Background: Vascular dysfunction and hypertension caused by RGS2 deficiency occur by poorly understood mechanisms.
Results: Endothelial RGS2 deficiency impaired endothelium-derived hyperpolarizing factor-mediated relaxation of resistance arteries by a pertussis toxin-sensitive mechanism, without increasing blood pressure significantly.
Conclusion: Endothelial dysfunction, a common feature of hypertension, can be caused by RGS2 deficiency.
Significance: RGS2 deficiency in several cell types may be required to increase blood pressure.
Keywords: Endothelial Dysfunction, G Proteins, Hypertension, RGS Proteins, Vascular Biology, EDHF
Abstract
Regulator of G protein signaling 2 (RGS2) is a GTPase-activating protein for Gq/11α and Gi/oα subunits. RGS2 deficiency is linked to hypertension in mice and humans, although causative mechanisms are not understood. Because endothelial dysfunction and increased peripheral resistance are hallmarks of hypertension, determining whether RGS2 regulates microvascular reactivity may reveal mechanisms relevant to cardiovascular disease. Here we have determined the effects of systemic versus endothelium- or vascular smooth muscle-specific deletion of RGS2 on microvascular contraction and relaxation. Contraction and relaxation of mesenteric resistance arteries were analyzed in response to phenylephrine, sodium nitroprusside, or acetylcholine with or without inhibitors of nitric oxide (NO) synthase or K+ channels that mediate endothelium-derived hyperpolarizing factor (EDHF)-dependent relaxation. The results showed that deleting RGS2 in vascular smooth muscle had minor effects. Systemic or endothelium-specific deletion of RGS2 strikingly inhibited acetylcholine-evoked relaxation. Endothelium-specific deletion of RGS2 had little effect on NO-dependent relaxation but markedly impaired EDHF-dependent relaxation. Acute, inducible deletion of RGS2 in endothelium did not affect blood pressure significantly. Impaired EDHF-mediated vasodilatation was rescued by blocking Gi/oα activation with pertussis toxin. These findings indicated that systemic or endothelium-specific RGS2 deficiency causes endothelial dysfunction resulting in impaired EDHF-dependent vasodilatation. RGS2 deficiency enables endothelial Gi/o activity to inhibit EDHF-dependent relaxation, whereas RGS2 sufficiency facilitates EDHF-evoked relaxation by squelching endothelial Gi/o activity. Mutation or down-regulation of RGS2 in hypertension patients therefore may contribute to endothelial dysfunction and defective EDHF-dependent relaxation. Blunting Gi/o signaling might improve endothelial function in such patients.
Introduction
Agonists for G protein-coupled receptors (GPCRs)3 trigger arterial relaxation by stimulating endothelial production of NO, vasodilatory arachidonic acid metabolites, and endothelium-derived hyperpolarizing factor (EDHF) (1–5). Defects in these mechanisms contribute to aspects of endothelial dysfunction in several diseases, including hypertension, atherosclerosis, diabetes, and preeclampsia (6). Endothelial GPCRs such as M3 muscarinic and B2 bradykinin receptors couple to the Gq/11 class of heterotrimeric G proteins to activate phospholipase Cβ, which hydrolyzes phosphatidylinositol 4,5-bisphosphate to form diacylglycerol, which activates protein kinase C, and inositol 1,4,5-trisphosphate, which releases Ca2+ from intracellular stores. Increased intracellular Ca2+ levels activate endothelial nitric-oxide synthase (eNOS) to produce NO (2), phospholipase activity to produce arachidonic acid and its metabolites (7), and calcium-activated small (SKCa) and intermediate (IKCa) conductance potassium channels to hyperpolarize endothelium and vascular smooth muscle (1, 3). Indeed, mice lacking eNOS or IKCa and SKCa activity exhibit endothelial dysfunction and hypertension (8).
In contrast, pertussis toxin (PTX)-sensitive G proteins of the Gi/o class have poorly understood roles in vasodilatation, even though they couple to many GPCRs, inhibit adenylyl cyclase, and modulate activity of certain ion channels. Whereas PTX has been shown by certain studies to have no effect on endothelium-dependent vasodilatation (9–20), in others it inhibited NO-dependent (10, 11, 21–23) or EDHF-dependent (16) relaxation. However, effects of PTX must be interpreted cautiously, because this multifunctional toxin can impact cell and tissue function by blocking Gi/o activation or by affecting other processes (24). Whether PTX affects vasodilatation specifically by blocking Gi/o signaling has not been established.
Regulator of G protein signaling 2 (RGS2) has provided new insight into G protein regulatory mechanisms that impact vascular reactivity and blood pressure homeostasis. RGS2 homozygous or heterozygous knock-out mice are hypertensive (25, 26), indicating that this gene is a hypertension quantitative trait locus. In certain hypertension patients, RGS2 function or expression is impaired by missense mutations (27, 28), promoter mutations, or mRNA down-regulation (29–35). RGS2 potentially regulates blood pressure by several mechanisms because it is expressed in vasculature, kidney, and other tissues and has several biochemical functions (36, 37), including GTPase-activating protein (GAP) activity toward Gq/11 and Gi/o classes of Gα subunits; GAP-independent inhibition of certain adenylyl cyclase isoforms; phosphorylation and activation by type Iα cGMP-dependent protein kinase; and association with certain GPCRs and scaffold proteins. In vascular smooth muscle, RGS2 negatively regulates Gq/11 signaling because RGS2 deficiency augments Gq/11-mediated contraction of aortic rings and renal interlobar arteries (38, 39). Furthermore, RGS2 promotes relaxation by serving as an effector of the NO/cGMP pathway to inhibit Gq/11-dependent vasoconstriction because RGS2 deficiency blunts the effects of NO donors on blood pressure and cGMP analogs on aortic ring relaxation (26, 40). RGS2 deficiency in transplanted kidney is sufficient to elevate blood pressure (41), suggesting that renal nephron and vascular regulation are crucial functions of RGS2.
Although RGS2 is expressed in endothelium and vascular smooth muscle, its respective functions in these compartments of the resistance vasculature have not been established. Here we have addressed this question by analyzing mesenteric resistance arteries derived from conventional, smooth muscle- and endothelium-specific RGS2 knock-out mice. In addition to discovering that endothelial RGS2 deficiency causes endothelial dysfunction characterized by impaired EDHF-dependent vasodilatation, our studies uncover a new role for endothelial Gi/o signaling and suggest means of ameliorating endothelial dysfunction caused by RGS2 deficiency, which may be relevant to hypertension patients when RGS2 is mutated or down-regulated.
EXPERIMENTAL PROCEDURES
Animals
Studies were performed in accordance with protocols approved by the Washington University Animal Studies Committee. The mice were provided access to food and water ad libitum in our institution's animal facility at constant temperature of 22 °C and a 12-h light/dark cycle. All of the experiments were performed using 3–6-month-old mice backcrossed more than seven generations into the C57BL/6 background (Charles River Laboratories). Conventional RGS2−/− mice have been described (42). The methods used to generate RGS2fl/fl mice are described in the supplemental materials (supplemental Fig. S1A). Endothelium-specific RGS2 knock-out mice were generated as follows. RGS2fl/fl mice were crossed with mice bearing the Tie2-CreERT2 transgene in the C57BL/6 background (43) (provided by Dr. Kyunghee Choi, Washington University School of Medicine). RGS2fl/+ Tie2-CreERT2 offspring were crossed with RGS2fl/fl mice to obtain RGS2fl/fl Tie2-CreERT2 mice, which at 3 months of age received intraperitoneal injection of tamoxifen (2 mg/day) for 5 days to generate endothelium-specific RGS2-deficient mice (RGS2EC−/−). Smooth muscle-specific RGS2 knock-out mice (RGS2SM−/−) were generated by a similar procedure using smooth muscle-specific SMMHC-CreERT2 transgenic mice in the C57BL/6 background (44) (provided by Dr. Stefan Offermanns, University of Heidelberg, Germany). Quantitative RT-PCR was used to assess the extent of gene inactivation using total RNA extracted from lung microvascular endothelial cells freshly isolated by fluorescent activated cell sorting or from aorta. Experiments were performed with mesenteric arteries isolated 2 weeks following the last tamoxifen injection. Tamoxifen-treated mice carrying only the Tie2-CreERT2 or SMMHC-CreERT2 transgene provided negative controls.
Vessel Isolation and Preparation
The relevant methods are described in the supplemental materials.
Vessel Reactivity Assays
Our published methods were used (45, 46). Excised mesenteric arteries were transferred into an organ bath containing MOPS and mounted on the stage of an inverted microscope (Zeiss Axiovert S100TV). Arteries were cannulated on one end with a glass perfusion pipette and occluded on the other with a collecting pipette. No luminal flow was allowed. Vessel diameter changes in response to vasoactive agents were recorded with a video camera (MTI CCD-72). The base-line internal diameter was measured at 60 mm Hg and 37 °C with a computerized diameter tracking system (sampling rate of 10 Hz; Diamtrak 3 Plus, Montech Pty, Ltd.). After the vessels were equilibrated >60 min, the bath solution was replaced with warmed MOPS buffer containing phenylephrine (PE; 100 μmol/liter) to preconstrict vessels. After constriction reached steady state (<1 min), the bath solution was changed to MOPS buffer containing 100 μmol/liter PE and acetylcholine (ACh) at various concentrations (10−9 to 10−4 mol/liter). After the highest ACh concentration was applied and the data were recorded, the bath solution was replaced with MOPS buffer to allow relaxation. To inhibit eNOS or cyclooxygenase, l-NAME (0.5 mmol/liter) or indomethacin (10 μmol/liter) was applied to the vessel bath for 30 min. To inhibit SKCa and IKCa channels that mediate EDHF-dependent vasodilatation, apamin and Tram-34 dissolved in Me2SO were added to 1% w/v of BSA-MOPS buffer, applied in the vessel lumen, and allowed to incubate for 30 min. The final concentration of Me2SO was 0.02%, which had no effect on its own (data not shown). As indicated, vessels were incubated intraluminally for 2 h with pertussis toxin or the toxin B oligomer (200 ng/ml).
Radiotelemetric Analysis of Cardiovascular Function
Published methods were used to analyze cardiovascular function in control and endothelium-specific RGS2 knock-out mice by radiotelemetry (47). Detailed methods are presented in the supplemental materials.
RESULTS
RGS2 Deficiency Causes Endothelial Dysfunction Characterized by Impairment of Acetylcholine-evoked Vasodilatation of Mesenteric Resistance Arteries
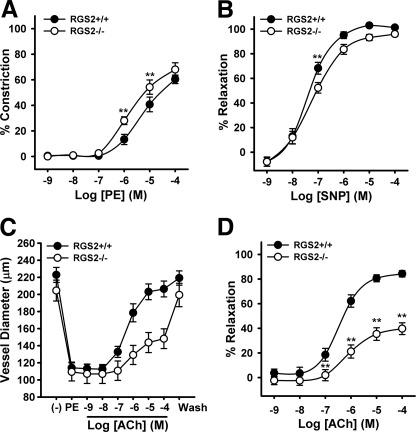
We used second order mesenteric arteries (MA; ∼220 μm in diameter and containing two or three smooth muscle cell layers) from WT and conventional RGS2−/− mice to determine whether RGS2 influences contraction or relaxation of resistance arteries. First, we determined whether the RGS2 deficiency affects vasoconstriction induced by PE, which activates Gq/11-coupled α1-adrenergic receptors. Results indicated that PE potency was augmented somewhat by the absence of RGS2 (Fig. 1A; pEC50 WT = 5.1 ± 0.25 versus RGS2−/− = 5.6 ± 0.2; p = 0.18). PE efficacy also trended slightly higher in RGS2−/− MA (WT = 61% contraction ± 2 versus RGS2−/− = 75% ± 10) but was below the significance threshold (p = 0.24). Second, we determined whether RGS2 deficiency affects endothelium-independent vasodilatation. Because MA do not establish spontaneous tone, we preconstricted them with PE (100 μmol/liter) and then added an NO donor (sodium nitroprusside (SNP)) at increasing concentrations. The results indicated that SNP-elicited vasodilatation was slightly impaired in RGS2−/− MA (Fig. 1B), in contrast to the profound deficit in SNP-evoked relaxation observed with RGS2−/− aortic rings (26). Thus, the relative importance of RGS2 in NO-mediated relaxation apparently differs between conducting and resistance arteries, perhaps because NO is the dominant endothelium-produced vasodilator in conducting arteries. Third, we determined whether RGS2 deficiency affects endothelium-dependent vasodilatation in response to increasing concentrations of ACh. Using MA preconstricted with PE (100 μmol/liter), we found that RGS2 deficiency dramatically impaired ACh efficacy (WT = 86% relaxation ± 2 versus RGS2−/− = 42% ± 5; p < 0.001; Fig. 1, C and D) and modestly blunted ACh potency (pEC50 WT = 6.4 ± 0.1 versus RGS2−/− = 5.3 ± 0.5; p < 0.05). Therefore, the most striking defect observed in RGS2−/− MA was impaired endothelium-dependent vasodilatation.
FIGURE 1.
Vascular reactivity of wild type and RGS2-deficient MA. A, vasoconstriction of WT and RGS2−/− MA to PE (n = 6–10 animals (2 vessels/animal) per group). The data shown are the mean percentages of change in diameter of arteries superfused with MOPS containing PE at indicated concentrations. The data are expressed as the percentages of change in vessel diameter relative to base line. B, vasodilatation of WT and RGS2−/− MA to the NO donor, SNP, following constriction with PE. The data are expressed as percentages of diameter increase. C, changes in WT and RGS2−/− arterial diameter after application of PE and increasing concentrations of ACh. D, concentration response of ACh-induced vasodilation expressed as percentages of increase in arterial diameter after constriction with PE (100 μmol/liter). The data are expressed as the means ± S.E. **, p < 0.01 versus WT (two-way analysis of variance).
Vasodilatatory Defect of RGS2−/− Mesenteric Arteries Is Independent of Endothelium-derived Vasoconstrictors ET-1 or Prostanoids
Because ACh and other vasodilatory agonists can stimulate vascular endothelium to produce both vasodilatory and vasoconstrictor substances (48), the relaxation defect observed in RGS2−/− MA could be due to augmented production of endothelium-derived vasoconstrictors and/or to impaired production or action of vasodilatory factors. To probe the role of endothelium-produced vasoconstrictors, we measured ACh-evoked vasodilatation of WT and RGS2−/− MA in the presence of indomethacin to inhibit cyclooxygenase activity that produces arachidonic acid-derived vasoconstrictor prostanoids or the ETA receptor antagonist BQ123 to block endothelin-1 action. As shown in Fig. 2, neither BQ123 (5 μmol/liter; Panel A) nor indomethacin (10 μmol/liter; Panel B) affected the vasodilatory defect of RGS2−/− MA. Thus, the vasodilatory defect of RGS2−/− MA appeared to be independent of ET-1 receptor or vasoconstrictor prostanoid action.
FIGURE 2.
Effect of endothelin ETA receptor and cyclooxygenase blockade on ACh-induced vasodilatation of WT and RGS2-deficient MA. A, vasodilatation of WT (n = 5) and RGS2−/− (n = 6) MA to ACh in the presence or absence of the ETA receptor antagonist, BQ123 (5 μmol/liter). B, vasodilatation of WT (n = 6) and RGS2−/− (n = 6) MA incubated with or without indomethacin (10 μmol/liter). The data shown are the percentages of change in vessel diameter ± S.E. after application of increasing concentrations of ACh in the presence of 100 μmol/liter PE.
RGS2 Deficiency Impairs EDHF-dependent Vasodilatation
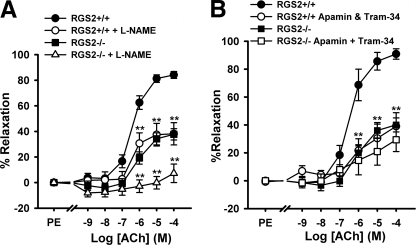
Next we investigated whether impaired vasorelaxation of RGS2−/− MA is associated with defective endothelium-derived vasodilatory factors. The results described above (Fig. 2B) indicated that ACh-induced vasodilatation of RGS2−/− MA is insensitive to cyclooxygenase inhibition, excluding a role for vasodilatory prostanoids. We therefore determined whether the absence of RGS2 impairs vasodilatation via NO- and/or EDHF-dependent mechanisms. In WT MA (Fig. 3), treatment with either the eNOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 500 μmol/liter) or EDHF inhibitors (apamin (50 nmol/liter), which blocks SKCa channels, plus Tram-34 (1 μmol/liter), which blocks IKCa channels) blunted the maximal extent of ACh-evoked relaxation by ∼50%. Therefore, ACh relaxes WT MA by employing NO- and EDHF-dependent mechanisms to similar extent, consistent with prior studies of wild type second order MA (49). In RGS2−/− MA, the maximal extent of ACh-evoked relaxation was blunted much more by l-NAME (∼75% inhibition) than by EDHF inhibitors (∼25% inhibition) (Fig. 3). These results indicated that the absence of RGS2 impairs EDHF-dependent relaxation (i.e. relaxation occurring in the presence of l-NAME) more strongly than it impairs NO-dependent vasodilatation (i.e. relaxation occurring in the presence of EDHF inhibitors).
FIGURE 3.
Effect of eNOS and EDHF inhibitors on ACh-induced vasodilatation of WT and RGS2−/− MA. A, ACh-induced vasodilatation of WT and RGS2−/− MA in the presence (WT, n = 7; RGS2−/−, n = 7) and absence (WT, n = 10; RGS2−/−, n = 10) of eNOS inhibitor, l-NAME (500 μmol/liter). B, vasodilatation of WT and RGS2−/− MA in the presence (WT, n = 4; RGS2−/−, n = 5) and absence (WT, n = 8; RGS2−/−, n = 8) of EDHF inhibitors, apamin (50 nmol/liter) and Tram-34 (1 μmol/liter). The data are the means ± S.E. **, p < 0.01 versus WT and RGS2−/− controls, respectively.
Endothelium-specific Depletion of RGS2 Is Sufficient to Cause Endothelial Dysfunction with Minimal Effect on Resting Blood Pressure and Heart Rate
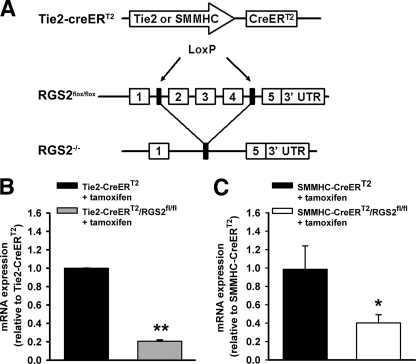
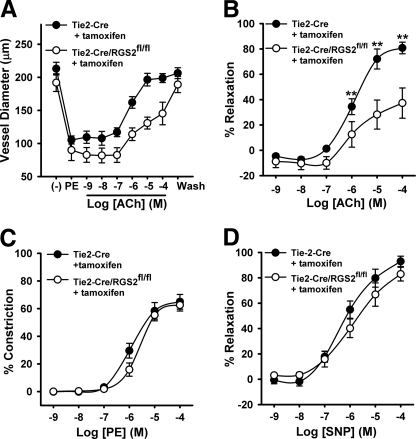
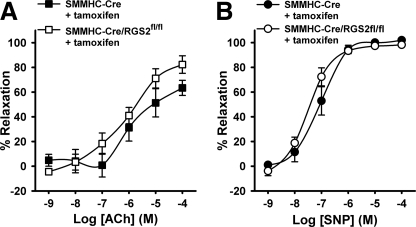
To determine whether the vasodilatory defect of RGS2−/− MA occurs at the level of endothelium and/or vascular smooth muscle, we generated mice bearing floxed alleles of the Rgs2 gene (RGS2fl/fl), whereby exons required for GAP activity and stable protein expression could be removed in a cell type-specific manner (supplemental Fig. S1 and Fig. 4A). Endothelium-specific deletion of RGS2 was achieved by crossing RGS2fl/fl mice with congenic transgenic mice expressing tamoxifen-regulated CreERT2 from the endothelium-specific Tie2 promoter (Tie2-CreERT2) (43). Following tamoxifen administration, RGS2 mRNA expression in endothelial cells was reduced ∼80% relative to tamoxifen-treated RGS2+/+ Tie2-CreERT2 controls (Fig. 4B), indicating that the Rgs2 gene was deleted efficiently. We analyzed vasoconstrictor and vasodilatory responses of MA derived from tamoxifen-treated mice bearing RGS2fl/fl Tie2-CreERT2 (termed RGS2EC−/− mice) or tamoxifen-treated control mice bearing only Tie2-CreERT2. MA from RGS2EC−/− mice exhibited ACh-induced vasodilatory defects similar to those observed in MA from conventional RGS2−/− mice (Fig. 5, A and B). RGS2EC−/− vessels showed marked decrease in ACh efficacy relative to controls (Tie2-CreERT2 control = 83% relaxation ± 4; RGS2EC−/− = 54% ± 9, p < 0.05). In contrast, vascular smooth muscle function was not affected significantly, as indicated by dose-response profiles for PE-induced vasoconstriction and SNP-induced vasodilatation (Fig. 5, C and D).
FIGURE 4.
Generation and assessment of endothelium- and smooth muscle-specific RGS2 knock-out mice. A, to generate endothelium- or smooth muscle-specific RGS2−/− mice, transgenic mice carrying tamoxifen-sensitive, CreERT2 gene driven by the Tie2 or SMMHC promoter (top) were bred with RGS2fl/fl knock-in mice in which exons 2–4 of the Rgs2 gene were flanked by loxP sites (middle). When CreERT2 was expressed following tamoxifen treatment, exons between the LoxP sites were removed, leaving one LoxP site and a nonfunctional Rgs2 gene (bottom). B, quantitation of RGS2 mRNA in freshly isolated lung endothelial cells from WT (Tie2-CreERT2 + tamoxifen) and endothelium-specific RGS2−/− (Tie2-CreERT2/RGS2fl/fl + tamoxifen) mice by quantitative RT-PCR. C, quantitation of RGS2 mRNA in aorta of WT (SMMHC-CreERT2 + tamoxifen) and smooth muscle-specific RGS2−/− (SMMHC-CreERT2/RGS2fl/fl + tamoxifen) mice by qRT-PCR. mRNA expression levels were normalized to samples from CreERT2 control mice treated with tamoxifen. The data are expressed as the means ± S.E. *, p < 0.05 versus CreERT2 control; **, p < 0.01 versus CreERT2 control (Student's t test).
FIGURE 5.
Vascular reactivity of wild type and endothelium-specific RGS2-deficient MA. A, changes in WT (Tie2-CreERT2 + tamoxifen) (n = 6) and RGS2EC−/− (Tie2-CreERT2/RGS2fl/fl + tamoxifen) (n = 5) arterial diameter after application of PE and increasing concentrations of ACh as indicated. B, concentration response of ACh-induced vasodilatation in A expressed as percentages of increase in arterial diameter after application of PE (100 μmol/liter). C, vasoconstriction of WT and RGS2EC−/− MA to phenylephrine. D, vasodilatation of WT and RGS2EC−/− MA to SNP, following constriction with PE. The data are expressed as the means ± S.E. **, p < 0.01 versus WT (two-way analysis of variance).
To exclude that the ACh-evoked vasodilatory defect of RGS2−/− MA occurs at the level of vascular smooth muscle, we analyzed MA isolated from tamoxifen-treated RGS2fl/fl mice expressing CreERT2 from the smooth muscle-specific SMMHC promoter (SMMHC-CreERT2) (44) and tamoxifen-treated congenic control mice bearing only the SMMHC-CreERT2 transgene. Tamoxifen administration reduced RGS2 mRNA expression in aorta ∼65% relative to SMMHC-CreERT2 control mice (Fig. 4C). The results indicated that smooth muscle-specific depletion of RGS2 had statistically insignificant effects on ACh- or SNP-evoked vasodilatory responses in MA (Fig. 6). Taken together, these results indicated that endothelially expressed RGS2 is a novel component of signaling networks that promote ACh-evoked and EDHF-dependent vasodilatation.
FIGURE 6.
Vasodilatory responses of control and smooth muscle-specific RGS2-deficient MA. A, concentration response of ACh-induced vasodilatation in WT (SMMHC-CreERT2 + tamoxifen) (n = 5) and RGS2SM −/− (RGS2SM −/−: SMMHC-CreERT2/RGS2fl/fl + tamoxifen) (n = 6) MA expressed as percentages of increase in arterial diameter after application of PE (100 μmol/liter). B, vasodilatation of WT and RGS2SM −/− MA to SNP, following constriction with PE. The data are expressed as the means ± S.E.
To determine whether endothelium-specific deletion of RGS2 is sufficient to alter blood pressure homeostasis, we used radiotelemetry to compare arterial blood pressure in conscious, freely moving tamoxifen-treated RGS2EC−/− and control mice (Tie2-CreERT2) in the same genetic background (C57Bl/6) (supplemental materials). Because conventional RGS2−/− mice exhibit increased urinary excretion of the sympathetic neurotransmitter norepinephrine and decreased heart rate variability (50), we also measured indices of autonomic function in the RGS2EC−/− and control mice. One week following tamoxifen administration, daytime mean arterial blood pressure values in control (110 ± 2 mm Hg) and RGS2EC−/− (110 ± 4, p = 0.43) mice were indistinguishable. However, analysis of diurnal variation 9 days following tamoxifen treatment indicated a trend toward higher mean arterial blood pressure values during the dark phase (6:00 p.m. to 6:00 a.m.) in RGS2EC−/− mice (120 ± 2 mm Hg) relative to controls (115 ± 2 mm Hg; p = 0.12), albeit below the statistical threshold of significance. Corresponding mean arterial blood pressure measured during the light phase (6:00am-6:00 pm) between the two groups were indistinguishable (104 ± 2 versus 103 ± 3 mm Hg in control; p = 0.98). Deleting RGS2 in the endothelium had an undetectable effect on heart rate variability, baroreflex sensitivity, and other indices of autonomic function (supplemental Table S1). These data together indicated that depleting RGS2 in the endothelium is insufficient to cause persistent elevation of mean arterial blood pressure, in contrast to what is observed in conventional RGS2−/− mice (25, 26, 31, 41).
RGS2 Deficiency Impairs EDHF-dependent Vasodilatation by Mechanisms Requiring Gi/o Signaling
To determine the mechanism responsible for defective EDHF-dependent vasodilatation in RGS2−/− MA, we considered various biochemical functions of RGS2. Although RGS2 is a GAP that deactivates Gα subunits of the Gq/11 class, augmented Gq/11 signaling that might occur in the absence of RGS2 seemed an unlikely mechanism because this effect should enhance rather than impair ACh signaling via endothelial Gq/11-coupled M3 muscarinic receptors. Alternatively, because RGS2 also is a GAP that deactivates the Gi/o class of Gα subunits (51), augmented endothelial Gi/o signaling might occur in the absence of RGS2. However, whether augmented endothelial Gi/o activity would enhance or inhibit EDHF-dependent vasodilatation was unclear because prior studies had not firmly established the role of Gi/o signaling in endothelium.
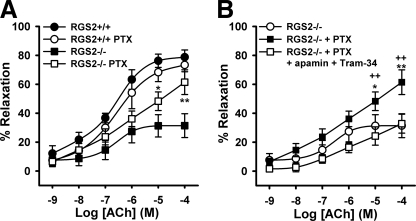
Accordingly, we determined whether Gi/o signaling affects ACh-evoked vasodilatation of WT or RGS2−/− MA. Whereas previous studies of artery rings treated both endothelium and vascular smooth muscle with pertussis toxin to block receptor-mediated Gi/o activation, we treated only the intraluminal space of cannulated WT and RGS2−/− MA with PTX (200 ng/ml, 2 h) to block Gi/o activation preferentially in endothelium. PTX treatment of WT MA had insignificant effects on ACh-evoked vasodilatation (Fig. 7A), indicating that endothelial Gi/o signaling is dispensable. In contrast, PTX treatment of RGS2−/− MA significantly improved ACh-evoked vasodilatation (Fig. 7B). The restorative effect of PTX was accompanied by recovery of EDHF-dependent vasodilatation, because relaxation of RGS2−/− MA regained sensitivity to EDHF inhibitors (apamin (50 nmol/liter) plus Tram-34 (1 μmol/liter)) (Fig. 7B).
FIGURE 7.
Effect of intraluminal application of pertussis toxin on ACh-induced vasodilatation. A, concentration-response curves of WT and RGS2−/− MA with (WT, n = 4; RGS2−/−, n = 8) and without (WT, n = 10; RGS2−/−, n = 10) PTX treatment. B, effect of EDHF inhibitors apamin (50 nmol/liter) and Tram-34 (1 μmol/liter) on ACh-induced vasodilatation of RGS2−/− (n = 5) MA, following 2 h of incubation with PTX. *, p < 0.05; **, p < 0.01 versus RGS2−/− control; ++, p < 0.01 versus RGS2−/− PTX + EDHF inhibitors.
Lastly, we determined the mechanism(s) whereby PTX restores EDHF-dependent relaxation of RGS2−/− MA. This was a critical question because components of pertussis holotoxin exert biological effects by distinct mechanisms: the A protomer ADP-ribosylates Gi/oα subunits to block GPCR-evoked Gi/o activation, whereas the membrane-binding B oligomer uses other mechanisms to elicit various effects including elevation of intracellular Ca2+ levels (24). Accordingly, we determined whether the A protomer is required to rescue EDHF-dependent relaxation by treating RGS2−/− MA intraluminally with only the B oligomer at a concentration (200 ng/ml) identical to that used for studies of holotoxin. The results showed that ACh-evoked vasodilatation was unaffected by B oligomer (supplemental Fig. S2), indicating that the ability of PTX holotoxin to improve EDHF-evoked vasodilatation RGS2−/− MA was specifically due to blockade of Gi/o activation by the A protomer. Taken together, these results indicated that endothelial Gi/o signaling inhibits EDHF-dependent relaxation of RGS2−/− MA, whereas it does not significantly affect relaxation of wild type MA.
DISCUSSION
Here we have shown that RGS2 deficiency in mesenteric resistance arteries causes endothelial dysfunction and impaired EDHF-dependent vasodilatation as a consequence of dysregulated Gi/o signaling. As discussed below, these findings reveal new functions for RGS2 and Gi/o signaling in resistance arteries that may be relevant in hypertension patients when RGS2 is mutated or down-regulated.
As expected for a multifunctional regulatory protein (26, 51–53), RGS2 now appears to have at least three roles in the vasculature. As shown previously, RGS2 functions in vascular smooth muscle as a GAP that (i) negatively regulates Gq/11-coupled α1-adrenergic receptor-mediated contraction (25, 26, 38) and (ii) promotes relaxation of aortic rings by undergoing cGKIα-mediated phosphorylation and activation to inhibit contraction evoked by Gq/11-coupled vasoconstrictor receptors (26, 40). Our findings here indicate a third role in which RGS2 acts in endothelium of resistance arteries to promote EDHF-dependent vasodilatation by blunting Gi/o-dependent processes, consistent with the ability of RGS2 to function as a GAP for Gi/oα subunits (51). In contrast, endothelium-expressed RGS2 apparently does not provide substantial negative regulation of relaxation evoked by M3 muscarinic acetylcholine receptors coupled to Gq/11, even though RGS2 is a more potent GAP for Gq/11α than Gi/oα subunits (52–54). Factors such as specialized compartmentalization or association with certain GPCRs, effectors, or scaffold proteins may determine in a cell type-specific manner whether RGS2 negatively regulates Gq/11- and/or Gi/o-mediated processes (37, 55–57).
In addition to providing new understanding of RGS2 function in the vasculature, our studies reveal a novel role for Gi/o signaling in endothelium-dependent vasodilatation. By comparing the effects of luminally delivered pertussis holotoxin or its B oligomer on ACh-evoked relaxation, we have found that RGS2 deficiency enables endothelial Gi/o signaling to inhibit EDHF-dependent vasodilatation, whereas RGS2 sufficiency precludes this inhibitory effect. Our findings support prior studies of wild type artery rings indicating that ACh-evoked relaxation is insensitive to pertussis toxin (9–20). Whereas other investigations have reported that ACh-evoked relaxation is sensitive to pertussis toxin (10, 11, 21–23), they did not, to our knowledge, establish whether this effect occurred by blocking Gi/o activation versus other toxin-sensitive processes (24). Furthermore, they did not establish whether pertussis toxin exerted its effects on endothelium and/or vascular smooth muscle. In contrast, we have shown that endothelium-specific depletion of RGS2 is sufficient to impair ACh-evoked relaxation, as expected if dysregulated endothelial Gi/o signaling is responsible.
What mechanism(s) potentially enable dysregulated endothelial Gi/o signaling to inhibit EDHF-dependent vasodilatation? One possibility comes from evidence indicating that endothelial GPCRs such as M3 muscarinic receptors can couple to Gi/o (58) even though they couple preferentially to Gq/11. The absence of RGS2-encoded Gi/o GAP activity therefore potentially could augment Gi/o activation by M3 or other receptors (Fig. 8), which potentially could lead to greater inhibition of adenylyl cyclase activity, lower cAMP levels, and blunted cAMP-evoked activation of IKCa channels or assembly of gap junctions that mediate EDHF-dependent vasodilatation (19, 59–61). Indeed, impaired cAMP signaling blunts EDHF-dependent vasodilatation in mesenteric arteries from diabetic rats (60, 61). However, detailed studies will be required to determine how dysregulated Gi/o signaling impairs EDHF-mediated vasodilatation in RGS2-deficient arteries.
FIGURE 8.
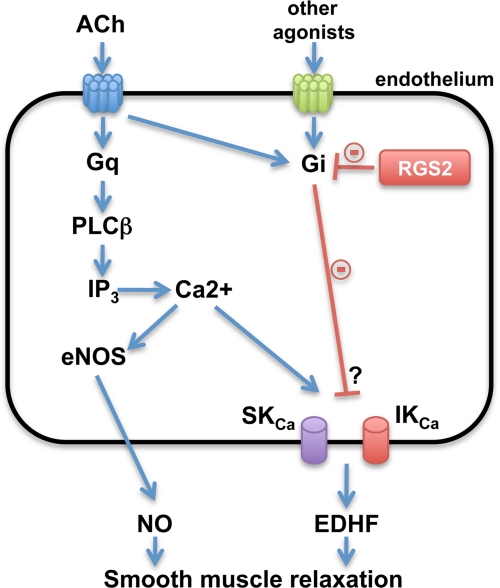
Model for regulation of resistance artery relaxation by RGS2 in endothelium. Vasodilatory agonists such as ACh activate Gq-coupled receptors in endothelial cells of resistance arteries to evoke Ca2+ transients that stimulate eNOS for producing NO and K channels (SKCa and IKCa) for producing endothelium-derived hyperpolarizing factor (EDHF). RGS2 accelerates G protein deactivation by functioning as a GAP for Gα subunits of the Gi or Gq classes. RGS2 deficiency in endothelium is proposed to impair vascular relaxation by augmenting Gi signaling, which inhibits EDHF production by mechanisms yet to be determined. IP3, inositol 1,4,5-trisphosphate.
Our results indicated that acute ablation of RGS2 in endothelium does not significantly elevate mean arterial blood pressure, despite causing marked impairment of GPCR-induced, EDHF-mediated vasodilatation of resistance arteries. These findings differ from those obtained with conventional RGS2 knock-out mice, which are hypertensive (25, 26, 31, 41). The lack of hypertension despite significant endothelial dysfunction in mice lacking RGS2 in endothelium may be explained in several ways. First, RGS2 deficiency in multiple cell types or organ systems such as vascular smooth muscle cells, the nervous system, renal nephron, or elsewhere in the kidney may be required to cause persistent increases in blood pressure. Second, RGS2 deficiency from early development onward, as occurs in conventional but not inducible RGS2 knock-out mice, may be required to elicit hypertension. Third, endothelium-specific RGS2 deficiency may require combination with other risk factors, such as age, diabetes, or atherosclerosis, to elicit hypertension. Regardless of which factors prove to be relevant, RGS2 function in kidney is likely to be crucial, because renal transplantation studies have shown that RGS2 deficiency in kidney is sufficient to elevate blood pressure (41).
In conclusion, our studies indicate that RGS2 deficiency in vascular endothelium leads to dysregulated Gi/o signaling that inhibits EDHF-dependent vasodilatation. Because RGS2 hypomorphic alleles or down-regulated gene expression occurs in certain hypertension patients (29–35), our findings suggest that such patients may exhibit endothelial dysfunction in part as a consequence of dysregulated endothelial Gi/o signaling. Blunting endothelial Gi/o signaling therefore may provide a novel avenue to explore for improving endothelial function in such patients.
Supplementary Material
Acknowledgments
We thank members of the Blumer, Dietrich, and Chapleau labs for providing advice and support.
This work was supported, in whole or in part, by National Institutes of Health Grants HL075632 and GM44592 (to K. J. B.); NS30555, HL041250, and NS32636 (to H. H. D.); and HL14388 (to M. W. C.). This work was also supported by American Heart Association Postdoctoral Fellowship 09POST2260099 (to P. O.-O.).

This article contains supplemental text, references, Table S1, and Figs. S1 and S2.
- GPCR
- G protein-coupled receptor
- ACh
- acetylcholine
- CreERT2
- fusion protein of Cre recombinase and tamoxifen-stabilized estrogen receptor
- EDHF
- endothelium-derived hyperpolarizing factor
- eNOS
- endothelial nitric-oxide synthase
- IKCa
- intermediate conductance Ca2+-activated K+ channels
- l-NAME
- Nω-nitro-l-arginine methyl ester
- MA
- second order mesenteric arteries
- PTX
- pertussis toxin
- RGS2
- regulator of G protein signaling 2
- SKCa
- small conductance Ca2+-activated K+ channels
- SNP
- sodium nitroprusside
- GAP
- GTPase-activating protein
- PE
- phenylephrine.
REFERENCES
- 1. Busse R., Edwards G., Félétou M., Fleming I., Vanhoutte P. M., Weston A. H. (2002) EDHF. Bringing the concepts together. Trends Pharmacol. Sci. 23, 374–380 [DOI] [PubMed] [Google Scholar]
- 2. Christopoulos A., El-Fakahany E. E. (1999) The generation of nitric oxide by G protein-coupled receptors. Life Sci. 64, 1–15 [DOI] [PubMed] [Google Scholar]
- 3. Edwards G., Félétou M., Weston A. H. (2010) Endothelium-derived hyperpolarising factors and associated pathways. A synopsis. Pflugers Arch. Eur. J. Physiol. 459, 863–879 [DOI] [PubMed] [Google Scholar]
- 4. Félétou M., Köhler R., Vanhoutte P. M. (2010) Endothelium-derived vasoactive factors and hypertension. Possible roles in pathogenesis and as treatment targets. Curr. Hypertens. Rep. 12, 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michel T., Vanhoutte P. M. (2010) Cellular signaling and NO production. Pflugers Arch. Eur. J. Physiol. 459, 807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Endemann D. H., Schiffrin E. L. (2004) Endothelial dysfunction. J. Am. Soc. Nephrol. 15, 1983–1992 [DOI] [PubMed] [Google Scholar]
- 7. Himmel H. M., Whorton A. R., Strauss H. C. (1993) Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension 21, 112–127 [DOI] [PubMed] [Google Scholar]
- 8. Brähler S., Kaistha A., Schmidt V. J., Wölfle S. E., Busch C., Kaistha B. P., Kacik M., Hasenau A. L., Grgic I., Si H., Bond C. T., Adelman J. P., Wulff H., de Wit C., Hoyer J., Köhler R. (2009) Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119, 2323–2332 [DOI] [PubMed] [Google Scholar]
- 9. Shimokawa H., Aarhus L. L., Vanhoutte P. M. (1987) Porcine coronary arteries with regenerated endothelium have a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin. Circ. Res. 61, 256–270 [DOI] [PubMed] [Google Scholar]
- 10. Flavahan N. A., Shimokawa H., Vanhoutte P. M. (1989) Pertussis toxin inhibits endothelium-dependent relaxations to certain agonists in porcine coronary arteries. J. Physiol. 408, 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimokawa H., Flavahan N. A., Vanhoutte P. M. (1989) Natural course of the impairment of endothelium-dependent relaxations after balloon endothelium removal in porcine coronary arteries. Possible dysfunction of a pertussis toxin-sensitive G protein. Circ. Res. 65, 740–753 [DOI] [PubMed] [Google Scholar]
- 12. Hohlfeld J., Liebau S., Förstermann U. (1990) Pertussis toxin inhibits contractions but not endothelium-dependent relaxations of rabbit pulmonary artery in response to acetylcholine and other agonists. J. Pharmacol. Exp. Ther. 252, 260–264 [PubMed] [Google Scholar]
- 13. Adeagbo A. S., Malik K. U. (1990) Endothelium-dependent and BRL 34915-induced vasodilatation in rat isolated perfused mesenteric arteries. Role of G-proteins, K+ and calcium channels. Br. J. Pharmacol. 100, 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller V. M., Flavahan N. A., Vanhoutte P. M. (1991) Pertussis toxin reduces endothelium-dependent and independent responses to α-2-adrenergic stimulation in systemic canine arteries and veins. J. Pharmacol. Exp. Ther. 257, 290–293 [PubMed] [Google Scholar]
- 15. Bryan R. M., Jr., Eichler M. Y., Swafford M. W., Johnson T. D., Suresh M. S., Childres W. F. (1996) Stimulation of α2 adrenoceptors dilates the rat middle cerebral artery. Anesthesiology 85, 82–90 [DOI] [PubMed] [Google Scholar]
- 16. White R., Hiley C. R. (1997) A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 122, 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boulanger C. M., Vanhoutte P. M. (1997) G proteins and endothelium-dependent relaxations. J. Vasc Res. 34, 175–185 [DOI] [PubMed] [Google Scholar]
- 18. Lembo G., Iaccarino G., Vecchione C., Barbato E., Morisco C., Monti F., Parrella L., Trimarco B. (1997) Insulin enhances endothelial α2-adrenergic vasorelaxation by a pertussis toxin mechanism. Hypertension 30, 1128–1134 [DOI] [PubMed] [Google Scholar]
- 19. Balolu E., Kiziltepe O., Gürdal H. (2007) The role of Gi proteins in reduced vasorelaxation response to β-adrenoceptor agonists in rat aorta during maturation. Eur. J. Pharmacol. 564, 167–173 [DOI] [PubMed] [Google Scholar]
- 20. Ng K. F., Leung S. W., Man R. Y., Vanhoutte P. M. (2008) Endothelium-derived hyperpolarizing factor mediated relaxations in pig coronary arteries do not involve Gi/o proteins. Acta Pharmacol. Sin. 29, 1419–1424 [DOI] [PubMed] [Google Scholar]
- 21. Flavahan N. A., Shimokawa H., Vanhoutte P. M. (1991) Inhibition of endothelium-dependent relaxations by phorbol myristate acetate in canine coronary arteries. Role of a pertussis toxin-sensitive G-protein. J. Pharmacol. Exp. Ther. 256, 50–55 [PubMed] [Google Scholar]
- 22. Nakaike R., Shimokawa H., Owada M. K., Tokunaga O., Yasutake H., Kishimoto T., Imada C., Shiraishi T., Egashira K., Takeshita A. (1996) Vanadate causes synthesis of endothelium-derived NO via pertussis toxin-sensitive G protein in pigs. Am. J. Physiol. 271, H296–H302 [DOI] [PubMed] [Google Scholar]
- 23. Shimokawa H., Flavahan N. A., Vanhoutte P. M. (1991) Loss of endothelial pertussis toxin-sensitive G protein function in atherosclerotic porcine coronary arteries. Circulation 83, 652–660 [DOI] [PubMed] [Google Scholar]
- 24. Wong W. S., Rosoff P. M. (1996) Pharmacology of pertussis toxin B-oligomer. Can. J. Physiol. Pharmacol. 74, 559–564 [DOI] [PubMed] [Google Scholar]
- 25. Heximer S. P., Knutsen R. H., Sun X., Kaltenbronn K. M., Rhee M. H., Peng N., Oliveira-dos-Santos A., Penninger J. M., Muslin A. J., Steinberg T. H., Wyss J. M., Mecham R. P., Blumer K. J. (2003) Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J. Clin. Invest. 111, 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang K. M., Wang G. R., Lu P., Karas R. H., Aronovitz M., Heximer S. P., Kaltenbronn K. M., Blumer K. J., Siderovski D. P., Zhu Y., Mendelsohn M. E. (2003) Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat. Med. 9, 1506–1512 [DOI] [PubMed] [Google Scholar]
- 27. Bodenstein J., Sunahara R. K., Neubig R. R. (2007) N-terminal residues control proteasomal degradation of RGS2, RGS4, and RGS5 in human embryonic kidney 293 cells. Mol. Pharmacol. 71, 1040–1050 [DOI] [PubMed] [Google Scholar]
- 28. Gu S., Tirgari S., Heximer S. P. (2008) The RGS2 gene product from a candidate hypertension allele shows decreased plasma membrane association and inhibition of Gq. Mol. Pharmacol. 73, 1037–1043 [DOI] [PubMed] [Google Scholar]
- 29. Yang J., Kamide K., Kokubo Y., Takiuchi S., Tanaka C., Banno M., Miwa Y., Yoshii M., Horio T., Okayama A., Tomoike H., Kawano Y., Miyata T. (2005) Genetic variations of regulator of G-protein signaling 2 in hypertensive patients and in the general population. J. Hypertens. 23, 1497–1505 [DOI] [PubMed] [Google Scholar]
- 30. Riddle E. L., Rana B. K., Murthy K. K., Rao F., Eskin E., O'Connor D. T., Insel P. A. (2006) Polymorphisms and haplotypes of the regulator of G protein signaling-2 gene in normotensives and hypertensives. Hypertension 47, 415–420 [DOI] [PubMed] [Google Scholar]
- 31. da Costa Goncalves A. C., Luft F. C., Gross V. (2008) Fine tuning of blood pressure by the regulator of G protein signaling (RGS) 2. J. Am. Soc. Hypertens. 2, 403–409 [DOI] [PubMed] [Google Scholar]
- 32. Li N. F., Zhang J. H., Yang J., Zhou L., Luo W. L., Guo Y. Y., Yao X. G., Wang H. M., Chang J. H. (2010) Association of genetic variations of regulator of G-protein signaling 2 with hypertension in the general Xinjiang Kazakh population. Clin. Exp. Hypertens. 32, 256–261 [DOI] [PubMed] [Google Scholar]
- 33. Watanabe Y., Metoki H., Ohkubo T., Katsuya T., Tabara Y., Kikuya M., Hirose T., Sugimoto K., Asayama K., Inoue R., Hara A., Obara T., Nakura J., Kohara K., Totsune K., Ogihara T., Rakugi H., Miki T., Imai Y. (2010) Accumulation of common polymorphisms is associated with development of hypertension. A 12-year follow-up from the Ohasama study. Hypertens. Res. 33, 129–134 [DOI] [PubMed] [Google Scholar]
- 34. Semplicini A., Strapazzon G., Papparella I., Sartori M., Realdi A., Macchini L., Calò L. A., Ceolotto G. (2010) RGS2 expression and aldosterone. Renin ratio modulate response to drug therapy in hypertensive patients. J. Hypertens. 28, 1104–1108 [DOI] [PubMed] [Google Scholar]
- 35. Sugimoto K., Katsuya T., Kamide K., Fujisawa T., Shimaoka I., Ohishi M., Morishita R., Ogihara T., Rakugi H. (2010) Promoter polymorphism of RGS2 gene is associated with change of blood pressure in subjects with antihypertensive treatment. The azelnidipine and temocapril in hypertensive patients with type 2 diabetes study. Int. J. Hypertens. 2010, 196307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu S., Cifelli C., Wang S., Heximer S. P. (2009) RGS proteins. Identifying new GAPs in the understanding of blood pressure regulation and cardiovascular function. Clin. Sci. 116, 391–399 [DOI] [PubMed] [Google Scholar]
- 37. Heximer S. P., Blumer K. J. (2007) RGS proteins. Swiss army knives in seven-transmembrane domain receptor signaling networks. Sci. STKE 2007, pe2. [DOI] [PubMed] [Google Scholar]
- 38. Hercule H. C., Tank J., Plehm R., Wellner M., da Costa Goncalves A. C., Gollasch M., Diedrich A., Jordan J., Luft F. C., Gross V. (2007) Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp. Physiol. 92, 1014–1022 [DOI] [PubMed] [Google Scholar]
- 39. Tank J., Obst M., Diedrich A., Brychta R. J., Blumer K. J., Heusser K., Jordan J., Luft F. C., Gross V. (2007) Sympathetic nerve traffic and circulating norepinephrine levels in RGS2-deficient mice. Auton. Neurosci. 136, 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun X., Kaltenbronn K. M., Steinberg T. H., Blumer K. J. (2005) RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol. Pharmacol. 67, 631–639 [DOI] [PubMed] [Google Scholar]
- 41. Gurley S. B., Griffiths R. C., Mendelsohn M. E., Karas R. H., Coffman T. M. (2010) Renal actions of RGS2 control blood pressure. J. Am. Soc. Nephrol. 21, 1847–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oliveira-Dos-Santos A. J., Matsumoto G., Snow B. E., Bai D., Houston F. P., Whishaw I. Q., Mariathasan S., Sasaki T., Wakeham A., Ohashi P. S., Roder J. C., Barnes C. A., Siderovski D. P., Penninger J. M. (2000) Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc. Natl. Acad. Sci. U.S.A. 97, 12272–12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forde A., Constien R., Gröne H. J., Hämmerling G., Arnold B. (2002) Temporal Cre-mediated recombination exclusively in endothelial cells using Tie2 regulatory elements. Genesis 33, 191–197 [DOI] [PubMed] [Google Scholar]
- 44. Wirth A., Benyó Z., Lukasova M., Leutgeb B., Wettschureck N., Gorbey S., Orsy P., Horváth B., Maser-Gluth C., Greiner E., Lemmer B., Schütz G., Gutkind J. S., Offermanns S. (2008) G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14, 64–68 [DOI] [PubMed] [Google Scholar]
- 45. Horiuchi T., Dietrich H. H., Hongo K., Dacey R. G. (2003) Comparison of P2 receptor subtypes producing dilation in rat intracerebral arterioles. Stroke 34, 1473–1478 [DOI] [PubMed] [Google Scholar]
- 46. Dietrich H. H., Abendschein D. R., Moon S. H., Nayeb-Hashemi N., Mancuso D. J., Jenkins C. M., Kaltenbronn K. M., Blumer K. J., Turk J., Gross R. W. (2010) Genetic ablation of calcium-independent phospholipase A2β causes hypercontractility and markedly attenuates endothelium-dependent relaxation to acetylcholine. Am. J. Physiol. Heart Circ. Physiol. 298, H2208–H2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sabharwal R., Zhang Z., Lu Y., Abboud F. M., Russo A. F., Chapleau M. W. (2010) Receptor activity-modifying protein 1 increases baroreflex sensitivity and attenuates angiotensin-induced hypertension. Hypertension 55, 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michel F. S., Man G. S., Man R. Y., Vanhoutte P. M. (2008) Hypertension and the absence of EDHF-mediated responses favour endothelium-dependent contractions in renal arteries of the rat. Br. J. Pharmacol. 155, 217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ceroni L., Ellis A., Wiehler W. B., Jiang Y. F., Ding H., Triggle C. R. (2007) Calcium-activated potassium channel and connexin expression in small mesenteric arteries from eNOS-deficient (eNOS−/−) and eNOS-expressing (eNOS+/+) mice. Eur. J. Pharmacol. 560, 193–200 [DOI] [PubMed] [Google Scholar]
- 50. Gross V., Tank J., Obst M., Plehm R., Blumer K. J., Diedrich A., Jordan J., Luft F. C. (2005) Autonomic nervous system and blood pressure regulation in RGS2-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1134–R1142 [DOI] [PubMed] [Google Scholar]
- 51. Ingi T., Krumins A. M., Chidiac P., Brothers G. M., Chung S., Snow B. E., Barnes C. A., Lanahan A. A., Siderovski D. P., Ross E. M., Gilman A. G., Worley P. F. (1998) Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J. Neurosci. 18, 7178–7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heximer S. P., Srinivasa S. P., Bernstein L. S., Bernard J. L., Linder M. E., Hepler J. R., Blumer K. J. (1999) G protein selectivity is a determinant of RGS2 function. J. Biol. Chem. 274, 34253–34259 [DOI] [PubMed] [Google Scholar]
- 53. Heximer S. P., Watson N., Linder M. E., Blumer K. J., Hepler J. R. (1997) RGS2/G0S8 is a selective inhibitor of Gqα function. Proc. Natl. Acad. Sci. U.S.A. 94, 14389–14393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kimple A. J., Soundararajan M., Hutsell S. Q., Roos A. K., Urban D. J., Setola V., Temple B. R., Roth B. L., Knapp S., Willard F. S., Siderovski D. P. (2009) Structural determinants of G-protein α subunit selectivity by regulator of G-protein signaling 2 (RGS2). J. Biol. Chem. 284, 19402–19411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abramow-Newerly M., Roy A. A., Nunn C., Chidiac P. (2006) RGS proteins have a signalling complex. Interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 18, 579–591 [DOI] [PubMed] [Google Scholar]
- 56. Neitzel K. L., Hepler J. R. (2006) Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin. Cell Dev. Biol. 17, 383–389 [DOI] [PubMed] [Google Scholar]
- 57. Xie G. X., Palmer P. P. (2007) How regulators of G protein signaling achieve selective regulation. J. Mol. Biol. 366, 349–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burford N. T., Tobin A. B., Nahorski S. R. (1995) Coupling of muscarinic m1, m2 and m3 acetylcholine receptors, expressed in Chinese hamster ovary cells, to pertussis toxin-sensitive/insensitive guanine nucleotide-binding proteins. Eur. J. Pharmacol. 289, 343–351 [DOI] [PubMed] [Google Scholar]
- 59. Griffith T. M., Chaytor A. T., Taylor H. J., Giddings B. D., Edwards D. H. (2002) cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc. Natl. Acad. Sci. U.S.A. 99, 6392–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matsumoto T., Kobayashi T., Kamata K. (2003) Alterations in EDHF-type relaxation and phosphodiesterase activity in mesenteric arteries from diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 285, H283–H291 [DOI] [PubMed] [Google Scholar]
- 61. Matsumoto T., Kobayashi T., Kamata K. (2006) Mechanisms underlying the impaired EDHF-type relaxation response in mesenteric arteries from Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Eur. J. Pharmacol. 538, 132–140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.