Background: Syk is increased in effector T cells and SLE T cells and contributes to increased TCR activation-induced intracytoplasmic calcium flux.
Results: c-Jun in cooperation with Ets2 increases the transcription of SYK and the intracytoplasmic calcium flux.
Conclusion: c-Jun overexpression may cause the increase in calcium flux in SLE.
Significance: c-Jun represents an important diagnostic and treatment target for SLE.
Keywords: Autoimmunity, Signaling, T Cell, T Cell Receptor, Transcription Factors, AP-1, Ets2, SLE, Syk, c-Jun
Abstract
Effector T cells and T cells from patients with systemic lupus erythematosus (SLE) express increased levels of the spleen tyrosine kinase (Syk). Syk binds to the T cell receptor (TCR)-CD3 complex and transduces the TCR-mediated signal in the cell more efficiently than the canonical CD3ζ chain. The reasons for the increased expression of Syk are unclear. In the present study, we found that Syk is regulated by the transcription factor c-Jun in cooperation with Ets2. c-Jun and Ets2 bound to the SYK promoter in close proximity and increased the promoter activity in a specific manner. Disruption of c-Jun and Ets2 expression by siRNA resulted in decreased expression of Syk. Overexpression of c-Jun but not Ets2 resulted in increase in Syk protein. c-Jun and Ets2 co-immunoprecipitated and had an additive effect on Syk expression. c-Jun-driven SYK promoter activation showed a similar pattern in B cells; however, as expected, basal promoter activity was much higher in B cells as compared with T cells. Overexpression of c-Jun led to increase in intracytoplasmic calcium flux following TCR stimulation. Moreover, we found that SLE T cells had increased levels of c-Jun at baseline and phosphorylated c-Jun upon activation. Finally, disruption of c-Jun and Ets2 in SLE T cells resulted in a decrease in calcium flux upon TCR stimulation. In conclusion, c-Jun in cooperation with Ets2 increases the expression of Syk and contributes to Syk-mediated heightened calcium responses in SLE T cells.
Introduction
The spleen tyrosine kinase (Syk)2 is increased in total amount and level of phosphorylation in systemic lupus erythematosus (SLE) T cells and associates with the T cell receptor (TCR)-CD3 complex, serving as a main signal transducer (1). This event contributes to the hyperexcitable phenotype of SLE T cells, i.e. the robust intracytoplasmic flux of calcium following the engagement of TCR. Contrary to SLE T cells, resting T cells from healthy volunteers do not express Syk at significant levels. Once activated, however, CD4 T cells acquire an effector phenotype that, not unlike SLE T cells, is characterized by expression of Syk (2). In both SLE T cells and effector CD4 T cells, Syk binds to the FcRγ chain that replaces the CD3ζ chain as the preferred signal-transducing molecule of the TCR-CD3 complex.
The significance of this rewiring of the main signal sensor of T cells with Syk becoming its preferred signaling partner has been well established. Inhibition of Syk in SLE T cells in vitro leads to normalization of calcium flux in these cells (1). Similarly, an oral Syk inhibitor improved nephritis in two different strains of lupus-prone mice (3, 4) and showed promising results in clinical trials in rheumatoid arthritis patients (5).
Nevertheless, the mechanisms that underlie the up-regulation of Syk in both effector and SLE T cells are largely unknown. Our previous work showed that the expression of Syk in T cells (6) is controlled at the promoter level, with the transcriptional repressor cAMP-responsive element modulator-α (CREM-α) tonically preventing the transcription of SYK in normal but not in SLE T cells. We therefore hypothesized that other trans factors bind to the SYK promoter and dictate the expression of Syk in SLE T cells and effector T cells overcoming the effect of transcriptional repressors. In this study, we show that the SYK promoter contains sites that bind c-Jun and Ets2. The two transcription factors cooperate to increase the expression of Syk, contributing to higher calcium influx in T cells.
EXPERIMENTAL PROCEDURES
Patients
12 patients who fulfilled the American College of Rheumatology criteria for the diagnosis of SLE (7) donated 40–50 ml of blood for our studies. Samples were collected in heparin-lithium tubes. All but one patient were female with a mean age of 39.6 (29–60) years old. 25% of the patients were African-Americans, and 75% were white. The disease activity was calculated using the SLE disease activity index (8). At the time of the blood draw, 42% of the patients were taking oral prednisone at a mean dose of 9.5 (2.5–15) mg, 75% were taking hydroxychloroquine, and 50% were on Mycophenolate Mofetil. One patient was on tacrolimus, one was on azathioprine, and one patient was taking weekly methotrexate at the time of the study. Prednisone was held for at least 12 h prior to the blood draw. The institutional review board of Beth Israel Deaconess Medical Center approved the study protocol, and informed consents were obtained from all the study subjects.
Antibodies
Mouse monoclonal antibody against Syk (clone 4D10), c-Jun antibody (sc-74543), Ets2 antibody, goat anti-rabbit HRP, and goat anti-mouse HRP were procured from Santa Cruz Biotechnology. An antibody against phosphorylated Syk (Tyr-348) was purchased from Pharmingen. Antibody against phosphorylated c-Jun was purchased from Cell Signaling Technology (Danvers, MA). The anti-human CD3 antibody clone OKT3 was purchased from BioXcell, and anti-human CD28 was procured from BioLegend. β-Actin antibody was purchased from Sigma-Aldrich.
Expression Vectors
A SYK promoter luciferase reporter construct (product identifier 108157_CHR9_P0393_R1-SYK) that was cloned in pSGG vector (modified pGL4) was purchased from Switch Gear Genomics (Menlo Park, CA). The construct length was −782 from the transcription initiation site. pRSV-c-Jun was a gift from Michael Blanar (University of California, San Francisco ). The Rous sarcoma virus promoter/enhancer-c-Jun cassette was described before (9). Ets2 expression vector was a gift from Michael C. Ostrowski (Department of Molecular Genetics, Ohio State University, Columbus, OH) (10). Jun D cDNA in an expression vector was purchased from OriGene Technologies (Rockville, MD). CREB and c-Fos constructs were described before (11, 12).
Two deletion mutants of SYK promoter of −385 and −73 were constructed in luciferase expression vector by directional cloning of amplified PCR product using XhoI and HindIII restriction sites as linkers. The primers used in the study are −385 sense, TAACTCGAGTCCAGCACAGTAGCTTGGAGCTT and −73 sense ATTAACTCGAGCTTCAGCCGATTCCCGCCCAGCTCC in combination with an antisense primer, CGCAAGCTTTAGAACAAGAAAGGGCATGAAAGCA.
T Cell Isolation
T cells from healthy donors and SLE patients were isolated and purified from peripheral blood by using a RosetteSep kit (Stem Cell Technologies Inc.) following the manufacturer's instructions. T cells were separated from Rosetted cell complexes by Lymphoprep gradient (Fresenius Kabi Norge AS, Oslo, Norway) and then cultured in RPMI 1640 with 5% CO2 supplemented with 10% fetal bovine serum for 2 h prior to experimental use.
B Cell Isolation
B cells were isolated from peripheral blood of healthy individuals by using CD19 MicroBeads procured from Miltenyi Biotech (Cambridge, MA) following company provided protocol.
Transfection/Luciferase Experiments
5 × 106 purified T cells were resuspended in T cell Amaxa nucleofector solution (Lonza Walkersville Inc., Walkersville, MD), and transient transfection of 2.0 μg of SYK reporter plasmids was performed along with 0.5 μg of c-Jun or Ets2 cDNA in expression vector either alone or together. 50 ng of Renilla luc gene driven by the cytomegalovirus (CMV) promoter (pRL-CMV; Promega, Madison, WI) was used as an internal control in all transfection experiments. After 24 h of incubation, cells were washed with PBS and lysed with Passive Lysis Buffer, and luciferase activities were measured by injecting luciferase assay reagent (Promega) into cell extracts and recording luminescence (a 15-s light output) in a TD20/20 luminometer (Turner Biosystems, Mountain View, CA). The Renilla luciferase activity as internal control was assayed with the Dual-Luciferase reporter assay system (Promega). The relative luciferase activity was then calculated according to the formula: relative luciferase activity = firefly luciferase activity/Renilla luciferase activity.
Mutagenesis
The QuikChange site-directed mutagenesis method was performed using PfuTurbo® DNA polymerase in a temperature cycler. The basic procedure utilizes a supercoiled double-stranded DNA vector with the insert of interest and two synthetic oligonucleotide primers containing the desired mutation. The oligonucleotide primers that were used to mutate the activator protein-1 (AP-1) site were M-AP-1 sense, AGCTTGTTGGTTTGGATGTCAAGATACACTCCAGG, and M-AP-1 antisense, CCTGGAGTGTATCTTGACATCCAAACCAACAAGCT. The primers were extended during temperature cycling by PfuTurbo DNA polymerase. Incorporation of the oligonucleotide primers generated a mutated plasmid containing staggered nicks. Following temperature cycling, the product was treated with DpnI. The nicked vector DNA containing the desired mutations was then transformed into One Shot® TOP10 cells (Invitrogen) supercompetent cells. The final clone was sequenced and used in transfection. Luciferase activity was measured following the procedure as discussed before.
Western Blot
Total protein was extracted from T cells by radioimmunoprecipitation assay (RIPA) buffer (Boston BioProducts) supplemented with protease and phosphatase inhibitors (5 mm sodium fluoride (NaF), 4 mm sodium vanadate (Na3VO4), aprotinin, leupeptin, 1 mm dithiothreitol, and 1 mm 4-(2-aminoethyl) benzenesulfonyl fluoride). An equal amount of proteins was resolved on a 4–12% Bis-Tris NuPAGE precast gel at 200 V for 1 h and transferred to polyvinylidene difluoride (PVDF) membrane at 30 V for 2 h, blocked in 5% nonfat milk in Tris-buffered saline with Tween 20 (TBS-T) buffer for 3 h, incubated with primary antibody (1:1000) overnight, washed three times with TBS-T, incubated with horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibody for 3 h, washed three times with TBS-T buffer, incubated for 2–5 min with ECL reagents (Amersham Biosciences), and visualized by a Fuji LAS-3000 scanner. Respective blots were stripped, blocked again, and developed with β-actins following a similar procedure.
siRNA Transfection
Predesigned and validated siRNAs were purchased from Applied Biosystems (Carlsbad, CA): c-Jun siRNA, sense, GGCACAGCUUAAACAGAAATT, and antisense, UUUCUGUUUAAGCUGUGCCAC; Ets2 siRNA, sense, GAGCUGCUAUCAGACAAAUTT, and antisense, AUUUGUCUGAUAGCAGCUCCA); and Syk siRNA, sense, CGCUCUUAAAGAUGAGUUATT, and antisense, UAACUCAUCUUUAAGAGCGGG. Negative control siRNA-Silencer Negative Control #1 siRNA was purchased from Applied Biosystems. Two different concentrations of siRNAs were transfected by electroporation in 5 × 106 primary T cells by using Amaxa nucleofector solution as described above. siRNA-transfected cells were plated in RPMI 1640 media without any antibiotic and incubated for 72 h. Where stated, transfected cells were exposed to anti-CD3/CD28 antibodies for 30 min. Cells were lysed with RIPA buffer and analyzed by Western blots and appropriate antibodies. To visualize better Syk protein expression in T cells, 50 μg of total protein was loaded during separation on SDS-PAGE.
Chromatin Immunoprecipitation Assay (ChIP)
3 × 106 T cells were stimulated with 1 μg of anti-CD3/CD28 antibodies or left untreated and cross-linked with 1% formaldehyde for 10 min at 37 °C. Cells were washed twice with ice-cold PBS containing protease inhibitors, dissolved in SDS Lysis Buffer to obtain the appropriate number of cells/150 μl, and incubated for 10 min on ice, and DNA was sheared to lengths between 200 and 1000 bp by sonication. Sonicated DNA was diluted with ChIP dilution buffer, precleared with 30 μl of salmon sperm DNA-protein A-agarose 50% slurry for 1 h at 4 °C with agitation, and subjected to precipitation using anti-c-Jun antibody overnight. Immunoprecipitated DNA was incubated with 50 μl of salmon sperm DNA/protein A-agarose slurry for 6 h followed by gentle centrifugation (∼1000 rpm for 5 min). Protein A-agarose/antibody/protein complex was washed sequentially by low salt, high salt, LiCl immune complex, and Tris-EDTA wash buffer (Millipore, Billerica, MA). Freshly prepared elution buffer (1% SDS, 0.1 m NaHCO3) was used to elute DNA-protein complex, purified by using a QIAamp DNA purification kit (Qiagen), and analyzed by PCR. The primers used for amplification of the SYK promoter were: sense, GAGTGCTCCAGCACAGTAGCTT, and antisense, TTCATGCCCTTTCTTGTTCT.
Electrophoretic Mobility Shift Assay (EMSA)
5 pmol of double-stranded synthetic oligonucleotides encompassing one potential AP-1 site or Ets binding sites on SYK promoter was annealed and labeled with [32P]ATP by using T4 polynucleotide kinase. The primers used were as follows: AP-1 wild type probe, sense, GTTTGGTGACCAAGATACAC, and antisense: GTGTATCTTGGTCACCAAAC; AP-1 mutated probe, sense, GTTTGGTCACTAGGATACAC, and antisense, GTGTATCCTAGTGACCAAAC; Ets2 wild type probe, sense, CACTCCAGGGAATATGCCATGC, and antisense, GCATGGCATATTCCCTGGAGTG; Ets2 mutated probe, sense, CACTCCAGTTCATATGCCATGC, and antisense: GCATGGCATATGAACTGGAGTG. Briefly, 5 μg of T cell nuclear extracted protein (13) was incubated with labeled probe in the presence of binding buffer, 1 μg of poly(dI·dC), and different concentrations of cold competitor for 1 h at 20 °C. The DNA-protein complex was isolated on 6% DNA retardation gel at 110 V for 1 h. In a separate binding reaction, 2 μg of c-Jun antibody (Santa Cruz Biotechnology and BD Biosciences) was used to verify the specificity of complex. An additional binding reaction was performed with a probe where AP-1 sites or Ets binding sites were disrupted by three bases alongside the wild type probe. Gels were fixed in 10% acetic acid and 10% methanol for 10 min, dried, exposed overnight to a phosphor screen, and scanned using a PhosphorImager scanner (GE Healthcare).
Co-immunoprecipitation
10 × 106 T cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors. Immunoprecipitation was performed using specific antibody and 100 μg of total cell lysates in radioimmunoprecipitation buffer overnight. The immune complexes were collected by incubating the cell extracts with protein G-agarose beads for 5–6 h at 4C. Protein G-agarose beads were washed three times with radioimmunoprecipitation buffer supplemented with protease inhibitors and 500 mm sodium chloride and eluted by SDS protein sample buffer without any reducing agent, separated in denaturing gel, and analyzed by Western blot.
Measurement of Free Cytosolic Calcium
5 × 103 primary T cells isolated from healthy donors and SLE patients were transfected with 0.9 μm c-Jun, Ets2, Syk, and control siRNA in separate groups, and after appropriate incubation, the cells were washed with RPMI 1640 with 1% FBS followed by labeling with 1 μg/ml Indo-1 (Molecular Probes, Eugene, OR) for 30 min at 37 °C, washed with RPMI 1640, and kept on 37 °C water bath. Cells were analyzed using a BD Biosciences LSRII flow cytometer. Samples were run and at 40 s, and then either anti-CD3 (10 μg/ml) or the isotype control goat anti-mouse IgG was added; after running for another 10 s, goat anti-mouse cross-linker was added. The ratio of the violet to blue Indo-1 fluorescence, which is directly proportional to free cytosolic Ca2+, was recorded for a period of 8 min. Data were analyzed in FlowJo software (Tree Star, Inc. Ashland, OR). In a separate experiment, T cells were transfected with 0.5 μg of c-Jun in expression vector or empty vector, and cytosolic Ca2+ was measured following the same procedure as stated above.
Statistical Analysis
Statistical analyses were performed using GraphPad® Prism. Student's t test and analysis of variance were used for statistical comparisons. Results with p ≤ 0.05 were regarded as statistically significant.
RESULTS
c-Jun Binds to SYK Promoter at Position −354
Previous work (1, 2) has shown that resting T cells express low levels of Syk, but when they become activated, Syk expression increases. We postulated that transcription factors that are activated following T cell stimulation contribute to the up-regulation of Syk.
One such important factor is c-Jun, which forms the AP-1 complex either as a homodimer or as a heterodimer with c-Fos. AP-1 alone or in cooperation with other transcription factors such as nuclear factor of activated T cells (NF-AT) increases the expression of multiple pro-inflammatory genes.
We found that the promoter of SYK contains a potential AP-1 binding site at position −354 (Fig. 1A). We performed ChIP assays using nuclear lysates from normal T cells under resting conditions or following stimulation with anti-CD3 and anti-CD28 antibodies. We found that (Fig. 1B) c-Jun binds to the SYK promoter in a specific manner. We then constructed a 32P-labeled probe that mirrored the AP-1 binding site of the SYK promoter (Fig. 1C). Incubation of this probe with nuclear extracts from T cells resulted in a specific protein-DNA complex that was dissolved in the presence of anti-Jun antibody (Fig. 1C, left panel) or excess unlabeled probe (Fig. 1C, right panel). Moreover, mutation of three nucleotides in the AP-1 probe resulted in significant decrease in binding (Fig. 1D). Taken together, these findings suggest that c-Jun binds to SYK promoter at the −354 site. Of note, c-Jun binding to SYK promoter increases with stimulation of the T cells, consistent with the recognized increase in Syk expression in preactivated effector T cells.
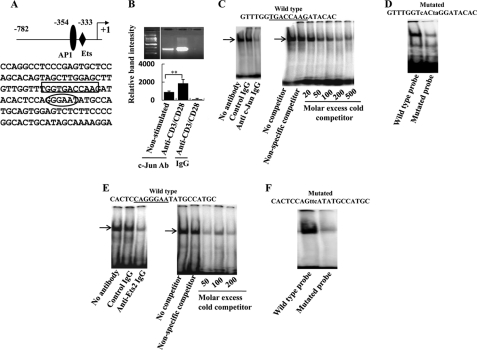
FIGURE 1.
c-Jun and Ets2 bind to the proximal SYK promoter. A, the SYK proximal promoter area is depicted here. The proposed AP-1 and Ets binding sites are marked. B, 3 × 106 normal T cells were cultured in the presence or absence of anti-CD3/CD28 for 30 min and then lysed and used for ChIP experiments with anti-c-Jun or control antibody (Ab) as described under “Experimental Procedures.” The immunoprecipitated DNA was amplified using primers specific for the SYK proximal promoter. A representative experiment and cumulative data from three independent experiments are shown here. C, resting normal T cells were lysed, and the nuclear extract was incubated with a double-stranded oligonucleotide spanning the −354 region of the proximal SYK promoter (−354 probe). In the left panel, we show an EMSA assay of the nuclear protein/−354 probe solution in the absence of antibody or in the presence of control IgG or anti-c-Jun antibodies. In the right panel, the same EMSA nuclear protein/−354 probe solution was incubated in the presence of various concentrations of nonradiolabeled −354 probe, and the autoradiographic picture is shown. D, resting normal T cells were lysed, and the nuclear extract was incubated with the −354 probe or a mutated double-stranded oligonucleotide spanning the −354 region of the proximal SYK promoter (mutated −354 probe); the autoradiographic picture is shown here. E, resting normal T cells were lysed, and the nuclear extract was incubated with a double-stranded oligonucleotide spanning the −333 region of the proximal SYK promoter (−333 probe). In the left panel, we show an EMSA assay of the nuclear protein/−333 probe solution in the absence of antibody or in the presence of control IgG or anti-Ets2 antibodies. In the right panel, the same EMSA nuclear protein/−333 probe solution was incubated in the presence of various concentrations of nonradiolabeled −333 probe, and the autoradiographic picture is shown. F, resting normal T cells were lysed, and the nuclear extract was incubated with the −333 probe or a mutated double-stranded oligonucleotide spanning the −333 region of the proximal SYK promoter (mutated −333 probe); the autoradiographic picture is shown here.
Ets2 Binds to SYK Promoter
Complexes formed with c-Jun act in cooperation with other factors to increase the transcription of several genes. In the case of SYK promoter, we noted that it contained several Ets binding sites, one of which, at position −333, was in close proximity to the −354 12-O-tetradecanoylphorbol-13-acetate response element site. We therefore postulated that Ets binds to the SYK promoter and may cooperate with c-Jun in its transcription.
We constructed Ets binding radiolabeled probes (Fig. 1E) that formed a complex with T cell-derived nuclear protein. The complex was disrupted in the presence of anti-Ets2 antibody (Fig. 1E, left panel) or excess unlabeled probe (Fig. 1E, right panel). In Fig. 1F, we show that mutation of three nucleotides in the −333 probe prevented the formation of the specific DNA-protein complex. We therefore concluded that Ets2 binds to the SYK promoter in close proximity to the c-Jun binding site.
c-Jun and Ets2 Regulate Transcription of Syk
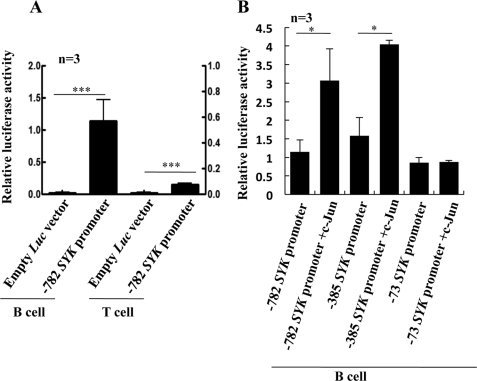
Given that c-Jun and Ets2 bind to the SYK promoter, we asked whether they regulate the expression of Syk in T cells. To this end, we transfected T cells with a luciferase construct driven by the proximal SYK promoter (see “Experimental Procedures”) together with a c-Jun expressing vector that indeed increased the expression of c-Jun (Fig. 2A, upper panel). We found that increase in c-Jun expression resulted in a 15-fold increase in luciferase activity (Fig. 2B). Mutation of the SYK promoter-luciferase construct at the −354 site attenuated but did not abolish completely the effect of c-Jun on the promoter activity. In addition to the −782 SYK promoter luciferase construct, we tested several other deletion mutants. We found that elimination of the cAMP-response element site at position −539 increased 30-fold the promoter activity with c-Jun overexpression as compared with −782 as expected based on our previous observations (6). Conversely, a shorter (starting at the −73 nucleotide) luciferase construct that did not contain the −354 site had almost no activity with c-Jun overexpression (Fig. 2C).
FIGURE 2.
c-Jun and Ets2 increase SYK promoter activity in T cells. A, overexpression of c-Jun and Ets2. Upper panel, 5 × 106 T cells from healthy donors were transfected with 1 μg of c-Jun expression vector or empty vector, and at 24 h after transfection, cells were lysed in RIPA buffer and subjected to Western blot with an antibody against total c-Jun. The lower panel is the same as the upper panel except that expression vector was used for Ets2 and Western blot was performed with Ets2 antibody. The same membranes were stripped, blocked, and reprobed with β-actin. B, 5 × 106 T cells from peripheral blood of healthy donors were transfected with wild type −782 SYK promoter (left side) or an AP-1 site mutated −782 SYK promoter construct (right side). c-Jun-overexpressing vector was used to increase c-Jun expression. After 24 h of incubation, the cells were lysed, and the luciferase activity was recorded (see “Experimental Procedures”). The mean luciferase activity ± S.E. from 10 independent experiments is shown here. C, 5 × 106 T cells from peripheral blood of healthy donors were transfected with SYK promoter of −782, −385, and −73 lengths, and c-Jun-overexpressing vector was used to increase c-Jun expression. After 24 h of incubation, the cells were lysed, and the luciferase activity was recorded (see “Experimental Procedures”). The mean luciferase activity ± S.E. from three independent experiments is shown here. CRE, cAMP-response element. D, 5 × 106 T cells from peripheral blood of healthy donors were transfected with wild type SYK promoter and c-Jun- and Ets2-overexpressing vectors or control vectors as indicated. After 24 h of incubation, the cells were lysed, and the luciferase reading was recorded. The mean luciferase activity ± S.E. from 10 independent experiments is shown here. E, 5 × 106 T cells from healthy donors were transfected with 1 μg of c-Jun expression vector or empty vector. After 24 h of incubation, the cells were lysed in RIPA buffer and subjected to Western blot with an antibody against total Syk. The band intensity was calculated using Bio-Rad Quantity One, and the ratio of Syk/actin was calculated. A representative experiment and cumulative data from three separate experiments are presented here. F, 5 × 106 T cells from peripheral blood of healthy donors transfected with −782 SYK promoter were also cotransfected with c-Jun-, JunD-, c-Fos-, and CREB-expressing vectors as indicated. After 24 h of incubation, the cells were lysed, and the luciferase activity was recorded (see “Experimental Procedures”). The mean luciferase activity ± S.E. from three independent experiments is shown here. (The error bars represent S.E.; *, p < 0.05, **, p < 0.01, ***, p < 0.001.)
Contrary to the effect of c-Jun, we observed a mild effect of Ets2 overexpression (Fig. 2A, lower panel) on SYK promoter activity (Fig. 2D). Nevertheless, overexpression of both c-Jun and Ets2 resulted in an additive effect with the luciferase activity increasing 25-fold over the activity when only c-Jun was overexpressed (Fig. 2D).
In a different set of experiments, we observed that overexpression of c-Jun greatly increased the expression of Syk protein (Fig. 2E) in T cells. Contrary to that, Ets2 overexpression did not lead to detectable Syk protein increase (data not shown). These results suggested that c-Jun binds to the −354 site of the SYK promoter and increases its activity, resulting in increased levels of Syk protein in T cells. Moreover, although Ets2 is a weak activator of SYK, it does have an additive effect with c-Jun in increasing Syk expression. We did not observe any increase in the SYK luciferase construct activity by overexpressing other members of the AP-1 family of transcription factors; namely, overexpression of c-Fos, JunD, and CREB in T cells had no effect on the promoter activity (Fig. 2F). Overexpression of c-Jun in primary B cells had also similar effect as in T cells, with an increase in the SYK promoter activity (Fig. 3B). It has to be noted that baseline SYK promoter activity was significantly higher in B cells than in T cells as expected (B cells versus T cells relative luciferase activity ± S.E.: 1.14 ± 0.32 versus 0.153 ± 0.02, Fig. 3A). In T cells, basal SYK promoter activity was ∼7-fold over empty vector, contrary to B cells, in which activity was ∼50-fold (Fig. 3A).
FIGURE 3.
c-Jun increases SYK promoter activity in B cells. A, 5 × 106 B cells and T cells were isolated from peripheral blood of healthy donors and transfected with −782 SYK promoter or empty luciferase vector. After 24 h of incubation, the luciferase activity was recorded. The mean luciferase activity ± S.E. from three independent experiments is shown here. B, 5 × 106 B cells were isolated from peripheral blood of healthy donors and transfected with SYK promoter of −782, −385, and −73 lengths, and c-Jun-overexpressing vector was used to increase c-Jun expression. After 24 h of incubation, the cells were lysed, and the luciferase activity was recorded (see “Experimental Procedures”). The mean luciferase activity ± S.E. from three independent experiments is shown here. *, p < 0.05, ***, p < 0.001.
c-Jun and Ets2 Cooperate in Regulating Expression of Syk
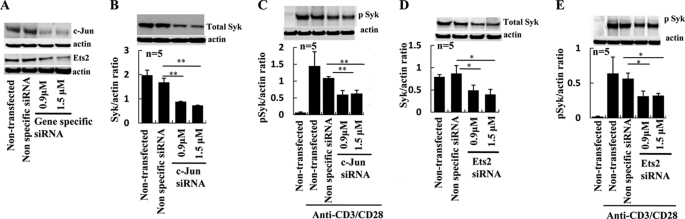
To further understand the effect of c-Jun and Ets2 in the expression and activation of Syk, we used siRNA that specifically targeted c-Jun (Fig. 4A, top panel) and Ets2 (Fig. 4A, lower panel) expression. The cells that were transfected with siRNA showed a significant (>50%) reduction in the expression of c-Jun and Ets2. T cells treated with c-Jun siRNA displayed a significant decrease in Syk expression (control versus c-Jun siRNA-treated T cells Syk/actin ratio ± S.E.: 1.68 ± 0.163 versus 0.73 ± 0.009; p ≤ 0.01, Fig. 4B). More importantly T cells treated with c-Jun siRNA showed a decrease in the expression of the activated p-Syk following TCR stimulation (control versus c-Jun siRNA-treated T cells p-Syk/actin ratio ± S.E.: 1.1 ± 0.04 versus 0.62 ± 0.09; p ≤ 0.05, Fig. 4C). Similarly, T cells transfected with Ets2 siRNA displayed decreased expression of Syk (control versus Ets2 siRNA-treated T cells Syk/actin ratio ± S.E.: 0.87 ± 0.15 versus 0.4 ± 0.11, p ≤ 0.05, Fig. 4D) and p-Syk following activation (control versus Ets2 siRNA-treated T cells p-Syk/actin ratio ± S.E.: 0.56 ± 0.07 versus 0.32 ± 0.032; p ≤ 0.05, Fig. 4E).
FIGURE 4.
Silencing of c-Jun and Ets2 in T cells abrogated Syk protein expression. A, 5 × 106 T cells were transfected with two different concentrations of c-Jun siRNA, Ets2 siRNA, or nonspecific siRNA. After 72 h, the cells were lysed in RIPA buffer and subjected to Western blot with c-Jun antibody (upper panel) and Ets2 antibody (lower panel). B, 5 × 106 T cells were transfected with two different concentrations of c-Jun siRNA or nonspecific siRNA. After 72 h, the cells were lysed in RIPA buffer and subjected to Western blot with total Syk antibody. The band density was quantified, and the Syk/actin ratio was calculated. One representative experiment and cumulative data from five independent experiments are shown here. C, 5 × 106 T cells were transfected with two different concentrations of c-Jun siRNA or nonspecific siRNA. After 72 h, the cells were treated with anti-CD3/anti-CD28 antibodies (treated) or control antibody (untreated) for 30 min. The cells were subsequently lysed in RIPA buffer, and Western blot was performed with antibody against phosphorylated Syk. The band density was quantified, and the p-Syk/actin ratio was calculated. One representative experiment and cumulative data from five independent experiments are shown here. D, 5 × 106 T cells were transfected with two different concentrations of Ets2 siRNA or nonspecific siRNA. After 72 h, the cells were lysed in RIPA buffer and subjected to Western blot with total Syk antibody. The band density was quantified, and the Syk/actin ratio was calculated. One representative experiment and cumulative data from five independent experiments are shown here. E, 5 × 106 T cells were transfected with two different concentrations of Ets2 siRNA or nonspecific siRNA. After 72 h, the cells were treated with anti-CD3/anti-CD28 antibodies (treated) or control antibody (untreated) for 30 min. The cells were subsequently lysed in RIPA buffer, and Western blot was performed with antibody against phosphorylated Syk. The band density was quantified, and the p-Syk/actin ratio was calculated. One representative experiment and cumulative data from five independent experiments are shown here. (The error bars represent S.E.; *, p < 0.05, **, p < 0.01.)
Next, we transfected resting T cells with both c-Jun and Ets2 siRNA. As can be seen in Fig. 5A, we noted a profound decrease in Syk expression in resting T cells. More importantly, following TCR stimulation, T cells transfected with c-Jun and Ets2 displayed a blunted Syk phosphorylation (Fig. 5B). Finally, in co-immunoprecipitation experiments shown in Fig. 5C, c-Jun and Ets2 co-precipitate, especially after T cell activation. It has to be noted that we used >50 μg of protein when evaluating the expression of Syk as Syk concentration is low in resting T cells. These results suggest that c-Jun and Ets2 cooperate in the transcriptional regulation of Syk.
FIGURE 5.
c-Jun and Ets2 cooperate to inhibit Syk expression. A, 5 × 106 T cells were transfected with equal concentrations of c-Jun and Ets2 siRNA or nonspecific siRNA. 72 h after transfection, the cells were lysed in RIPA buffer and used in Western blots with total Syk antibody. The band density was quantified, and the Syk/actin ratio was calculated. One representative experiment and cumulative data from five independent experiments are shown here. B, 5 × 106 T cells were transfected with equal concentrations of c-Jun and Ets2 siRNA or nonspecific siRNA. After 72 h, the cells were treated with anti-CD3/anti-CD28 antibodies (treated) or control antibody (untreated) for 30 min. The cells were then lysed and used in Western blots with p-Syk antibody. The band density was quantified, and the p-Syk/actin ratio was calculated. One representative experiment and cumulative data from five independent experiments are shown here. C, T cells were incubated with anti-CD3/anti-CD28 antibodies (treated) or control antibody (untreated). The cells were subsequently lysed with RIPA buffer supplemented with protease and phosphatase inhibitors. 100 μg of total proteins was immunoprecipitated with 4 μg of Ets-2 overnight. Immune complexes were recovered by using protein G-agarose beads, and Western blots were performed using antibody against c-Jun (upper panel). In the lower panel, the immunoprecipitation (IP) was performed using a c-Jun antibody, and the Western blot was done with Ets2 antibody. (The error bars represent S.E.; *, p < 0.05, **, p < 0.01, ***, p < 0.001.)
c-Jun and Ets2 Contribute to Syk-mediated Increase in Calcium Flux
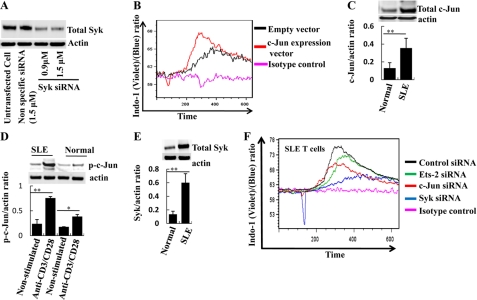
SLE T cells display increased flux of calcium following TCR stimulation. To understand whether c-Jun contributes to this observation, we transfected normal T cells with c-Jun and recorded the influx of calcium in the cytoplasm following TCR engagement. As shown in Fig. 6B, following cell activation, the c-Jun-overexpressing T cells had significantly more calcium concentration in their cytoplasm than control T cells. Conversely, we used SLE T cells to answer whether silencing c-Jun can decrease the expression of Syk and normalize the influx of calcium. T cells were isolated from patients with SLE (SLE disease activity index: 0–9). SLE T cells have higher levels of both c-Jun (control T cells versus SLE T cells: c-Jun/actin ratio ± S.E., 0.13 ± 0.06 versus 0.355 ± 0.11; p ≤ 0.01) (Fig. 6C) and phosphorylated c-Jun after anti-CD3/CD28 stimulation (control T cells versus SLE T cells: phospo-c-Jun/actin ratio ± S.E., 0.355 ± 0.040 versus 0.757 ± 0.029) (Fig. 6D). As previously published (1, 2), SLE T cells have increased Syk levels as compared with controls (control T cells versus SLE T cells; Syk/actin ratio ± S.E.: 0.12 ± 0.009 versus 0.597 ± 0.11; p ≤ 0.01) Fig. 6E). We then transfected SLE T cells with siRNA against c-Jun, Ets2, and Syk. As shown in Fig. 6F, c-Jun and Ets2 siRNA-treated SLE T cells had blunted calcium flux responses as compared with control-treated. Syk siRNA (Fig. 6A) had a more pronounced effect on calcium flux of SLE T cells. There was no apparent difference in the expression of c-Jun, phosphorylated c-Jun, or Syk between patients with different disease activities or different medication doses (data not shown). These results taken together suggest that c-Jun assisted by Ets2 plays a central role in the Syk-mediated increased calcium influx observed in SLE T cells.
FIGURE 6.
c-Jun and Ets2 influence TCR-induced calcium responses in normal and SLE T cells. A, silencing of Syk with siRNA. 5 × 106 T cells were transfected with two different concentrations of Syk siRNA and nonspecific siRNA. After 72 h after transfection, cells were lysed in RIPA buffer and subjected to Western blot with Syk antibody. B, 5 × 106 T cells were transfected with 0.5 μg of c-Jun expression vector or an empty vector for c-Jun. 18 h after transfection, the cells were labeled with Indo-1, and Ca2+ flux was measured by flow cytometer upon stimulation with anti-CD3 antibody and goat anti-mouse cross-linking IgG. A representative of four separate experiments is shown. C, T cells from 10 SLE patients and four sex/age-matched controls were lysed in RIPA buffer, and Western blot was performed with an antibody against c-Jun. The band density was quantified, and the c-Jun/actin ratio was calculated. One representative experiment and cumulative data are shown here. D, T cells from 10 SLE patients and four sex/age-matched controls were treated with anti-CD3/anti-CD28 antibodies and control antibody (untreated) for 10 min and then lysed in RIPA buffer. The extracts were used in Western blots with an antibody against phosphorylated c-Jun. The band density was quantified, and the phospho-c-Jun/actin ratio was calculated. One representative experiment and cumulative data are shown here. E, T cells from 10 SLE patients and four sex/age-matched controls were lysed in RIPA buffer, and Western blot was performed with an antibody against Syk. The band density was quantified, and the Syk/actin ratio was calculated. One representative experiment and cumulative data are shown here. F, 5 × 106 T cells were isolated from eight SLE patients and transfected with control siRNA, c-Jun, Ets2, or Syk siRNA as indicated. The transfected cells were labeled with Indo-1, and Ca2+ flux was measured after stimulation with anti-CD3 antibody and goat anti-mouse IgG. A representative experiment is shown. (The error bars represent S.E.; *, p < 0.05, **, p < 0.01, ***, p < 0.001.)
DISCUSSION
SLE and effector T cells express Syk in association with FcRγ chain that substitutes for CD3ζ-Zap-70 duet as the main signal transducer of the TCR-CD3 molecular complex. This rewiring of the T cell receptor empowers the T cells to sense and react vigorously to antigenic triggers. In SLE, several lines of evidence suggest that TCR rewiring is of pathophysiologic importance. First, reconstitution of the expression of CD3ζ in SLE T cells results in reversal of the inappropriate calcium flux and cytokine secretion (14). Second, inhibition of Syk attenuates the heightened calcium flux in SLE T cells (1). Therefore, understanding the pathophysiologic mechanisms that lead to TCR rewiring is of major interest.
Following our previous work on the regulation of the SYK promoter by cAMP-responsive element modulator-α (6) in T cells, we found that c-Jun increases the expression of Syk. This effect is augmented in the presence of Ets2. Perturbation of the expression of c-Jun and Ets2 resulted in changes in T cell responsiveness, as exhibited by reduced anti-CD3-induced intracytoplasmic calcium flux. Moreover, we showed for the first time that altered expression and activation of c-Jun in SLE T cells contributes to Syk-mediated increased calcium flux.
The reason for the increase in c-Jun in SLE T cells is unknown. c-Jun resides in the cytoplasm of resting T cells and becomes phosphorylated by Jun-N-terminal kinases (JNK) (reviewed in Ref. 15). Phosphorylated c-Jun associates with c-Fos, ATF2, or other Jun molecules to form AP-1 complexes that are potent pro-inflammatory gene activators. In addition, AP-1 binds to JUN promoter, increasing its expression in a positive feedback manner (16).
The most plausible explanation for the increased c-Jun in SLE T cells is increased JNK activity. JNK phosphorylation, which mirrors JNK activation status, has been reported as increased in SLE patients with active disease versus controls and patients with inactive SLE (17). JNK gets activated by several mechanisms including CD3/CD28 stimulation, cytokines such as TNF-α, and physical stimuli such as UV light (18). In SLE, repeated cycles of T cell activation, abnormal cytokine milieu, and/or UV light may explain the JNK and c-Jun activation. In the present study, the low number of patients limited us from exploring potential correlations between the expression of c-Jun and SLE disease activity, medications, or autoantibody profile. Larger studies following patients over time will be needed to establish a temporal relation between SLE flares and increased expression/phosphorylation of c-Jun.
Besides leading to increased Syk expression and transforming T cells into effector T cells, c-Jun activation plays a role in T cell polarization. It is established that Jun N-terminal kinases influence T cell polarization into Th1/Th2 effector cells (19). Specifically, JNK positively influences the production of Th1 cytokines while inhibiting the production of Th2 cytokines as exhibited by in vitro activation/differentiation of dnJNK1+Jnk2−/− cells. It is therefore possible that altered c-Jun activation polarizes T cells toward a Th1 program, and therefore enhanced production of IFN-γ, which is a major contributor to lupus nephritis.
In conclusion, herein we showed for the first time that c-Jun in cooperation with Ets2 regulates the expression of Syk in T cells and contributes to the abnormal early signaling events in SLE T cells. Future studies will address the usefulness of targeting the JNK/c-Jun pathway in SLE for diagnosis and/or treatment.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 AI42269 (to G. C. T.) and K23 AR055672 (to V. C. K.).
- Syk
- spleen tyrosine kinase
- SLE
- systemic lupus erythematosus
- TCR
- T cell receptor
- AP-1
- activator protein-1
- CREB
- cAMP-response element-binding protein
- RIPA
- radioimmunoprecipitation assay
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol
- p-SyK
- phospho-Syk.
REFERENCES
- 1. Krishnan S., Juang Y. T., Chowdhury B., Magilavy A., Fisher C. U., Nguyen H., Nambiar M. P., Kyttaris V., Weinstein A., Bahjat R., Pine P., Rus V., Tsokos G. C. (2008) Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J. Immunol. 181, 8145–8152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krishnan S., Warke V. G., Nambiar M. P., Tsokos G. C., Farber D. L. (2003) The FcR γ-subunit and Syk kinase replace the CD3 ζ-chain and ZAP-70 kinase in the TCR signaling complex of human effector CD4 T cells. J. Immunol. 170, 4189–4195 [DOI] [PubMed] [Google Scholar]
- 3. Bahjat F. R., Pine P. R., Reitsma A., Cassafer G., Baluom M., Grillo S., Chang B., Zhao F. F., Payan D. G., Grossbard E. B., Daikh D. I. (2008) An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum. 58, 1433–1444 [DOI] [PubMed] [Google Scholar]
- 4. Deng G. M., Liu L., Bahjat F. R., Pine P. R., Tsokos G. C. (2010) Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum. 62, 2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinblatt M. E., Kavanaugh A., Burgos-Vargas R., Dikranian A. H., Medrano-Ramirez G., Morales-Torres J. L., Murphy F. T., Musser T. K., Straniero N., Vicente-Gonzales A. V., Grossbard E. (2008) Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 58, 3309–3318 [DOI] [PubMed] [Google Scholar]
- 6. Ghosh D., Kis-Toth K., Juang Y. T., Tsokos G. C. (2012) CREM-α suppresses spleen tyrosine kinase expression in normal but not systemic lupus erythematosus T cells. Arthritis Rheum. 64, 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hochberg M. C. (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725. [DOI] [PubMed] [Google Scholar]
- 8. Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. (1992) Derivation of the SLEDAI: a disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 35, 630–640 [DOI] [PubMed] [Google Scholar]
- 9. Cross J. C., Wen P., Rutter W. J. (1993) Transactivation by hepatitis B virus X protein is promiscuous and dependent on mitogen-activated cellular serine/threonine kinases. Proc. Natl. Acad. Sci. U.S.A. 90, 8078–8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang B. S., Hauser C. A., Henkel G., Colman M. S., Van Beveren C., Stacey K. J., Hume D. A., Maki R. A., Ostrowski M. C. (1996) Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol. Cell. Biol. 16, 538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manna P. R., Eubank D. W., Lalli E., Sassone-Corsi P., Stocco D. M. (2003) Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP-response element-binding protein and steroidogenic factor 1. J. Mol. Endocrinol. 30, 381–397 [DOI] [PubMed] [Google Scholar]
- 12. Manna P. R., Eubank D. W., Stocco D. M. (2004) Assessment of the role of activator protein-1 on transcription of the mouse steroidogenic acute regulatory protein gene. Mol. Endocrinol. 18, 558–573 [DOI] [PubMed] [Google Scholar]
- 13. Dignam J. D., Lebovitz R. M., Roeder R. G. (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nambiar M. P., Fisher C. U., Warke V. G., Krishnan S., Mitchell J. P., Delaney N., Tsokos G. C. (2003) Reconstitution of deficient T cell receptor ζ-chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis Rheum. 48, 1948–1955 [DOI] [PubMed] [Google Scholar]
- 15. Manning A. M., Davis R. J. (2003) Targeting JNK for therapeutic benefit: from junk to gold? Nat. Rev. Drug Discov. 2, 554–565 [DOI] [PubMed] [Google Scholar]
- 16. Rozek D., Pfeifer G. P. (1993) In vivo protein-DNA interactions at the c-jun promoter: preformed complexes mediate the UV response. Mol. Cell. Biol. 13, 5490–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molad Y., Amit-Vasina M., Bloch O., Yona E., Rapoport M. J. (2010) Increased ERK and JNK activities correlate with disease activity in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 69, 175–180 [DOI] [PubMed] [Google Scholar]
- 18. Adler V., Schaffer A., Kim J., Dolan L., Ronai Z. (1995) UV irradiation and heat shock mediate JNK activation via alternate pathways. J. Biol. Chem. 270, 26071–26077 [DOI] [PubMed] [Google Scholar]
- 19. Dong C., Yang D. D., Tournier C., Whitmarsh A. J., Xu J., Davis R. J., Flavell R. A. (2000) JNK is required for effector T cell function but not for T cell activation. Nature 405, 91–94 [DOI] [PubMed] [Google Scholar]