Abstract
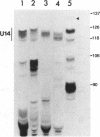
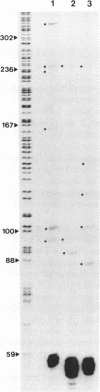
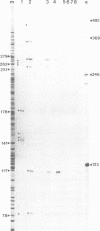
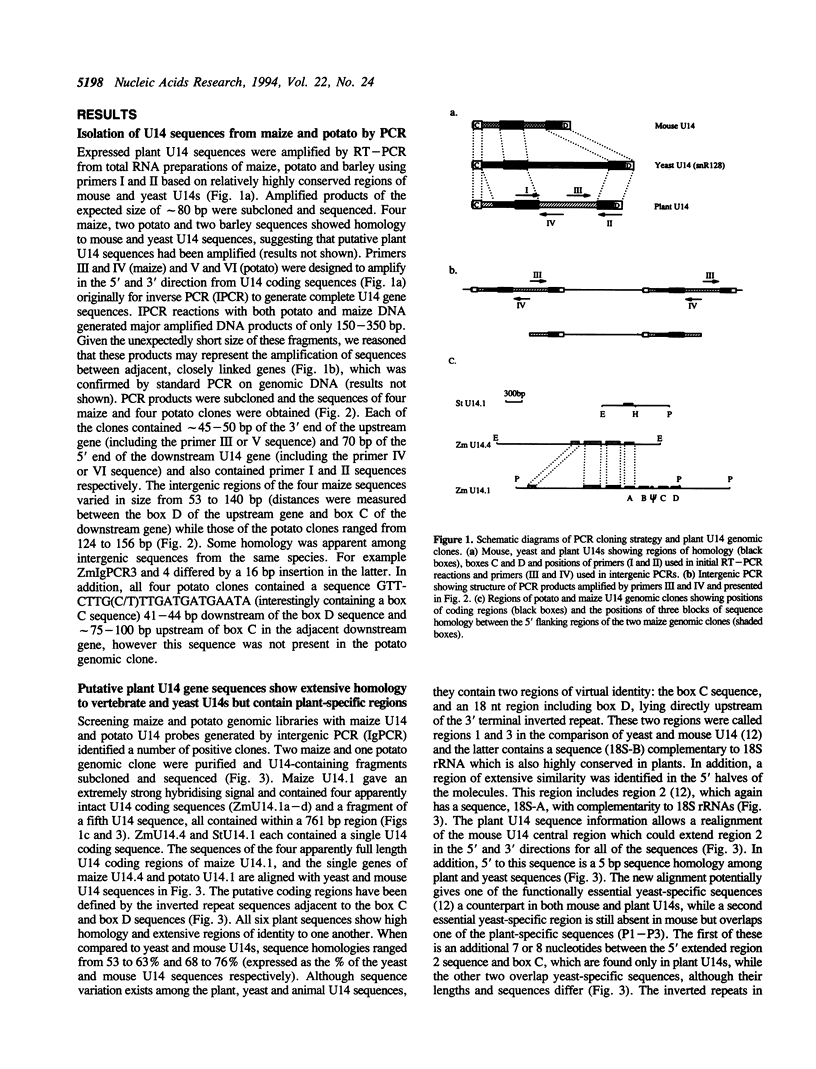
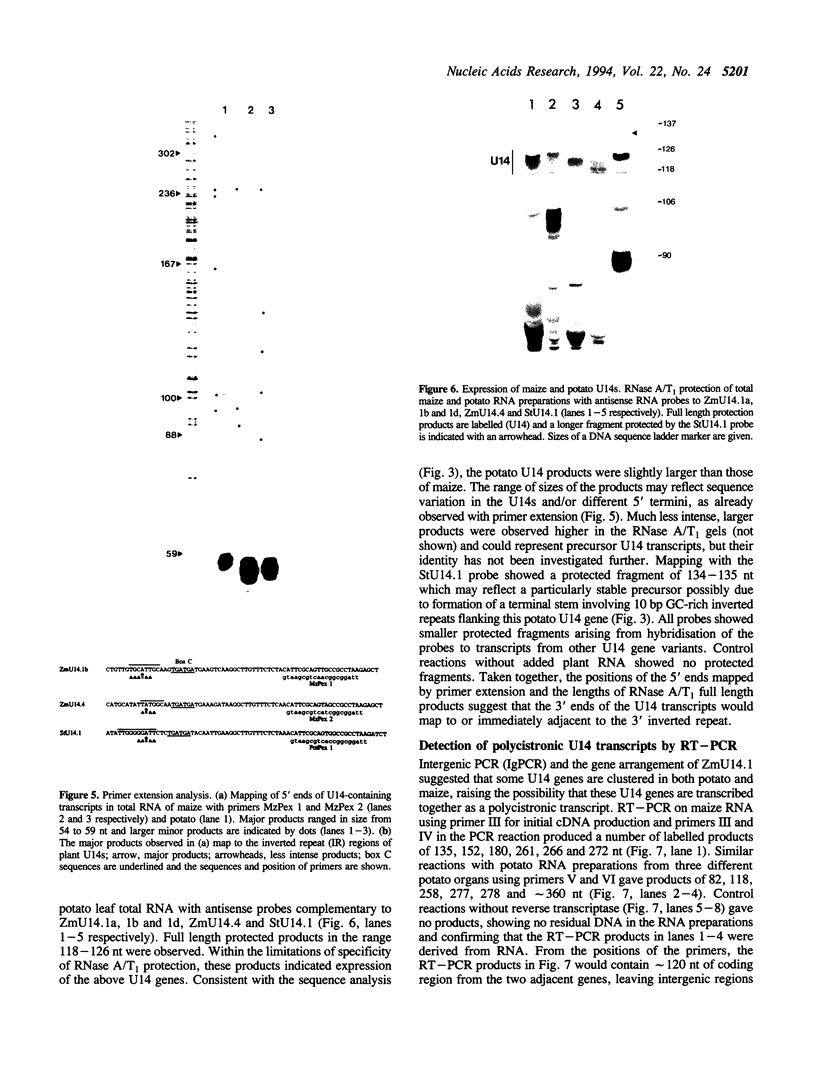
U14snoRNAs are highly conserved eukaryotic nucleolar small RNAs involved in precursor ribosomal RNA processing. In vertebrates, U14snoRNAs and a number of other snoRNAs are transcribed within introns of protein coding genes and are released by processing. We have isolated potato and maize genomic U14 clones using PCR-amplified plant U14 probes. Plant U14s show extensive homology to those from yeast and animals but contain plant-specific sequences. One of the isolated maize clones contains a cluster of four U14 genes in a region of only 761 bp, confirming the close linkage of U14 genes in maize, potato and barley as established by PCR. The absence of known plant promoter elements, the proximity of the genes and the detection of transcripts containing linked U14s by RT-PCR indicates that some plant U14snoRNAs are transcribed as precursor RNAs which are then processed to release individual U14s. Whether plant U14snoRNAs are intron-encoded or transcribed from novel promoter sequences, remains to be established.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balakin A. G., Lempicki R. A., Huang G. M., Fournier M. J. Saccharomyces cerevisiae U14 small nuclear RNA has little secondary structure and appears to be produced by post-transcriptional processing. J Biol Chem. 1994 Jan 7;269(1):739–746. [PubMed] [Google Scholar]
- Baserga S. J., Yang X. D., Steitz J. A. An intact Box C sequence in the U3 snRNA is required for binding of fibrillarin, the protein common to the major family of nucleolar snRNPs. EMBO J. 1991 Sep;10(9):2645–2651. doi: 10.1002/j.1460-2075.1991.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffarelli E., Arese M., Santoro B., Fragapane P., Bozzoni I. In vitro study of processing of the intron-encoded U16 small nucleolar RNA in Xenopus laevis. Mol Cell Biol. 1994 May;14(5):2966–2974. doi: 10.1128/mcb.14.5.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi F., Mariottini P., Loreni F., Pierandrei-Amaldi P., Campioni N., Amaldi F. U17XS8, a small nucleolar RNA with a 12 nt complementarity to 18S rRNA and coded by a sequence repeated in the six introns of Xenopus laevis ribosomal protein S8 gene. Nucleic Acids Res. 1994 Mar 11;22(5):732–741. doi: 10.1093/nar/22.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Kiss T. Structure and function of nucleolar snRNPs. Mol Biol Rep. 1993 Aug;18(2):149–156. doi: 10.1007/BF00986770. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Maxwell E. S. The nucleolar snRNAs: catching up with the spliceosomal snRNAs. Trends Biochem Sci. 1993 Apr;18(4):131–135. doi: 10.1016/0968-0004(93)90020-n. [DOI] [PubMed] [Google Scholar]
- Fragapane P., Caffarelli E., Lener M., Prislei S., Santoro B., Bozzoni I. Identification of the sequences responsible for the splicing phenotype of the regulatory intron of the L1 ribosomal protein gene of Xenopus laevis. Mol Cell Biol. 1992 Mar;12(3):1117–1125. doi: 10.1128/mcb.12.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragapane P., Prislei S., Michienzi A., Caffarelli E., Bozzoni I. A novel small nucleolar RNA (U16) is encoded inside a ribosomal protein intron and originates by processing of the pre-mRNA. EMBO J. 1993 Jul;12(7):2921–2928. doi: 10.1002/j.1460-2075.1993.tb05954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highett M. I., Beven A. F., Shaw P. J. Localization of 5 S genes and transcripts in Pisum sativum nuclei. J Cell Sci. 1993 Aug;105(Pt 4):1151–1158. doi: 10.1242/jcs.105.4.1151. [DOI] [PubMed] [Google Scholar]
- Huang G. M., Jarmolowski A., Struck J. C., Fournier M. J. Accumulation of U14 small nuclear RNA in Saccharomyces cerevisiae requires box C, box D, and a 5', 3' terminal stem. Mol Cell Biol. 1992 Oct;12(10):4456–4463. doi: 10.1128/mcb.12.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmolowski A., Zagorski J., Li H. V., Fournier M. J. Identification of essential elements in U14 RNA of Saccharomyces cerevisiae. EMBO J. 1990 Dec;9(13):4503–4509. doi: 10.1002/j.1460-2075.1990.tb07901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Filipowicz W. Small nucleolar RNAs encoded by introns of the human cell cycle regulatory gene RCC1. EMBO J. 1993 Jul;12(7):2913–2920. doi: 10.1002/j.1460-2075.1993.tb05953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Marshallsay C., Filipowicz W. 7-2/MRP RNAs in plant and mammalian cells: association with higher order structures in the nucleolus. EMBO J. 1992 Oct;11(10):3737–3746. doi: 10.1002/j.1460-2075.1992.tb05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Marshallsay C., Filipowicz W. Alteration of the RNA polymerase specificity of U3 snRNA genes during evolution and in vitro. Cell. 1991 May 3;65(3):517–526. doi: 10.1016/0092-8674(91)90469-f. [DOI] [PubMed] [Google Scholar]
- Kiss T., Solymosy F. Molecular analysis of a U3 RNA gene locus in tomato: transcription signals, the coding region, expression in transgenic tobacco plants and tandemly repeated pseudogenes. Nucleic Acids Res. 1990 Apr 25;18(8):1941–1949. doi: 10.1093/nar/18.8.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverette R. D., Andrews M. T., Maxwell E. S. Mouse U14 snRNA is a processed intron of the cognate hsc70 heat shock pre-messenger RNA. Cell. 1992 Dec 24;71(7):1215–1221. doi: 10.1016/s0092-8674(05)80069-8. [DOI] [PubMed] [Google Scholar]
- Li D., Fournier M. J. U14 function in Saccharomyces cerevisiae can be provided by large deletion variants of yeast U14 and hybrid mouse-yeast U14 RNAs. EMBO J. 1992 Feb;11(2):683–689. doi: 10.1002/j.1460-2075.1992.tb05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. D., Zagorski J., Fournier M. J. Depletion of U14 small nuclear RNA (snR128) disrupts production of 18S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Mar;10(3):1145–1152. doi: 10.1128/mcb.10.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Maxwell E. S. Mouse U14 snRNA is encoded in an intron of the mouse cognate hsc70 heat shock gene. Nucleic Acids Res. 1990 Nov 25;18(22):6565–6571. doi: 10.1093/nar/18.22.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshallsay C., Connelly S., Filipowicz W. Characterization of the U3 and U6 snRNA genes from wheat: U3 snRNA genes in monocot plants are transcribed by RNA polymerase III. Plant Mol Biol. 1992 Sep;19(6):973–983. doi: 10.1007/BF00040529. [DOI] [PubMed] [Google Scholar]
- Marshallsay C., Kiss T., Filipowicz W. Amplification of plant U3 and U6 snRNA gene sequences using primers specific for an upstream promoter element and conserved intragenic regions. Nucleic Acids Res. 1990 Jun 25;18(12):3459–3466. doi: 10.1093/nar/18.12.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell E. S., Martin T. E. A low-molecular-weight RNA from mouse ascites cells that hybridizes to both 18S rRNA and mRNA sequences. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7261–7265. doi: 10.1073/pnas.83.19.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S., Henikoff S. Gene organization: nested genes take flight. Curr Biol. 1993 Jun 1;3(6):372–374. doi: 10.1016/0960-9822(93)90205-3. [DOI] [PubMed] [Google Scholar]
- Nag M. K., Thai T. T., Ruff E. A., Selvamurugan N., Kunnimalaiyaan M., Eliceiri G. L. Genes for E1, E2, and E3 small nucleolar RNAs. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9001–9005. doi: 10.1073/pnas.90.19.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prislei S., Michienzi A., Presutti C., Fragapane P., Bozzoni I. Two different snoRNAs are encoded in introns of amphibian and human L1 ribosomal protein genes. Nucleic Acids Res. 1993 Dec 25;21(25):5824–5830. doi: 10.1093/nar/21.25.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prislei S., Sperandio S., Fragapane P., Caffarelli E., Presutti C., Bozzoni I. The mechanisms controlling ribosomal protein L1 pre-mRNA splicing are maintained in evolution and rely on conserved intron sequences. Nucleic Acids Res. 1992 Sep 11;20(17):4473–4479. doi: 10.1093/nar/20.17.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff E. A., Rimoldi O. J., Raghu B., Eliceiri G. L. Three small nucleolar RNAs of unique nucleotide sequences. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):635–638. doi: 10.1073/pnas.90.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B. Novel intron-encoded small nucleolar RNAs. Cell. 1993 Nov 5;75(3):403–405. doi: 10.1016/0092-8674(93)90374-y. [DOI] [PubMed] [Google Scholar]
- Séraphin B. How many intronic snRNAs? Trends Biochem Sci. 1993 Sep;18(9):330–331. doi: 10.1016/0968-0004(93)90067-w. [DOI] [PubMed] [Google Scholar]
- Tollervey D. A yeast small nuclear RNA is required for normal processing of pre-ribosomal RNA. EMBO J. 1987 Dec 20;6(13):4169–4175. doi: 10.1002/j.1460-2075.1987.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh-Rohlik Q., Maxwell E. S. Homologous genes for mouse 4.5S hybRNA are found in all eukaryotes and their low molecular weight RNA transcripts intermolecularly hybridize with eukaryotic 18S ribosomal RNAs. Nucleic Acids Res. 1988 Jul 11;16(13):6041–6056. doi: 10.1093/nar/16.13.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Steitz J. A. A small nucleolar RNA is processed from an intron of the human gene encoding ribosomal protein S3. Genes Dev. 1993 Jul;7(7A):1176–1190. doi: 10.1101/gad.7.7a.1176. [DOI] [PubMed] [Google Scholar]
- Vankan P., Filipowicz W. A U-snRNA gene-specific upstream element and a -30 'TATA box' are required for transcription of the U2 snRNA gene of Arabidopsis thaliana. EMBO J. 1989 Dec 1;8(12):3875–3882. doi: 10.1002/j.1460-2075.1989.tb08566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waibel F., Filipowicz W. RNA-polymerase specificity of transcription of Arabidopsis U snRNA genes determined by promoter element spacing. Nature. 1990 Jul 12;346(6280):199–202. doi: 10.1038/346199a0. [DOI] [PubMed] [Google Scholar]
- Waugh R., Clark G., Brown J. W. Sequence variation and linkage of potato U2snRNA-encoding genes established by PCR. Gene. 1991 Nov 15;107(2):197–204. doi: 10.1016/0378-1119(91)90319-7. [DOI] [PubMed] [Google Scholar]
- Waugh R., Clark G., Vaux P., Brown J. W. Sequence and expression of potato U2 snRNA genes. Nucleic Acids Res. 1991 Jan 25;19(2):249–256. doi: 10.1093/nar/19.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafarullah M., Wisniewski J., Shworak N. W., Schieman S., Misra S., Gedamu L. Molecular cloning and characterization of a constitutively expressed heat-shock-cognate hsc71 gene from rainbow trout. Eur J Biochem. 1992 Mar 1;204(2):893–900. doi: 10.1111/j.1432-1033.1992.tb16709.x. [DOI] [PubMed] [Google Scholar]
- Zagorski J., Tollervey D., Fournier M. J. Characterization of an SNR gene locus in Saccharomyces cerevisiae that specifies both dispensible and essential small nuclear RNAs. Mol Cell Biol. 1988 Aug;8(8):3282–3290. doi: 10.1128/mcb.8.8.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]