University of Kentucky Animal Care and Use Committee and/or the Institutional Animal Care and Use Committee of Hanyang University. With the exception of experiments using transgenic mice, female CD1 mice were used throughout. For the transgenic analysis, mice with a global deletion of ESR1 [ESR1 knockout (ESR1KO)] were generated on a C57BL6 background using the cre/loxP approach, and female littermates that expressed ESR1 served as wild-type (WT) controls (35). Genotypes were confirmed by the analysis of genomic DNA extracted from ear punches using the Easy DNA kit (Invitrogen, Carlsbad CA) according to the manufacturer's directions, as described previously (35, 36). For the analysis of oviducts collected on defined days of the estrous cycle, females were staged by analysis of vaginal cytology, as described before (37). Briefly, vaginal smears were collected daily, at the same time each day, with 0.9% sodium chloride using a bent, blunted borosilicate glass pipette. Vaginal cytology was analyzed under a Motic AE21 inverted microscope (Motic Instruments, Richmond, British Columbia, Canada) and classified according to well-established morphological criteria (38, 39). Digital images were recorded for later reference.
Immunohistochemistry
The spatial localization of HPGDS in the oviduct was determined in regularly cycling CD1 females, as well as immature females treated with 5 IU of pregnant mare's serum gonadotropin (PMSG) (Sigma-Aldrich, St. Louis, MO) to induce follicular development and the production of estradiol. In addition, the dependence of HPGDS on ESR1 was evaluated in 10-wk-old ESR1KO and WT females, killed on diestrus. Diestrus was chosen for consistency among genotypes, because the ESR1KO mice do not display regular estrous cycles (40). Oviducts were retrieved from all mice and fixed for 4 h in Bouin's fixative (Sigma-Aldrich), then embedded in paraffin. Sections were cut to 5 μm, mounted on poly-l-lysine-coated glass slides, deparaffinized, and rehydrated. Antigen retrieval was performed by autoclaving for 10 min in 10 mm citrate buffer (pH 6.0). The slides were then incubated for 15 min in 3% H2O2 in PBS to remove endogenous peroxidase activity, blocked for 60 min in 3% BSA in PBS, and incubated overnight at 4 C with a primary (rat monoclonal) antibody against HPGDS (no. MBS601438; MyBioSource, San Diego, CA) at a dilution of 1:1000. After washing in PBS, the slides were then incubated for 60 min at room temperature in a 1:500 dilution of rabbit antirat IgG (Abcam, Cambridge, MA) in PBS with 1% BSA. After further rinses in PBS, the coloring reaction was conducted with ImmPACT diaminobenzidine-based peroxidase substrate for 10 min at room temperature. Sections were rinsed again in PBS, then dehydrated in a graded ethanol series, dipped in xylenes, and permanently mounted. Images were obtained using a digital imaging system (DFC320; Leica Microsystems, Wetzlar, Germany), as described previously (41, 42). Immunohistochemistry was performed on multiple sections in at least two independent experiments for any genotype and/or time of collection.
Western blotting
Western blotting was used to determine the temporal expression of HPGDS in whole oviducts collected from 8- to 10-wk-old mice killed on each day of the estrous cycle. Oviducts were collected from mice killed between 1300 and 1400 h on each day of the estrous cycle. Single oviducts collected from each of four mice were pooled to generate a sample; the analysis was replicated in three experiments. Oviducts were lysed by homogenization in radioimmunoprecipitation assay buffer (Cell Signaling Technology, Danvers, MA). The concentration of protein in each sample was determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL) and protein (50 μg per lane) separated by 10% SDS-PAGE before being transferred to nitrocellulose membranes (Whatman, Kent, UK) using a semidry transfer. Membranes were blocked with 5% nonfat milk at room temperature for 2 h to reduce nonspecific binding and then incubated at 4 C overnight with the same antibody used for the immunohistochemical localization of HPGDS at a dilution of 1:1000. The immunoreaction was determined by incubation for 1 h at room temperature with a horseradish peroxidase-conjugated antirat IgG (Abcam, Cambridge, UK). Subsequent incubation with antiglyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Lab Frontier, Seoul, Korea) and anti-β-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) polyclonal rabbit antibodies, each diluted at 1:5000, was used to verify protein loading, with all images acquired for subsequent quantification using an enhanced chemiluminescence kit (Amersham Pharmacia, Freiburg, Germany), as described before (37).
Gene expression I
Real-time PCR was performed to determine the level of expression of mRNA encoding the two receptors that bind PGD2, PGD receptor (Ptgdr), and G protein-coupled receptor 44 (Gpr44). Gene expression was determined in isolated oviductal EPI and residual oviductal stroma collected from immature CD1 females killed at 0 and 48 h after treatment with 5 IU of PMSG. Briefly, oviductal EPI were separated from the residual stroma (RES) by gently squeezing small sections of the oviduct with fine forceps under a dissecting microscope, as described before (41). The EPI or RES from three or four mice was pooled to generate a single sample, and a total of three samples were generated at each time point. Total RNA was then extracted using TRIzol (Invitrogen) and purified with RNeasy (QIAGEN, Valencia, CA). Oligonucleotide primers (Table 1) were generated for Ptgdr and Gpr44 as well as L19 as an endogenous reference gene. The specificity of each primer set was confirmed by running the PCR products on a 1.5% agarose gel as well as analyzing the melting (dissociation) curve after each PCR reaction. Real-time PCR was performed with a total volume of 25 μl per reaction, with each reaction containing 5 μl of cDNA, 1 μl of a 10 μm stock of each primer (forward and reverse), 12.5 μl of 2× SYBR Green PCR Master Mix, and 5.5 μl of diethylpyrocarbonate-treated water. Real-time PCR were performed on an Eppendorf ep realplex2 Thermal Cycler (Eppendorf, Hamburg, Germany), as described previously (37, 41). The relative amount of each transcript was calculated using the 2−ΔΔCT method (43).
Table 1.
Primer sequences (forward and reverse) used for independent real-time PCR analyses
| Name | Primer sequence |
|---|---|
| Ccl3 | 5′-CCTCTGTCACCTGCTCAACA-3′ |
| 5′-GATGAATTGGCGTGGAATCT-3′ | |
| Ccl4 | 5′-TCCCACTTCCTGCTGTTTCT-3′ |
| 5′-GAGGAGGCCTCTCCTGAAGT-3′ | |
| Ccl11 #1 | 5′-TTCCATCTGTCTCCCTCCAC-3′ |
| 5′-CTATGGCTTTCAGGGTGCAT-3′ | |
| Ccl11 #2 | 5′-CTCCACAGCGCTTCTATTCC-3′ |
| 5′-CTTCTTCTTGGGGTCAGCAC-3′ | |
| Ccl11 #3 | 5′-CTCCACAGCGCTTCTATTCC-3′ |
| 5′-CTATGGCTTTCAGGGTGCAT-3′ | |
| Ccl11 #4 | 5′-CTCCACAGCGCTTCTATTCC-3′ |
| 5′-CTTCTTCTTGGGGTCAGCAC-3′ | |
| Ccl19 | 5′-CAGGAAACCAAGGACCAGAA-3′ |
| 5′-CGGCTTTATTGGAAGCTCTG-3′ | |
| Cxcl11 | 5′-CAGCTGCGACAAAGTTGAAG-3′ |
| 5′-GCATGTTCCAAGACAGCAGA-3′ | |
| Cxcl12 | 5′-CTCCCTCTCTTCCCTTTGCT-3′ |
| 5′-TCAGAGCCCATAGAGCCACT-3′ | |
| Gapdh | 5′-CCCCCAATGTGTCCGTCGTGG-3′ |
| 5′-TGAGAGCAATGCCAGCCCCG-3′ | |
| Gpr44 | 5′-AGAGACACCATCCCGCGGCT-3′ |
| 5′-ACAGAATGGGCACGCGCCTC-3′ | |
| Hpgds | 5′-GCGCCAAACCCAGAAGGCCT-3′ |
| 5′-GCTTGGGCCTGGGCCACATT-3′ | |
| Il13 | 5′-GGTTCTCTCACTGGCTCTGG-3′ |
| 5′-CCACACTCCATACCATGCTG-3′ | |
| Il17b | 5′-CTGACTTGGTGGGATGGACT-3′ |
| 5′-CTCTTCCATTCGAGCGTAGG-3′ | |
| L19 | 5′-TGGTTGGATCCCAATGAGAC-3′ |
| 5′-GTCTGCCTTCAGCTTGTGGAT-3′ | |
| Ptgdr | 5′-CTTCGGTGCGGGGCTTCTGG-3′ |
| 5′-CCAGCAAAGGGGAGCGCACA-3′ | |
| Tnfrsf1b | 5′-AAGGACTGTTCCTGGGTGTG-3′ |
| 5′-GTAAAGGTGGGATGGGAGGT-3′ |
Gene expression II
To determine whether inhibition of HPGDS in vivo affected the expression of genes regulating inflammation in the oviduct, a real-time PCR-based superarray approach was used. Beginning on the day of diestrus, 8-wk-old CD1 mice were treated orally, twice daily for 4 d, with 30 mg/kg body weight of an inhibitor of HPGDS (HQL-79; Cayman Chemical, Ann Arbor, MI) in 2% methyl cellulose, or 2% methyl cellulose as the vehicle control. Treatment with HQL-79 will specifically inhibit HPGDS-catalyzed production of PGD2; treatment will have minimal effect on the production of any other PG (44). This dose was chosen based upon reports by others (45–48). At 1300 h on the fifth day, mice were killed and the oviducts collected. Oviducts were collected from four mice for each treatment, and the paired oviducts from each mouse were handled as individual samples for this analysis. Total RNA was isolated from each sample and purified, as described above. The mouse Inflammatory Cytokines and Receptors RT2 Profiler PCR arrays and RT2 Real-Timer SYBR Green reagent were purchased from SuperArray Bioscience Corp. (Frederick, MD) and used, as described before (37). Briefly, each superarray (96-well plate) contains the primers to identify 84 key genes involved in the inflammatory response as well as housekeeping genes. A genomic DNA contamination control, RT controls and positive PCR controls are also included. The RT2 First Strand kit contained all the reagents required to reverse transcribe the total RNA to cDNA and eliminate potential genomic DNA. The level of expression of those genes identified by the superarray analysis as having a probability of being affected by treatment (P < 0.10) was then examined by real-time PCR using independently designed primers. Oligonucleotide primers were generated and tested (Table 1) and real-time PCR performed, as described above. For this analysis, ROX dye, a stabilized conjugate of caboxy-X-rhodamine in water (at a 1:500 dilution) was also included in each PCR, and consistent with analysis of the superarray dataset, the relative level of expression of each mRNA was standardized against GAPDH as a housekeeping gene (43).
Gene expression III
To determine whether the expression of mRNA encoding Hpgds in the oviduct was dependent upon ESR1, oviducts were collected from transgenic mice bearing a global deletion of ESR1 for analysis by real-time PCR. Immature female mice (ESR1KO and WT) were killed at 23 d of age or treated with 5 IU of PMSG and killed 48 h later. Whole oviducts were collected for subsequent extraction of RNA, and the oviducts from three or four mice of each genotype were pooled to generate a single sample. A total of three samples were generated for each group. Oligonucleotide primers (Table 1) for Hpgds were generated, tested, and real-time PCR performed, as described above.
EPI viability analysis
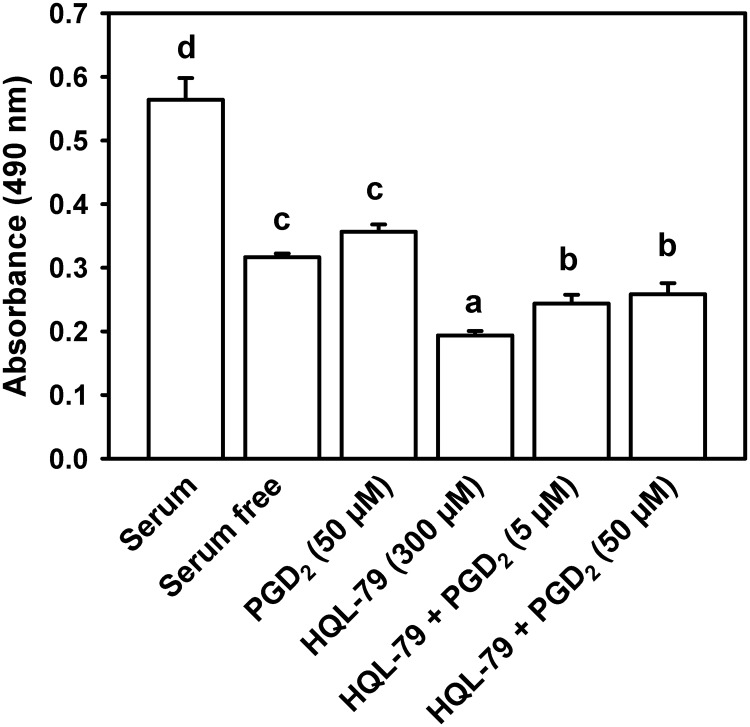
Inflammation-induced oviductal EPI death is a common precursor to oviductal disease (8–13). We therefore sought to determine whether inhibition of HPGDS in vitro affected oviductal EPI viability using a well-characterized, immortalized line (OE-E6/E7) of hOEC (49) and the CellTiter 96 AQueous One Solution (MTS, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt]) Cell Proliferation Assay system (Promega, Madison, WI). The cell line was a generous gift from William S.B. Yeung at the University of Hong Kong and was used according to reported guidelines (49–52). Cells were maintained in T-25 (25 cm2) or T-75 (75 cm2) flasks in DMEM/F12 medium with 10% fetal bovine serum plus antibiotics at 5% CO2, 37 C (all from Invitrogen). For the cell viability analysis, cells were subcultured to 96-well plates at 2 × 104 cells per well. Six hours before experimental treatment, culture medium was changed to serum-free DMEM/F12. Cells were then treated with vehicle, 50 μm PGD2, 300 μm HQL-79, 300 μm HQL-79 plus 5 μm PGD2, or 300 μm HQL-79 plus 50 μm PGD2 (Cayman Chemical). After overnight incubation, cell viability was determined using the MTS assay according to the manufacturer's protocol. Twenty microliters of the MTS reagent were added to each well, the cells incubated for an additional 3 h, and then absorbance measured at 492 nm using an Infinite F200 plate reader (Tecan Group, Männedorf, Switzerland), as described before (53). As described by the manufacturer, cell viability is directly proportional to the quantity of formazan product as measured by absorbance.
Statistical analysis
Datasets were first tested for normality and homogeneity of variance. When appropriate, data were transformed before statistical analysis. Nontransformed data are depicted in all the figures. One-way ANOVA using SigmaStat 3.5 (Systat Software, Inc., Point Richmond, CA) was used to determine differences in EPI viability, levels of mRNA for the analyses of Hpgds in WT and ESR1KO mice, and for the quantification of HPGDS in the oviducts of cycling mice. If differences were detected, Tukey's test was used to determine which means differed. The Student's t test was used to determine the effect of treatment with HQL-79 on gene expression in the superarray and independent real-time PCR analyses, to determine whether levels of mRNA for Ptgdr and Gpr44 differed in EPI vs. RES and to determine whether HPGDS differed in the oviducts of immature mice vs. those treated with PMSG.
Results
Expression of HPGDS in the oviduct
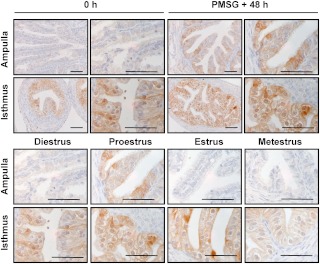
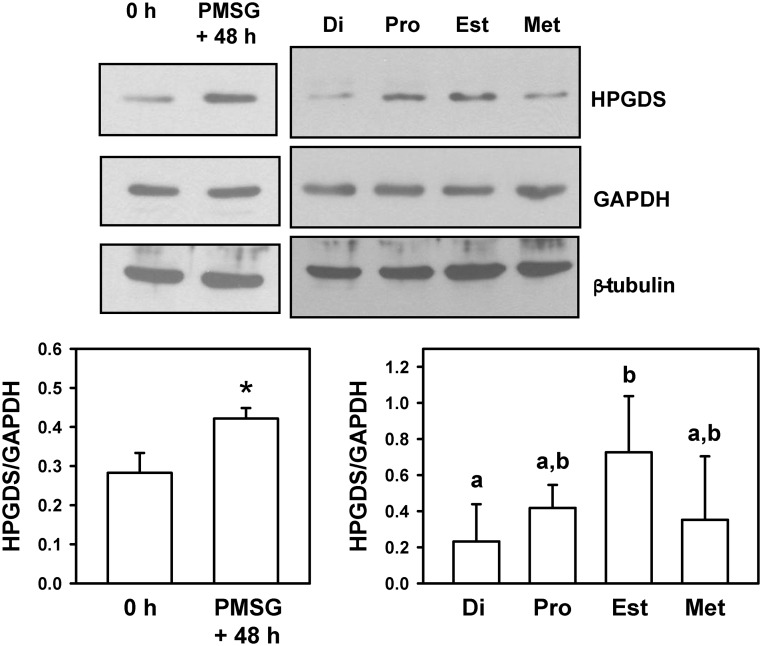
The oviduct is known as a dominant site for the expression of mRNA encoding Hpgds (29). However, no analysis of the spatial or temporal patterns of expression had been performed, an analysis that could provide clues as to the functional significance of what appears to be an active modulator of oviductal function. Hence, immunohistochemistry was used to determine the cellular and spatial localization of HPGDS, as well as provide an indication of whether the expression of this synthase changes in response to treatment with exogenous gonadotropins, or over the course of the estrous cycle. In the immature, prepubertal mouse, HPGDS was consistently expressed in the EPI lining the isthmus of the oviduct (Fig. 1). Forty-eight hours after follicular development was stimulated in these immature mice by treatment with PMSG, staining for HPGDS was also readily observed in the oviduct's ampullary EPI layer. Our analysis of HPGDS in the oviducts of mature, naturally cycling mice proved consistent with these results. Epithelial localization of HPGDS was observed in the isthmus of mice killed on all days of the estrous cycle, with an induction of HPGDS in the EPI of the ampulla observed in mice killed at proestrus. As a complement to the analysis of HPGDS by immunohistochemistry, Western blotting was performed on lysates of whole oviducts collected from mice at the same stages of development. The hematopoetic form of PGDS was detectable in whole oviducts of immature (0 h) mice and increased after treatment with PMSG (P < 0.01) (Fig. 2). The level of expression of HPGDS also differed over the course of the estrous cycle; HPGDS was increased in whole oviducts collected on the day of estrus vs. the day of diestrus (P < 0.05) (Fig. 2). Because differences in the spatial localization of HPGDS were observed by immunohistochemistry, especially an induction of HPGDS in the EPI of the ampulla in mice killed at PMSG + 48 h and at proestrus, the quantification of HPGDS in whole oviducts should be interpreted accordingly.
Fig. 1.
Immunohistochemical localization of HPGDS in the ampulla and isthmus of the mouse oviduct. Representative images are shown. Upper panels, Immature mice were killed at 23 d of age (0 h) or treated with 5 IU of PMSG to induce follicular development and killed 48 h later. Lower panel, Eight- to ten-week-old regularly cycling mice were killed on each day of the estrous cycle. Tissues were fixed in Bouin's fixative and embedded in paraffin. Scale bars, 50 μm.
Fig. 2.
Detection of HPGDS in whole oviducts of mice. Upper panels, Images of representative Western blottings are shown. Upper left, Immature mice were killed at 23 d of age (0 h) or treated with 5 IU of PMSG to induce follicular development and killed 48 h later. Upper right, Eight- to ten-week-old regularly cycling mice were killed on each day of the estrous cycle. Di, Diestrus; Pro, proestrus; Est, estrus; Met, metestrus. GAPDH and β-tubulin were used as loading controls. Lower panels, Quantification of Western blottings (HPGDS/GAPDH). Lower left, An asterisk indicates differences in gene expression (P < 0.01). Lower right, Values with different superscript letters differ (P < 0.05).
Expression of Ptgdr and Gpr44 in the oviduct
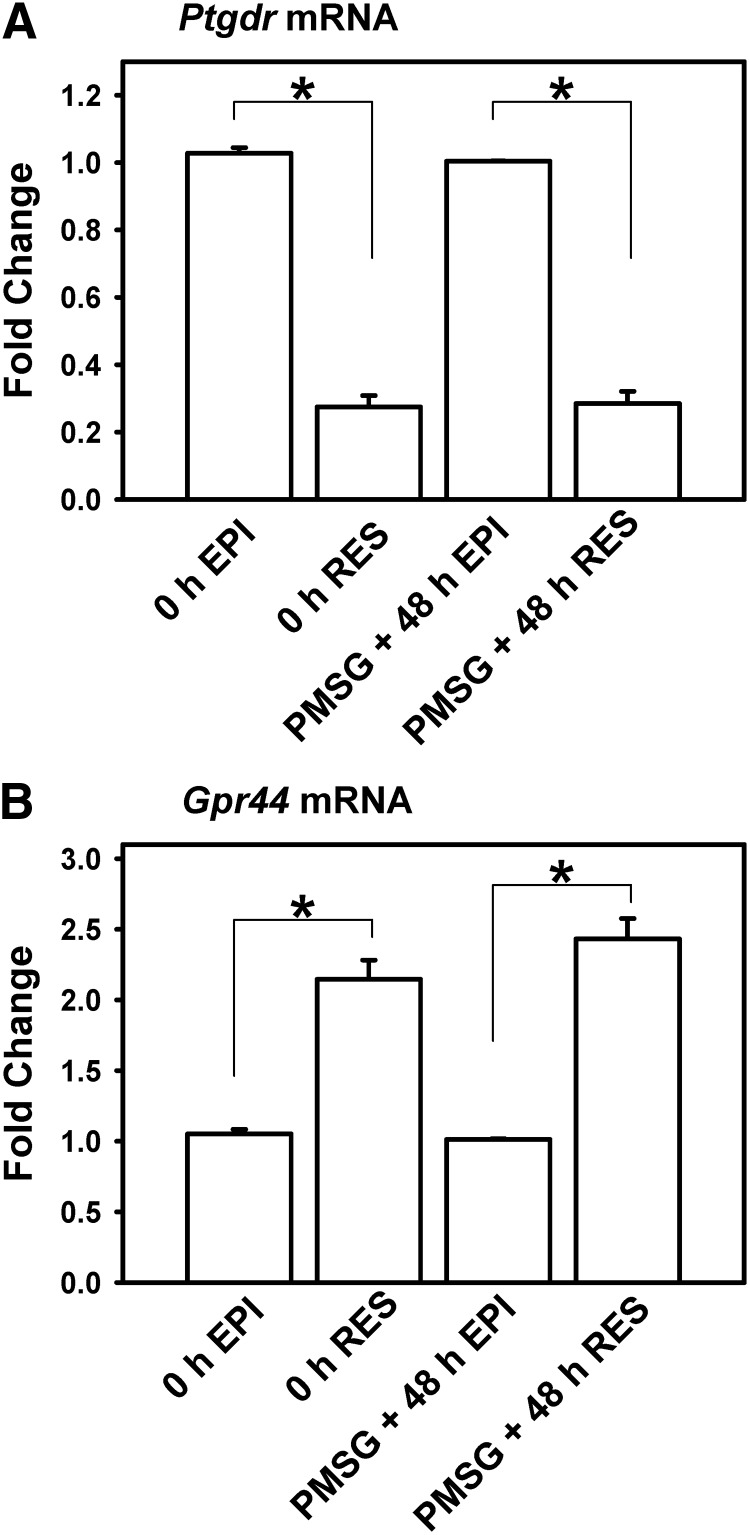
Two receptor subtypes are reported to bind PGD2, PTGDR and GPR44, which is also known as chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2). To begin to understand the receptor-mediated signaling pathways involved in EPI-catalyzed PGD2, a real-time PCR analysis was performed to determine levels of mRNA encoding Ptgdr and Gpr44 in the oviducts of immature mice, before and 48 h after treatment with exogenous PMSG. On a per microgram of total RNA basis, the expression of mRNA for Ptgdr was higher in oviductal EPI than RES (P < 0.05) (Fig. 3A). In contrast, mRNA for Gpr44 was higher in the RES than isolated EPI (P < 0.05) (Fig. 3B). This was observed in mice killed at 0 h and at PMSG + 48 h. Overall, these results suggest diversity to the effects of PGD2 on oviductal function, with biological action(s) apparently not confined to the dominant, epithelial site of HPGDS expression.
Fig. 3.
Expression of mRNA encoding Ptgdr and Gpr44 in EPI and RES collected from whole oviducts of mice. Immature mice were treated with 5 IU of PMSG to induce follicular development and killed 48 h later. Data are the means ± sem for real-time PCR analysis of three samples for each cell type, with EPI and RES cells pooled from three to four mice for each sample. Levels of mRNA were obtained by real-time PCR and are expressed as fold changes. For each time point within a panel, an asterisk indicates differences in gene expression (P < 0.05).
Dependence of HPGDS on ESR1
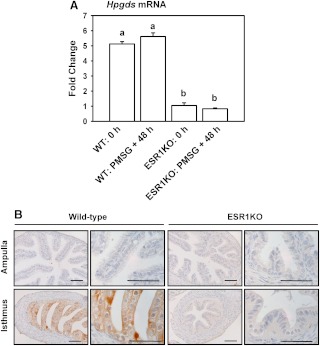
Immunohistochemistry indicated an induction of HPGDS in the EPI of the ampulla from immature mice treated with PMSG and cycling mice killed at proestrus. With these results suggesting possible regulation through estradiol and ESR1, the expression of mRNA encoding Hpgds and its protein was determined in oviducts collected from mice with or without a global deletion of ESR1. Real-time PCR revealed readily detectable levels of mRNA for Hpgds in whole oviducts collected from immature WT mice, regardless of treatment with PMSG (Fig. 4A), with the lack of any induction of Hpgds by PMSG likely reflecting the dominance of EPI in the isthmus vs. the ampulla of the oviduct (where the differential staining for HPGDS was observed). The expression of mRNA encoding Hpgds was decreased to approximately 20% of WT controls in ESR1KO females, again regardless of treatment with PMSG (P < 0.05). This apparent dependence on ESR1 was confirmed by immunohistochemistry, with HPGDS readily detectable in the EPI of the isthmus in 10-wk-old WT mice but not in 10-wk-old ESR1KOs (Fig. 4B).
Fig. 4.
A, Expression of mRNA encoding Hpgds in whole oviducts collected from WT and ESR1KO mice. Immature mice were killed at 23 d of age (0 h) or treated with 5 IU of PMSG to induce follicular development and killed 48 h later. Data are the means ± sem for real-time PCR analysis of three samples for each treatment and genotype, with oviducts pooled from three to four mice for each sample. Levels of mRNA were obtained by real-time PCR and are expressed as fold changes. Values with different superscript letters differ (P < 0.05). B, Localization of HPGDS in the ampulla and isthmus of 10-wk-old WT, and ESR1KO mice killed on diestrus. Representative images are shown. Tissues were fixed in Bouin's fixative and embedded in paraffin. Scale bars, 50 μm.
Regulation of inflammatory gene expression in vivo
To test our hypothesis that HPGDS is regulating inflammatory processes in the oviduct, we treated mice for 4 d with an orally active inhibitor of HPGDS (HQL-79) before collecting their oviducts for a superarray, real-time PCR-based analysis of inflammatory gene expression. The expression of nine key genes identified in the superarray analysis (P < 0.10) was then reevaluated by independent real-time PCR. With the exception of chemokine (C-C motif) ligand 11 (Ccl11), the results of the two real-time PCR analyses were consistent (Table 2). Expression of mRNA's encoding chemokine (C-C motif) ligands 3, 4 and 19 (Ccl3, Ccl4, and Ccl19) were each increased by 2- to 3-fold after treatment of mice with HQL-79 (P < 0.02 for each mRNA by independent real-time PCR). Expression of mRNA encoding chemokine (C-X-C motif) ligands 11 and 12 (Cxcl11 and Cxcl12, respectively) was also increased by inhibition of HPGDS in vivo; however, our secondary analysis of mRNA for Cxcl11 indicated a reduced magnitude of change for the expression of that gene (3.03- vs. 1.69-fold, P = 0.082 by independent real-time PCR). Similarly, expression of mRNA encoding IL-13 and IL-17B (Il13 and Il17b), and TNF receptor superfamily, member 1b (Tnfrsf1b), was increased by approximately 2-fold by treatment of mice with HQL-79 (P < 0.02 for each mRNA by independent PCR). Given the −87-fold (P < 0.001) effect of treatment on the expression of mRNA encoding Ccl11 in the superarray analysis, we generated a total of four sets of primers to validate those results. We did not confirm the superarray data for Ccl11, and our secondary, independent analysis was consistent using all four primer pairs that were generated. Although levels of mRNA for Ccl11 in the oviducts of HQL-79-treated mice appeared to average approximately 5-fold increased levels of expression, this was largely due to a dramatic induction of mRNA for this gene in the oviducts of one animal alone. Overall, the results of this experiment suggest that HPGDS does regulate inflammation in the oviduct of the mouse. Inhibition of HPGDS by treatment of mice with HQL-79 increased the expression of mRNA for eight of the inflammatory genes examined.
Table 2.
Effect of treatment with HQL-79 on inflammatory gene expression in vivo
| Gene name | Symbol | Fold change HQL-79/Vehicle |
P value (t test) |
||
|---|---|---|---|---|---|
| Array | PCR | Array | PCR | ||
| Chemokine (C-C motif) ligand 3 | Ccl3 | 2.04 | 2.31 | 0.036 | 0.008 |
| Chemokine (C-C motif) ligand 4 | Ccl4 | 1.97 | 2.78 | 0.083 | 0.004 |
| Chemokine (C-C motif) ligand 11 | Ccl11 | −87.4 | 4.97 | 0.001 | .120 |
| Chemokine (C-C motif) ligand 19 | Ccl19 | 2.14 | 2.42 | 0.026 | 0.015 |
| Chemokine (C-X-C motif) ligand 11 | Cxcl11 | 3.03 | 1.69 | 0.013 | 0.082 |
| Chemokine (C-X-C motif) ligand 12 | Cxcl12 | 2.03 | 2.93 | 0.063 | 0.018 |
| IL-13 | II13 | 2.52 | 2.27 | 0.097 | 0.012 |
| IL-17B | II17b | 2.31 | 2.24 | 0.033 | 0.005 |
| TNF receptor superfamily, member 1b | Tnfrsf1b | 1.95 | 1.83 | 0.057 | 0.006 |
Fold change in gene expression and P values are indicated after analysis by superarray (array) and by independent real-time PCR (PCR).
Regulation of oviductal EPI viability in vitro
Inflammation-induced oviductal EPI death is known to precede tubal blockage and infertility (8–13). To address the functional significance of identifying HPGDS as a regulator of inflammation in the oviduct, we sought to determine whether treatment of an immortalized line of hOEC with this same inhibitor affected EPI viability in vitro. Withdrawal of serum decreased the viability of hOEC (P < 0.05) (Fig. 5). Treatment of serum-free hOEC with HQL-79 decreased the viability of EPI to approximately 60% of serum-free controls (P < 0.05). Treatment of hOEC with PGD2 alone did not affect EPI viability (P > 0.05); however, concurrent treatment of hOEC with HQL-79 plus either dose of PGD2 was partially able to reverse the effect of HQL-79, suggesting that the effect of treatment with HQL-79 was specific to the HPGDS-PGD2 pathway and not a deleterious effect of treatment with this inhibitor. Real-time PCR confirmed that mRNA for Ptgdr and Gpr44 was expressed in cultured hOEC (data not shown).
Fig. 5.
Effect of HQL-79 and PGD2 on the viability of hOEC in vitro. Viability is expressed as absorbance, using the Cell Titer MTS assay system. Data are the means ± sem from six replicates per treatment. Values with different superscript letters differ (P < 0.05).
Discussion
Our results suggest that HPGDS-catalyzed PGD2 may act as a regulator of inflammation in the oviduct. Inhibition of HPGDS in vivo consistently increased the expression of mRNA for inflammation-regulating chemokines and ILs. Inhibition of HPGDS in vitro decreased the viability of hOEC. We found that HPGDS was abundant in the EPI of the isthmus, even in the immature mouse, and appeared largely dependent upon functional expression of ESR1. In addition, some temporal regulation of HPGDS was observed over the course of the estrous cycle, especially an induction in the EPI of the ampulla under an estrogen-dominant hormonal environment (at PMSG + 48 h and at proestrus). Considering that freshly ovulated cumulus-oocyte complexes, associated follicular debris, spermatozoa, seminal fluids, and possibly an array of foreign pathogens must traverse the oviduct after each ovulation or after mating, it can be postulated that high levels of HPGDS are constitutively expressed in the oviduct as part of a mechanism that ensures this organ the ability to rapidly respond to an inflammatory insult and, therefore, maintain homeostasis and continued function, i.e. we hypothesize that upon an insult to the oviduct, the expression of HPGDS is decreased, allowing the inflammatory response to proceed rapidly.
When first cloned, mRNA for Hpgds was detected in the oviduct but not in the ovary or uterus (29). This appears to be due to the sensitivity of the methods employed rather than total specificity of this synthase to the oviduct as a female reproductive organ. In cultures of luteinizing granulosa cells collected from rats, the expression of mRNA for Hpgds was reported to increase after treatment with human chorionic gonadotropin (53). mRNA for Gpr44 has also been localized to the granulosa cells of the ovarian follicle (54). In addition, Zelinski-Wooten et al. (55, 56) have performed analyses of all the major PG, including PGD2, on various aspects of luteal function. Most pertinent to the results described here, treatment of granulosa cells collected from rats with PGD2 was reported to increase cell viability (53). Although we did not observe an increase in oviductal EPI viability after treatment with PGD2 alone, the effect of PGD2 on HQL-79-treated oviductal EPI viability can be considered consistent with this. The ovary must therefore be considered a potential site of action for HPGDS-catalyzed PGD2; however, from a functional viewpoint, the abundance of HPGDS to the oviduct needs to be recognized. We evaluated the relative level of expression of mRNA for Hpgds in the reproductive tract of the mouse and consistently found that the level of mRNA for Hpgds was approximately 15-fold higher in the oviduct than the associated ovary (Bridges, P.J. and M. Jeoung, unpublished data).
When considering the inflammatory process and the potential regulation within the oviduct by HPGDS, cytokines can be broadly described as signaling molecules that regulate immune function. Chemokines and ILs are both members of the family of cytokines; chemokines are recognized for their chemotactic activity toward leukocytes (57); however, the breadth in the immune response to ILs makes them harder to classically define. Interestingly, chemotactic recruitment of leukocytes into the oviduct appears consistent with the endocrine-regulated immune response to the physiological inflammatory process that is ovulation. Leukocytes are well-established infiltrators of the ovary around ovulation (58, 59), and in this study, we observed an increase in expression of mRNA for chemokine (C-C motif) ligands 3, 4, and 19 and chemokine (C-X-C motif) ligands 11 and 12 in the oviducts of mice treated with HQL-79. Overall, the extent of chemokine regulation that we observed suggests a role for HPGDS in the regulation of leukocyte infiltration in preparation for EPI damage after ovulation and/or mating. Additional research specifically investigating inflammatory infiltration after inhibition of HPGDS is warranted to delineate the precise mechanisms involved. Given that the spleen appears to acts as a reservoir for the leukocytes released around ovulation (58, 59), the specificity of dominant sites of expression for Hpgds, i.e. to the spleen plus oviduct (29), is intriguing.
An increase in mRNA for Il13 and Il17b was also observed after inhibition of HPGDS. IL-17 is reported to contribute to the influx of neutrophils after genital infection of mice with Chlamydia muridarum (60), and an increase in IL-2, IL-6, and IL-10 was observed after infection of pig-tailed macaques with Chlamydia trachomatis (61). Infection with C. trachomatis is also reported to induce chemokine (C-X-C motif) ligand 13 within the human oviduct (62). In addition to HPGDS-regulated inflammation as a mechanism to ensure oviductal homeostasis after ovulation and mating, potential regulation of cytokines induced in response to pathological infection appears likely. Given that the incidence of infection with C. trachomati is currently at record high levels in the United States (63) and that treatment of Neisseria gonorrhoeae, the second most reported of these notifiable sexually transmitted diseases, is becoming increasingly plagued by resistance to antibiotic therapy (64), further examination of HPGDS as a potential regulator of the immune response to pathological infection is warranted.
If HPGDS does indeed act as a primary regulator of inflammation within the oviduct, the apparent dependence of this synthase on ESR1 is interesting. This is especially true when considering that the subtle temporal regulation of HPGDS over the course of the estrous cycle, and apparent induction of this synthase in the EPI of the ampulla in response to PMSG and at proestrus suggests only minor regulation by ovarian-produced estradiol. Considering that HPGDS was also found to be highly expressed in the oviducts of immature, prepubertal mice, it appears that this requirement for ESR1 is a developmental phenomenon rather than an active postpubertal steroid/receptor signaling mechanism. Within the oviduct, ESR1 is distributed in a homogenous manner. Immunostaining reveals distinct nuclear localization to both ciliated and secretory EPI, as well as to the nuclei of the oviduct's smooth muscle (65). This nuclear receptor is also abundant in the oviducts of prepubertal mice (65). It is important to note, however, that in contrast to the well-recognized phenotype of the ovaries collected from ESR1KO mice (66, 67), sections of both the ampulla and isthmus appear to be morphologically normal in these ESR1KOs (Fig. 4).
Further examination of gene expression in the oviducts of ESR1KO mice also revealed what appears to be a partial mechanism to compensate for decreased HPGDS activity. In the oviducts of mice devoid of ESR1 and therefore HPGDS, we observed an increase in the expression of mRNA for a genetically distinct, alternate synthase that can also catalyze the production of PGD2 (68, 69), lipocalin-type PGDS (LPGDS). This synthase is recognized for its role in the central nervous system (70, 71), especially in the regulation of sleep-wake cycles (72). Although the overall level of expression of mRNA for Lpgds in the oviduct is low when compared with Hpgds, the expression of mRNA for Lpgds in the oviducts of ESR1KO mice was approximately 1.5-fold greater than for their WT littermates (Bridges, P.J. and M. Jeoung, unpublished data). LPGDS is established as an estrogen-regulated gene (73, 74), yet consistent with our analysis of mRNA for Hpgds, we observed no affect of treatment with gonadotropins on the expression of mRNA for Lpgds in WT or ESR1KOs.
Overall, our results support the hypothesis that HPGDS is a regulator of inflammation in the oviduct, an effect likely required to maintain homeostasis and function of this key reproductive organ. Given the prevalence of oviductal dysfunction causing infertility in both women (3–5) and domestic species (75), further investigation of not only HPGDS-regulated inflammation but how the oviduct responds to pathological challenges appears to be needed. Our understanding of oviductal biology remains limited as a whole, and delineating mechanisms such as HPGDS-regulated inflammation can only increase our ability to manage for optimal reproductive health.
Acknowledgment
This work was supported by Start-up funds from the University of Kentucky (P.J.B.), National Institutes of Health Grants P20 RR15592 (P.J.B., C.K., M.Jo) and K12 DA014040 (P.J.B.), and the Korean Government Grant NRF 2011-0017084 (to M.C.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ccl11
- Chemokine (C-C motif) ligand 11
- EPI
- epithelial cell
- ESR1
- estrogen receptor-α
- ESR1KO
- ESR1 knockout
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Gpr44
- G protein-coupled receptor 44
- hOEC
- human oviductal EPI
- HPGDS
- hematopoietic form of PGD synthase
- LPGDS
- lipocalin-type PGDS
- MTS
- [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt]
- PG
- prostaglandin
- PMSG
- pregnant mare's serum gonadotropin
- Ptgdr
- PGD receptor
- RES
- residual stroma
- WT
- wild type.
References
- 1. Confino E, Radwanska E. 1992. Tubal factors in infertility. Curr Opin Obstet Gynecol 4:197–202 [PubMed] [Google Scholar]
- 2. Kodaman PH, Arici A, Seli E. 2004. Evidence-based diagnosis and management of tubal factor infertility. Curr Opin Obstet Gynecol 16:221–229 [DOI] [PubMed] [Google Scholar]
- 3. Wright VC, Chang J, Jeng G, Macaluso M. 2006. Assisted reproductive technology surveillance—United States, 2003. MMWR Surveill Summ 55:1–22 [PubMed] [Google Scholar]
- 4. Wright VC, Chang J, Jeng G, Chen M, Macaluso M. 2007. Assisted reproductive technology surveillance—United States, 2004. MMWR Surveill Summ 56:1–22 [PubMed] [Google Scholar]
- 5. Wright VC, Chang J, Jeng G, Macaluso M. 2008. Assisted reproductive technology surveillance—United States, 2005. MMWR Surveill Summ 57:1–23 [PubMed] [Google Scholar]
- 6. Goldner TE, Lawson HW, Xia Z, Atrash HK. 1993. Surveillance for ectopic pregnancy—United States, 1970–1989. MMWR CDC Surveill Summ 42:73–85 [PubMed] [Google Scholar]
- 7. Creanga AA, Shapiro-Mendoza CK, Bish CL, Zane S, Berg CJ, Callaghan WM. 2011. Trends in ectopic pregnancy mortality in the United States: 1980–2007. Obstet Gynecol 117:837–843 [DOI] [PubMed] [Google Scholar]
- 8. Aziz N. 2010. Laparoscopic evaluation of female factors in infertility. J Coll Physicians Surg Pak 20:649–652 [DOI] [PubMed] [Google Scholar]
- 9. Perfettini JL, Darville T, Gachelin G, Souque P, Huerre M, Dautry-Varsat A, Ojcius DM. 2000. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor α secretion on apoptosis in the murine genital tract. Infect Immun 68:2237–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Ulloa AC, Arrieta O. 2005. Tubal occlusion causing infertility due to an excessive inflammatory response in patients with predisposition for keloid formation. Med Hypotheses 65:908–914 [DOI] [PubMed] [Google Scholar]
- 11. Steffl M, Schweiger M, Sugiyama T, Amselgruber WM. 2008. Review of apoptotic and non-apoptotic events in non-ciliated cells of the mammalian oviduct. Ann Anat 190:46–52 [DOI] [PubMed] [Google Scholar]
- 12. Donnez J, Casanas-Roux F. 1988. Histology: a prognostic factor in proximal tubal occlusion. Eur J Obstet Gynecol Reprod Biol 29:33–38 [DOI] [PubMed] [Google Scholar]
- 13. Punnonen R, Söderström KO, Alanen A. 1984. Isthmic tubal occlusion: etiology and histology. Acta Eur Fertil 15:39–42 [PubMed] [Google Scholar]
- 14. Scher JU, Pillinger MH. 2009. The anti-inflammatory effects of prostaglandins. J Investig Med 57:703–708 [DOI] [PubMed] [Google Scholar]
- 15. Bertolini A, Ottani A, Sandrini M. 2001. Dual acting anti-inflammatory drugs: a reappraisal. Pharmacol Res 44:437–450 [DOI] [PubMed] [Google Scholar]
- 16. Scher JU, Pillinger MH. 2005. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol 114:100–109 [DOI] [PubMed] [Google Scholar]
- 17. Narumiya S. 2009. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med 87:1015–1022 [DOI] [PubMed] [Google Scholar]
- 18. Crosby LM, Waters CM. 2010. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298:L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Card JW, Carey MA, Bradbury JA, Graves JP, Lih FB, Moorman MP, Morgan DL, DeGraff LM, Zhao Y, Foley JF, Zeldin DC. 2006. Cyclooxygenase-1 overexpression decreases basal airway responsiveness but not allergic inflammation. J Immunol 177:4785–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perini RF, Ma L, Wallace JL. 2003. Mucosal repair and COX-2 inhibition. Curr Pharm Des 9:2207–2211 [DOI] [PubMed] [Google Scholar]
- 21. Sinicrope FA. 2006. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Mol Carcinog 45:447–454 [DOI] [PubMed] [Google Scholar]
- 22. Khan SR. 2004. Role of renal epithelial cells in the initiation of calcium oxalate stones. Nephron Exp Nephrol 98:e55–e60 [DOI] [PubMed] [Google Scholar]
- 23. Zhang MZ, Wang JL, Cheng HF, Harris RC, McKanna JA. 1997. Cyclooxygenase-2 in rat nephron development. Am J Physiol 273:F994–F1002 [DOI] [PubMed] [Google Scholar]
- 24. Jana B, Kozłowska A, Koszykowska M, Majewski M. 2009. Expression of cyclooxygenase-2 in the inflammatory changed porcine uterus. Pol J Vet Sci 12:1–8 [PubMed] [Google Scholar]
- 25. Stjernholm-Vladic Y, Stygar D, Mansson C, Masironi B, Akerberg S, Wang H, Ekman-Ordeberg G, Sahlin L. 2004. Factors involved in the inflammatory events of cervical ripening in humans. Reprod Biol Endocrinol 2:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrer R, Moreno JJ. 2010. Role of eicosanoids on intestinal epithelial homeostasis. Biochem Pharmacol 80:431–438 [DOI] [PubMed] [Google Scholar]
- 27. Oba M, Miwa K, Fujimura T, Harada S, Sasaki S, Oyama K, Ohta T, Hattori T. 2009. A selective cyclooxygenase-2 inhibitor prevents inflammation-related squamous cell carcinogenesis of the forestomach via duodenogastric reflux in rats. Cancer 115:454–464 [DOI] [PubMed] [Google Scholar]
- 28. Wang D, Dubois RN. 2010. Eicosanoids and cancer. Nat Rev Cancer 10:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanaoka Y, Ago H, Inagaki E, Nanayama T, Miyano M, Kikuno R, Fujii Y, Eguchi N, Toh H, Urade Y, Hayaishi O. 1997. Cloning and crystal structure of hematopoietic prostaglandin D synthase. Cell 90:1085–1095 [DOI] [PubMed] [Google Scholar]
- 30. Kanaoka Y, Fujimori K, Kikuno R, Sakaguchi Y, Urade Y, Hayaishi O. 2000. Structure and chromosomal localization of human and mouse genes for hematopoietic prostaglandin D synthase. Conservation of the ancestral genomic structure of σ-class glutathione S-transferase. Eur J Biochem 267:3315–3322 [DOI] [PubMed] [Google Scholar]
- 31. Gerashchenko D, Beuckmann CT, Kanaoka Y, Eguchi N, Gordon WC, Urade Y, Bazan NG, Hayaishi O. 1998. Dominant expression of rat prostanoid DP receptor mRNA in leptomeninges, inner segments of photoreceptor cells, iris epithelium, and ciliary processes. J Neurochem 71:937–945 [DOI] [PubMed] [Google Scholar]
- 32. Bouwmans CA, Lintsen BA, Al M, Verhaak CM, Eijkemans RJ, Habbema JD, Braat DD, Hakkaart-Van Roijen L. 2008. Absence from work and emotional stress in women undergoing IVF or ICSI: an analysis of IVF-related absence from work in women and the contribution of general and emotional factors. Acta Obstet Gynecol Scand 87:1169–1175 [DOI] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention, ASRM, Society for Assisted Reproductive Technology 2010. 2008 Assisted reproductive technology success rates: national summary and fertility clinic reports. Atlanta: United States Department of Health and Human Services [Google Scholar]
- 34. Gleicher N. 2000. Cost-effective infertility care. Hum Reprod Update 6:190–199 [DOI] [PubMed] [Google Scholar]
- 35. Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C. 2008. Pituitary gonadotroph estrogen receptor-α is necessary for fertility in females. Endocrinology 149:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bridges PJ, Koo Y, Kang DW, Hudgins-Spivey S, Lan ZJ, Xu X, DeMayo F, Cooney A, Ko C. 2008. Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor α (Esr1) from the ovary and testis. Genesis 46:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeoung M, Bridges PJ. 2011. Cyclic regulation of apoptotic gene expression in the mouse oviduct. Reprod Fertil Dev 23:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldman JM, Murr AS, Cooper RL. 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97 [DOI] [PubMed] [Google Scholar]
- 39. Caligioni CS. 2009. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4:Appendix 4I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. 2000. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- 41. Jeoung M, Lee S, Hawng HK, Cheon YP, Jeong YK, Gye MC, Iglarz M, Ko C, Bridges PJ. 2010. Identification of a novel role for endothelins within the oviduct. Endocrinology 151:2858–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shin I, Kim HJ, Lee JE, Gye MC. 2006. Aquaporin7 expression during perinatal development of mouse brain. Neurosci Lett 409:106–111 [DOI] [PubMed] [Google Scholar]
- 43. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 44. Aritake K, Kado Y, Inoue T, Miyano M, Urade Y. 2006. Structural and functional characterization of HQL-79, an orally selective inhibitor of human hematopoietic prostaglandin D synthase. J Biol Chem 281:15277–15286 [DOI] [PubMed] [Google Scholar]
- 45. Redensek A, Rathore KI, Berard JL, López-Vales R, Swayne LA, Bennett SA, Mohri I, Taniike M, Urade Y, David S. 2011. Expression and detrimental role of hematopoietic prostaglandin D synthase in spinal cord contusion injury. Glia 59:603–614 [DOI] [PubMed] [Google Scholar]
- 46. Liu M, Eguchi N, Yamasaki Y, Urade Y, Hattori N, Urabe T. 2009. Protective role of hematopoietic prostaglandin D synthase in transient focal cerebral ischemia in mice. Neuroscience 163:296–307 [DOI] [PubMed] [Google Scholar]
- 47. Mohri I, Aritake K, Taniguchi H, Sato Y, Kamauchi S, Nagata N, Maruyama T, Taniike M, Urade Y. 2009. Inhibition of prostaglandin D synthase suppresses muscular necrosis. Am J Pathol 174:1735–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsushita N, Hizue M, Aritake K, Hayashi K, Takada A, Mitsui K, Hayashi M, Hirotsu I, Kimura Y, Tani T, Nakajima H. 1998. Pharmacological studies on the novel antiallergic drug HQL-79: I. Antiallergic and antiasthmatic effects in various experimental models. Jpn J Pharmacol 78:1–10 [DOI] [PubMed] [Google Scholar]
- 49. Lee YL, Lee KF, Xu JS, Wang YL, Tsao SW, Yeung WS. 2001. Establishment and characterization of an immortalized human oviductal cell line. Mol Reprod Dev 59:400–409 [DOI] [PubMed] [Google Scholar]
- 50. Lee KF, Chow JF, Xu JS, Chan ST, Ip SM, Yeung WS. 2001. A comparative study of gene expression in murine embryos developed in vivo, cultured in vitro, and cocultured with human oviductal cells using messenger ribonucleic acid differential display. Biol Reprod 64:910–917 [DOI] [PubMed] [Google Scholar]
- 51. Lee YL, Lee KF, Xu JS, Kwok KL, Luk JM, Lee WM, Yeung WS. 2003. Embryotrophic factor-3 from human oviductal cells affects the messenger RNA expression of mouse blastocyst. Biol Reprod 68:375–382 [DOI] [PubMed] [Google Scholar]
- 52. Ling L, Lee YL, Lee KF, Tsao SW, Yeung WS, Kan FW. 2005. Expression of human oviductin in an immortalized human oviductal cell line. Fertil Steril 84(Suppl 2):1095–1103 [DOI] [PubMed] [Google Scholar]
- 53. Park ES, Lind AK, Dahm-Kähler P, Brännström M, Carletti MZ, Christenson LK, Curry TE, Jr, Jo M. 2010. RUNX2 transcription factor regulates gene expression in luteinizing granulosa cells of rat ovaries. Mol Endocrinol 24:846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Farhat A, Philibert P, Sultan C, Poulat F, Boizet-Bonhoure B. 2011. Hematopoietic-prostaglandin D2 synthase through PGD2 production is involved in the adult ovarian physiology. J Ovarian Res 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zelinski-Wooten MB, Stouffer RL. 1990. Intraluteal infusions of prostaglandins of the E, D, I, and A series prevent PGF2α-induced, but not spontaneous, luteal regression in rhesus monkeys. Biol Reprod 43:507–516 [DOI] [PubMed] [Google Scholar]
- 56. Zelinski-Wooten MB, Sargent EL, Molskness TA, Stouffer RL. 1990. Disparate effects of the prostaglandin synthesis inhibitors, meclofenamate, and flurbiprofen on monkey luteal tissue in vitro. Endocrinology 126:1380–1387 [DOI] [PubMed] [Google Scholar]
- 57. Kitaya K, Yamada H. 2011. Pathophysiological roles of chemokines in human reproduction: an overview. Am J Reprod Immunol 65:449–459 [DOI] [PubMed] [Google Scholar]
- 58. Oakley OR, Kim H, El-Amouri I, Lin PC, Cho J, Bani-Ahmad M, Ko C. 2010. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology 151:4551–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oakley OR, Frazer ML, Ko C. 2011. Pituitary-ovary-spleen axis in ovulation. Trends Endocrinol Metab 22:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scurlock AM, Frazer LC, Andrews CW, Jr, O'Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. 2011. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun 79:1349–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. 1997. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun 65:2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. King M, Poya H, Rao J, Natarajan S, Butch AW, Aziz N, Kok S, Chang MH, Lyons JM, Ault K, Kelly KA. 2009. CXCL13 expression in Chlamydia trachomatis infection of the female reproductive tract. Drugs Today 45(Suppl B):125–134 [PMC free article] [PubMed] [Google Scholar]
- 63. Centers for Disease Control and Prevention 2010. Sexually transmitted disease surveillance 2009. Atlanta: United States Department of Health and Human Services [Google Scholar]
- 64. Deguchi T, Nakane K, Yasuda M, Maeda S. 2010. Emergence and spread of drug resistant Neisseria gonorrhoeae. J Urol 184:851–858; quiz 1235 [DOI] [PubMed] [Google Scholar]
- 65. Shao R, Egecioglu E, Weijdegård B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. 2007. Dynamic regulation of estrogen receptor-α isoform expression in the mouse fallopian tube: mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab 293:E1430–E1442 [DOI] [PubMed] [Google Scholar]
- 66. Lindzey J, Korach KS. 1997. Developmental and physiological effects of estrogen receptor gene disruption in mice. Trends Endocrinol Metab 8:137–145 [DOI] [PubMed] [Google Scholar]
- 67. Korach KS. 1994. Insights from the study of animals lacking functional estrogen receptor. Science 266:1524–1527 [DOI] [PubMed] [Google Scholar]
- 68. Gerashchenko DY, Beuckmann CT, Marcheselli VL, Gordon WC, Kanaoka Y, Eguchi N, Urade Y, Hayaishi O, Bazan NG. 1998. Localization of lipocalin-type prostaglandin D synthase (β-trace) in iris, ciliary body, and eye fluids. Invest Ophthalmol Vis Sci 39:198–203 [PubMed] [Google Scholar]
- 69. Beuckmann CT, Gordon WC, Kanaoka Y, Eguchi N, Marcheselli VL, Gerashchenko DY, Urade Y, Hayaishi O, Bazan NG. 1996. Lipocalin-type prostaglandin D synthase (β-trace) is located in pigment epithelial cells of rat retina and accumulates within interphotoreceptor matrix. J Neurosci 16:6119–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Urade Y, Fujimoto N, Hayaishi O. 1985. Purification and characterization of rat brain prostaglandin D synthetase. J Biol Chem 260:12410–12415 [PubMed] [Google Scholar]
- 71. Urade Y, Kitahama K, Ohishi H, Kaneko T, Mizuno N, Hayaishi O. 1993. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc Natl Acad Sci USA. 90:9070–9074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hayaishi O. 1991. Molecular mechanisms of sleep-wake regulation: roles of prostaglandins D2 and E2. FASEB J 5:2575–2581 [PubMed] [Google Scholar]
- 73. Otsuki M, Gao H, Dahlman-Wright K, Ohlsson C, Eguchi N, Urade Y, Gustafsson JA. 2003. Specific regulation of lipocalin-type prostaglandin D synthase in mouse heart by estrogen receptor β. Mol Endocrinol 17:1844–1855 [DOI] [PubMed] [Google Scholar]
- 74. Devidze N, Fujimori K, Urade Y, Pfaff DW, Mong JA. 2010. Estradiol regulation of lipocalin-type prostaglandin D synthase promoter activity: evidence for direct and indirect mechanisms. Neurosci Lett 474:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tummaruk P, Kesdangsakonwut S, Kunavongkrit A. 2009. Relationships among specific reasons for culling, reproductive data, and gross morphology of the genital tracts in gilts culled due to reproductive failure in Thailand. Theriogenology 71:369–375 [DOI] [PubMed] [Google Scholar]