Abstract
A role of Rho-associated coiled-coil-containing protein kinase (ROCK)1 in regulating whole-body glucose homeostasis has been reported. However, cell-autonomous effects of ROCK1 on insulin-dependent glucose transport in adipocytes and muscle cells have not been elucidated. To determine the specific role of ROCK1 in glucose transport directly, ROCK1 expression in 3T3-L1 adipocytes and L6 myoblasts was biologically modulated. Here, we show that small interfering RNA-mediated ROCK1 depletion decreased insulin-induced glucose transport in adipocytes and myoblasts, whereas adenovirus-mediated ROCK1 expression increased this in a dose-dependent manner, indicating that ROCK1 is permissive for glucose transport. Inhibition of ROCK1 also impaired glucose transporter 4 translocation in 3T3-L1 adipocytes. Importantly, the ED50 of insulin for adipocyte glucose transport was reduced when ROCK1 was expressed, leading to hypersensitivity to insulin. These effects are dependent on actin cytoskeleton remodeling, because inhibitors of actin polymerization significantly decreased ROCK1's effect to promote insulin-stimulated glucose transport. Unlike ROCK2, ROCK1 binding to insulin receptor substrate (IRS)-1 was not detected by immunoprecipitation, although cell fractionation demonstrated both ROCK isoforms localize with IRS-1 in low-density microsomes. Moreover, insulin's ability to increase IRS-1 tyrosine 612 and serine 632/635 phosphorylation was attenuated by ROCK1 suppression. Replacing IRS-1 serine 632/635 with alanine reduced insulin-stimulated phosphatidylinositol 3-kinase activation and glucose transport in 3T3-L1 adipocytes, indicating that phosphorylation of these serine residues of IRS-1, which are substrates of the ROCK2 isoform in vitro, are crucial for maximal stimulation of glucose transport by insulin. Our studies identify ROCK1 as an important positive regulator of insulin action on glucose transport in adipocytes and muscle cells.
A key function of insulin is to stimulate glucose transport in muscle and adipose cells by eliciting the translocation of glucose transporter 4 (Glut4), the insulin-glucose transporter, from intracellular storage compartments to the plasma membrane (PM) and transverse tubules (1). Signal transduction from the insulin receptor (IR) is essential for this effect (1). Accumulated evidence suggests that Rho-associated coiled-coil-containing protein kinase (ROCK) isoforms play a pivotal role in the regulation of insulin signaling and insulin-dependent glucose homeostasis in cells and tissues (2–6). Overexpression of dominant negative (DN)-ROCK2 (also known as RhoA-binding kinase, ROKα) decreases insulin-stimulated glucose transport in 3T3-L1 adipocytes and L6 muscle cells by impairing phosphatidylinositol 3-kinase (PI3K) activity, likely due to ROCK2-mediated IR substrate (IRS)-1 serine phosphorylation (2). In addition, genetic deletion of ROCK1 (also known as ROKβ) causes systemic insulin resistance by impairing insulin signaling in skeletal muscle (3). Therefore, ROCK isoforms are likely to be important regulators of insulin action on glucose transport. The exact role of ROCK1 in glucose transport and insulin sensitivity has not been identified.
ROCK isoforms have been implicated in a variety of cellular functions, including smooth muscle contraction, actin cytoskeleton organization (7), cell adhesion and motility (8), cytokinesis (9), and gene expression (10). Of particular interest is that ROCK isoforms regulate the reorganization of the actin cytoskeleton through the activation of LIM (Lin-11, Isl1, Mec3) kinase, which leads to the phosphorylation and inactivation of cofilin (11). Actin cytoskeleton reorganization is required for the regulation of insulin-mediated glucose uptake and Glut4 translocation in adipocytes and muscle cells (12–18), as revealed by the fact that F-actin-disrupting agents, such as cytochalasin D (16, 19–21), latrunculin A/B, (13, 19, 21, 22), or jasplakinolide (13), abolished insulin's effect on glucose transport. Recent studies demonstrate that inhibition of ROCK1 results in loss of stress fibers and focal adhesions in rat-embryo fibroblasts, whereas suppression of ROCK2 enhances microfilament cytoskeleton and fibronectin matrix assembly (23, 24). In addition, ROCK isoforms are found to have different binding partners (25–30), raising the possibility that they may have distinct roles in regulating glucose metabolism. However, little is known as to whether actin cytoskeletal remodeling is necessary for ROCK1-mediated glucose transport regulation in insulin-sensitive cells. Furthermore, the effects of cofilin signaling and ROCK isoform regulation on insulin-dependent glucose transport are not known.
In the present study, we investigated the role of ROCK1 in the regulation of glucose transport and actin cytoskeletal remodeling in adipocytes and myoblasts. Here, we demonstrate that activation of ROCK1 is necessary and sufficient to control insulin-induced Glut4 translocation and glucose transport in adipocytes and myoblasts, which is mainly controlled by actin polymerization.
Materials and Methods
Cell culture for 3T3-L1 adipocytes and L6 myoblasts
3T3-L1 adipocytes were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and penicillin-streptomycin (Cellgro, Manassas, VA) in a humidified incubator with 5% CO2 at 37 C. 3T3-L1 adipocytes were differentiated as described (2). Cells were used for experiments 10–12 d after induction of differentiation. L6 myoblasts (a gift from Amira Klip, Hospital for Sick Children, Toronto, Canada) were maintained as described (31).
Transduction of 3T3-L1 adipocytes
Transduction of differentiated 3T3-L1 adipocytes was performed as described (2). Recombinant adenoviruses encoding either the wild-type (WT) ROCK1 isoform, β-galactosidase (β-Gal), WT-IRS-1, phosphor-mimic mutant, IRS-1 protein containing glutamic acid substitution for serine 632/635 (S632/635E-IRS-1), or inactive mutant, IRS-1 protein containing alanine substitution for serine 632/635 (S632/635A-IRS-1) were transduced into cells at a concentration of 1 × 109 PFU/ml. The virus was removed after 14 h, fresh medium was added, and experiments were performed 72 h later.
Transfection of 3T3-L1 adipocytes and L6 myoblasts
Small interfering RNA (siRNA) were introduced into cells by transient transfection with a Lipofectamine RNAiMAX reagent (Invitrogen). For 3T3-L1 adipocytes, cells were transfected 6 d after the onset of differentiation. After 72 h, cells were used for glucose uptake and insulin signaling analysis. 3T3-L1 preadipocytes and L6 myoblasts were transfected at 2 d after plating. After 72 h, cells were used for the glucose uptake and insulin signaling analysis. For overexpression of ROCK1 in L6 myoblasts, WT-ROCK1 cDNA was transiently transfected with Lipofectamine LTX reagent (Invitrogen). Cells were further incubated for 3 d and then harvested for studies.
Plasmid vectors and synthesis of siRNA
The WT-ROCK1 plasmid construct was generously provided by L. Wei (Indiana University, Indianapolis, IN), and the ROCK2 plasmid construct was provided by K. Kaibuchi (Nagoya University, Nagoya, Japan). RhoA siRNA (sc-29471) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), the luciferase reporter control siRNA was purchased from Invitrogen, and siRNA for murine ROCK1, ROCK2, v-akt murine thymoma viral oncogene homolog 2 (Akt2), and phosphatase and tensin homolog (PTEN) were synthesized as Stealth siRNA duplexes (Invitrogen). The sequences used are as follows: ROCK1, 5′-UCCAAGUCACAAGCAGACAAGGAUU-3′; ROCK2, 5′-CCGGACCCAUGG AUCAGAGAUAAUU-3′; Akt2, 5′-UGGAGAGUCUACAUGGAAGGUCCUC-3′; and PTEN, 5′-GAGGA UGGAUUCGACUUAGACUU GA-3′.
Glucose uptake
Glucose transport was measured as described previously (2). Briefly, 3T3-L1 adipocytes were serum starved for 3 h and then treated with 0–100 nm insulin or vehicle for 30 min at 37 C. [3H]-2-deoxy-D-glucose was added to the medium, cells were incubated for 10 min, and the level of [3H]-2-deoxy-D-glucose uptake into cells was expressed as a percentage of basal glucose transport in control cells. For inhibitor studies, cells were pretreated with 2 μm latrunculin B (Calbiochem, San Diego, CA), 2 μm cytochalasin D (Sigma-Aldrich, St. Louis, MO), 50 nm wortmannin (Millipore, Bedford, MA), or with vehicle for 0.5–3 h, followed by stimulation with insulin as indicated before measurement of glucose uptake.
PI3K and Akt2 activity
Cell lysates (100 μg of protein) were subjected to immunoprecipitation for 4 h at 4 C with 5 μl of a polyclonal IRS-1 antibody (a gift from Morris White, Children's Hospital, Boston, MA), coupled to protein A-Sepharose beads (Sigma-Aldrich) or 1 μg of a polyclonal Akt antibody (Millipore) coupled to protein G-agarose beads (Roche, Indianapolis, IN). The immune complex was washed, and PI3K and Akt2 activity was determined as described (2).
Subcellular fractionation
Differentiated 3T3-L1 adipocytes were serum starved followed by stimulation with insulin (100 nm) or vehicle for 15 min. Cells were washed twice with HEPES/EDTA/sucrose (HES) buffer [250 mm sucrose, 10 mm HEPES (pH 7.4), and 5 mm EDTA], harvested by scraping, pelleted by centrifugation, and resuspended in HES buffer. Cells were homogenized in a Potter homogenizer with a Teflon pestle and homogenates subjected to an initial ultracentrifugation at 15,000 × g for 20 min. PM in the resultant pellet were resuspended in HES buffer overlaid on a 1.12 m sucrose cushion and then isolated from the interphase of the gradient obtained after centrifugation at 35,000 rpm for 30 min in TLS-55 rotor (Beckman, Brea, CA). The pellet from this step constituted nuclei and mitochondria. The supernatant from the 15,000 × g centrifugation was centrifuged a second time at 28,000 rpm for 20 min to yield a pellet of high-density microsomes (HDM). The supernatant of the 28,000 rpm step was centrifuged a third time at 60,000 rpm for 75 min using a Beckman TLA 100.2 rotor to obtain a pellet of low-density microsomes (LDM). The supernatant of the 60,000 rpm ultracentrifugation step was considered the cytosol. All pellets were resuspended in lysis buffer [20 mm Tris (pH 7.5), 5 mm EDTA, 10 mm Na4P2O7, 100 mm NaF, 2 mm Na3VO4, 1% Nonidet P-40, 1 mm phenylmethanesulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin], and proteins (20 μg) of each fraction were separated by SDS-PAGE, followed by immunoblotting, as below.
Coimmunoprecipitation of proteins
For identification of the interaction between ROCK isoforms and IRS-1, cell lysates protein (100 μg) were subjected to immunoprecipitation with 1 μg of a polyclonal ROCK1 or ROCK2 antibody coupled to protein G-Sepharose (Amersham Biosciences, Piscataway, NJ). Immunoprecipitates were washed and bound proteins separated by SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were incubated with a polyclonal IRS-1 antibody. The bands were visualized by enhanced chemiluminescence. Reciprocal association was identified by immunoprecipitation with a polyclonal IRS-1 antibody, followed by immunoblotting with ROCK1 or ROCK2 antibodies, as described above.
Immunoblotting analysis
Cell lysate proteins (20–50 μg) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated with polyclonal antibodies against IRS-1 (a gift from Morris White); phosphor-Y612 IRS-1 (Invitrogen); phospho-Ser632/635IRS-1 (Cell Signaling, Beverly, MA); phospho-Ser473Akt (Cell Signaling); phospho-Thr308Akt (Cell Signaling); phospho (pTEpY) MAPK (Promega, Madison, WI); ROCK1 (H-85 and C-19; Santa Cruz Biotechnology, Inc.); ROCK2 (H-85 and C-20; Santa Cruz Biotechnology, Inc.); IR (Santa Cruz Biotechnology, Inc.); Akt (Santa Cruz Biotechnology, Inc.); Glut4 (Millipore); phospho-Ser3 cofilin-1 (Cell Signaling); and cofilin-1 (Santa Cruz Biotechnology, Inc.) or monoclonal antibodies specific for RhoA (26C4; Santa Cruz Biotechnology, Inc.), RhoE (Millipore), or Sodium Potassium ATPase α1 (Novus Biologicals, Littleton, CO). The bands were visualized with enhanced chemiluminescence and quantified by densitometry (32). All phosphoprotein data were normalized to the total level of the respective protein.
Confocal microscopy
Cells on coverslips were washed twice with PBS and fixed on ice with 3% paraformaldehyde/PBS for 10 min, and then washed with PBS. Residual paraformaldehyde was quenched by incubation with 0.1 m glycine for 10 min. After washing with PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 3 min, washed with PBS, and incubated in blocking solution (5% milk) for 10 min. Cells were stained for F-actin by Alexa Fluor 546 phalloidin (Invitrogen) and incubated with anti-ROCK1 (C-19), anti-ROCK2 (C-20), anti-RhoA (26C4), anti-β-tubulin (9F3), anti-Ras-related proteins in the brain (Rab5) (C8B1), or anticytochrome C oxidase IV (3E11) antibodies (as sourced above) diluted 1:100 in blocking buffer overnight at 4 C. Actin was visualized using a Zeiss LSM 510 confocal fluorescence microscope (Zeiss, Oberkochen, Germany), and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Statistical analysis
Data are presented as means ± sem. Statistical analyses were performed using StatView (Abacus Concepts, Inc., Berkeley, CA). Statistical significance among the groups was determined with ANOVA and unpaired Student's t tests, when appropriate.
Results
ROCK1 is a key positive regulator of glucose transport in 3T3-L1 adipocytes and L6 myoblasts
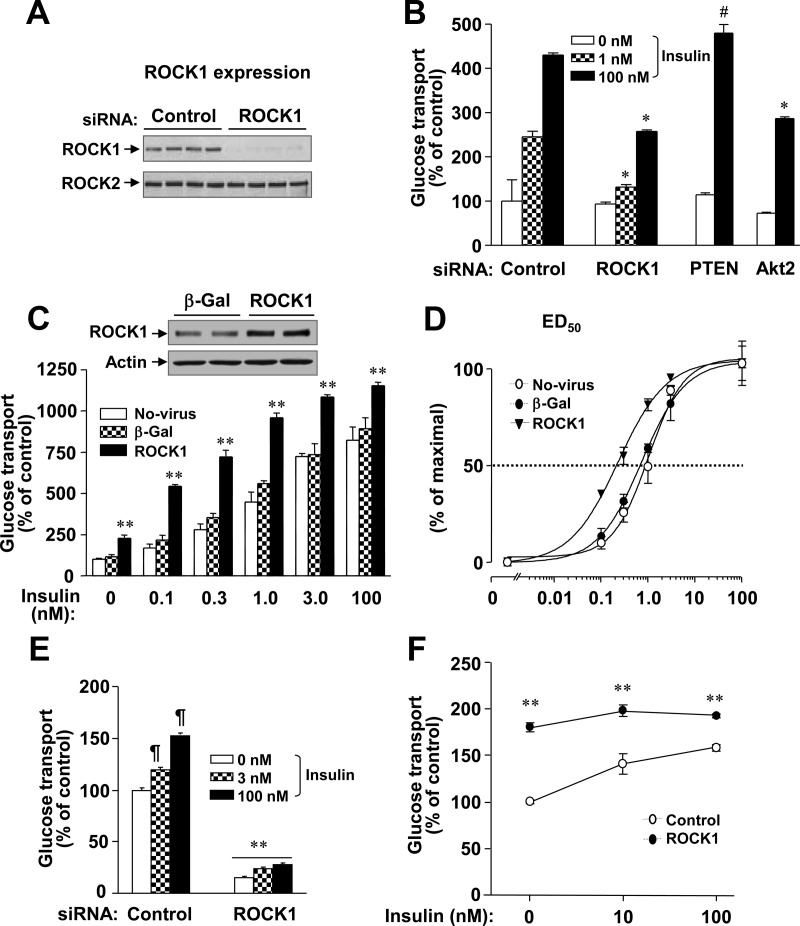
Rho and ROCK signaling play an important role in regulating insulin-mediated glucose metabolism in insulin-sensitive cells (2, 3, 33, 34). Our previous work has demonstrated that deficiency of ROCK1 causes insulin resistance in mice (3). To determine the role of ROCK1 in insulin-stimulated glucose transport in 3T3-L1 adipocytes, we suppressed endogenous ROCK1 expression by siRNA-mediated gene silencing. Endogenous ROCK1 expression was reduced by approximately 85% after transfection of siRNA, whereas ROCK2 expression was unchanged (Fig. 1A). 2-Deoxy glucose uptake in ROCK1 siRNA transfected cells was compared with that of cells transfected with siRNA for Akt2 or PTEN, which are reported to decrease or increase insulin-stimulated glucose transport into 3T3-L1 adipocytes, respectively (35, 36). Consistent with these previous findings, Akt2 siRNA decreased insulin-stimulated glucose transport, whereas PTEN siRNA increased it in 3T3-L1 adipocytes (Fig. 1B). Suppression of ROCK1 expression caused a more than 45% decrease in insulin stimulation of glucose transport in 3T3-L1 adipocytes (Fig. 1B). These data suggest that ROCK1 activation is required for normal activation of glucose transport by insulin in 3T3-L1 adipocytes.
Fig. 1.
ROCK1 regulates glucose transport in 3T3-L1 adipocytes and L6 myoblasts. A, The expression levels of ROCK1 and ROCK2 in 3T3-L1 adipocytes. Cells were transfected with ROCK1 siRNA or luciferase (control) siRNA. ROCK were visualized by immunoblotting with a polyclonal ROCK1 or ROCK2 antibody. B, Effects of ROCK1 inhibition on glucose transport in 3T3-L1 adipocytes. Cells were serum starved and then stimulated with insulin (0, 10, or 100 nm) for 30 min. As control experiments, cells were also transfected with Akt2 siRNA or PTEN siRNA. [3H]-2-deoxy-D-glucose uptake was measured and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem; #, P < 0.05; *, P < 0.01 vs. the corresponding condition with control. C, Effects of ROCK1 overexpression on glucose transport in 3T3-L1 adipocytes. Cells were transduced with recombinant adenovirus encoding β-Gal or ROCK1 as described in Materials and Methods. ROCK1 was visualized by immunoblotting with a polyclonal ROCK1 antibody. Cells were serum starved and then stimulated with insulin, as indicated, for 30 min. [3H]-2-deoxy-D-glucose uptake was measured, and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem; **, P < 0.001 vs. the corresponding condition with β-Gal or no-virus. D, Effects of ROCK1 on dose response of glucose transport in 3T3-L1 adipocytes. ED50 for glucose uptake was calculated from the data of C. E, Effects of ROCK1 inhibition on glucose transport in L6 myoblasts. Cells were transfected with ROCK1 siRNA, serum starved, and then stimulated with insulin (3 or 100 nm) for 30 min. [3H]-2-deoxy-D-glucose uptake was measured, and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem; ¶, P < 0.01 vs. basal condition in control cells; **, P < 0.001 vs. the corresponding condition with control siRNA. F, Effects of ROCK1 overexpression on glucose transport in L6 myoblasts. Cells were transfected with ROCK1 cDNA or control cDNA. Cells were serum starved and then incubated without and with insulin (100 nm) for 30 min. [3H]-2-deoxy-D-glucose uptake was measured, and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem; **, P < 0.01 vs. the corresponding condition of the control. All data are representative of three independent experiments.
To further define the effects of ROCK1 on glucose transport, we overexpressed ROCK1 in 3T3-L1 adipocytes via an adenovirus-mediated gene-transfer system. In 3T3-L1 adipocytes expressing the ROCK1 transgene, the total level of ROCK1 protein was approximately 2.5-fold higher than in 3T3-L1 adipocytes expressing a β-Gal transgene (Fig. 1C). In adipocytes expressing β-Gal or no transgene, insulin's stimulation of glucose transport increased in a dose-dependent manner (Fig. 1C). In adipocytes overexpressing ROCK1, basal glucose transport was increased approximately 2.3-fold compared with control cells (Fig. 1C). Importantly, ROCK1 overexpression significantly increased insulin-induced glucose transport compared with cells expressing β-Gal or no transgene at the indicated dose of insulin (Fig. 1C). The insulin dose mediating ED50 in 3T3-L1 adipocytes expressing ROCK1 was significantly decreased compared with control adipocytes expressing β-Gal (Fig. 1D), suggesting increased sensitivity to insulin. The EC50 for nontransduced cells was 1.02 ± 0.11 nm; for cells expressing β-Gal, 0.74 ± 0.10 nm; and for cells overexpressing ROCK1, 0.23 ± 0.05 nm (P < 0.01). These data strongly suggest that increased ROCK1 expression is sufficient to enhance insulin action on glucose transport in 3T3-L1 adipocytes. Of note, enhanced basal glucose transport was not due to increased Glut1 expression, because its protein levels were unaltered by ROCK1 overexpression (data not shown). Possibly, enhanced glucose transport could be due to increased Glut4 activity at the PM.
In L6 myoblasts, ROCK1 suppression decreased basal glucose transport, whereas ROCK1 overexpression increased this. However, insulin's effect on glucose transport was modest when ROCK1 was overexpressed or inhibited in myoblasts (Fig. 1, E and F). The ability of insulin to increase glucose transport in these cells was minimal (Fig. 1, E and F), as previously reported by us (2) and others (37, 38). This is most likely due to the fact that insulin-responsive Glut4 transport is not the major glucose transporter isoform in L6 muscle cells (39). Possibly, ROCK1 overexpression may saturate insulin-stimulated PM glucose transport in this cell line. Similar findings were observed in cells overexpressing either Akt or the catalytic subunit 110α of PI3K (40, 41). Because transfection efficiency is markedly higher L6 myoblasts than L6 myotubes (data not shown), we used L6 myoblasts for the current studies. These cells are responsive to insulin's action to stimulate glucose transport.
ROCK1 induces insulin-stimulated IRS-1 activation and is permissive for PM Glut4 translocation
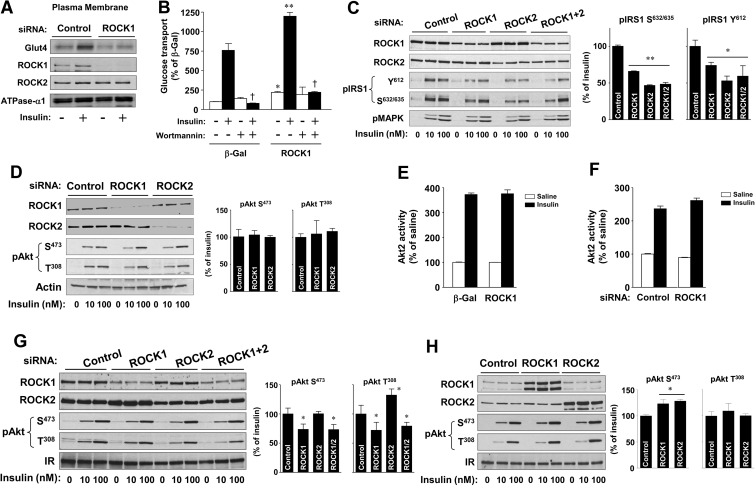
To determine whether the decreased insulin-stimulated glucose transport seen with ROCK1 inhibition was due to impaired translocation of Glut4 to the PM, we measured the amount of Glut4 on the cell surface in 3T3-L1 adipocytes upon ROCK1 suppression using cell fractionation. The expression level of N+, K+-ATPase-α1, a PM marker, was similar between control adipocytes and ROCK1-suppressing adipocytes. As expected, insulin increased the amount of Glut4 on the cell surface approximately 2.2-fold over basal levels in luciferase siRNA-transfected 3T3-L1 adipocytes. However, insulin's ability to increase PM Glut4 translocation was impaired in ROCK1 siRNA-treated 3T3-L1 adipocytes (Fig. 2A), suggesting that the reduction of insulin-induced glucose transport caused by ROCK1 suppression results from decreased Glut4 translocation to the PM.
Fig. 2.
Regulation of insulin signaling by ROCK isoforms in 3T3-L1 adipocytes and L6 myoblasts. A, Effects of ROCK1 on insulin-dependent Glut4 translocation in 3T3-L1 adipocytes. Cells were transfected with ROCK1 siRNA or luciferase (control) siRNA. Cells were serum starved and then stimulated with insulin (100 nm) for 30 min. The PM fraction was harvested as described in Materials and Methods. Glut4, ROCK1, ROCK2, and N+,K+-APTase α1 were visualized by immunoblotting with indicated antibodies. B, Effects of wortmannin on ROCK1-mediated glucose transport in 3T3-L1 adipocytes. Cells were transduced with recombinant adenovirus encoding β-Gal or ROCK1 as described in Materials and Methods. Cells were pretreated with a PI3K inhibitor wortmannin (50 nm) or with a vehicle for 30 min and then stimulated with insulin (100 nm) for 30 min. [3H]-2-deoxy-D-glucose uptake was measured, and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem and representative of three independent experiments. +, P < 0.001 vs. insulin-stimulated condition within same group; *, P < 0.01; **, P < 0.001 vs. the corresponding condition with β-Gal. C and D, Effects of ROCK isoforms on insulin signaling in 3T3-L1 adipocytes. Cells were transfected with ROCK1, ROCK2, or luciferase (control) siRNA. Cells were serum starved and then stimulated with insulin (10 or100 nm) for 15 min. The bands for insulin signaling molecules were visualized by immunoblotting with phospho-specific antibodies or total antibodies as indicated. The bands were quantitated using densitometry. Data are means ± sem and representative of three independent experiments. *, P < 0.05; **, P < 0.01 vs. control siRNA. E and F, Effects of ROCK1 on Akt2 activity in 3T3-L1 adipocytes. E, Cells were transduced with recombinant adenovirus encoding β-Gal or ROCK1. F, Cells were transfected with ROCK1 or luciferase siRNA (control). Cells were serum starved and then stimulated with insulin (100 nm) for 20 min. Akt2 activity was measured and represented compared with control in the basal condition. Data are means ± sem. G, Effects of ROCK inhibition on insulin signaling in L6 myoblasts. Cells were transfected with ROCK1, ROCK2, or luciferase (control) siRNA. Cells were serum starved and then stimulated with insulin (10 or 100 nm) for 15 min. The bands for insulin signaling molecules were visualized by immunoblotting with phospho-specific antibodies or total antibodies as indicated. The Akt bands were quantitated using densitometry. Data are means ± sem and representative of three independent experiments. *, P < 0.05 vs. control siRNA. H, Effects of ROCK overexpression on insulin signaling in L6 myoblasts. Cells were transfected with ROCK1 cDNA or control cDNA. Cells were serum starved and then stimulated with insulin (10 or100 nm) for 15 min. The bands for insulin signaling molecules were visualized by immunoblotting with phospho-specific antibodies or total antibodies as indicated. The Akt bands were quantitated using densitometry. Data are means ± sem and representative of three independent experiments. *, P < 0.05 vs. control.
Because PI3K signaling is a key regulator of insulin-induced Glut4 translocation (1), we tested whether activation of PI3K is required for the regulation of ROCK1-mediated Glut4 translocation. In the absence of wortmannin, a PI3K chemical inhibitor, adenovirus-mediated overexpression of ROCK1 in 3T3-L1 adipocytes significantly increased both basal and insulin-stimulated glucose transport, and this effect was completely blocked by wortmannin treatment (Fig. 2B). These results show that intact PI3K activity is required for the enhanced insulin-stimulated Glut4 translocation observed with ROCK1 overexpression.
To investigate whether ROCK isoforms may have differential effects on the insulin signaling pathway in 3T3-L1 adipocytes or L6 muscle cells, we used siRNA to suppress endogenous expression of ROCK1 or ROCK2. As shown in Fig. 2C, insulin-stimulated phosphorylation of IRS-1 at tyrosine 612 and serine 632/635 was diminished by both ROCK1 and ROCK2 suppression in 3T3-L1 adipocytes. No additive effects of ROCK isoforms on IRS-1 phosphorylation inhibition were seen (Fig. 2C). However, Akt phosphorylation at serine 473 and threonine 308 in response to insulin was normal in cells with or without blockade of ROCK1 or ROCK2 expression (Fig. 2D). Moreover, insulin-stimulated Akt2 activity in 3T3-L1 adipocytes was also unaltered by ROCK1 overexpression or suppression (Fig. 2, E and F). These data suggest that maximal stimulation of Akt by insulin may not require the full activation of upstream signaling, such as IRS-1 and PI3K, or ROCK isoform signaling.
In L6 myoblasts, ROCK1 inhibition, but not ROCK2, decreased Akt phosphorylation at serine 473 and threonine 308 during insulin stimulation. There was no additive effect on Akt phosphorylation with inhibition of both of ROCK isoforms (Fig. 2G). When ROCK1 or ROCK2 was overexpressed in L6 myoblasts, insulin-induced Akt serine 473 phosphorylation, but not threonine 308, was modestly increased (Fig. 2H). We further tested the effects of ROCK inhibition on Akt activation in other cell types, such as CHO cells expressing the IR and IRS-1. Inhibition of ROCK1 or ROCK2 significantly decreased Akt serine 473 phosphorylation in response to insulin (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). It is therefore likely that Akt activation could be regulated in a cell type-specific manner under conditions where ROCK expression is altered. Nevertheless, these results are consistent with our previous findings that deficiency of ROCK1 impairs insulin-stimulated Akt phosphorylation in skeletal muscle in vivo (3).
IRS-1 binds ROCK2 but not ROCK1
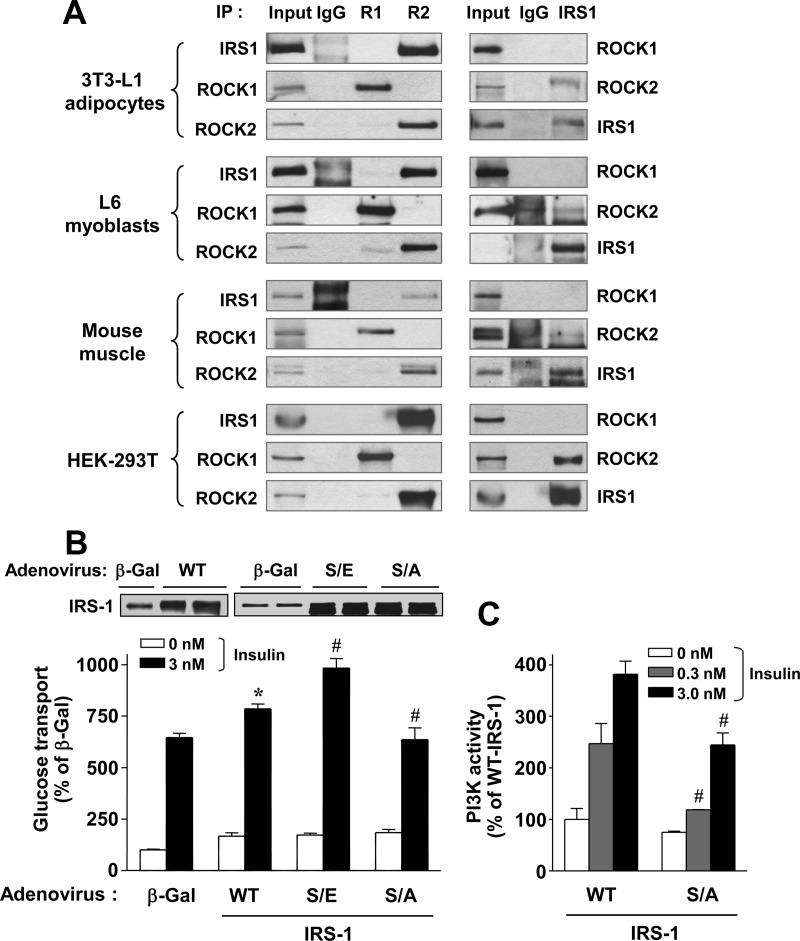
Although xROKα, the Xenopus ROCK2 orthologue, was reported to bind to xIRS-1 (5), and some reports have demonstrated that the mammalian ROCK2 protein binds to IRS-1 (4, 42), it is still unknown whether ROCK1 binds IRS-1 in mammalian cells. To address this question, we performed coimmunoprecipitations using antibodies for ROCK1 or ROCK2 followed by immunoblot analysis with an IRS-1 antibody. Consistent with previous findings (4, 5, 42), ROCK2 was found to bind to IRS-1 in all cell types or tissues examined, including 3T3-L1 adipocytes, murine skeletal muscle, L6 myoblasts, and HEK-293T cells (Fig. 3A, left). Interestingly, however, the binding of ROCK1 to IRS-1 was not detected (Fig. 3A, left). These data were confirmed by reciprocal binding analyses: immunoprecipitation with anti-IRS1 antibody followed by immunoblot analysis with either a ROCK1 or a ROCK2 antibody (Fig. 3A, right). These results suggest that these two ROCK isoforms differ in their binding capacities for IRS-1. Collectively, these findings indicate that ROCK isoforms may have differential roles in regulating insulin-stimulated glucose metabolism.
Fig. 3.
Functional link between ROCK isoforms and IRS-1. A, Physical interaction between ROCK isoforms and IRS-1 in culture cell lines and mouse muscle. Cell lysates from 3T3-L1 adipocytes, L6 myoblasts, mouse muscle tissue, and HEK-293T were subjected to immunoprecipitation (IP) with a ROCK1 (R1), ROCK2 (R2), or IRS-1 antibody. Immunoprecipitated proteins were resolved by PAGE, and ROCK and IRS-1 were visualized by immunoblotting with a ROCK1, ROCK2, or IRS-1 antibody. C, Effects of IRS-1 serine 632/635 phosphorylation on insulin-stimulated glucose transport and PI3K activation in 3T3-L1 adipocytes. Cells were transduced with recombinant adenovirus encoding β-Gal, WT-IRS-1, S632/635E-IRS-1 (S/E), or S632/635A-IRS-1 (S/A) as described in Materials and Methods. IRS-1 was visualized by immunoblotting with a polyclonal IRS-1 antibody. Cells were serum starved and then stimulated with insulin, as indicated, for 30 min. [3H]-2-deoxy-D-glucose uptake (B) and PI3K activity (C) was measured, and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem; #, P < 0.01 vs. the corresponding condition with WT-IRS-1; *, P < 0.01 vs. the corresponding condition with β-Gal.
IRS-1 serine 632/635 phosphorylation is a key mediator of insulin-stimulated glucose transport and PI3K activity
Our previous work showed that ROCK2 directly phosphorylates IRS-1 serine residues at 632/635 in vitro, and mutation of these sites caused a significant impairment in IRS-1 tyrosine phosphorylation and PI3K activity (2). To determine the effects of IRS-1 serine 632/635 phosphorylation itself on glucose transport and PI3K activity, S632/635A-IRS-1 (inactive) or S632/6345E-IRS-1 mutant (phosphor mimic) was overexpressed in 3T3-L1 adipocytes via an adenovirus-gene transfer system. Insulin-stimulated glucose transport was increased in WT-IRS-1-expressing adipocytes compared with control adipocytes expressing transgenic β-Gal (P < 0.01) (Fig. 3B). Importantly, expression of the S632/635E-IRS-1 active mutant significantly increased insulin-stimulated glucose transport in 3T3-L1 adipocytes, whereas expression of the S632/635A-IRS-1 inactive mutant decreased this compared with that observed with expression of WT-IRS-1 (P < 0.01, respectively) (Fig. 3B). As expected, insulin significantly increased IRS-1-associated PI3K activity in WT-IRS-1-expressing adipocytes in a dose-dependent fashion. However, the ability of insulin to increase PI3K activity was decreased approximately 40% in S632/635A-IRS-1 inactive mutant-expressing adipocytes compared with WT-IRS-1-expressing adipocytes (Fig. 3C). These data suggest that IRS-1 serine 632/635 phosphorylation, which is positively regulated by ROCK1 as well as ROCK2, plays a positive role in regulating insulin-dependent glucose transport and insulin signaling.
ROCK1 colocalizes to LDM with IRS-1 and ROCK2
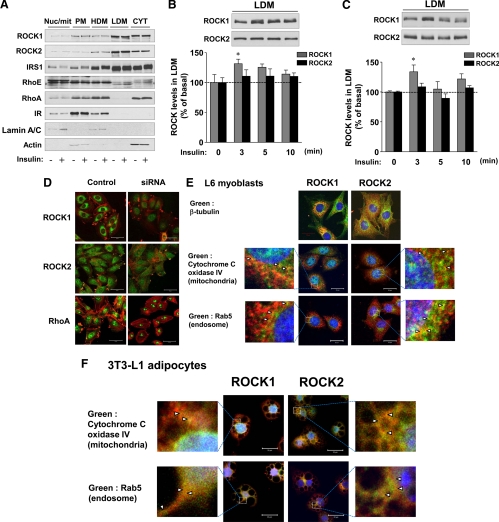
To define the mechanics of ROCK1- and ROCK2-mediated glucose transport, we examined the subcellular localization of ROCK isoforms and IRS-1 in 3T3-L1 adipocytes. Localization of both ROCK isoforms was prominent in the LDM fraction and the cytosolic fraction, with scarce distribution in the nuclear/mitochondrial, PM, and HDM fractions. Importantly, the distributional pattern of ROCK localization was similar to that of IRS-1 but was distinct from that of RhoA or RhoE, key affecters of ROCK functions (26, 27, 43). Redistribution of ROCK1 or ROCK2 was not detected in response to insulin at 15 min (Fig. 4A).
Fig. 4.
Subcellular localization of ROCK isoforms in 3T3-L1 adipocytes and L6 myoblasts. A, Subcellular localization of ROCK isoforms and IRS-1 in 3T3-L1 adipocytes. Cells were serum starved and stimulated with 100 nm insulin for 15 min when indicated. The nucleus/mitochondria (Nuc/mit), PM, HDM, LDM, and cytosol (CYT) fractions were harvested as described in Materials and Methods, and equal portions were used for immunoblotting. The bands for all molecules were visualized by immunoblotting with specific antibodies as indicated. Lamin A/C was used as a marker for the nuclear fraction, IR for the PM fraction, and β-actin for the cytosolic fraction, respectively. B and C, Localization of ROCK isoforms in LDM fraction of 3T3-L1 adipocytes (C) and L6 myoblasts (D) during insulin signaling. Cells were serum starved and stimulated with 100 nm insulin for the indicated times. LDM fractions were harvested and subjected to immunoblotting with antibodies to ROCK1 and ROCK2. The ROCK isoform bands were quantitated using densitometry. Data are means ± sem; *, P < 0.05 vs. 0 time point. D, Analysis of antibody specificity for immunocytochemical studies and subcellular localization of ROCK isoforms and RhoA in L6 myoblasts. Cells were transfected with ROCK1, ROCK2, RhoA siRNA, or luciferase siRNA (control) and were fixed with paraformaldehyde. Immunocytochemical analyses were performed with a polyclonal ROCK1, ROCK2, or RhoA antibody (green). ROCK isoforms and RhoA were visualized by scanning confocal microscopy (left panel). F-actin (red) in cells was stained using Alexa Fluor 546 phalloidin. Scale bar, 50 μm. E and F, Localization of ROCK isoforms in L6 myoblasts and 3T3-L1 adipocytes. Cells were fixed with paraformaldehyde, and immunocytochemical analyses were performed with a polyclonal ROCK1 or ROCK2 antibody (red). β-Tubulin, cytochrome C IV, or Rab5 antibodies were used to visualize microtubules, mitochondria, or endosomes (green), respectively. Nuclei were stained with DAPI (blue). Scale bar, 20 μm.
Because Glut4 vesicle translocation to the PM in response to insulin is rapid, we further examined the subcellular localization of ROCK isoforms for changes in distribution at very early intervals after insulin stimulation. Interestingly, in 3T3-L1 adipocytes and L6 myoblasts, the amount of ROCK1, but not ROCK2, was increased by approximately 30% in the LDM fraction at 3 min after insulin stimulation (P < 0.05) and thereafter gradually decreased (Fig. 4, B and C). However, changes in the level of ROCK isoforms in the cytosolic fraction in response to insulin were not seen in these cells (data not shown). These data identify different patterns of subcellular movement by ROCK1 and ROCK2 in response to insulin, providing support for the hypothesis that ROCK1 and ROCK2 have distinct functions in regulating insulin-stimulated glucose transport.
The subcellular distribution of ROCK1 differs from that of ROCK2
We also investigated the subcellular distributions of ROCK isoforms in 3T3-L1 adipocytes and L6 myoblasts using confocal laser scanning microscopy. Antibodies for detection of ROCK isoforms and RhoA by fluorescent microscopy were validated using siRNA knockdown (Fig. 4D). Confocal images revealed that ROCK1 and ROCK2 are mainly localized as granular forms in the cytoplasm in L6 myoblasts. Compared with ROCK2, ROCK1 was more condensed in the perinuclear region (Fig. 4, D and E). In contrast, RhoA, which was evenly distributed in the cytoplasm and nucleus, showed intense immunoreactivity in cell membrane protrusions (Fig. 4D).
To further clarify the subcellular localization of ROCK isoforms in 3T3-L1 adipocytes and L6 myoblasts, we compared the localization of ROCK isoforms with cytochrome C oxidase IV, a mitochondrial marker, and Rab5, an early endosomal marker. In L6 cells, a majority of ROCK1 (red) was observed in the cytoplasm with discrete distribution from both mitochondrial and endosomal markers (green), whereas ROCK2 is widely distributed in the cytoplasm (Fig. 4E). Under high magnification, some of ROCK1 and ROCK2 were found to be colocalized with these organelle markers as indicated by arrows (Fig. 4E, magnified images). The distribution pattern of each ROCK isoform in 3T3-L1 adipocytes was similar to that observed in L6 muscle cells (Fig. 4F). Colocalization of ROCK isoforms with cytochrome C oxidase IV or Rab5 was also detected in 3T3-L1 adipocytes (Fig. 4F, magnified images). These data suggest that in addition to regulating insulin-stimulated glucose transport, ROCK isoforms could play a role in modulating the IRS-1 signaling at endosomes and mitochondria that occurs in response to insulin (44).
Actin cytoskeletal remodeling is required for ROCK1-mediated glucose transport
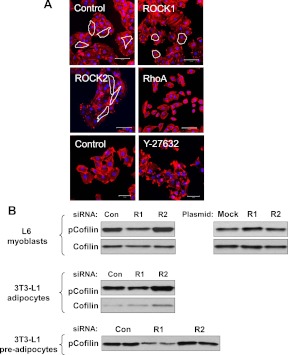
Actin polymerization (F-actin/G-actin transition) is prerequisite for Glut4 translocation in response to insulin in 3T3-L1 adipocytes and L6 cells (13–18). ROCK isoforms have been implicated in the regulation of actin polymerization (7). To explore the effects of ROCK isoforms on the actin cytoskeleton, we assessed the effect of alterations in ROCK isoform expression on cell morphology of L6 myoblasts. ROCK1 suppression resulted in a less-protruded, rounded shape of cell morphology, whereas L6 myoblasts with ROCK2 suppression displayed an elongated shape relative to control myoblasts (Fig. 5A). No significant changes in the quantity of stress fibers were detected with suppression of either ROCK isoform. In contrast, inhibition of RhoA by siRNA knockdown or treatment with ROCK inhibitor Y-27632 caused a decrease in L6 myoblast stress fibers (Fig. 5A). RhoA knockdown resulted in a shrunken cell morphology, but cell morphology was unchanged by Y-27632 treatment (Fig. 5A). These data suggest that ROCK1 may regulate actin dynamics in a way that is different from ROCK2.
Fig. 5.
Regulation of actin cytoskeleton by ROCK1. A, The structure of F-actin in L6 myoblasts. Cells were transfected with ROCK1, ROCK2, RhoA, or luciferase (control) siRNA. Cells were treated with the ROCK chemical inhibitor Y-27632 (10 μm) for 30 min. F-actin (red) in cells was stained using Alexa Fluor 546 phalloidin. Nuclei were stained with DAPI (blue color). Representative cell morphological shapes are outlined in white. Scale bar, 100 μm (upper and middle) and 50 μm (bottom). B, Effects of ROCK isoforms on confilin-1 phosphorylation in L6 myoblasts, and 3T3-L1 adipocytes and preadipocytes. Cells were transfected with ROCK1 (R1), ROCK2 (R2), RhoA, or luciferase (Con) siRNA. Cofilin was visualized by immunoblotting with a phosphor-specific cofilin-1 Ser 3 antibody or total cofilin antibody.
To further determine the effects of ROCK isoforms on the ROCK→LIM→cofilin signaling cascade that mediates insulin-induced actin polymerization, we measured cofilin serine 3 phosphorylation in L6 myoblasts, 3T3-L1 preadipocytes, and adipocytes. Phosphorylation of cofilin-1 at serine 3 by LIM motif-containing protein kinase is critical in controlling its activity (45–47). In L6 myoblasts, ROCK1 inhibition decreased cofilin phosphorylation, whereas ROCK1 overexpression increased it. However, ROCK2 had no effect on cofilin phosphorylation in these cells (Fig. 5B). Similar results were seen in 3T3-L1 adipocytes and preadipocytes (Fig. 5B). These data suggest that ROCK1 acts as an upstream regulator of the LIMK-cofilin signaling pathway in insulin-responsive muscle and adipocyte cell lines and further support that ROCK isoforms may have divergent functions in the control of insulin-stimulated actin cytoskeletal reorganization involving glucose transport and Glut4 translocation (13–18).
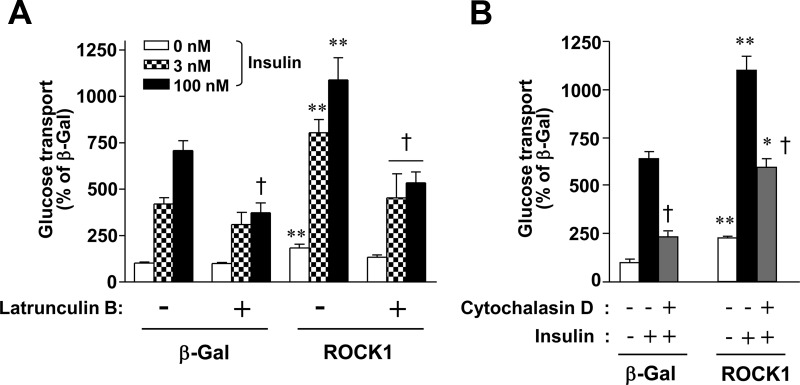
To address whether ROCK1's effects on actin polymerization are required for ROCK1-mediated stimulation of glucose transport, 3T3-L1 adipocytes expressing exogenous β-gal or ROCK1 were treated with inhibitors of actin polymerization, and insulin-stimulated glucose transport was measured. In the absence of latrunculin B or cytochalasin D, potent cell permeable inhibitors of actin polymerization, overexpression of ROCK1 significantly increased basal and insulin-stimulated glucose transport in 3T3-L1 adipocytes (Fig. 6, A and B). However, the ability of insulin to increase glucose transport in ROCK1-overexpressing 3T3-L1 adipocytes was impaired by treatment with latrunculin B or cytochalasin D (Fig. 6, A and B). These results suggest that insulin-stimulated actin polymerization is required for the regulation of ROCK1-mediated glucose transport in 3T3-L1 adipocytes, establishing the new role for ROCK1 in glucose metabolism.
Fig. 6.
Intact actin cytoskeletal reorganization is required for ROCK1-mediated glucose transport in 3T3-L1 adipocytes. A, Effects of latrunculin B on ROCK1-mediated glucose transport in 3T3-L1 adipocytes. Cells were transduced with recombinant adenovirus encoding β-Gal or ROCK1 as described in Materials and Methods. Cells were pretreated with latrunculin B (2 μm) or with vehicle for 3 h and then stimulated with insulin (3 or 100 nm) for 30 min. [3H]-2-deoxy-D-glucose uptake was measured, and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem and representative of three independent experiments. +, P < 0.001 vs. insulin-stimulated condition within same group; **, P < 0.001 vs. the corresponding condition of β-Gal. B, Effects of cytochalasin D on ROCK1-mediated glucose transport in 3T3-L1 adipocytes. Cells were transduced with recombinant adenovirus encoding β-Gal or ROCK1 as described in Materials and Methods. Cells were pretreated with cytochalasin D (2 μm) or with vehicle for 3 h and then stimulated with insulin (100 nm) for 30 min. [3H]-2-deoxy-D-glucose uptake was measured, and the results are expressed as a percentage of basal glucose transport in control cells. Data are means ± sem and representative of three independent experiments. +, P < 0.001 vs. insulin-stimulated condition within same group; *, P < 0.01; **, P < 0.001 vs. the corresponding condition of β-Gal.
Discussion
The impairment in insulin-dependent glucose transport in skeletal muscle and adipose tissue is a key feature of insulin resistance (48), and trying to identify the molecular mechanism for this phenomenon has been a major concern in the physiologic metabolism field. Although ROCK isoforms have not previously been suspected to be involved in glucose metabolism, our previous work demonstrated that the ability of insulin to activate glucose transport and signaling was impaired when ROCK2 was inhibited with a DN isoform (2). However, because DN-ROCK2 inhibited the activity of both ROCK1 and ROCK2 (49, 50), the specific effects of each ROCK isoform on glucose transport could not be determined. The present study focused on determining the exact role of ROCK1 in the regulation of insulin action on glucose transport, with particular emphasis on the involvement of actin cytoskeletal remodeling. One of the major findings of this study is that inhibition (or overexpression) of ROCK1 expression decreases (or increases) insulin-stimulated glucose transport in adipocytes and muscle cells. These data suggest that activation of ROCK1 is essential for the metabolic action of insulin on glucose transport in adipocytes and muscle cells.
Our data show that siRNA-mediated suppression of endogenous ROCK1 expression causes a significant decrease in insulin-stimulated glucose transport in 3T3-L1 adipocytes and L6 myoblasts. This effect is most likely due to defective Glut4 translocation induced by insulin. These data are in agreement with our previous work showing that inhibition of ROCK2 decreases insulin's effect on glucose transport and Glut4 translocation in adipocytes and muscle cells (2). In contrast, overexpression of ROCK1 is sufficient to enhance insulin-stimulated glucose transport in these cell types. However, these effects are completely abolished by treatment with a PI3K inhibitor, indicating that ROCK1-mediated glucose transport is regulated via a PI3K-dependent mechanism. Importantly, the insulin-sensitizing effect for glucose transport assessed by ED50 is significantly increased when ROCK1 expression is elevated. Together, these data suggest that ROCK1 regulates insulin-dependent glucose transport and Glut4 translocation through the PI3K pathway in adipocytes and muscle cells.
We see a discrepancy between ROCK effects on phosphorylation of IRS-1 and activation of Akt, a downstream mediator of IRS-1/PI3K signaling, in 3T3-L1 adipocytes. A possible explanation for this discrepancy is that only partial stimulation of IRS-1 phosphorylation may be needed for maximal phosphorylation and activation of Akt. These results are also consistent with previous results demonstrating that Akt activation is normal despite impaired upstream insulin signaling when DN-ROCK is expressed (2). Other possible mechanisms causing discordance could involve regulation distal to IRS-1-associated PI3K activation. For example, increased activity of phosphoinositide-dependent kinases that activate Akt (51) or decreased activity of phosphatases that dephosphorylate phospholipid products of PI3K could amplify the signal from IRS-1/PI3K (52). We also cannot rule out the possibility that the pool of IRS-1/PI3K regulated by ROCK may not be the pool of IRS-1/PI3K that signals to Akt activation. Whether such alterations are present in cells lacking ROCK1 when IRS-1 phosphorylation is impaired are important questions for future studies.
ROCK2 binds to IRS-1 and directly phosphorylates IRS-1 serine residues at 632/635, 936, and 972 in vitro (2, 4, 5, 42). Consistent with this, coimmunoprecipitation analysis revealed that ROCK2 strongly binds to IRS-1 in 3T3-L1 adipocytes, L6 myoblasts, HEK293 cells, and mouse muscle. In contrast, ROCK1 binding to IRS-1 is limited under these conditions. Although the mechanisms mediating the differential binding of ROCK isoforms to IRS-1 are unclear at this time, the ROCK isoforms have relatively low similarity of their carboxyl-terminal region where IRS-1 physically binds to ROCK2 (5). In the carboxyl-terminal region of ROCK1 and ROCK2, the pleckstrin homology domain has 72% identity, and coiled-coil region has 55% identity. In contrast, there is more than 90% similarity in the catalytic kinase domain of ROCK isoforms. In this regard, some other studies have demonstrated that ROCK1 preferentially interacts with Gem (immediate early gene expressed in mitogen-stimulated T-cells) (30), caspase-3 (25, 29), RhoE (27), and pyruvate dehydrogenase kinase 1 (26), whereas ROCK2 strongly binds with granzyme B (28) and Rad (Ras associated with diabetes) (30), leading to divergent functions in a variety of cellular events and consistent with our data showing that ROCK isoforms have nonoverlapping roles in regulating insulin action on glucose transport.
Experimental evidence from the study of IRS-1 mutants indicated that IRS-1 serine 632/635 phosphorylation is required for insulin-induced glucose transport and PI3K activity in 3T3-L1 adipocytes. This is thought to be regulated via ROCK2, as revealed by the fact that IRS-1 serine 632/635 is a substrate for ROCK2 (2). However, it is unlikely that alteration in IRS-1 phosphorylation by ROCK1 is a major mechanism in regulating glucose transport in 3T3-L1 adipocytes. This could be due to changes in actin cytoskeleton reorganization indirectly, and further studies will be sure to clarify this important issue.
The subcellular distribution patterns of ROCK isoforms are similar to those of IRS-1, with abundant localization in the LDM and cytosolic fraction of 3T3-L1 adipocytes. In contrast, RhoA and RhoE, both of which are upstream mediators of ROCK, are mainly found in the compartment of PM and HDM. Colocalization of ROCK isoforms with IRS-1 in L6 myoblasts by confocal microscopic analysis was limited due to the unavailability of an IRS-1 antibody for immunocytochemistry. However, the subcellular distribution pattern of ROCK isoforms observed in L6 myoblasts supports results from cell fractionation studies of 3T3-L1 adipocytes. ROCK1, but not ROCK2, is abundantly localized in the perinuclear region of the cytosol compartment in L6 myoblasts, suggesting that subtle changes in cellular distribution of ROCK isoforms may cause divergent effects, including actin cytoskeleton reorganization. The lack of interaction between ROCK1 and IRS-1 in binding assays does not rule out that these proteins interact in vivo. Indeed, that ROCK1 and IRS-1 localize to the same subcellular compartment and that a strong interaction between ROCK2 and IRS-1 is closely associated with similar localization in adipocytes and muscle cells suggest molecular interaction between ROCK1 and substrates of insulin signaling (2, 4–6).
A dynamic actin cytoskeleton is required for insulin-stimulated glucose transport and Glut4 translocation in adipocytes and muscle cell membranes (13–18). Our data demonstrate that treatment of adipocytes with latrunculin B or cytochalasin D impairs insulin's ability to increase glucose transport, indicating that actin cytoskeletal remodeling is critical in regulation of the ROCK1-mediated glucose transport in 3T3-L1 adipocytes. Interestingly, however, cytochalasin D is not sufficient to fully inhibit glucose transport in response to insulin, as latrunculin B does under the conditions used here. Although both chemicals are widely used as potent inhibitors of actin polymerization, they control actin filaments in a somewhat different way, as indicated by the findings that cytochalasin D returns the F-actin to its basal level by inhibiting the addition of actin subunits to the barbed end of F-actin (53), but latrunculin B causes a decrease in the resting F-actin level by depolymerizing it (54). It is thus possible that such structural changes of actin filaments may cause different effects on glucose transport in adipocytes. Another possible explanation for this is that cytochalasin D does not completely disrupt the actin filaments that are responsible for the movement of Glut4 vesicles in adipocytes. In view of the evidence that microfilaments have several distinct structures, including lamellipodia, filopodia, stress fibers, focal complexes, and focal adhesions (55, 56), it is unclear which structures are involved in this specific event. Nevertheless, these data highlight the important role of actin skeletal reorganization in glucose transport induced by ROCK1.
In examining the effects of ROCK1 isoforms on F-actin structures in L6 muscle cells, confocal microscopic images revealed that ROCK1-suppressed cells have a less-protruding, rounded cell shape, whereas inhibition of ROCK2 causes an elongated cell shape. Unlike previous findings (23), a significant loss of stress fibers in ROCK1-deficient L6 muscle cells was not detected under these experimental conditions. Considering the results showing loss of stress fibers in RhoA-depleted or Y27632-treated L6 muscle cells, it is conceivable that a dynamic of F-actin reorganization is not simply controlled by the ROCK pathway. Moreover, we found that phosphorylation of cofilin-1, a key downstream modulator of the ROCK pathway, is specifically regulated in a ROCK1-dependent manner. Collectively, these data suggest that ROCK isoforms could play divergent roles in the regulation of actin cytoskeletal remodeling that is critical for movement of Glut4 vesicles to the PM.
In conclusion, this is the first demonstration of a role of ROCK1 in the regulation of glucose transport and insulin sensitivity in adipocytes and muscle cells. Our data suggest that activation of ROCK1 is necessary for the regulation of Glut4 translocation and glucose transport in response to insulin in insulin-sensitive cells. The major mechanism responsible for this involves changes in actin cytoskeletal reorganization and insulin signaling. Thus, our studies identify ROCK1 as an important regulator of insulin action on glucose transport. The emergence of ROCK1 as a key effecter of insulin action on glucose metabolism may lead to new treatment approaches and elucidation of new therapeutic targets for obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
We thank Dr. Amira Klip for valuable advice and technical assistance, Dr. L. Wei for ROCK1 cDNA and Dr. K. Kaibuchi for ROCK2 cDNA, and Dr. M. White for IRS-1 antibodies.
This work was supported by National Institutes of Health Grants 1R01DK083567 (to Y.-B.K.) and 5R01CA127247 (to S.W.L.), the American Diabetes Association Grant 1-09-RA-87 (to Y.-B.K.), the Korea Science and Engineering Foundation Grant M10642140004-06N4214-0040 (to K.S.P.), and the 21C Frontier Microbial Genomics and Applications Center Program from the Ministry of Education, Science and Technology Grant 11-2008-18-001-00 (to B.-C.O.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Akt2
- v-Akt murine thymoma viral oncogene homolog 2
- DAPI
- 4′,6-diamidino-2-phenylindole
- DN
- dominant negative
- β-Gal
- β-galactosidase
- Glut4
- glucose transporter 4
- HDM
- high-density microsome
- HES
- HEPES/EDTA/sucrose
- IR
- insulin receptor
- IRS
- IR substrate
- LDM
- low-density microsome
- LIM
- Lin-11, Isl1, Mec3
- PI3K
- phosphatidylinositol 3-kinase
- PM
- plasma membrane
- PTEN
- phosphatase and tensin homolog
- Rab
- Ras-related proteins in the brain
- ROCK
- Rho-associated coiled-coil-containing protein kinase
- ROK
- RhoA-binding kinase
- S632/635A-IRS-1
- inactive mutant, IRS-1 protein containing alanine substitution for serine 632/635
- S632/635E-IRS-1
- phosphor-mimic mutant, IRS-1 protein containing glutamic acid substitution for serine 632/635
- siRNA
- small interfering RNA
- WT
- wild type.
References
- 1. Bryant NJ, Govers R, James DE. 2002. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3:267–277 [DOI] [PubMed] [Google Scholar]
- 2. Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. 2005. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2:119–129 [DOI] [PubMed] [Google Scholar]
- 3. Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, White MF, Wei L, Kim YB. 2009. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem 284:11776–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. 2002. Active Rho kinase (ROK-α) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem 277:6214–6222 [DOI] [PubMed] [Google Scholar]
- 5. Farah S, Agazie Y, Ohan N, Ngsee JK, Liu XJ. 1998. A rho-associated protein kinase, ROKα, binds insulin receptor substrate-1 and modulates insulin signaling. J Biol Chem 273:4740–4746 [DOI] [PubMed] [Google Scholar]
- 6. Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K, Saruta T. 2006. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 20:169–171 [DOI] [PubMed] [Google Scholar]
- 7. Schmandke A, Schmandke A, Strittmatter SM. 2007. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 13:454–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Narumiya S, Tanji M, Ishizaki T. 2009. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev 28:65–76 [DOI] [PubMed] [Google Scholar]
- 9. Madaule P, Furuyashiki T, Eda M, Bito H, Ishizaki T, Narumiya S. 2000. Citron, a Rho target that affects contractility during cytokinesis. Microsc Res Tech 49:123–126 [DOI] [PubMed] [Google Scholar]
- 10. Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, McGonigle S, Paradise E, Sweetnam P, Fink LM, Vozenin-Brotons MC, Hauer-Jensen M. 2008. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinolysis 19:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. 1999. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285:895–898 [DOI] [PubMed] [Google Scholar]
- 12. Khan AH, Pessin JE. 2002. Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia 45:1475–1483 [DOI] [PubMed] [Google Scholar]
- 13. Kanzaki M, Pessin JE. 2001. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem 276:42436–42444 [DOI] [PubMed] [Google Scholar]
- 14. Török D, Patel N, Jebailey L, Thong FS, Randhawa VK, Klip A, Rudich A. 2004. Insulin but not PDGF relies on actin remodeling and on VAMP2 for GLUT4 translocation in myoblasts. J Cell Sci 117:5447–5455 [DOI] [PubMed] [Google Scholar]
- 15. Tong P, Khayat ZA, Huang C, Patel N, Ueyama A, Klip A. 2001. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J Clin Invest 108:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsakiridis T, Vranic M, Klip A. 1994. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem 269:29934–29942 [PubMed] [Google Scholar]
- 17. Brozinick JT, Jr, Hawkins ED, Strawbridge AB, Elmendorf JS. 2004. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J Biol Chem 279:40699–40706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu XJ, Yang C, Gupta N, Zuo J, Chang YS, Fang FD. 2007. Protein kinase C-ζ regulation of GLUT4 translocation through actin remodeling in CHO cells. J Mol Med 85:851–861 [DOI] [PubMed] [Google Scholar]
- 19. Tsakiridis T, Bergman A, Somwar R, Taha C, Aktories K, Cruz TF, Klip A, Downey GP. 1998. Actin filaments facilitate insulin activation of the src and collagen homologous/mitogen-activated protein kinase pathway leading to DNA synthesis and c-fos expression. J Biol Chem 273:28322–28331 [DOI] [PubMed] [Google Scholar]
- 20. Wang Q, Bilan PJ, Tsakiridis T, Hinek A, Klip A. 1998. Actin filaments participate in the relocalization of phosphatidylinositol3-kinase to glucose transporter-containing compartments and in the stimulation of glucose uptake in 3T3-L1 adipocytes. Biochem J 331(Pt 3):917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omata W, Shibata H, Li L, Takata K, Kojima I. 2000. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem J 346(Pt 2):321–328 [PMC free article] [PubMed] [Google Scholar]
- 22. Emoto M, Langille SE, Czech MP. 2001. A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3-L1 adipocytes. J Biol Chem 276:10677–10682 [DOI] [PubMed] [Google Scholar]
- 23. Yoneda A, Multhaupt HA, Couchman JR. 2005. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. 2007. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell 18:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. 2001. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345 [DOI] [PubMed] [Google Scholar]
- 26. Pinner S, Sahai E. 2008. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol 10:127–137 [DOI] [PubMed] [Google Scholar]
- 27. Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. 2003. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol 23:4219–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sebbagh M, Hamelin J, Bertoglio J, Solary E, Bréard J. 2005. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med 201:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J. 2001. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 3:346–352 [DOI] [PubMed] [Google Scholar]
- 30. Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. 2002. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol 157:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huppertz C, Fischer BM, Kim YB, Kotani K, Vidal-Puig A, Slieker LJ, Sloop KW, Lowell BB, Kahn BB. 2001. Uncoupling protein 3 (UCP3) stimulates glucose uptake in muscle cells through a phosphoinositide 3-kinase-dependent mechanism. J Biol Chem 276:12520–12529 [DOI] [PubMed] [Google Scholar]
- 32. Kim YB, Shulman GI, Kahn BB. 2002. Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase Cλ/ζ but not on glycogen synthase kinase-3. J Biol Chem 277:32915–32922 [DOI] [PubMed] [Google Scholar]
- 33. Karnam P, Standaert ML, Galloway L, Farese RV. 1997. Activation and translocation of Rho (and ADP ribosylation factor) by insulin in rat adipocytes. Apparent involvement of phosphatidylinositol 3-kinase. J Biol Chem 272:6136–6140 [DOI] [PubMed] [Google Scholar]
- 34. Standaert M, Bandyopadhyay G, Galloway L, Ono Y, Mukai H, Farese R. 1998. Comparative effects of GTPγS and insulin on the activation of Rho, phosphatidylinositol 3-kinase, and protein kinase N in rat adipocytes. Relationship to glucose transport. J Biol Chem 273:7470–7477 [DOI] [PubMed] [Google Scholar]
- 35. Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100:7569–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang X, Powelka AM, Soriano NA, Czech MP, Guilherme A. 2005. PTEN, but not SHIP2, suppresses insulin signaling through the phosphatidylinositol 3-kinase/Akt pathway in 3T3-L1 adipocytes. J Biol Chem 280:22523–22529 [DOI] [PubMed] [Google Scholar]
- 37. Robinson R, Robinson LJ, James DE, Lawrence JC., Jr 1993. Glucose transport in L6 myoblasts overexpressing GLUT1 and GLUT4. J Biol Chem 268:22119–22126 [PubMed] [Google Scholar]
- 38. Ueyama A, Yaworsky KL, Wang Q, Ebina Y, Klip A. 1999. GLUT-4myc ectopic expression in L6 myoblasts generates a GLUT-4-specific pool conferring insulin sensitivity. Am J Physiol 277:E572–E578 [DOI] [PubMed] [Google Scholar]
- 39. Mitsumoto Y, Burdett E, Grant A, Klip A. 1991. Differential expression of the GLUT1 and GLUT4 glucose transporters during differentiation of L6 muscle cells. Biochem Biophys Res Commun 175:652–659 [DOI] [PubMed] [Google Scholar]
- 40. Kohn AD, Summers SA, Birnbaum MJ, Roth RA. 1996. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 271:31372–31378 [DOI] [PubMed] [Google Scholar]
- 41. Frevert EU, Kahn BB. 1997. Differential effects of constitutively active phosphatidylinositol 3- kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol Cell Biol 17:190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim MJ, Choi KJ, Ding Y, Kim JH, Kim BS, Kim YH, Lee J, Choe W, Kang I, Ha J, Yoon KS, Kim SS. 2007. RhoA/Rho kinase blocks muscle differentiation via serine phosphorylation of insulin receptor substrate-1 and -2. Mol Endocrinol 21:2282–2293 [DOI] [PubMed] [Google Scholar]
- 43. Fujisawa K, Fujita A, Ishizaki T, Saito Y, Narumiya S. 1996. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J Biol Chem 271:23022–23028 [DOI] [PubMed] [Google Scholar]
- 44. Cheng Z, Tseng Y, White MF. 2010. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab 21:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393:805–809 [DOI] [PubMed] [Google Scholar]
- 46. Endo M, Ohashi K, Sasaki Y, Goshima Y, Niwa R, Uemura T, Mizuno K. 2003. Control of growth cone motility and morphology by LIM kinase and Slingshot via phosphorylation and dephosphorylation of cofilin. J Neurosci 23:2527–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. 1998. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393:809–812 [DOI] [PubMed] [Google Scholar]
- 48. DeFronzo RA. 1997. Pathogenesis of type 2 diabetes : metabolic and molecular implications for identifying diabetes. Diabetes Rev 5:177–269 [Google Scholar]
- 49. Kobayashi K, Takahashi M, Matsushita N, Miyazaki J, Koike M, Yaginuma H, Osumi N, Kaibuchi K, Kobayashi K. 2004. Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J Neurosci 24:3480–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shirai H, Autieri M, Eguchi S. 2007. Small GTP-binding proteins and mitogen-activated protein kinases as promising therapeutic targets of vascular remodeling. Curr Opin Nephrol Hypertens 16:111–115 [DOI] [PubMed] [Google Scholar]
- 51. Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR. 1998. Activation of protein kinase Bβ and γ isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase Bα. Biochem J 331:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29–39 [DOI] [PubMed] [Google Scholar]
- 53. Cooper JA. 1987. Effects of cytochalasin and phalloidin on actin. J Cell Biol 105:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cassimeris L, McNeill H, Zigmond SH. 1990. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J Cell Biol 110:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lavelin I, Geiger B. 2005. Characterization of a novel GTPase-activating protein associated with focal adhesions and the actin cytoskeleton. J Biol Chem 280:7178–7185 [DOI] [PubMed] [Google Scholar]
- 56. Edlund M, Lotano MA, Otey CA. 2001. Dynamics of α-actinin in focal adhesions and stress fibers visualized with α-actinin-green fluorescent protein. Cell Motil Cytoskeleton 48:190–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.