Background: Wnt signaling blocks adipocyte development and is implicated in diabetes and the metabolic syndrome.
Results: Wnt stimulates insulin mediators via an insulin/IGF-1 receptor-dependent process; conversely, Wnt co-receptor LRP5 is essential to normal insulin signaling in preadipocytes.
Conclusion: Insulin and Wnt signaling pathways interact and are both dependent on LRP5.
Significance: Altered Wnt/LRP5 activity can play a role in obesity and insulin resistance.
Keywords: Adipogenesis, Insulin, Insulin-like Growth Factor (IGF), Signal Transduction, Wnt Signaling, LDL Receptor-related Protein-5 (LRP-5), Insulin Action, Signaling Cross-talk
Abstract
Disturbed Wnt signaling has been implicated in numerous diseases, including type 2 diabetes and the metabolic syndrome. In the present study, we have investigated cross-talk between insulin and Wnt signaling pathways using preadipocytes with and without knockdown of the Wnt co-receptors LRP5 and LRP6 and with and without knock-out of insulin and IGF-1 receptors. We find that Wnt stimulation leads to phosphorylation of insulin signaling key mediators, including Akt, GSK3β, and ERK1/2, although with a lower fold stimulation and slower time course than observed for insulin. These Wnt effects are insulin/IGF-1 receptor-dependent and are lost in insulin/IGF-1 receptor double knock-out cells. Conversely, in LRP5 knockdown preadipocytes, insulin-induced phosphorylation of IRS1, Akt, GSK3β, and ERK1/2 is highly reduced. This effect is specific to insulin, as compared with IGF-1, stimulation and appears to be due to an inducible interaction between LRP5 and the insulin receptor as demonstrated by co-immunoprecipitation. These data demonstrate that Wnt and insulin signaling pathways exhibit cross-talk at multiple levels. Wnt induces phosphorylation of Akt, ERK1/2, and GSK3β, and this is dependent on insulin/IGF-1 receptors. Insulin signaling also involves the Wnt co-receptor LRP5, which has a positive effect on insulin signaling. Thus, altered Wnt and LRP5 activity can serve as modifiers of insulin action and insulin resistance in the pathophysiology of diabetes and metabolic syndrome.
Introduction
Insulin signal transduction involves a complex network of diverging and converging pathways (1). In vivo, as well as in vitro, these pathways are modified and regulated by cross-talk with other signaling systems. One signaling system that interacts and modulates the insulin signaling network is the Wnt signaling pathway.
The Wnt signaling system in mammals involves 19 Wnt ligands, 10 Wnt receptors called Frizzleds (Fzs), and a family of co-receptors including low density lipoprotein receptor-related proteins 5 and 6 (LRP5 and LRP6)3 (2, 3). Following activation Wnt signaling proceeds along two pathways: the canonical classical Wnt pathway and the atypical pathway (2, 4). The canonical pathway involves activation of Fzs and LRP5/6 followed by inhibition of GSK3β, which results in stabilization and increased levels of β-catenin. Cytoplasmic β-catenin then translocates to the nucleus where it works as a co-factor in conjunction with lymphoid enhancer factor/T cell factor transcription factors to regulate the transcription of Wnt target genes (4–7). The atypical Wnt signaling pathway is more poorly characterized but involves activation of Fzs and the atypical Wnt receptors Ryk and Ror, leading to regulation of cell polarity and calcium signaling (8). Canonical and noncanonical Wnt ligands link the various Fzs to different pathways but compete for the same Fzs receptor activation sites, thereby having the potential to inhibit each other's actions (9).
Wnt signaling is crucial for healthy development. Among its many actions, in vivo Wnt signaling promotes bone and muscle formation and blocks the development of fat (10–15). In vitro studies show that the latter effect is due to Wnt acting as a molecular switch keeping preadipocytes in an undifferentiated state (16). Unbalanced Wnt signaling also plays a role in cancer and has been associated with an increased risk of diabetes (4, 17). The latter includes a strong association between genetic variants in a downstream target in the Wnt signaling pathway, the transcription factor TCF7L2, and increased risk of type 2 diabetes (18–21). Some of this effect on the risk of diabetes has been attributed to effects of Wnt signaling on β-cell proliferation, acting both directly and by up-regulation of GLP-1 expression (22–24). Other proteins in the Wnt signaling pathway have also been shown to have up-regulated expression in type 2 diabetic islets (25). Finally, polymorphisms and mutations in various Wnts and the Wnt co-receptors LRP5 and LRP6 have been associated with increased risks of obesity, osteoporosis, and the metabolic syndrome (4, 13, 26–29). Possible effects of Wnt signaling on insulin action have received less attention.
Cross-talk between the insulin and Wnt signaling pathways has been suggested to occur at different levels and in different cellular contexts. It is well known that the action of glycogen synthase kinase-3β (GSK3β) is inhibited by phosphorylation of the enzyme on serine 9 following insulin activation (1, 30). GSK3β is also inhibited in response to Wnt stimulation, but in this case, the mechanism is less clear (31). Interestingly, both pathways appear to lead to activation of the mTOR signaling pathway and regulate translation (32). β-Catenin has also been shown to accumulate in response to insulin in intestinal L cells (22, 33), and both insulin and insulin-like growth factor 1 (IGF-1) stimulation activate the β-catenin signaling pathway in hepatoma cells (34). In human embryonic kidney cells, however, insulin stimulation was found to have no effect on β-catenin accumulation (30), suggesting that insulin action on β-catenin is cell type-specific, and whether insulin signaling in general leads to nuclear β-catenin translocation remains to be debated. FOXO transcription factors, which are known to be regulated by insulin signaling, have been shown to compete with Wnt-regulated transcription factors for available β-catenin in control of gene transcription (28, 35). In myotubes, activation of Wnt signaling appears to increase effects of insulin on glucose transport via activation of the Akt and AMPK pathways (36). Furthermore, using stable isotope labeling, we have previously shown that some of the Wnt co-receptors are tyrosine-phosphorylated following stimulation of preadipocytes with either insulin or IGF-1 (37).
In the light of these data indicating cross-talk between the Wnt and insulin signaling pathways and the evidence of a role for Wnt signaling in regulation of adipocyte development, we have investigated the impact of Wnt signaling on insulin signal transduction and adipocyte differentiation. We have especially focused on the role of Wnt co-receptors LRP5 and LRP6, which are known to be important in initiation of the Wnt signal (38). LRP6 has been shown to be essential for embryonic development (39), whereas LRP5 KO mice appear relatively normal at birth but have low bone mass and altered vascularization of the eye because of a failure of macrophage-mediated apoptosis (40). A similar phenotype is found in patients with mis-sense mutations in the LRP5 gene (41). LRP5 KO mice also have impaired glucose tolerance and increased plasma cholesterol levels when fed a high fat diet (42).
In the present study, we show that insulin and IGF-1 receptors are required for Wnt signaling effects on Akt, GSK3β, and ERK1/2 and that LRP5 is required for normal insulin signaling in preadipocytes. Furthermore, LRP5 co-immunoprecipitates in an inducible manner with the insulin receptor, which was not the case for LRP5/IGF1 receptor association. These data indicate the importance of direct cross-talk between insulin and Wnt signaling at the receptor/co-receptor level and define a feature of differential signaling between insulin and IGF-1 receptors.
EXPERIMENTAL PROCEDURES
Antibodies
Primary antibodies anti-LRP6 (antibody 3395), anti-pLRP6 Ser-1490 (antibody 2568), anti-pGSK3β Ser-9 (antibody 9336), anti-pAkt Ser-473 (antibody 9271), anti-pERK1/2 Thr-202/Tyr-204 (antibody 9101), anti-pmTOR Ser-2481 (antibody 2974), anti-pp70S6kinase Thr-421/424 (antibody 9204), and anti-pMEK Ser-217/221 (antibody 9121) were purchased from Cell Signaling. Anti-LRP5 (C-20 sc-21390), anti-β-catenin (H102 sc-7199), anti-IR β-subunit (C19 sc-711), and anti-IGF-1 receptor (IGF1R) β-subunit (C20 sc-713) were purchased from Santa Cruz. Anti-pIRS1 Tyr-612 (antibody 44–816) was from Invitrogen, and anti-phosphotyrosine antibody 4G10 (antibody 05-321) was from Millipore. Secondary antibodies: HRP-conjugated anti-mouse (NA934V) and anti-rabbit (NA931V) were from GE Healthcare. HRP-conjugated anti-goat (sc-2350) and HRP-conjugated actin (sc-1616) were from Santa Cruz.
3T3-L1 Cell Culture and Manipulation
3T3-L1 cells were maintained in standard DMEM containing 10% FBS, streptomycin-penicillin, and Normocin (InvivoGen) at 37 °C in a humidified 5% CO2 atmosphere. Stable KD LRP5 and LRP6 cell lines were created using a lentiviral vector approach and shRNAs purchased from Open Biosystems (RMM4534-NM_001106321 and RMM4534-NM_001107892). In brief, construction of the KD cell lines involved co-transfection of nonconfluent 293 FT packaging cells with pLKO (shRNA containing plasmid), pmpAX2 plasmid, and pmp2G plasmid using TransIT-express transfection reagent (Mirus) adding sodium butyrate (final concentration, 10 mm) after 4 h. The medium was changed the following morning to DMEM containing 20 mm HEPES. After 2 additional days, the lentivirus was collected and used to infect 40% confluent 3T3-L1 preadipocytes. 48 h after infection, the cells were changed to medium containing puromycin (2.5 μg/ml; Sigma) to select cells expressing the shRNA of interest. The cells were kept in the selection medium containing puromycin until right before the beginning of an experiment. A control cell line was made in parallel using a scrambled shRNA sequence. Importantly, LRP5 and LRP6 KD cell lines were created twice to confirm key experiments.
Stimulation Studies of 3T3-L1 Cells
The cells were plated in 6-well plates and used for experiments when grown to confluence or after differentiating for a selected time period. Prior to any stimulation experiment, the cells were serum-starved for 3 h (with 0.5% FBS added to the medium). The cells were then stimulated with either 100 nm human insulin (Sigma), 100 nm human IGF-1 (Peprotech), or 75 ng/ml mouse Wnt3a (R & D Systems). At the end of stimulation, the medium was aspirated, and ice-cold PBS was added, after which plates were kept on ice until extraction. Stimulations of LRP5 and LRP6 KD cells were always performed in parallel at the same passage number, and independent stimulation studies were performed three times. The stimulation dose of 75 ng/ml Wnt3a was chosen after doing an initial dose-response experiment of β-catenin accumulation. Statistical significance was determined by Student's t test analysis.
Stimulation Studies of IR-KO, IGF1R-KO, IR/IGF1R-KO Brown Preadipocytes
Brown preadipocytes lacking either the IR, IGF1R, or both receptors were created as previously described (43, 44). The cells were plated in 6-well plates and used for experiments when grown to confluence. The cells were serum-starved for 3 h and then treated with either 100 nm human insulin (Sigma), 75 ng/ml mouse Wnt3a (R & D Systems), or serum for 5, 15, or 30 min. At the end of stimulation, the medium was aspirated, and ice-cold PBS was added, after which plates were kept on ice until protein extraction. The experiments were performed in the different KO cell lines using IRlox, IGFRlox, IRlox/IGFRlox, or wild type cell lines as a control. The data obtained using the different control cell lines showed similar results and were therefore pooled and referred to as WT throughout the manuscript. Statistical significance was determined by Student's t test analysis.
Adipocyte Differentiation
Preadipocytes were grown to confluence in 6-well plates and left for 1 additional day before adding an induction mixture to the medium containing 100 nm insulin, 500 μm isobutylmethylxanthine, 10 μm dexamethasone, and 1 μm rosiglitazone (day 0). After 48 h (day 2), the cells were kept in medium containing 100 nm insulin, and after 2 additional days (day 4), the cells were maintained in 10% FBS DMEM without supplements. At day 7, the cells were fixed with 10% formaldehyde (Sigma) and then stained with a filtered oil red O solution (Sigma) for at least 1 h to assess differentiation. The cells were washed in water and left to dry.
Western Blot Analysis and Immunoprecipitation
Total cell protein extracts were made using radioimmune precipitation assay (Upstate) extraction buffer containing NaF (1 mm), protease inhibitor mixture, and phosphatase inhibitor cocktails 1 and 2 (all from Sigma). Total protein concentrations were determined by Bradford assay, and ∼10 μg of protein was loaded on 4–12% gradient gels from Invitrogen. Proteins were transferred to a PVDF membrane (Thermo Scientific) either overnight at 30 V or for 100 min at 100 V. The membranes were blocked in Starting Block (Pierce) for at least 30 min before the addition of primary antibody. All of the primary antibodies were used at a 1:1000 dilution except for anti-LRP5, which was diluted 1:500. All secondary antibodies were diluted 1:5000. Proteins were visualized using Supersignal West Pico (Pierce) or Immobilon Western Chemiluminescent Substrate (Millipore). The intensity of protein bands was quantified using the Image J software.
For immunoprecipitation 400 μg of total protein in a volume of 500 μl was incubated overnight with LRP5 antibody (1:50) or LRP6 antibody (1:200). 30 μl of A/G Plus agarose beads (Santa Cruz sc-2003) were subsequently added for another 45 min. The beads were washed in extraction buffer, and the samples were boiled for 10 min in Laemmli buffer before being loaded to protein gels for further analysis.
Analysis of Gene Expression by Quantitative RT-PCR
Total RNA was isolated using the RNeasy Mini kit from Qiagen. 1 μg of RNA was used for reverse transcription using the high capacity cDNA reverse transcription kit from Applied Biosystems. cDNA was diluted 1:20 and used for quantitative PCR employing gene-specific primers and SYBR Green reaction mix from Fermentas. Fluorescence was monitored and analyzed in an ABI Prism 7900 HT sequence detection system (Applied Biosystems). Relative expression for each condition was calculated using expression of Tbp (TATA box-binding protein) as the endogenous control.
RESULTS
Insulin and Wnt Stimulation Leads to Phosphorylation of the Same Signaling Proteins but with Different Magnitude and Kinetics
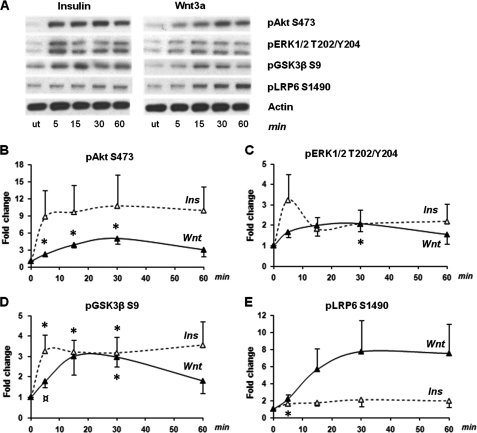
Previous studies have shown that both insulin and Wnt signaling lead to inhibition of GSK3β activity but via different mechanisms (1, 30–32). Interestingly, insulin stimulation induces serine 9 phosphorylation of GSK3β, which has been found not to be the case for Wnt activation in human embryonic kidney cells and other cell types (1, 30). In 3T3-L1 preadipocytes, both insulin and Wnt3a stimulation lead to phosphorylation of GSK3β on serine 9, as well as phosphorylation of Akt on serine 473 and ERK1/2 on threonine 202 and tyrosine 204 (Fig. 1, A–D, and supplemental Fig. S1). However, in each case Wnt3a-stimulated phosphorylation reached a lower maximum level and peaked at a later time point than seen after insulin stimulation, suggesting that the phosphorylation in response to Wnt3a stimulation acts through a different pathway and may be a secondary, rather than a primary, effect. As expected, stimulation of 3T3-L1 cells with Wnt3a also resulted in phosphorylation of LRP6 on serine 1490, which reached a plateau after 30 min with an 8-fold increase (Fig. 1, A and E). Insulin had a minor but significant effect on LRP6 serine phosphorylation after 5 min (Fig. 1E), whereas treatment of cells with insulin and Wnt3a in combination slowed the rate of LRP6 serine phosphorylation (supplemental Fig. S2).
FIGURE 1.
Insulin and Wnt phosphorylation time courses in 3T3-L1 preadipocytes. Preadipocytes were treated with either 100 nm insulin (Ins) or 75 ng/ml Wnt3a in parallel at various time points for 60 min. A, representative Western blots and phosphorylation levels of Akt (serine 473), ERK1/2 (threonine 202 and tyrosine 204), GSK3β (serine 9), and LRP6 (serine 1490). B–E, band intensities were determined using the Image J software, and fold changes compared with untreated cells were calculated for all proteins. The error bars show S.E. (n = 3). * indicates a p value < 0.05 for basal versus stimulated values. ¤ indicates a p value < 0.06 for basal versus stimulated values. Total protein levels for each insulin and Wnt3a time point can be seen in supplemental Fig. S1. ut, untreated.
Wnt-induced Akt Phosphorylation Is Insulin/IGF-1 Receptor-dependent
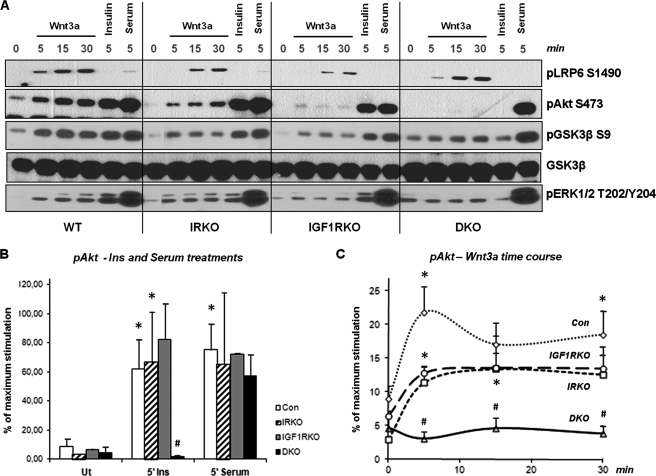
To determine whether Wnt-stimulated phosphorylation of proteins in the insulin signaling cascade is dependent on the insulin/IGF-1 receptors, we performed Wnt and insulin stimulation studies in brown preadipocyte cell lines lacking either the insulin receptor (IR), the IGF-1 receptor (IGF1R), or both receptors (double knock-out (DKO) cells) (43, 44) (Fig. 2). Wnt3a-stimulated phosphorylation of LRP6 was comparable in all cell lines, indicating intact canonical Wnt signaling in these cells. By comparison, Wnt3a-stimulated phosphorylation of Akt and to a lesser extent GSK3β and ERK1/2 were reduced in the single receptor knock-out cells and almost completely absent in the DKO cells (Fig. 2A). In contrast, the insulin response was lost only in the DKO cells, and all of the cell lines were fully responsive to serum stimulation of Akt, GSK3β, and ERK1/2 phosphorylation (Fig. 2, A and B). The data on Wnt3a-induced Akt phosphorylation are quantitated in Fig. 2C and show that the single receptor KO cells had a compromised Wnt3a response, whereas the DKO cells lost their Wnt3a response altogether.
FIGURE 2.
Phosphorylation response in Wnt stimulated IR/IGF1R knock-out cells. Brown preadipocytes lacking either the IR, the IGF1R, or both (DKO) were treated with 75 ng/ml Wnt3a for various time points, as well as 100 nm insulin and serum for 5 min. A, phosphorylation levels of LRP6, Akt, GSK3β, and ERK1/2 were determined by Western blot analysis. The band intensities for the phosphorylation of Akt were determined using Image J software, and the percentage of the maximum stimulation was calculated for all time points in the various cell lines. B, Akt phosphorylation in response to insulin and serum treatments for 5 min. The error bars show S.D. (n = 2–4). C, Akt phosphorylation time course in response to Wnt3a. The error bars show S.E. (n = 3–4). * indicates a p value < 0.05 for basal versus stimulated values within one cell line. # indicates a p value < 0.05 for differences between control cells and a KO cell line for a given treatment and time point. Con, control; Ut, untreated.
LRP5 and LRP6 Knockdown Cell Lines Show Impaired Differentiation and Still Respond to Wnt Inhibition of Adipogenesis
LRP5 and LRP6 are co-receptors that work together with the Fz receptors to initiate the Wnt signaling response (38). LRP6 has also been shown to be present in phosphotyrosine immunoprecipitates after insulin stimulation using a global proteomic approach (37), although direct links between the LRP family members and insulin signaling have not been studied.
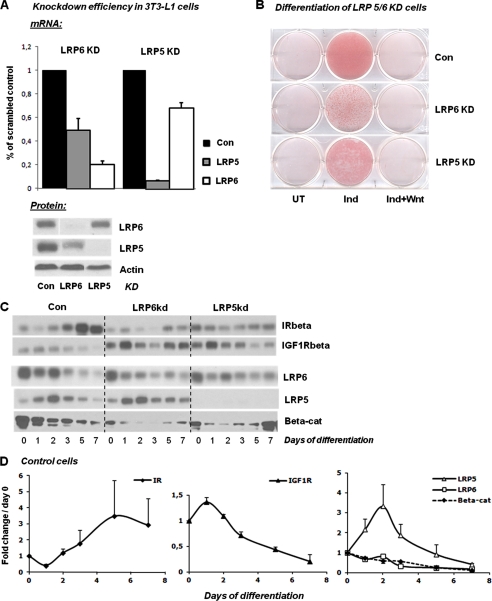
To investigate the role of these LRPs in insulin action, we created 3T3-L1 cell lines with stable KD of LRP5 and LRP6 using a lentiviral shRNA approach. This resulted in a 93% KD of LRP5 at mRNA level and an almost complete loss of LRP5 protein (Fig. 3A). The KD of LRP6 was less efficient and less stable through cell passage. Thus, immediately after selection of KD cells, there was an ∼80% reduction in LRP6 mRNA, and LRP6 protein was almost nondetectable; however, after serial passage, the LRP6 protein levels began to increase, such that the knockdown of LRP6 was on average less effective than that of LRP5.
FIGURE 3.
Knockdown efficiency and adipocyte differentiation of control, LRP5 KD, and LRP6 KD cells. Knockdown cell lines were created as stated under “Experimental Procedures” using a lentiviral shRNA approach. A control cell line, expressing a shRNA with a scrambled sequence, was created in parallel with the KD cells and is labeled Con. A, mRNA and protein levels of LRP5 and LRP6 in 3T3-L1 LRP5 and LRP6 KD preadipocytes as observed immediately after puromycin withdrawal (n = 2). B, the level of differentiation was determined for each cell line by oil red O staining at day 7 in untreated cells (UT), the cells were given a standard induction mixture (Ind), and the cells were given the induction mixture plus Wnt3a at 75 ng/ml (Ind+Wnt). C, protein levels of receptors and β-catenin were determined by Western blotting at different days of differentiation in all cell lines. D, band intensities for protein levels in control cells were determined using the Image J software, and fold changes compared with day 0 were calculated (n = 2). The error bars show S.D. Con, control; UT, untreated; Beta-cat, β-catenin.
Because stimulation of the Wnt/β-catenin signaling pathway is known to block the process of adipocyte differentiation (16), we expected that reduced levels of LRP5 and LRP6 might lead to an increase in preadipocyte differentiation and fat accumulation. To our surprise, the opposite effect was observed in the LRP5 and LRP6 KD cells. Thus, both LRP5- and LRP6-deficient 3T3-L1 cells had decreased fat accumulation when induced to differentiate compared with the control cells as demonstrated by reduced oil red O staining (Fig. 3B). Furthermore, although there was a marked decrease in LRP6 and especially LRP5 in these cells, treatment with Wnt3a was still able to completely block the ability of the KD 3T3-L1 cells to differentiate, indicating that this effect of Wnt signaling is largely independent of these important co-receptors.
As expected, in the control 3T3-L1 cells, IR expression increased, whereas IGF1R decreased during the process of differentiation. By comparison, the components of the Wnt signaling system showed complex patterns of expression (Fig. 3, C and D). Thus, LRP6 and β-catenin expression steadily decreased during differentiation, whereas the levels of LRP5 increased more than 3-fold during early stages of differentiation and then decreased, falling below basal levels at approximately day 7. These results suggest that LRP5 and LRP6 play different roles in the differentiation process.
In the LRP5 and LRP6 KD cells, expression of the IR was reduced. Conversely, the expression of the IGF1R was increased in the LRP6 KD cells as compared with control cells, albeit with a high level of fluctuation throughout the differentiation process (Fig. 3C). β-Catenin levels were decreased in both KD cell lines from day 0, decreased further during the middle of differentiation, and then rebounded toward initial levels as differentiation was completed.
Wnt-induced β-Catenin Accumulation and Phosphorylation Response Is Impaired in LRP5-deficient Preadipocytes
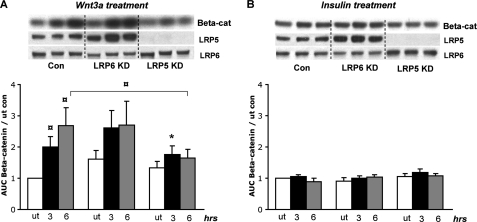
Accumulation of β-catenin is one of the key events in the Wnt signaling pathway and has been shown to be important for the Wnt inhibitory effect on adipocyte differentiation (13). Although not well studied in classical insulin responsive tissues, insulin has also been shown to stimulate an increase in β-catenin levels in intestinal L cells (33). As expected, treatment of control 3T3-L1 cells with Wnt3a produced a significant 2–3-fold increase in β-catenin levels at 3 and 6 h after stimulation (Fig. 4A). Insulin stimulation of 3T3-L1 preadipocytes, on the other hand, had no effect on β-catenin levels (Fig. 4B). The Wnt effect on β-catenin was unchanged in the LRP6 KD cells, whereas β-catenin accumulation in response to Wnt stimulation was significantly decreased in the LRP5 KD cells compared with controls (Fig. 4A). Because Wnt stimulation is still able to block differentiation in the LRP5 KD cells (Fig. 3B), the latter finding indicates that β-catenin accumulation in the nucleus is not required for the Wnt inhibitory effect on fat differentiation and that other pathways must be involved.
FIGURE 4.
β-Catenin accumulation in LRP5 and LRP6 KD preadipocytes. Confluent 3T3-L1 preadipocytes were treated with either 100 nm insulin or 75 ng/ml Wnt3a for 3 or 6 h, and total protein was extracted for Western blot analysis. Band intensities for β-catenin levels were determined using Image J software, and fold changes were calculated as compared with untreated (ut) cells. A, Wnt3a stimulation. The error bars show S.E. (n = 4). * indicates a p value < 0.05, and ¤ indicates a p value < 0.06. B, insulin stimulation. The error bars show S.E. (n = 5). Con, control; ut, untreated; Beta-cat, β-catenin.
Both LRP5 and LRP6 are known to play the role of co-receptors in the Wnt/β-catenin signaling pathway (38). Not surprisingly, in 3T3-L1 cells, the overall Wnt3a phosphorylation response was reduced in LRP6 KD cells. Basal phosphorylation of LRP6 on serine 1490 was reduced by 40–50% compared with controls, and although KD cells responded to Wnt stimulation, they never reached the same maximum level of serine phosphorylation as seen in controls (Fig. 5A). Decreased signaling in the LRP5 KD cells was even greater with marked reduction in Akt, ERK, and GSK3β phosphorylation (Fig. 5A). The bigger reduction in LRP6, Akt, ERK1/2, and GSK3β phosphorylation found in the LRP5 KD cells suggests that LRP5 is more important in propagating the Wnt signal in preadipocytes than LRP6.
FIGURE 5.
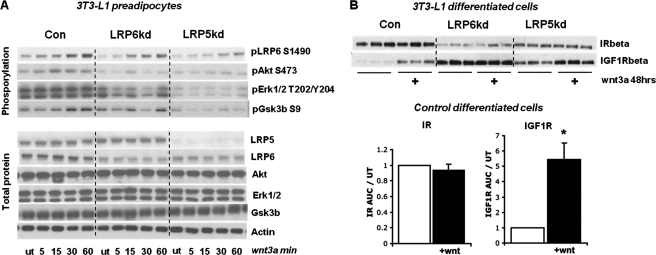
Wnt signaling in LRP5 and LRP6 KD preadipocytes and regulation of the insulin/IGF-1 signaling system. A, all of the 3T3-L1 cell lines were treated with 75 ng/ml Wnt3a for various times for 60 min, and the phosphorylation response was determined by Western blot analysis (n = 3). B, differentiated 3T3-L1 cells were grown with or without Wnt3a (75 ng/ml) added to the medium for 48 h. The proteins were extracted and subjected to Western blot analysis for IR and IGF1R levels. The error bars show S.E. (n = 3). Con, control; ut, untreated.
Wnt Treatment of Differentiated Adipocytes Leads to Significant Up-regulation of IGF1R but Not IR
As shown above, the Wnt signaling pathway interacts with the insulin/IGF-1 signaling pathway on numerous levels. In addition, chronic Wnt3a treatment of differentiated 3T3-L1 cells led to a significant increase of IGF1R (5.5-fold), but not insulin receptor, protein (Fig. 5B). The up-regulation of IGF1R was also found in the LRP5 KD cells, but not in the LRP6 KD cells. The regulation of IGF1R protein levels occurred with no change in IGF1R mRNA levels, suggesting regulation at the post-transcriptional level (supplemental Fig. S3).
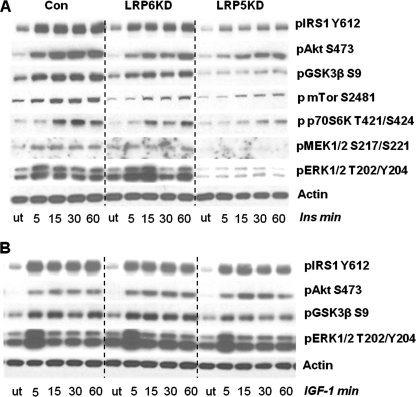
LRP5-deficient Preadipocytes Have Greatly Impaired Signaling Response to Insulin but Not IGF-1
As shown above, Wnt signaling leads to phosphorylation of several proteins known to be key mediators in the insulin signaling network. Insulin signaling in preadipocytes with knockdown of the Wnt co-receptors LRP5 and LRP6 was also altered (Fig. 6). In control cells, stimulation by 100 nm insulin produced robust phosphorylation of IRS1, MEK1/2, Akt, ERK1/2, GSK3β, mTOR, and p70S6K, as assessed by Western blotting (Fig. 6A). In contrast, in the LRP5 KD cells, both basal and maximal insulin-stimulated phosphorylation of all major insulin signaling proteins was markedly decreased. This was illustrated by up to 85% reductions in basal phosphorylation of IRS1, Akt, GSK3β, and ERK1/2 and an almost absent insulin response. These changes occurred with no consistent change in IR tyrosine phosphorylation. Basal phosphorylation of insulin signaling proteins was also reduced in LRP6 KD cells. These cells were still responsive to insulin, although never reaching the same maximum phosphorylation as seen for the control. Differences in phosphorylation were not due to differences in total protein levels as these levels were unaltered in the KD cells (supplemental Fig. S4). By contrast, knockdown of LRP5 or LRP6 had no effect on the response to IGF-1 stimulated phosphorylation responses (Fig. 6B). Thus, the decrease in signaling in LRP5 and LRP6 knockdown cells was specific to the insulin signaling pathway and did not impact upon IGF-1 action.
FIGURE 6.
Insulin and IGF-1 signaling in LRP5 and LRP6 KD preadipocytes. 3T3-L1 preadipocytes were stimulated with 100 nm insulin or IGF-1 at various time points for 60 min. Total cell extracts were made and used directly for Western blot analysis. A, the insulin phosphorylation response of proteins in the insulin signaling pathway (n = 3). B, the IGF-1 phosphorylation response of proteins in the insulin signaling pathway (n = 3). Total protein levels for the various insulin time points can be seen in supplemental Fig. S4. Con, control; ut, untreated.
Although both KD cell lines had a lower capacity to differentiate than control cells, the major effect of LRP5 KD on insulin signal transduction was reversed after differentiation of the 3T3-L1 cells to adipocytes (supplemental Fig. S5). In addition, in the KD cells, the changes in IR and IGF1R expression were divergent from what is normally seen during differentiation. Thus, in the LRP6 KD cells, which had the lowest differentiation capacity, the insulin receptor levels were lower than in control differentiated cells, and the IGF1R was more than 4-fold increased. Consistent with this, the response to IGF-1 in the LRP6 KD cells was greatly increased compared with control cells and LRP5 KD cells.
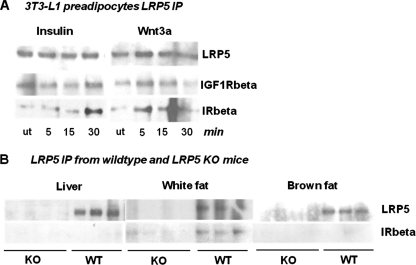
The Insulin Receptor and LRP5 Co-immunoprecipitate in Inducible Manner
To determine whether the insulin/IGF-1 receptor might physically interact with LRP5 in the plasma membrane, we immunoprecipitated cell extracts with anti-LRP5 and Western blotted the precipitates with anti-IR or anti-IGF1R antibody at different times after insulin and Wnt3a stimulation. As shown in Fig. 7A, LRP5 co-immunoprecipitated with both the IR and the IGF1R in the basal state. More importantly, the association between LRP5 and the insulin receptor, but not the IGF1R, was further increased after insulin and Wnt3a stimulation, reaching a peak at ∼30 min. IR and LRP5 co-immunoprecipitation was also observed using freshly isolated white adipose tissue from wild type mice (Fig. 7B) but was not detected in adipose tissue from LRP5 knock-out mice, indicating that the co-immunoprecipitation was specific. Furthermore, the LRP5/IR interaction appeared to be specific to white fat because the co-immunoprecipitation was not observed in extracts of liver or brown fat.
FIGURE 7.
LRP5 and insulin/IGF-1 receptor immunoprecipitation studies. A, LRP5 was immunoprecipitated from 3T3-L1 preadipocytes after insulin (100 nm) or Wnt3a (75 ng/ml) stimulations at various time points and blotted with anti-LRP5 and anti-IR/IGF1R antibodies. B, liver, white adipose, and brown adipose tissue samples from WT and LRP5 KO mice were immunoprecipitated with anti-LRP5 antibody and blotted with anti-LRP5 and anti-IR antibody. n = 4 for KO and n = 3 for WT. ut, untreated.
DISCUSSION
Adipose tissue is one of the main targets for insulin action and a major contributor to insulin resistance in states of obesity. Both insulin and Wnt signaling play important roles in adipose development: the former by acting to induce adipocyte differentiation and the latter acting to inhibit it (10, 16, 45). Although these two pathways have opposite effects on adipose development, the insulin and Wnt signaling pathways are also known to have convergent effects, such as their ability to inhibit GSK3β activity (46). Furthermore, one of the main transcription factors downstream the Wnt signaling pathway, TCF7L2, has been implicated as an important gene in the development of type 2 diabetes (18). In the present study, we have explored how the insulin and Wnt signaling pathways interact in adipose tissue to both complement and inhibit each other's actions.
We find that activation of the Wnt signaling pathway in 3T3-L1 preadipocytes leads to phosphorylation of many of the key proteins involved in insulin signal transduction, including Akt, ERK1/2, and GSK3β, on the same residues as insulin, albeit with lower fold stimulation and slower kinetics. In agreement with this, others have shown that Wnt stimulation can increase Akt phosphorylation in NIH-3T3 and C2C12 cells (47, 48) and can increase insulin sensitivity and action in myotubes (36). Interestingly, we find that this insulin-like effect of Wnt is lost in cells lacking both insulin receptors and IGF-1 receptors but not in cells lacking only one of the two receptor types. Thus, this effect is dependent on the presence of either the insulin or IGF-1 receptor.
Previous studies have suggested that Wnt induced GSK3β inhibition is independent of GSK3β serine 9 phosphorylation (30, 31). However, our studies show that Wnt3a stimulates serine 9 phosphorylation of GSK3β in preadipocytes, and this is accompanied by increased phosphorylation of Akt and ERK1/2, resembling insulin stimulation but with a slower time course and smaller magnitude of response. These differences in kinetics and magnitude of stimulation could suggest that phosphorylation of GSK3β in response to insulin and Wnt3a occurs in different intracellular compartments, which could be linked to different signaling pathways (31). In Xenopus laevis, differences in magnitude of β-catenin activation have been shown to link to different biological effects (49). Differences in kinetics may, however, also suggest that the Wnt effect on GSK3β, Akt, and ERK1/2 phosphorylation is indirect, which is supported by the loss of this Wnt response in brown preadipocytes lacking both the IR and the IGF1R. Hence, we suggest that that the insulin/IGF-1 receptors essentially work as components of this newly discovered part of the Wnt signaling pathway. A level of redundancy in this system is suggested by the observation that Wnt-induced pAkt phosphorylation is reduced in the single receptor KO cells and completely lost in the DKO cells.
One site of interaction between the insulin and Wnt signaling pathways is at the level of the Wnt co-receptors LRP5 and LRP6. This was initially suggested by our previous proteomic studies showing that LRPs are present in the anti-phosphotyrosine immunoprecipitates from preadipocytes following insulin stimulation (37). Indeed, LRP5 KD cells, and to a lesser extent LRP6 KD cells, have a reduced basal and Wnt-stimulated phosphorylation level of several proteins found in the insulin signaling pathway, including IRS1, GSK3β, and especially ERK1/2. In addition, we find that reduced levels of LRP5 lead to impaired Wnt stimulation of LRP6 serine phosphorylation, suggesting that LRP5 is upstream of LRP6 in these early Wnt signaling events.
Reduction of either LRP5 but especially LRP6 also has a negative effect on adipocyte differentiation and triglyceride accumulation. Because Wnt signaling normally inhibits adipogenesis (10, 16), one might have expected the opposite effect. Furthermore, although knockdown of LRP5 markedly reduces Wnt3a stimulated β-catenin accumulation in 3T3-L1 preadipocytes, Wnt3a is still able to completely block adipocyte differentiation in the LRP5 KD cells. Previous studies have suggested that Wnt activation represses adipocyte differentiation through both β-catenin-dependent and -independent pathways (13, 50). Our results suggest that the β-catenin-independent pathway is sufficient for the anti-adipogenic effect of Wnt. Reducing levels of either co-receptor was also found to significantly up-regulate IGF1R levels, but not IR, levels in the 3T3-L1 cells. It has been previously shown that Wnt signaling inhibits apoptosis and induces expression of anti-apoptotic genes in 3T3-L1 preadipocytes, such as IGF-1 and IGF-2 (51). Taken together with our results, it is possible that up-regulation of the IGF1R in adipocytes is involved in Wnt-induced suppression of apoptosis.
The most striking result in the LRP study, however, is the profound reduction in the phosphorylation response to insulin stimulation in the LRP5 KD cells. The role of LRPs in insulin signaling demonstrates several interesting features. First, LRP5 has a pronounced effect on basal phosphorylation of insulin signaling proteins and the insulin response in preadipocytes. Second, this mechanism is specific to insulin, as compared with IGF-1, stimulation and thus is one of the few clear differences between insulin and IGF-1 signaling identified in 3T3-L1 cells. Third, the positive effects of LRP5 on insulin signaling involve an insulin/Wnt-inducible interaction between LRP5 and the IR as demonstrated by co-immunoprecipitation. This interaction was shown to be both physiological and specific, because the co-immunoprecipitation of LRP5 and IR was also seen in normal white adipose tissue from wild type mice but was not seen in fat from LRP5 KO mice. Interestingly, this interaction appears to be white adipose-specific, because it was not observed in liver or brown adipose tissue. It is not clear at this point whether the interaction is directly between the IR and LRP5 or whether the two proteins are docking to some scaffolding protein common to both signaling pathways. These observations make it reasonable to speculate that LRP5 could work as a co-receptor, not only in Wnt signaling, but also in insulin signaling, and that the IR/LRP5 interaction plays a role in the Wnt-induced phosphorylation of insulin signaling molecules. This positive role of LRP5 on insulin signaling is only observed in preadipocytes and is lost after the cells differentiate and the insulin receptors markedly increase in number. This suggests that LRP5 potentiates the action of insulin when receptor number is low, such as in early adipocyte development when Wnt signaling is most important.
In the context of insulin signaling, there appears to be a clear difference in the function of LRP5 compared with LRP6. Part of this difference could be due to the lower efficiency of LRP6 knockdown compared with LRP5. It is also possible that reduced levels of LRP5 or LRP6 lead to changes in levels of other Wnt components and that these changes add to the effects of LRP5 or LRP6 reduction. However, we also find differences between LRP5 and LRP6 in nonmanipulated wild type cells, especially in terms of adipocyte differentiation. Thus, during differentiation, LRP5 levels initially increase more than 3-fold in wild type cells, whereas LRP6 and β-catenin expression steadily decrease. Contradicting our results, a previous study has found that LRP5 and LRP6 are constitutively expressed throughout differentiation (52); however, this was only investigated at the mRNA level and might differ from what we see on the protein level. Taken together, these data suggest that LRP5 and LRP6 have different roles in the regulation of fat development and insulin signaling.
Insulin and Wnt signaling interactions could also play a role in the association of these pathways with conditions like obesity, type 2 diabetes, and cancer. Increased activity of the Wnt system has been identified in several types of cancer (17, 53), whereas decreased Wnt signaling has been suggested to play a role in the metabolic syndrome (54). Wnt signaling tends to decrease with age as the risk of the metabolic syndrome increases. This is in part due to increased competition between canonical Wnt and FOXO signaling for available β-catenin (24). Increased FOXO transcriptional activity and decreased Wnt activity have been suggested to play a role in the formation of β-amyloid plaques in Alzheimer's disease, as well as age-related heart disease (55, 56). Furthermore, Wnt signaling has been shown to regulate the balance between myogenesis and adipogenesis and regulate insulin sensitivity in myoblasts (36). Recently a family was described with mutations in LRP5 that was strongly associated with diabetes or impaired glucose tolerance (57). Although one component of diabetes in this family was β cell dysfunction, there was no specific assessment of insulin resistance in these individuals. Our results suggest that decreased LRP5 expression can explain the phenotype of this family because this would lead to insulin resistance in preadipocytes, changing the balance of the pro- and anti-adipogenic signals and altering glucose metabolism.
In summary, we have shown that Wnt activation leads to phosphorylation of key insulin signaling proteins, including GSK3β, in an insulin and IGF-1 receptor-dependent manner. This cross-talk between insulin and Wnt signaling occurs at least in part at the level of the Wnt co-receptor LRP5, which has a profound positive effect on insulin signaling in preadipocytes. This involves a direct interaction between the insulin receptor and LRP5, which occurs in an insulin/Wnt-inducible manner. The inducible nature of this interaction is specific to the insulin receptor and is not observed with the IGF-1 receptor. In this context, LRP5 appears to serve as a co-receptor, not only in Wnt signaling, but also in insulin signaling. Notably, the IR/LRP5 interaction could also be a mode of action in the Wnt effect on Akt, ERK1/2, and GSK3β phosphorylation, which would explain the role of insulin/IGF-1 receptors in this Wnt-induced phosphorylation response. Thus, the IR/LRP5 interaction acts as a mechanistic bridge between the two pathways that could play a role in the pathogenesis of insulin resistance and obesity.
Supplementary Material
Acknowledgments
The IR/IGF1R knock-out cell lines were previously created and characterized by Jeremie Boucher and Kristina Kriauciunas.
This work was supported, in whole or in part, by National Institutes of Health Grants DK31036, DK33201, and DK55545.

This article contains supplemental Figs. S1–S5.
- LRP
- low density lipoprotein receptor-related proteins
- IGF
- insulin-like growth factor
- GSK
- glycogen synthase kinase
- IRS
- insulin receptor substrate
- IGF1R
- IGF-1 receptor
- KD
- knockdown
- DKO
- double knock-out.
REFERENCES
- 1. Taniguchi C. M., Emanuelli B., Kahn C. R. (2006) Critical nodes in signalling pathways. Insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 [DOI] [PubMed] [Google Scholar]
- 2. Kikuchi A., Yamamoto H., Kishida S. (2007) Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 19, 659–671 [DOI] [PubMed] [Google Scholar]
- 3. Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., He X. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 [DOI] [PubMed] [Google Scholar]
- 4. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling. Components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yum S., Lee S. J., Piao S., Xu Y., Jung J., Jung Y., Oh S., Lee J., Park B. J., Ha N. C. (2009) The role of the Ser/Thr cluster in the phosphorylation of PPPSP motifs in Wnt coreceptors. Biochem. Biophys. Res. Commun. 381, 345–349 [DOI] [PubMed] [Google Scholar]
- 6. Piao S., Lee S. H., Kim H., Yum S., Stamos J. L., Xu Y., Lee S. J., Lee J., Oh S., Han J. K., Park B. J., Weis W. I., Ha N. C. (2008) Direct inhibition of GSK3β by the phosphorylated cytoplasmic domain of LRP6 in Wnt/β-catenin signaling. PLoS One 3, e4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sugimura R., Li L. (2010) Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res. 90, 243–256 [DOI] [PubMed] [Google Scholar]
- 9. Grumolato L., Liu G., Mong P., Mudbhary R., Biswas R., Arroyave R., Vijayakumar S., Economides A. N., Aaronson S. A. (2010) Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 24, 2517–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Longo K. A., Wright W. S., Kang S., Gerin I., Chiang S. H., Lucas P. C., Opp M. R., MacDougald O. A. (2004) Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 279, 35503–35509 [DOI] [PubMed] [Google Scholar]
- 11. Wright W. S., Longo K. A., Dolinsky V. W., Gerin I., Kang S., Bennett C. N., Chiang S. H., Prestwich T. C., Gress C., Burant C. F., Susulic V. S., MacDougald O. A. (2007) Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes 56, 295–303 [DOI] [PubMed] [Google Scholar]
- 12. Christodoulides C., Lagathu C., Sethi J. K., Vidal-Puig A. (2009) Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 20, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prestwich T. C., Macdougald O. A. (2007) Wnt/β-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 19, 612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Day T. F., Guo X., Garrett-Beal L., Yang Y. (2005) Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750 [DOI] [PubMed] [Google Scholar]
- 15. Glass D. A., 2nd, Bialek P., Ahn J. D., Starbuck M., Patel M. S., Clevers H., Taketo M. M., Long F., McMahon A. P., Lang R. A., Karsenty G. (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 [DOI] [PubMed] [Google Scholar]
- 16. Ross S. E., Hemati N., Longo K. A., Bennett C. N., Lucas P. C., Erickson R. L., MacDougald O. A. (2000) Inhibition of adipogenesis by Wnt signaling. Science 289, 950–953 [DOI] [PubMed] [Google Scholar]
- 17. Rey J. P., Ellies D. L. (2010) Wnt modulators in the biotech pipeline. Dev. Dyn. 239, 102–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grant S. F., Thorleifsson G., Reynisdottir I., Benediktsson R., Manolescu A., Sainz J., Helgason A., Stefansson H., Emilsson V., Helgadottir A., Styrkarsdottir U., Magnusson K. P., Walters G. B., Palsdottir E., Jonsdottir T., Gudmundsdottir T., Gylfason A., Saemundsdottir J., Wilensky R. L., Reilly M. P., Rader D. J., Bagger Y., Christiansen C., Gudnason V., Sigurdsson G., Thorsteinsdottir U., Gulcher J. R., Kong A., Stefansson K. (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 38, 320–323 [DOI] [PubMed] [Google Scholar]
- 19. Florez J. C., Jablonski K. A., Bayley N., Pollin T. I., de Bakker P. I., Shuldiner A. R., Knowler W. C., Nathan D. M., Altshuler D., and Diabetes Prevention Program Research Group (2006) TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 355, 241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin T., Liu L. (2008) The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol. Endocrinol. 22, 2383–2392 [DOI] [PubMed] [Google Scholar]
- 21. Doria A., Patti M. E., Kahn C. R. (2008) The emerging genetic architecture of type 2 diabetes. Cell Metab. 8, 186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi F., Sun J., Lim G. E., Fantus I. G., Brubaker P. L., Jin T. (2008) Cross talk between the insulin and Wnt signaling pathways. Evidence from intestinal endocrine L cells. Endocrinology 149, 2341–2351 [DOI] [PubMed] [Google Scholar]
- 23. Welters H. J., Kulkarni R. N. (2008) Wnt signaling. Relevance to β-cell biology and diabetes. Trends Endocrinol. Metab. 19, 349–355 [DOI] [PubMed] [Google Scholar]
- 24. Manolagas S. C., Almeida M. (2007) Gone with the Wnts. β-Catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol. Endocrinol. 21, 2605–2614 [DOI] [PubMed] [Google Scholar]
- 25. Lee S. H., Demeterco C., Geron I., Abrahamsson A., Levine F., Itkin-Ansari P. (2008) Islet specific Wnt activation in human type II diabetes. Exp. Diabetes Res. 2008, 728763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo Y. F., Xiong D. H., Shen H., Zhao L. J., Xiao P., Guo Y., Wang W., Yang T. L., Recker R. R., Deng H. W. (2006) Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J. Med. Genet. 43, 798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mani A., Radhakrishnan J., Wang H., Mani A., Mani M. A., Nelson-Williams C., Carew K. S., Mane S., Najmabadi H., Wu D., Lifton R. P. (2007) LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315, 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin T. (2008) The WNT signalling pathway and diabetes mellitus. Diabetologia 51, 1771–1780 [DOI] [PubMed] [Google Scholar]
- 29. Riancho J. A., Olmos J. M., Pineda B., García-Ibarbia C., Pérez-Núñez M. I., Nan D. N., Velasco J., Cano A., García-Pérez M. A., Zarrabeitia M. T., González-Macías J. (2011) Wnt receptors, bone mass, and fractures. Gene-wide association analysis of LRP5 and LRP6 polymorphisms with replication. Eur. J. Endocrinol. 164, 123–131 [DOI] [PubMed] [Google Scholar]
- 30. Ding V. W., Chen R. H., McCormick F. (2000) Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275, 32475–32481 [DOI] [PubMed] [Google Scholar]
- 31. Wu D., Pan W. (2010) GSK3. A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 35, 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., Wang C. Y., He X., MacDougald O. A., You M., Williams B. O., Guan K. L. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 [DOI] [PubMed] [Google Scholar]
- 33. Sun J., Jin T. (2008) Both Wnt and mTOR signaling pathways are involved in insulin-stimulated proto-oncogene expression in intestinal cells. Cell Signal. 20, 219–229 [DOI] [PubMed] [Google Scholar]
- 34. Desbois-Mouthon C., Cadoret A., Blivet-Van Eggelpoël M. J., Bertrand F., Cherqui G., Perret C., Capeau J. (2001) Insulin and IGF-1 stimulate the β-catenin pathway through two signalling cascades involving GSK-3β inhibition and Ras activation. Oncogene 20, 252–259 [DOI] [PubMed] [Google Scholar]
- 35. Jin T., George Fantus I., Sun J. (2008) Wnt and beyond Wnt. Multiple mechanisms control the transcriptional property of β-catenin. Cell Signal. 20, 1697–1704 [DOI] [PubMed] [Google Scholar]
- 36. Abiola M., Favier M., Christodoulou-Vafeiadou E., Pichard A. L., Martelly I., Guillet-Deniau I. (2009) Activation of Wnt/β-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PLoS One 4, e8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krüger M., Kratchmarova I., Blagoev B., Tseng Y. H., Kahn C. R., Mann M. (2008) Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc. Natl. Acad. Sci. U.S.A. 105, 2451–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cselenyi C. S., Jernigan K. K., Tahinci E., Thorne C. A., Lee L. A., Lee E. (2008) LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of β-catenin. Proc. Natl. Acad. Sci. U.S.A. 105, 8032–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pinson K. I., Brennan J., Monkley S., Avery B. J., Skarnes W. C. (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538 [DOI] [PubMed] [Google Scholar]
- 40. Kato M., Patel M. S., Levasseur R., Lobov I., Chang B. H., Glass D. A., 2nd, Hartmann C., Li L., Hwang T. H., Brayton C. F., Lang R. A., Karsenty G., Chan L. (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gong Y., Slee R. B., Fukai N., Rawadi G., Roman-Roman S., Reginato A. M., Wang H., Cundy T., Glorieux F. H., Lev D., Zacharin M., Oexle K., Marcelino J., Suwairi W., Heeger S., Sabatakos G., Apte S., Adkins W. N., Allgrove J., Arslan-Kirchner M., Batch J. A., Beighton P., Black G. C., Boles R. G., Boon L. M., Borrone C., Brunner H. G., Carle G. F., Dallapiccola B., De Paepe A., Floege B., Halfhide M. L., Hall B., Hennekam R. C., Hirose T., Jans A., Jüppner H., Kim C. A., Keppler-Noreuil K., Kohlschuetter A., LaCombe D., Lambert M., Lemyre E., Letteboer T., Peltonen L., Ramesar R. S., Romanengo M., Somer H., Steichen-Gersdorf E., Steinmann B., Sullivan B., Superti-Furga A., Swoboda W., van den Boogaard M., Van Hul W., Vikkula M., Votruba M., Zabel B., Garcia T., Baron R., Olsen B. R., Warman M. L. (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 [DOI] [PubMed] [Google Scholar]
- 42. Fujino T., Asaba H., Kang M. J., Ikeda Y., Sone H., Takada S., Kim D. H., Ioka R. X., Ono M., Tomoyori H., Okubo M., Murase T., Kamataki A., Yamamoto J., Magoori K., Takahashi S., Miyamoto Y., Oishi H., Nose M., Okazaki M., Usui S., Imaizumi K., Yanagisawa M., Sakai J., Yamamoto T. T. (2003) Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc. Natl. Acad. Sci. U.S.A. 100, 229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boucher J., Tseng Y. H., Kahn C. R. (2010) Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J. Biol. Chem. 285, 17235–17245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boucher J., Macotela Y., Bezy O., Mori M. A., Kriauciunas K., Kahn C. R. (2010) A kinase-independent role for unoccupied insulin and IGF-1 receptors in the control of apoptosis. Sci. Signal. 3, ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farmer S. R. (2006) Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forde J. E., Dale T. C. (2007) Glycogen synthase kinase 3. A key regulator of cellular fate. Cell Mol. Life Sci. 64, 1930–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fukumoto S., Hsieh C. M., Maemura K., Layne M. D., Yet S. F., Lee K. H., Matsui T., Rosenzweig A., Taylor W. G., Rubin J. S., Perrella M. A., Lee M. E. (2001) Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276, 17479–17483 [DOI] [PubMed] [Google Scholar]
- 48. Yoon J. C., Ng A., Kim B. H., Bianco A., Xavier R. J., Elledge S. J. (2010) Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 24, 1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goentoro L., Kirschner M. W. (2009) Evidence that fold-change, and not absolute level, of β-catenin dictates Wnt signaling. Mol. Cell 36, 872–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kennell J. A., MacDougald O. A. (2005) Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent mechanisms. J. Biol. Chem. 280, 24004–24010 [DOI] [PubMed] [Google Scholar]
- 51. Longo K. A., Kennell J. A., Ochocinska M. J., Ross S. E., Wright W. S., MacDougald O. A. (2002) Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J. Biol. Chem. 277, 38239–38244 [DOI] [PubMed] [Google Scholar]
- 52. Bennett C. N., Ross S. E., Longo K. A., Bajnok L., Hemati N., Johnson K. W., Harrison S. D., MacDougald O. A. (2002) Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277, 30998–31004 [DOI] [PubMed] [Google Scholar]
- 53. Schinner S. (2009) Wnt-signalling and the metabolic syndrome. Horm. Metab. Res. 41, 159–163 [DOI] [PubMed] [Google Scholar]
- 54. Vigneri P., Frasca F., Sciacca L., Pandini G., Vigneri R. (2009) Diabetes and cancer. Endocr. Relat. Cancer 16, 1103–1123 [DOI] [PubMed] [Google Scholar]
- 55. Manolopoulos K. N., Klotz L. O., Korsten P., Bornstein S. R., Barthel A. (2010) Linking Alzheimer's disease to insulin resistance. The FoxO response to oxidative stress. Mol. Psychiatry 15, 1046–1052 [DOI] [PubMed] [Google Scholar]
- 56. Naito A. T., Shiojima I., Komuro I. (2010) Wnt signaling and aging-related heart disorders. Circ. Res. 107, 1295–1303 [DOI] [PubMed] [Google Scholar]
- 57. Saarinen A., Saukkonen T., Kivelä T., Lahtinen U., Laine C., Somer M., Toiviainen-Salo S., Cole W. C., Lehesjoki A. E., Mäkitie O. (2010) Low density lipoprotein receptor-related protein 5 (LRP) mutations and osteoporosis, impaired glucose metabolism, and hypercholesterolaemia. Clin. Endocrinol. 72, 481–488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.