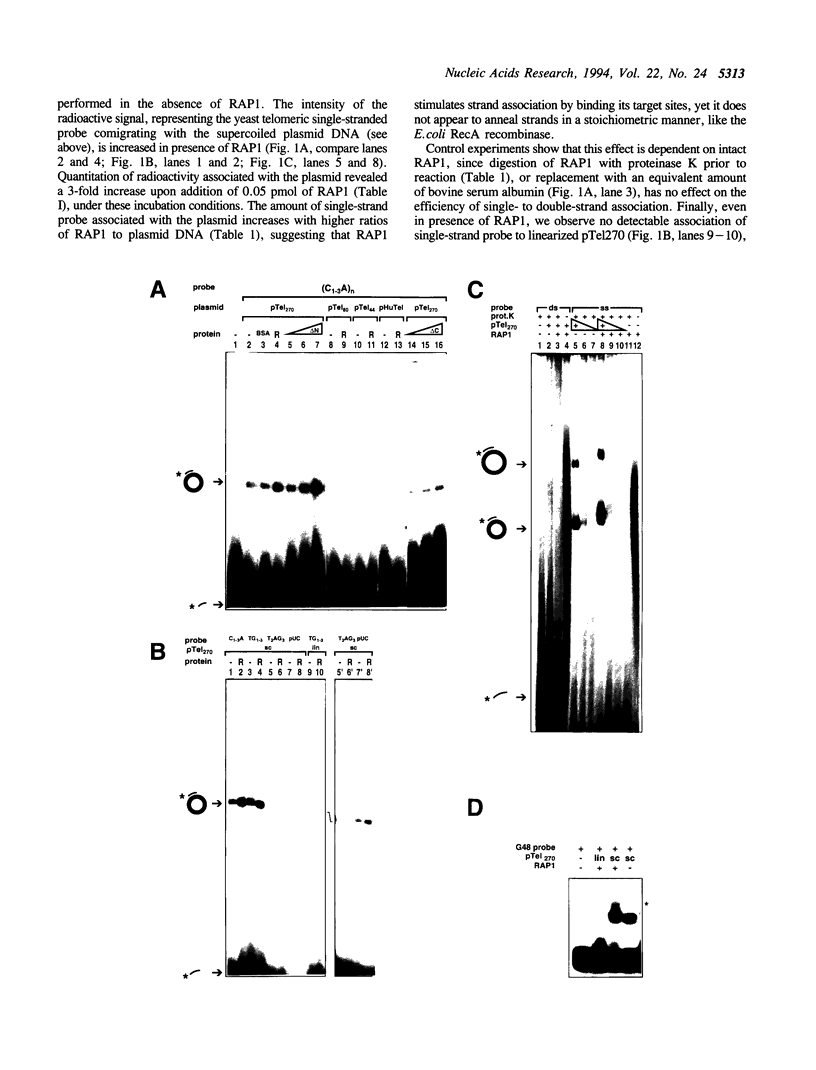
Abstract
Repressor Activator Protein 1 (RAP1) of Saccharomyces cerevisiae is an abundant nuclear protein implicated in telomere length maintenance, transactivation, and in the establishment of silent chromatin domains. The RAP1 binding site 5' of the yeast HIS4 gene is also a region of hyperrecombination in meiosis. We report here that as RAP1 binds its recognition consensus, it appears to untwist double-stranded DNA, which we detect as the introduction of a negative supercoil in circularization assays. Coincident with the RAP1-dependent untwisting, we observe stimulation of the association of a single-stranded yeast telomeric sequence with its homologous double-stranded sequence in a supercoiled plasmid. This unusual distortion of the DNA double helix by RAP1 may contribute to the RAP1-dependent enhancement of recombination rates and promote non-duplex strand interactions at telomeres.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., ten Heggeler-Bordier B., Wahli W., Stasiak A. Z., Stasiak A., Dubochet J. Direct visualization of supercoiled DNA molecules in solution. EMBO J. 1990 Dec;9(13):4551–4554. doi: 10.1002/j.1460-2075.1990.tb07907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Kintanar A., Henderson E. Human telomeric C-strand tetraplexes. Nat Struct Biol. 1994 Feb;1(2):83–88. doi: 10.1038/nsb0294-83. [DOI] [PubMed] [Google Scholar]
- Ashley T., Cacheiro N. L., Russell L. B., Ward D. C. Molecular characterization of a pericentric inversion in mouse chromosome 8 implicates telomeres as promoters of meiotic recombination. Chromosoma. 1993 Jan;102(2):112–120. doi: 10.1007/BF00356028. [DOI] [PubMed] [Google Scholar]
- Ashley T., Ward D. C. A "hot spot" of recombination coincides with an interstitial telomeric sequence in the Armenian hamster. Cytogenet Cell Genet. 1993;62(2-3):169–171. doi: 10.1159/000133464. [DOI] [PubMed] [Google Scholar]
- Belotserkovskii B. P., Krasilnikova M. M., Veselkov A. G., Frank-Kamenetskii M. D. Kinetic trapping of H-DNA by oligonucleotide binding. Nucleic Acids Res. 1992 Apr 25;20(8):1903–1908. doi: 10.1093/nar/20.8.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budarf M., Blackburn E. S1 nuclease sensitivity of a double-stranded telomeric DNA sequence. Nucleic Acids Res. 1987 Aug 11;15(15):6273–6292. doi: 10.1093/nar/15.15.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Hsieh P. Parallel DNA triplexes, homologous recombination, and other homology-dependent DNA interactions. Cell. 1993 Apr 23;73(2):217–223. doi: 10.1016/0092-8674(93)90224-e. [DOI] [PubMed] [Google Scholar]
- Devlin C., Tice-Baldwin K., Shore D., Arndt K. T. RAP1 is required for BAS1/BAS2- and GCN4-dependent transcription of the yeast HIS4 gene. Mol Cell Biol. 1991 Jul;11(7):3642–3651. doi: 10.1128/mcb.11.7.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G., Cech T. R. The beta subunit of Oxytricha telomere-binding protein promotes G-quartet formation by telomeric DNA. Cell. 1993 Sep 10;74(5):875–885. doi: 10.1016/0092-8674(93)90467-5. [DOI] [PubMed] [Google Scholar]
- Farr C., Fantes J., Goodfellow P., Cooke H. Functional reintroduction of human telomeres into mammalian cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7006–7010. doi: 10.1073/pnas.88.16.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard C., Strauss F. Association of poly(CA).poly(TG) DNA fragments into four-stranded complexes bound by HMG1 and 2. Science. 1994 Apr 15;264(5157):433–436. doi: 10.1126/science.8153633. [DOI] [PubMed] [Google Scholar]
- Gehring K., Leroy J. L., Guéron M. A tetrameric DNA structure with protonated cytosine.cytosine base pairs. Nature. 1993 Jun 10;363(6429):561–565. doi: 10.1038/363561a0. [DOI] [PubMed] [Google Scholar]
- Gilson E., Laroche T., Gasser S. M. Telomeres and the functional architecture of the nucleus. Trends Cell Biol. 1993 Apr;3(4):128–134. doi: 10.1016/0962-8924(93)90175-z. [DOI] [PubMed] [Google Scholar]
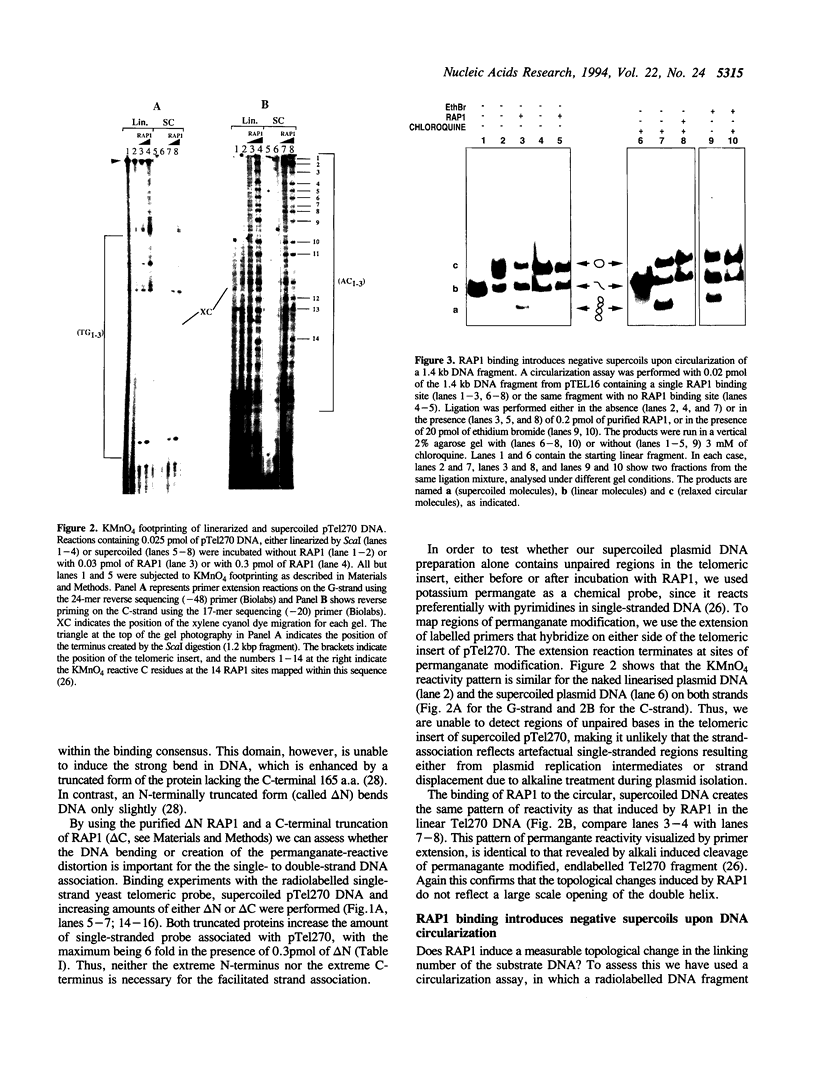
- Gilson E., Roberge M., Giraldo R., Rhodes D., Gasser S. M. Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J Mol Biol. 1993 May 20;231(2):293–310. doi: 10.1006/jmbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- Giraldo R., Rhodes D. The yeast telomere-binding protein RAP1 binds to and promotes the formation of DNA quadruplexes in telomeric DNA. EMBO J. 1994 May 15;13(10):2411–2420. doi: 10.1002/j.1460-2075.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990 Nov 16;63(4):751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Gralla J. D. Rapid "footprinting" on supercoiled DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3078–3081. doi: 10.1073/pnas.82.10.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Allshire R. C. Human telomeres: fusion and interstitial sites. Trends Genet. 1989 Oct;5(10):326–331. doi: 10.1016/0168-9525(89)90137-6. [DOI] [PubMed] [Google Scholar]
- Henderson S. T., Petes T. D. Instability of simple sequence DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Jun;12(6):2749–2757. doi: 10.1128/mcb.12.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Camerini-Otero R. D. Formation of joint DNA molecules by two eukaryotic strand exchange proteins does not require melting of a DNA duplex. J Biol Chem. 1989 Mar 25;264(9):5089–5097. [PubMed] [Google Scholar]
- IJdo J. W., Baldini A., Ward D. C., Reeders S. T., Wells R. A. Origin of human chromosome 2: an ancestral telomere-telomere fusion. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9051–9055. doi: 10.1073/pnas.88.20.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki J. E., Barnett M. A., MacCarthy A. B., Buckle V. J., Brown W. R., Porter A. C. Targeted breakage of a human chromosome mediated by cloned human telomeric DNA. Nat Genet. 1992 Dec;2(4):283–287. doi: 10.1038/ng1292-283. [DOI] [PubMed] [Google Scholar]
- Kang C., Zhang X., Ratliff R., Moyzis R., Rich A. Crystal structure of four-stranded Oxytricha telomeric DNA. Nature. 1992 Mar 12;356(6365):126–131. doi: 10.1038/356126a0. [DOI] [PubMed] [Google Scholar]
- Katinka M. D., Bourgain F. M. Interstitial telomeres are hotspots for illegitimate recombination with DNA molecules injected into the macronucleus of Paramecium primaurelia. EMBO J. 1992 Feb;11(2):725–732. doi: 10.1002/j.1460-2075.1992.tb05105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie D. A., Hultén M. A. Further studies on chiasma distribution and interference in the human male. Ann Hum Genet. 1985 Jul;49(Pt 3):203–214. doi: 10.1111/j.1469-1809.1985.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Guéron M., Mergny J. L., Hélène C. Intramolecular folding of a fragment of the cytosine-rich strand of telomeric DNA into an i-motif. Nucleic Acids Res. 1994 May 11;22(9):1600–1606. doi: 10.1093/nar/22.9.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Gilbert W. The yeast KEM1 gene encodes a nuclease specific for G4 tetraplex DNA: implication of in vivo functions for this novel DNA structure. Cell. 1994 Jul 1;77(7):1083–1092. doi: 10.1016/0092-8674(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Longtine M. S., Wilson N. M., Petracek M. E., Berman J. A yeast telomere binding activity binds to two related telomere sequence motifs and is indistinguishable from RAP1. Curr Genet. 1989 Oct;16(4):225–239. doi: 10.1007/BF00422108. [DOI] [PubMed] [Google Scholar]
- Louis E. J., Naumova E. S., Lee A., Naumov G., Haber J. E. The chromosome end in yeast: its mosaic nature and influence on recombinational dynamics. Genetics. 1994 Mar;136(3):789–802. doi: 10.1093/genetics/136.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V., Blackburn E. H. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993 Apr 23;73(2):347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Danilevskaya O. N., Voloshin O. N., Balatskaya S. V., Dobrynin V. N., Filippov S. A., Frank-Kamenetskii M. D. An unusual DNA structure detected in a telomeric sequence under superhelical stress and at low pH. Nature. 1989 Jun 22;339(6226):634–637. doi: 10.1038/339634a0. [DOI] [PubMed] [Google Scholar]
- Meyne J., Baker R. J., Hobart H. H., Hsu T. C., Ryder O. A., Ward O. G., Wiley J. E., Wurster-Hill D. H., Yates T. L., Moyzis R. K. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma. 1990 Apr;99(1):3–10. doi: 10.1007/BF01737283. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie A. I., Bowater R., Aboul-ela F., Lilley D. M. Helix opening transitions in supercoiled DNA. Biochim Biophys Acta. 1992 May 7;1131(1):1–15. doi: 10.1016/0167-4781(92)90091-d. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Claus T. E., Szostak J. W. Characterization of two telomeric DNA processing reactions in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4642–4650. doi: 10.1128/mcb.8.11.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino F., Laroche T., Gilson E., Axelrod A., Pillus L., Gasser S. M. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993 Nov 5;75(3):543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Zakian V. A. Recombination occurs during telomere formation in yeast. Nature. 1989 Feb 2;337(6206):429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- Porter S. E., White M. A., Petes T. D. Genetic evidence that the meiotic recombination hotspot at the HIS4 locus of Saccharomyces cerevisiae does not represent a site for a symmetrically processed double-strand break. Genetics. 1993 May;134(1):5–19. doi: 10.1093/genetics/134.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Pham M. H., Galas D. J. Plasmid permutation vectors to monitor DNA bending. Nucleic Acids Res. 1987 Dec 10;15(23):10060–10060. doi: 10.1093/nar/15.23.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B. J., Radding C. M. Formation of base triplets by non-Watson-Crick bonds mediates homologous recognition in RecA recombination filaments. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6161–6165. doi: 10.1073/pnas.91.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B. J., Radding C. M. Homologous recognition promoted by RecA protein via non-Watson-Crick bonds between identical DNA strands. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6646–6650. doi: 10.1073/pnas.90.14.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Gellert M., Thoma F., Maxwell A. Structure of the DNA gyrase-DNA complex as revealed by transient electric dichroism. J Mol Biol. 1987 Feb 5;193(3):555–569. doi: 10.1016/0022-2836(87)90266-x. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988 Jul 28;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Shore D., Baldwin R. L. Energetics of DNA twisting. I. Relation between twist and cyclization probability. J Mol Biol. 1983 Nov 15;170(4):957–981. doi: 10.1016/s0022-2836(83)80198-3. [DOI] [PubMed] [Google Scholar]
- Skarstad K., Baker T. A., Kornberg A. Strand separation required for initiation of replication at the chromosomal origin of E.coli is facilitated by a distant RNA--DNA hybrid. EMBO J. 1990 Jul;9(7):2341–2348. doi: 10.1002/j.1460-2075.1990.tb07406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Zakian V. A. Telomere-telomere recombination provides an express pathway for telomere acquisition. Nature. 1990 May 31;345(6274):456–458. doi: 10.1038/345456a0. [DOI] [PubMed] [Google Scholar]
- Wellinger R. J., Wolf A. J., Zakian V. A. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell. 1993 Jan 15;72(1):51–60. doi: 10.1016/0092-8674(93)90049-v. [DOI] [PubMed] [Google Scholar]
- White J. H., Gallo R. M., Bauer W. R. Closed circular DNA as a probe for protein-induced structural changes. Trends Biochem Sci. 1992 Jan;17(1):7–12. doi: 10.1016/0968-0004(92)90417-8. [DOI] [PubMed] [Google Scholar]
- White M. A., Dominska M., Petes T. D. Transcription factors are required for the meiotic recombination hotspot at the HIS4 locus in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6621–6625. doi: 10.1073/pnas.90.14.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
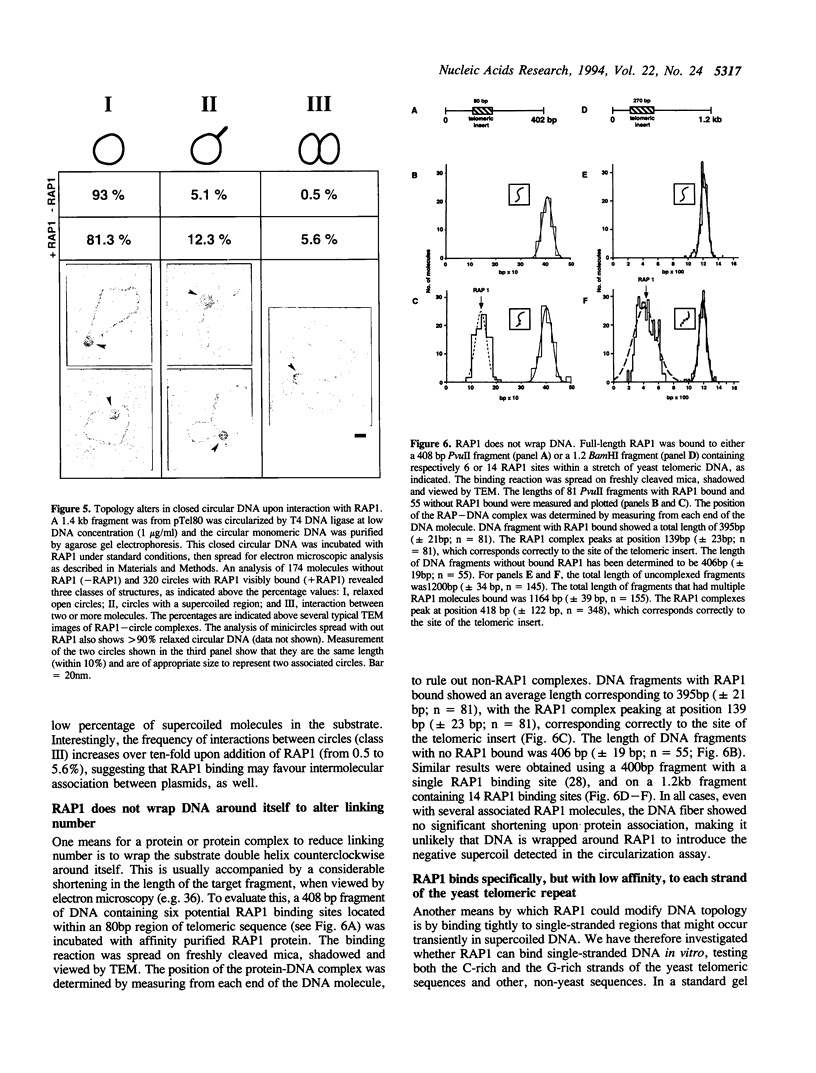
- White M. A., Wierdl M., Detloff P., Petes T. D. DNA-binding protein RAP1 stimulates meiotic recombination at the HIS4 locus in yeast. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9755–9759. doi: 10.1073/pnas.88.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]