Abstract
Transduction of exogenous T cell receptor (TCR) genes into patients’ activated peripheral blood T cells is a potent strategy to generate large numbers of specific T cells for adoptive therapy of cancer and viral diseases. However, the remarkable clinical promise of this powerful approach is still being overshadowed by a serious potential consequence: mispairing of the exogenous TCR chains with endogenous TCR chains. These “mixed” heterodimers can generate new specificities that result in graft-versus-host reactions. Engineering TCR constant regions of the exogenous chains with a cysteine promotes proper pairing and reduces the mispairing, but, as we show here, does not eliminate the formation of mixed heterodimers. By contrast, deletion of the constant regions, through use of a stabilized Vα/Vβ single-chain TCR (scTv), avoided mispairing completely. By linking a high-affinity scTv to intracellular signaling domains, such as Lck and CD28, the scTv was capable of activating functional T cell responses in the absence of either the CD3 subunits or the co-receptors, and circumvented mispairing with endogenous TCRs. Such transduced T cells can respond to the targeted antigen independent of CD3 subunits via the introduced scTv, without the transduced T cells acquiring any new undefined and potentially dangerous specificities.
Keywords: T cell receptors, peptide/MHC, retroviral transduction, adoptive T cell therapy
Introduction
The potential of T cells in eliminating cancer cells has been demonstrated in numerous studies. However, when cancer becomes clinically apparent, the patient’s T cells have clearly failed to effectively reject the malignancy. Active immunization is being attempted to make the patient’s own T cells recognize and destroy the cancer, but this approach is complicated by the fact that T cells specific for the cancer cells, particularly those with high avidity or affinity, may be suppressed or been deleted by central or peripheral tolerance. To improve the T cell response to cancer, recent approaches have combined adoptive T cell transfer with TCR gene therapy, in which a TCR of desired specificity and affinity is transduced into patients’ ex vivo activated T cells 1. This approach shows evidence of therapeutic promise 2,3 but also carries potential dangers 4.
T cells recognize foreign antigens, in the form of peptides bound to products of the major histocompatibility complex (pepMHC), through a heterodimeric T cell receptor (TCR) consisting of α and β chains 5. A recognized complication of TCR gene therapy is the ability of introduced TCR α and β chains to mismatch with endogenous T cell receptors 6–9. In two recent studies, TCR mispairing between introduced and endogenous TCR α and β chains led to undesirable reactivities. In the first study using a mouse model system, overt autoimmunity (graft-versus-host disease) was observed, to varying degrees, in five different TCR systems 4. The other study with human T cells showed that mismatched heterodimers caused off-target toxicity, with an estimate of one of every ten mixed TCR heterodimers causing neoreactivity 10. These findings reinforce the idea that TCR mispairing could have detrimental, potentially dangerous, consequences.
A number of approaches have been developed to minimize the extent of mispairing (reviewed in 8,9,11-14). These have included the use of hybrid human TCR chains containing mouse constant domains, which associate preferentially with each other rather than with the endogenous TCR chains containing human constant domains 15. Another strategy involved fusion of the TCR chains to CD3ζ transmembrane and signaling subunits, also leading to preferential pairing of the exogenous TCR chains 16. Finally, several laboratories have shown that the substitution of non-native cysteine residues within the constant domains of the introduced TCR 17 also promotes pairing of the exogenous chains, presumably through the formation of an additional disulfide bond at the Cα:Cβ interface 18–20, significantly reducing the extent of mispairing and graft-versus-host disease in mouse models of TCR gene therapy 4. However, the full extent to which these strategies can prevent TCR chain mispairing is unclear.
An additional challenge of the TCR gene therapy approach is that the levels of exogenous αβ TCRs expressed on the surface of T cells is reduced by the concurrent expression of endogenous αβ TCRs, since the total surface levels of TCR are controlled by the availability of the CD3 subunits, especially the ζ subunits 21,22. As CD3 subunits are limiting, even the optimized pairing strategies described above are likely to result in lower surface levels for some exogenous TCRs, compared to a single, homogenous αβ TCR. Because TCR surface levels directly impact antigen sensitivity 23, it would be useful to develop strategies that enhance the levels of the exogenous TCRs. To this end, a number of TCR modifications, such as codon and vector optimization have been shown to improve surface levels of exogenous TCRs 24–26.
Various single-chain TCR chimeras have been used in attempts to limit the problems associated with pairing of endogenous TCRs. These included three-domain TCRs that contain other signaling domains such as the CD3ζ intracellular domain (VαVβCβCD3ζ) 7,27,28 and three domain constructs along with a separate Cα domain 29. While these constructs mediate antigen-specific T cell activity, the extent of TCR mispairing and the quantitative comparisons of peptide activity with conventional two-chain αβ T cells has often not been examined. Single-chain T cell receptors consisting of only the TCR variable domains (VαVβ in principle would completely eliminate mispairing, but have been problematic due to instability of Vα and Vβ domains in the absence of constant domains 30.
Here we show that three domain TCR constructs (VαVβCβ) yield mispaired receptors in the presence of an endogenous α chain because of the contained Cβ domain. Stabilized two domain TCRs (VαVβ) with chimeric signaling domains avoid mispairing altogether and mediate T cell activity. The high-affinity scTv linked to intracellular signaling domains, Lck and CD28, was capable of activating T cells in the absence of either the CD3 subunits or the co-receptors CD4 or CD8. The scTv system also worked with a human construct specific for an HLA.A2 – restricted peptide derived from the HIV-Gag protein. Our novel approach preserves the endogenous TCRs to potentially be triggered via intentional immunization or homeostatic proliferative signals, while permitting the introduced scTv TCRs to respond to the targeted antigen independent of CD3 subunits, without the transduced T cells acquiring any new undefined and potentially dangerous specificities.
Results
Three-domain single-chain TCR (VαVβCβ) containing a non-native Cβ cysteine mispairs with endogenous TCR chains
Various studies have suggested that a single-chain TCR construct consisting of tandem VαVβCβ domains could minimize the potential for mispairing with endogenous TCRs. Inclusion of a non-native cysteine in C regions represents another strategy to minimize such mispairing. Since the Cα:Cβ interaction contributes significant binding energy to these pairing interactions, we decided to explore whether a single-chain TCR (VαVβCβ) that contains a non-native Cβ cysteine would mispair with an endogenous α chain (VαCα), presumably through the interaction of the Cβ with the Cα. To directly assess this, we took advantage of high-affinity probes for two different, properly associated VαVβ pairs. The first probe, for the 2C TCR, is a clonotypic antibody, denoted 1B2, that binds only to properly associated 2C VαVβ with an affinity of 1 nM 31. The second probe, for the mutated 2C TCR denoted m33 TCR, is the SIY/Kb ligand that binds only to properly associated m33 VαVβ with an affinity of 30 nM 32. The mutations that convert 2C to the high-affinity m33 reside in the Vα CDR3 region, and prevent binding of 1B2 33. In addition, since the affinity of the SIY/Kb complex for 2C is low (30 µM), this tetramer bound by m33 only binds to the 2C TCR in the presence of CD8 34.
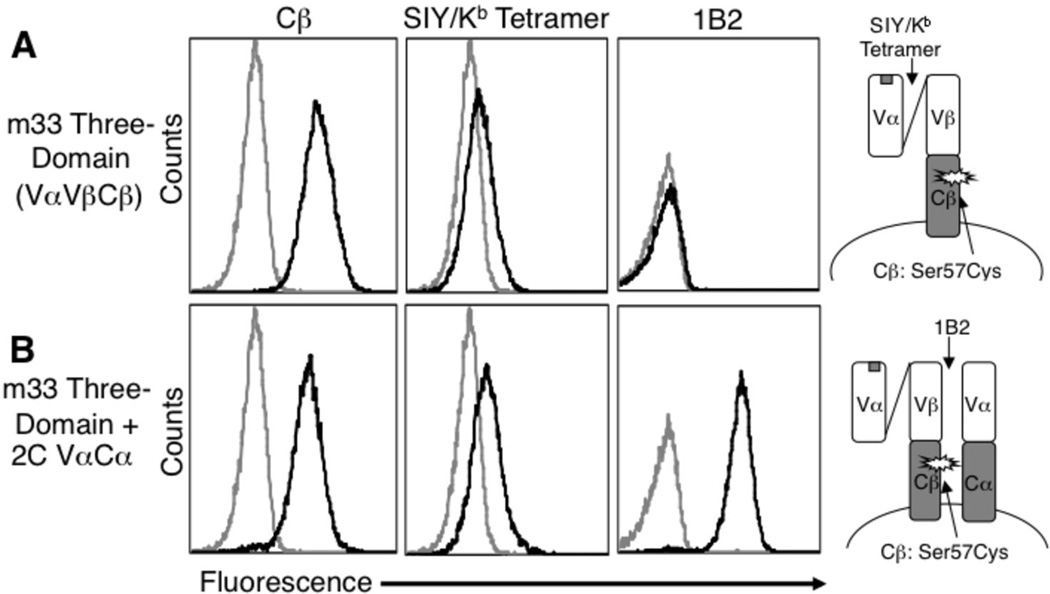
Expression of the m33 three-domain (VαVβCβ) construct (Figure 1) in the T cell hybridoma 58−/− resulted in cells that were cell surface positive for Cβ and weakly positive for staining with SIY/Kb (Figure 2A), but as expected were negative for staining with 1B2. The vector containing the m33 three-domain construct was then transduced into 58−/− cells together with the α chain from 2C (VαCα). These cells were not only positive for anti-Cβ and SIY/Kb, but were strongly positive for 1B2. The only way for the 2C α chain to reconstitute the 1B2-binding site is for the VβCβ region of the m33 three-domain construct to associate with the 2C α chain on the surface. As a consequence, it is likely that a fraction of cell surface receptors consist of the 2C α chain paired with the m33 β chain, while another fraction of cell surface receptors consists of the m33 Vα and Vβ domains of the three-domain construct properly associated. Thus, the three-domain construct can mispair with endogenous TCRs, and the nonnative Cβ cysteine did not prevent pairing with the endogenous α chain that lacks a non-native Cα cysteine. This result is consistent with a previous result that adding the C region cysteine mutation to a single introduced exogenous chain reduced but did not prevent mispairing with an endogenous chain 18.
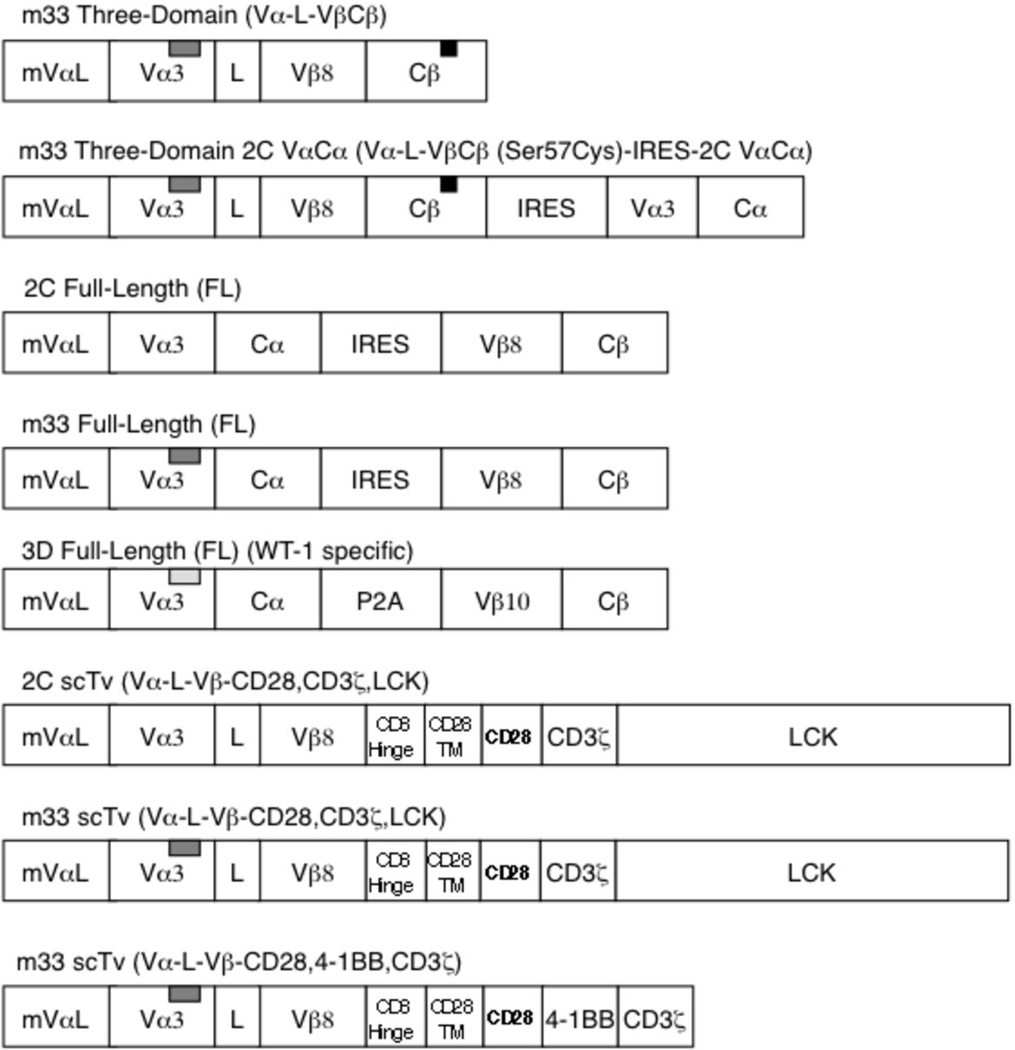
FIGURE 1.
Full length, three-domain, and single-chain VαVβ (Tv) TCR constructs. TCRs derived from the 2C or 3D TCR were introduced into the murine stem cell virus (MSCV) retroviral vector. The m33 high affinity variant of the 2C TCR differs only in the CDR3α sequence: 2C CDR3α-GFASA, m33 CDR3α-LHRPA (designated by gray insert rectangle). The 3D full-length receptor also contained CDR3α mutations that confer higher affinity to WT-1/Db. The m33 three domain constructs contained a non-native cysteine mutation (Cβ: Cys57), indicated by the black rectangle. IRES refers to internal ribosome entry site, CD28TM refers to the CD28 transmembrane domain, and mVαL refers to the murine Vα leader sequence.
FIGURE 2.
Three-domain m33 TCR mispairs with endogenous TCR α chains. T cell hybridoma 58−/− mock (no vector DNA) transduced cells (gray line) and transduced cells (black line) were analyzed by flow cytometry for surface expression after transduction with (A) the m33 three-domain construct or (B) the m33 three-domain construct and the 2C full α chain construct (2C VαCã̤ Transduced cells were examined with anti-Cβ antibody, SIY/Kb Tetramer (100 nM) or 1B2 clonotypic antibody. The m33 three-domain cassettes in both constructs contained a Cβ non-native cysteine while the 2C VαCα chain contained unmodified constant domains. The schematic in (B) depicts the binding sites of SIY/Kb tetramer and 1B2. A fraction of the receptors in (B) that mispair would bind to 1B2, and not bind to SIY/Kb tetramer, while other properly associated three-domain receptors on the same cell could bind to SIY/Kb tetramer.
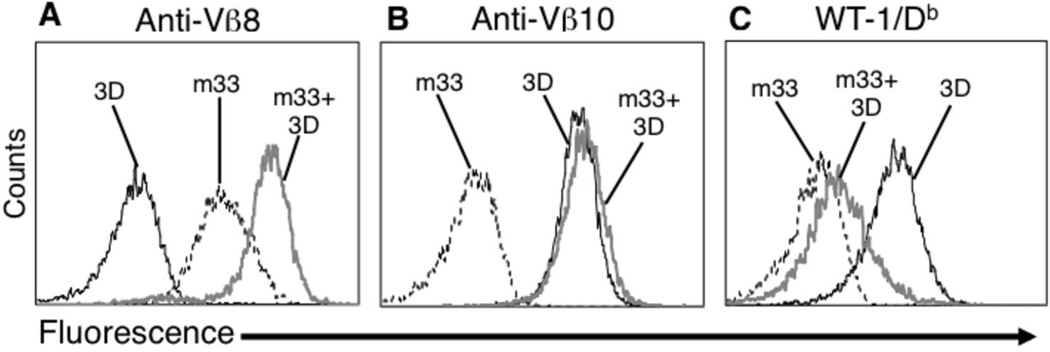
To verify that the three-domain construct was capable of mispairing with another TCR, when both the α and β chains were expressed, we used a mouse TCR called 3D that is specific for WT-1/Db. This TCR included mutations that improve the binding to WT-1/Db, as engineered by T cell display (unpublished results). The 3D TCR allowed detection of binding to a WT-1/Db dimer in the absence of co-receptor CD8, and thus, like m33 the pepMHC can be used directly to probe for properly associated 3D αβ. The 3D TCR was transduced with unmodified constant domains (Cα48:Thr, Cβ57:Ser) in order to provide a direct test of pairing of the three-domain m33 construct with the endogenous TCR chains. The m33 three-domain construct was then introduced into the 3D TCR expressing 58−/− T cells and cells were sorted with an antibody to Vβ8 (m33). Surface levels of (i) m33 three-domain construct alone, (ii) 3D TCR construct alone, or (iii) the combination of 3D TCR and m33 three-domain constructs were examined with anti-mouse Vβ8 (m33) (Figure 3A), anti-mouse Vβ10 (3D TCR) (Figure 3B), and WT-1/Db Ig dimmer (3D TCR) (Figure 3C). In cells co-expressing the m33 and 3D constructs, binding to the 3D ligand WT1/Db (Figure 3C) was significantly reduced, and binding to the m33 Vβ was significantly increased (Figure 3A). These results would appear to reflect mispairing of the m33 three-domain construct with the 3D α chain, resulting in a decrease in 3D heterodimers and the associated reduction of binding WT1/Db, and an increase in m33 Vβ expression from mispairing with the 3D α chain. The coexpressed 3D TCR and m33 three-domain construct showed similar levels of Vβ10 (Figure 3B) as the 3D TCR alone potentially because of ββ homodimers, as observed by others expressing murine constant domains 20.
FIGURE 3.
Three-domain m33 TCR mispairs with endogenous TCR 3D. The WT-1 specific TCR 3D, which contains unmodified murine constant domains, was used to mimic an endogenous TCR (3D TCR: mVα3, mVβ10). 3D TCR-positive T cell hybridoma 58−/− were transduced with the m33 three-domain TCR (Vβ8+) construct. Vβ8-sorted cells were analyzed with anti-Vβ8 (A), anti-Vβ10 (B) or WT-1/Db DimerX at 125 nM (C). Transduced 3D cells that co-express the m33 three-domain construct (gray line) were compared to cells that express only the 3D full-length TCR (black line), or only the m33 three-domain construct (black dashed line).
Expression of a scTv (VαVβ) avoids mispairing
As the three-domain construct mispaired with endogenous TCR chains, we reasoned it might be possible to use a single-chain TCR that lacked a Cβ region in order to completely avoid mispairing. Similar approaches have used scFv fragments as chimeric antigen receptors (CAR), consisting of various fused signaling domains 35. Although analogous single-chain TCR molecules are typically unstable without the Cβ domain, we have shown that it is possible to engineer scTv (VαVβ) by incorporating stabilizing mutations in the V regions 30,36,37.
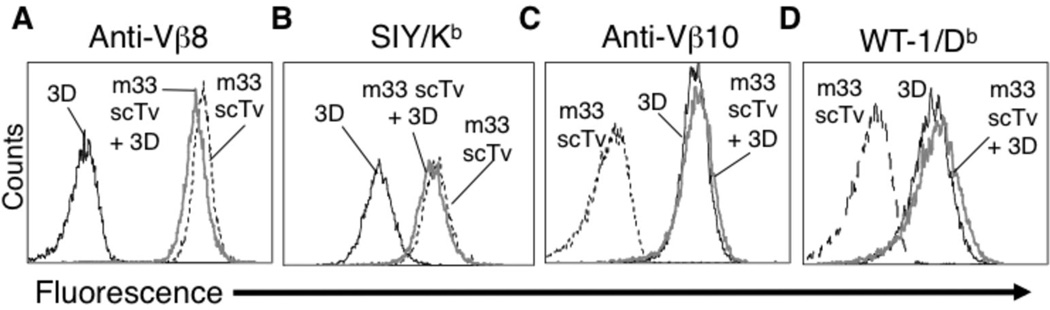
To test this strategy, we cloned the stabilized scTv of m33 as a fusion with CD28, CD3ζ, and Lck domains. The inclusion of Lck has been shown to improve T cell cytokine release in response to antigen with other chimeric antigen receptor constructs, where the extracellular binding domains were derived from MHC molecules 38. The m33 scTv fusion was introduced into 58−/− alone or with the 3D TCR and expressed at high levels on the surface of the 58−/− line, as detected with both the anti-Vβ8 antibody and the SIY/Kb (Figure 4). The binding of SIY/Kb indicated that the Vα and Vβ domains were associated appropriately at the cell surface. Furthermore, the expression of the m33 scTv was not influenced by expression of the 3D TCR, nor was the 3D TCR surface levels affected by the m33 scTv. These results suggest that significant mispairing was not occurring.
FIGURE 4.
A single-chain VαVβ TCR (scTv) construct avoids detectable mispairing with endogenous TCR 3D. 3D expressing T cells were transduced as in Fig. 3, but in this instance with the m33 scTv. Following sorting with anti-Vβ8 antibody, cells were analyzed with anti-Vβ8 (A), SIY/Kb tetramer at 100 nM (B) anti-Vβ10 (C), or WT-1/Db DimerX at 125 nM (D). Transduced 3D cells that co-express the m33 scTv construct (gray line) were compared to cells that express only the 3D full-length TCR (black line), or only the m33 scTv construct (black dashed line).
Surface levels of the stabilized scTv are higher than conventional TCR
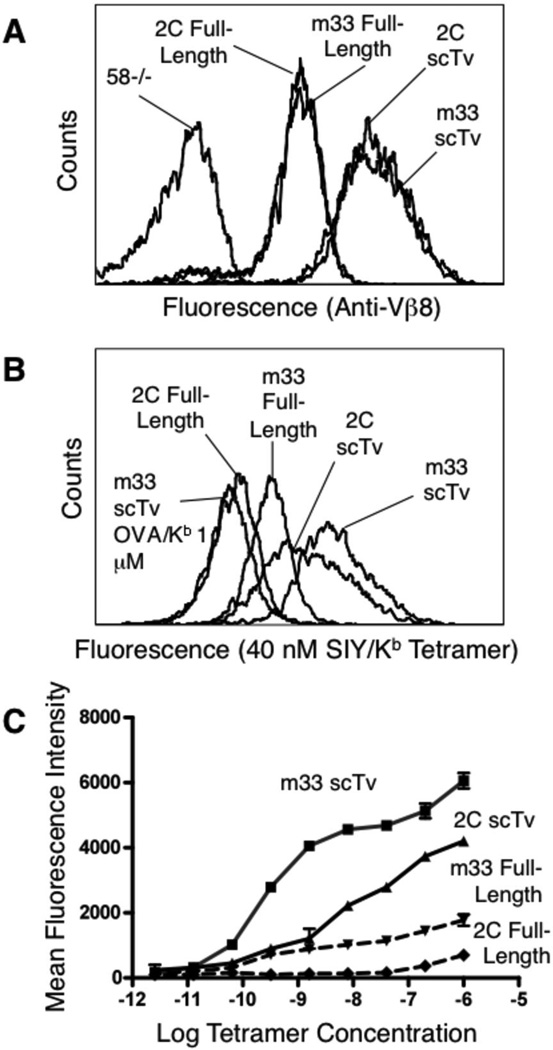
To determine whether the surface levels of the scTv fusions of the TCR were comparable to the conventional αβ TCR/CD3 complex, we examined transduced cells expressing the m33 scTv fusion or the full length m33 αβ chains in the absence of endogenous TCRs or the CD8 co-receptor. The scTv fusion was expressed at approximately five-times the surface level (based on mean fluorescence intensities) of the full-length αβ TCR, as detected with anti-Vβ8 antibody (Figure 5A). The wild type, low affinity 2C TCR expressed as either a scTv fusion or in the full-length form was detected at nearly identical levels to m33 (Figure 5A).
FIGURE 5.
Surface levels and SIY/Kb binding by scTv and full-length TCRs. scTv and full-length 2C (Kd=30 µM for SIY/Kb) and m33 (Kd=30 nM for SIY/Kb) constructs were introduced into 58−/− cells. Cell surface expression was monitored with anti-Vβ8 (A) or SIY/Kb SA:PE labeled tetramer at 40 nM (B) by flow cytometry. SIY/Kb tetramer binding to scTv and full-length constructs was examined at various concentrations of tetramer (C).
Since typical binding studies with pepMHC tetramers take advantage of the avidity achieved through the presence of many TCRs per cell, we next examined the binding of SIY/Kb tetramers to low (2C) and high-affinity (m33) forms of the TCR expressed as either a scTv fusion or full αβ TCR. As previously described 34,39,40, the αβ full-length, high-affinity TCR m33, but not the 2C, was capable of binding tetramers in the absence of CD8. The higher surface levels of the scTv forms yielded enhanced staining with the SIY/Kb tetramer, including significant binding by the 2C scTv despite its relatively low affinity (Kd = 30 µM). Full titrations with SIY/Kb tetramer revealed that the 2C low affinity scTv bound even higher levels than the high-affinity m33 expressed as a conventional αβ TCR, despite the 1000-fold higher affinity of m33 (Figure 5C). This finding highlights the importance of surface densities have on the binding of multimeric ligands.
Efficient T cell activation is mediated by the stabilized scTv fusions
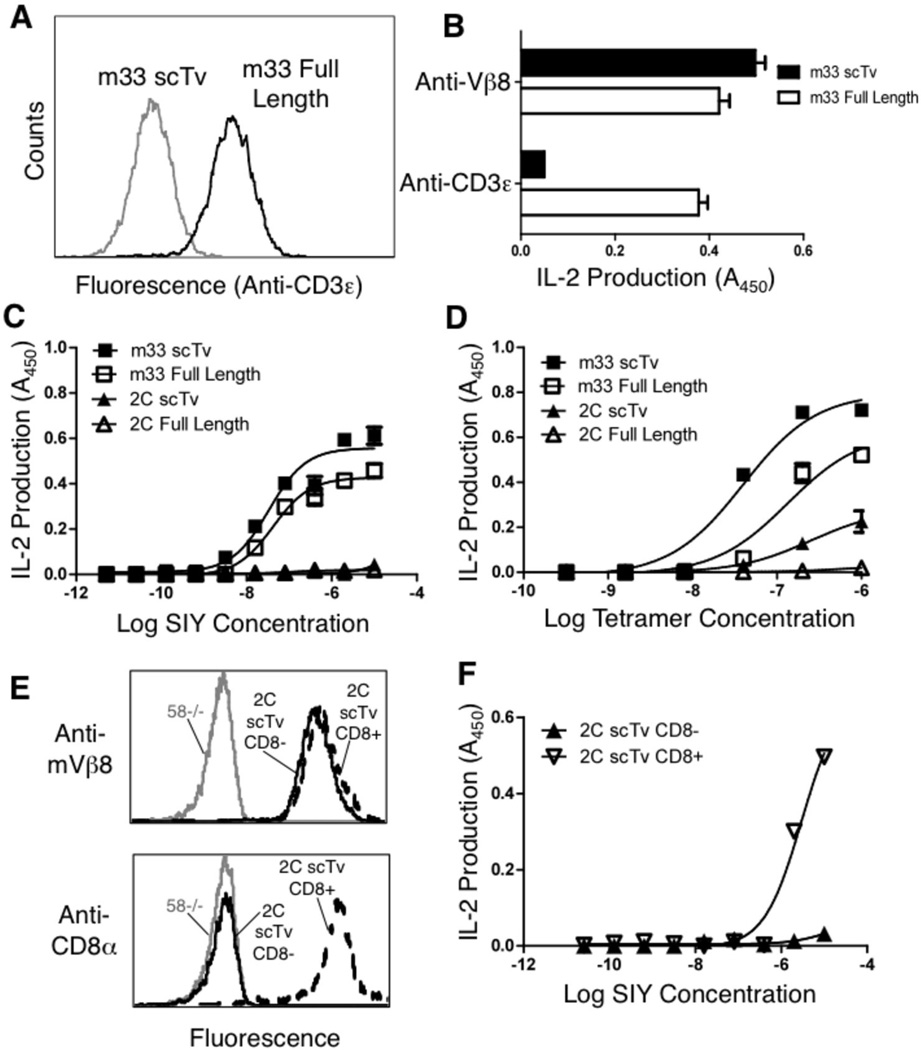
To examine the activation of T cells mediated by the scTv fusions, we examined IL-2 release by the transduced 58−/− lines using various stimulating ligands. As the 58−/− cell line was derived from an AKR mouse thymoma 41 and does not express Kb, it is unable to cross-present the SIY peptide. The full-length TCR was assembled with CD3 subunits, whereas surface expression of the scTv did not require CD3 as evidenced by the absence of staining with the anti-CD3ε antibody (Figure 6A). Despite the absence of CD3ε from the surface of cells transduced with scTv fusions, these cells were efficiently stimulated by anti-Vβ antibody immobilized on plates (Figure 6B). Cells that expressed the conventional αβ TCR were also stimulated by immobilized anti-CD3ε, whereas the scTv cells were not simulated by anti-CD3 antibodies (Figure 6B).
FIGURE 6.
Comparison of m33 (high affinity) and 2C (low affinity) full-length and scTv constructs for antigen specific T cell activation. (A) CD3ε surface expression of the m33 scTv (gray line) and full-length TCR (black line). (B) Activation of IL-2 release from m33 scTv and full-length expressing cells with plate bound anti-Vβ8 or anti-CD3ε antibodies. (C) Antigen specific activation of 2C and m33 scTv and full-length transduced cells using peptide-loaded T2-Kb cell line at various concentrations of exogenous SIY peptide. (D) Antigen specific activation of 2C and m33 scTv and full-length transduced cells using plate bound SIY/Kb tetramer. (E) Cell surface levels of 2C scTv (as monitored with anti-Vβ8) and CD8α in 2C scTv CD8αβ+ and CD8αβ- T cells. (F) Antigen specific activation of 2C scTv CD8− and CD8+ transduced cells with peptide-loaded T2-Kb cell line at various concentrations of exogenous SIY peptide. Data for B, C, and D, and F is representative of three independent experiments for each panel. OVA peptide (10 µM) loaded T2-Kb cells and OVA/Kb plate-bound pepMHC tetramer gave absorbance values from the IL-2 ELISA at background levels (not shown).
Antigen-presenting cells (T2-Kb) were used in the presence of various concentrations of the SIY peptide to determine if cells transduced with different forms of the TCRs mediated peptide dependent, CD8-independent activity. As described previously 32,40,42, CD8-negative cells transduced to express the m33 full-length TCR, but not the 2C TCR, responded to SIY peptide in the presence of T2-Kb (Figure 6C). The m33 scTv fusion stimulated activity at approximately the same concentration of peptide as the full-length m33 αβ TCR. In contrast, the 2C scTv was not stimulated by SIY-loaded T2-Kb cells, despite the high surface levels of the scTv form and the ability of the scTv to bind to SIY/Kb multimers (Figure 5C). Thus, in the absence of CD8, avidity did not overcome the requirement for an inherent binding threshold 40. However, immobilized SIY/Kb tetramers were capable of stimulating the 2C scTv fusions to a greater extent than the T2-Kb presented antigen (Figure 6D), consistent with our previous observations that low affinity TCRs can be stimulated under these conditions 40. Using a soluble high-affinity TCR as a probe, we have shown that the density of immobilized SIY/Kb is within a few fold of SIY/Kb on T2-Kb APCs. On the surface of T2-Kb cells, pulsed with 5 µM SIY, the estimated density was 2.5 × 108 molecules/µm2; at 250 nM tetramer immobilized on plastic, which is a stimulatory concentration for the 2C scTv T cells, the estimated density was 1.4 × 108 molecules/µm2 (40 and data not shown). Thus, the stimulation by pepMHC immobilized on plastic does not appear to be due to higher antigen density (although we can not rule out that it is due to higher localized densities of the ligand). A previous study has shown similar observations that immobilization can enhance T cell activity, but the mechanism is unknown 43. In our previous study, we showed that immobilized SIY/Kb elicited higher levels of pERK in the 2C full-length TCR 58−/− cells, but we have been unable to monitor Ca2+ levels in these T cell hybridoma lines 40.
To examine if CD8 can improve the response of the 2C scTv fusion to antigen, we introduced the 2C scTv into CD8αβ+ 58−/− cells (Figure 6E). T cell hybridomas with CD8 and the 2C scTv showed increased antigen sensitivity in response to T2-Kb cells (Figure 6F), presumably even without the assembled CD3 complex. This result suggests that the CD8 coreceptor might provide enhanced signaling through the scTv receptor, perhaps by recruiting the kinase Lck to the intracellular domains of the receptor through extracellular interactions between CD8 and cognate class I MHC molecules, and thereby also enhancing the avidity of the T cells for the target.
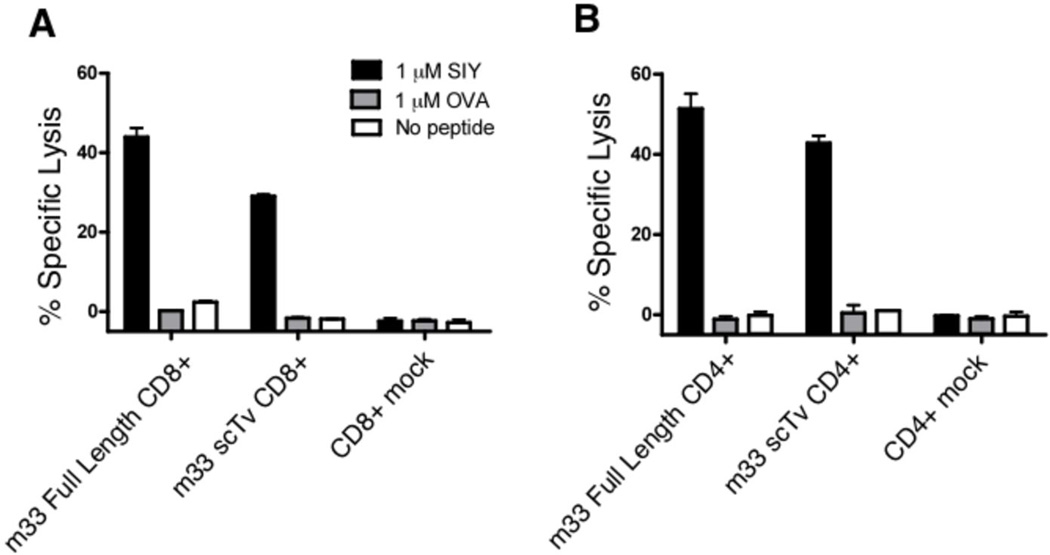
Finally, to evaluate the ability of the high-affinity m33 scTv constructs to redirect primary T cell activity, an m33 scTv receptor that contained intracellular signaling domains comprised of CD28, 4-1BB, and CD3ζ Figure 1, Vα−L-Vβ CD28,4-1BB,CD3ζ , was introduced into primary T cells and T cell cytotoxicity was assessed in a 51Cr release assay. Antigen-specific lysis of T2-Kb cells loaded with SIY at 1 µM was observed in both CD8+ (Figure 7A) and CD4+ (Figure 7B) polyclonal T cells, where activity was mediated by either the conventional full-length TCR or the scTv receptor.
FIGURE 7.
Comparison of m33 CD28, 4-1BB, CD3ζ scTv and m33 full-length TCR in T cell cytotoxicity directed against SIY+ APCs. Full-length m33 αβ TCR or m33 CD28, 4-1BB, CD3ζ scTv (m33 scTv) receptors were introduced into purified, activated CD8+ (A) or CD4+ (B) primary C57Bl/6 T cells and T cell cytotoxicity was assessed in a 4-hour 51Cr release assay with T2 target cells loaded with 1 µM exogenous SIY, OVA, or no peptide. T cell effector to T2 target cell ratio was fixed at 20:1. Mock – splenocytes activated without the addition of an endogenous receptor (untransduced).
T cell activity mediated through a stabilized human scTv fusion
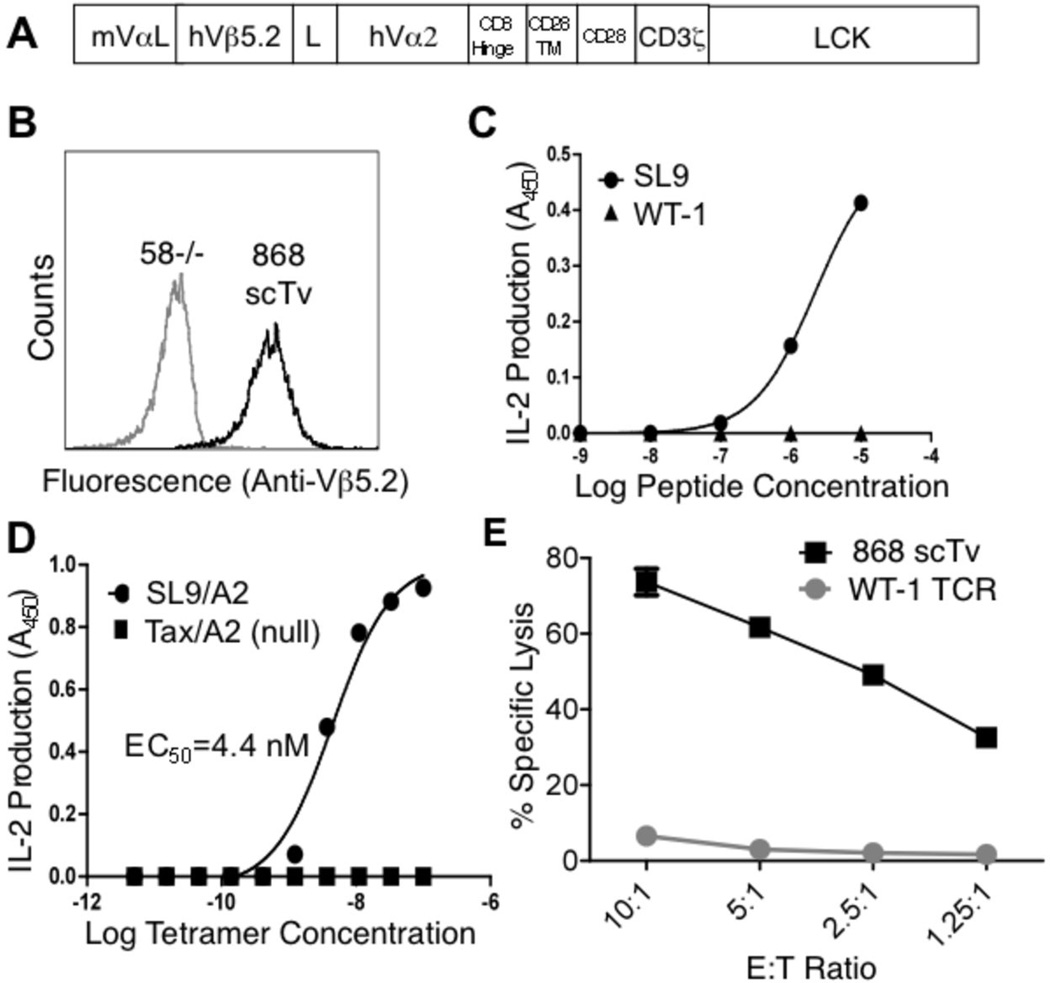
To examine the applicability of the scTv system to another TCR, a human TCR denoted 868, specific for HIV-Gag/HLA.A2 44, was introduced as an scTv construct. A stabilized scTv mutant of the parental αβ 868 TCR, engineered by yeast display, exhibited high-affinity for HIV-Gag (SL9)/HLA.A2 (Kd < 2 nM) 45. The human 868 scTv was fused to the same mouse intracellular signaling domains (Figure 8A) as with the 2C and m33 systems, the construct was transduced into the 58−/− T cell hybridoma, and the 868 scTv was detected on the surface as monitored by anti-human Vβ5.2 antibody (Figure 8B). The 868 scTv cell line was activated by SL9 peptide presented by T2 cells and by plate-bound SL9/HLA.A2 tetramer, but not the null peptide ligands WT1 or Tax (Figure 8C,D), consistent with our results with other TCR chains.
FIGURE 8.
The human high-affinity HIV-specific 868 scTv mediates antigen-specific activity in 58−/− T cells. (A) The leader from the 2C scTv was introduced upstream of the 868 scTv, and the human TCR was expressed as a fusion to murine intracellular signaling subunits. (B) Surface expression of 868 scTv in transduced cells was monitored by anti-human Vβ5.2. (gray line - 58−/− cells, black line – 868 scTv). To assess antigen specific activation, 868 scTv expressing cells were stimulated with (C) SL9 or WT-1 (null) peptide loaded T2 cells or (D) plate-bound SL9/A2 or Tax/A2 tetramers at various concentrations. Data in C and D are representative of two independent experiments for each panel. (E) T cell cytotoxicity with human PBMCs transduced with a WT-1 specific TCR (mock) or 868 scTv with T2 target cells pulsed with exogenous SL9 (10 µM) peptide. Data in E is representative of two experiments.
The 868 scTv, and a conventional full-length αβ TCR against the WT-1 peptide as control, were introduced into activated polyclonal peripheral human T cells, and examined in a 51Cr release assay (Figure 8E). Only T cells that expressed the 868 scTv were able to specifically lyse target cells that bear the SL9/HLA.A2 complex. Collectively, these experiments show that scTv chimeric receptors can be generated from various TCRs, that these receptors retain antigen specificity, and that this strategy can be used to generate antigen-specific T cells from a population of polyclonal human T cells that are capable of mediating effector functions against relevant pepMHC targets.
Discussion
Recent studies have introduced virus- or cancer-specific TCRs into T cells as a strategy to provide a patient with an enhanced T cell response, and ongoing studies are attempting to optimize this approach 1,2,9,13,14. In a related strategy to provide tumor-reactive T cells, transduced T cells expressing chimeric antigen receptors (CARs) that contain a scFv fused to intracellular T cell signaling domains (e.g. CD28, CD3ζ, and Lck) have entered clinical testing 35. Combining these approaches, several labs have attempted to use single-chain, three-domain TCRs (VαVβCβ) that can mediate proximal signaling through fused intracellular signaling domains 7,27,46. In principle, the advantages of these TCR fusions are that a single gene product could recognize target cell pepMHC antigens and stimulate the effector functions associated with T cells, independent of endogenous CD3 molecules 14. However, the three-domain strategy has been limited in some instances because the TCR surface levels are insufficient for recognition of low levels of antigen 28. Furthermore, while the three-domain TCR construct provides the stability necessary for surface expression, we show here that it does not avoid a major problem associated with use of exogenous TCRs in gene therapy, mispairing with endogenous TCR chains. This mispairing has recently been shown to result in neoreactivities 10, and T cell-induced graft-versus-host disease a mouse model 4. Possibly relevant to the mispairing issue, the expression of two TCRs in the same T cell allowed tolerance of T cells expressing the self-reactive TCR to be overcome, apparently by stimulation through the second TCR 47.
Improvements in surface levels of exogenous TCRs have been achieved by various methods, including codon optimization to enhance transcription levels 48, regulation of αβ chain stochiometry to improve proper pairing 49,50, inclusion of non-native cysteines 18,19, modification of other residues in the C regions 26,51,52, substitution of mouse C regions in human TCRs 15,53,54, or direct fusion of CD3ζ domains 16 to drive desired pairing of the introduced chains. However, the diversity of αβ TCRs makes it unlikely that mispairing can be completely avoided using these approaches. Therefore, attempts to avoid the problem altogether have involved use of different recipient cell types such as γδ T cells 55–57, NK cells 58, or oligoclonal populations of αβ T cells 4,59. However, these approaches also have limitations, including difficulty isolating and transducing a sufficient numbers of cells for adoptive transfer. A method to silence endogenous TCR has been developed using siRNA, but the duration and efficiency of endogenous TCR suppression is unclear 60. It is worth noting that because CD3ζ subunits are limiting in a T cell 22, several of these approaches will lead to competition between some exogenous and endogenous TCRs for CD3, resulting in lower levels of both receptors.
Our results show that the inclusion of a Cβ region with a non-native cysteine allowed association with endogenous α chains, and that deletion of the Cβ effectively eliminated pairing, within the limits of detection by flow cytometry (i.e. approximately 1,000 molecules/cell). The ability of α and β chains to pair is dependent on the interactions of the Vα:Vβ regions and the Cα:Cβ regions, but the relative contributions of each are not known. Assembly of the entire αβ/CD3 complex requires charged residues in the transmembrane domains of the various subunits 61. While the association of the Cα and Cβ domains presumably is the same among different TCRs, the Vα:Vβ interaction varies, and is controlled by multiple residues at the interface including regions within the CDR3α and CDR3β 30,62. Hence, TCR chain pairing studies often reveal “strong” or “weak” TCRs; and the efficiency of a particular αβ association helps determine the surface levels of a TCR pair in cells expressing multiple α and β chains 12,13,63. Our data demonstrates that a TCR with Vα and Vβ regions alone is insufficient to drive detectable association with an endogenous α and β TCR chains, suggesting that the dimerization constants of the V regions is low, and a large fraction of the association energy is driven by interactions between Cα and Cβ regions 29. As with the original principle of linking Vl and Vh to form scFv fragments 64, the scTv provides a strategy for forcing the association between the linked domains. In practice, this is not trivial, and single-chain VαVβ fragments (scTv) are considerably less stable than scFv fragments 30. Our lab has previously overcome this obstacle by engineering scTv with key stabilizing mutations in the V regions 30,36,37, and here we show that these scTv can be generated as fusions with intracellular signaling domains that can mediate T cell activation following antigen recognition. While chimeric antigen receptors directed by scFv fragments have been useful in targeting non-MHC restricted cell surface antigens, the recognition of MHC-associated antigens by scTv fragments makes it possible to target the vast array of intracellular antigens invisible to scFv on viable cancer or virally infected cells. In addition, scTv can recognize antigens released by cancer cells and cross-presented by tumor stroma, which can be critical for eradicating antigen-loss variants in a tumor mass and thereby prevent relapse after therapy 65,66. In principle, scFv fragments specific for MHC-restricted antigens could also be used for these purposes 67–70.
In order for a TCR, and presumably a scTv, to mediate activity in the absence of CD8 or CD4 binding to the presenting MHC molecules, its affinity needs to be above a threshold of about 1 µM 40. Because of this threshold limitation, the wild type TCR 2C (Kd = 30 µM) did not mediate activity against cell-presented antigen either as a scTv, or as a full-length TCR in the absence of CD8 (Figure 6C). Addition of the CD8 coreceptor provided some improvement in antigen sensitivity. Optimization of the fused signaling domains may be able to enhance the sensitivity of scTv, or possibly even overcome issues associated with tolerance when T cells must function in a suppressive tumor environment (e.g. 71,72). Nevertheless, it still remains questionable whether, in the absence of CD8, scTv that have not been modified to increase affinity will be capable of targeting the pepMHC levels found in many cancer or virus-infected cells. Fortunately, TCRs with affinities similar to those of antibodies can be engineered 73. We show here that scTv forms of engineered TCRs targeting a mouse pepMHC (SIY/Kb, m33 TCR) or the HIV Gag antigen (SL9/HLA.A2, 868 TCR) 44 can mediate T cell activation in a mouse T cell hybridoma line as well as polyclonal activated mouse and human T cells. Such high-affinity scTv could potentially improve TCR gene therapy formats, by avoiding the risk of TCR mispairing that is associated with receptors containing human or mouse Cα and Cβ regions, or their mutated variants. Furthermore, the higher affinity scTv would be capable of functioning independent of CD8 to mediate the activity of CD4+ Th cells 74,75.
Another potential advantage of the scTv-fusion approach is that the endogenous TCR/CD3 complexes remain at the same level on the transduced T cell as on the parental cell. In a study of T cells that expressed two transgenic TCRs, it was possible to overcome tolerance mediated by one of the TCRs through signaling mediated by the other TCR 71. It has also been shown that activation of T cells through the endogenous TCR can promote the persistence of transduced T cells, thereby enhancing their effectiveness against the target antigen of the transduced TCR 76,77. Thus, the ability of the scTv fusions to avoid mispairing could preserve efficient signaling through the endogenous TCR and avoid the formation of heterodimers of unknown specificity, yet allow the introduced scTv-fusion to mediate re-directed activity against tumor or viral epitopes.
Materials and Methods
Peptides, antibodies, and cell lines
Peptides SIY (SIYRYYGL), OVA (SIINFEKL), SL9 (HIV-Gag, SLYNTVATL), Tax (LLFGYPVYV) and WT-1 (RMFPNAPYL) peptides were synthesized by the Macromolecular Core Facility at Penn State College of Medicine (Hershey, PA). Peptides were purified by reverse-phase chromatography using a C18 column with mass confirmed by MALDI.
The following antibodies were used: PE-conjugated anti-mouse CD3ε (BD Pharmingen, Clone 145-2C11), anti-mouseVβ8.2 (Clone F23.2), 1B2 clonotypic antibody, anti-mouse Vβ10 (Clone B21.5, eBiosciences), DimerX Db (BD Biosciences), PE-conjugated anti-mouse CD8α (Clone 53–6.7, BD Pharmingen), goat anti-Mouse IgG AlexaFluor 647 (Molecular Probes) and anti-human Vβ5.2 (Clone 1C1, Pierce/Thermo Scientific). The 2C clonotypic antibody 1B2 was purified from hybridoma supernatant by ammonium sulfate precipitation followed by Protein G chromatography 78. Biotinylation of purified antibody was performed using the EZ-Link Suflo-NHS-LC-Biotinylation Kit (Pierce).
The Plat-E (Clonetech) retroviral packaging cell line was maintained in DMEM with 10% FCS, L-glutamine, penicillin and streptomycin. T2-Kb, a TAP-deficient cell line that can present exogenous peptides, and 58−/− T cell hybridoma derived from an AKR thymoma (H-2k) 41 were maintained in RPMI 1640 complete medium supplemented with 10% FCS, L-glutamine, penicillin and streptomycin. The 58−/− T cell line was transduced with retroviral supernatants of the full-length or scTv constructs as described 40.
Three-domain and two-domain (scTv) constructs
Various TCR constructs (Figure 1) were cloned into a murine stem cell virus (MSCV) retroviral vector 40. The two- and three-domain 2C and/or m33 single-chain TCR constructs contained six stabilizing mutations identified by yeast display (Vβ: Glu17Val, His47Tyr, Leu81Ser, Vα: Leu43Pro, Trp82Arg, Ile118Asn) 36,79. 2C TCR contains Vα3.1 (TRAV9-4) and Vβ8.2 (TRBV13-2) gene segments. The three-domain TCR gene was codon optimized (Genscript) in the Vα3-linker-Vβ8.2Cβ orientation with a nonnative cysteine at position 57 of the β chain constant region (Cβ: Ser57Cys), and introduced into the MSCV vector through AgeI/MluI restriction sites. For generation of the m33 Vα-linker-VβCβ-IRES-2CVαCα construct, the m33 three-domain construct was introduced into the 2C MSCV vector in the AgeI and XhoI restriction sites, replacing the 2C VβCβ chain 40.
For generation of scTv chimeric antigen receptor constructs, the murine CD8α hinge, CD28, CD3ζ, and LCK gene fusion was codon optimized (Genscript), based on sequences from the NIH database (GenBank accession #AAS07035.1) 27. The CD8α hinge was derived from scFv chimeric antigen receptor constructs previously described in murine systems 80, and the m33 scTCR (Vα-linker-Vβ) amplified by PCR from the three domain construct with a flanking 5’ AgeI restriction site. Subsequently, the scTv was fused to the CD8α hinge, CD28, CD3ζ gene by overlap extension PCR with a primer that added a 3’ MluI restriction site. The scTv gene fusion was introduced into the AgeI and MluI restriction sites of the MSCV vector. A XhoI site exists at the 3’ end of the m33 scTCR, prior to the start of the CD8α hinge region, allowing for introduction of other scTv genes with appropriate leader sequences into the scTv CD28,CD3ζ,LCK fusion protein. The 2C scTv with wild type CDR3α sequence (GFASA) was created by overlap extension PCR, and introduced into AgeI and XhoI sites in the m33 scTv construct.
The human TCR 868 against the HIV Gag peptide called SL9 44 was synthesized as a single-chain Tv 45. The 868 scTv gene was amplified by PCR from the pCT302 yeast display vector, with a 5’ primer that contained the leader sequence of the 2C scTv and an AgeI site, and a 3’ end primer which included a XhoI site. The 868 scTv was then introduced into the m33 scTv vector containing CD8α hinge, CD28, CD3ζ, LCK fusion in MSCV by the AgeI and XhoI sites, with the 868 scTCR replacing the m33 scTCR in the fusion construct. The 868 scTv gene contained high-affinity CDR mutations derived from phage-displayed, full-length TCR 44.
T cell hybridoma transductions
scTv and full-length TCR genes in MSCV were introduced into the Plat-E packaging line to produce cell supernatant containing retrovirus for introduction into 58−/− T cells. The retroviral packaging line was transfected with 40 µg of MSCV DNA with the lipofectamine 2000 reagent (Sigma) in Optimem serum-free media (Gibco). After four to six hours, cells were washed with supplemented DMEM and placed in 6 mL supplemented RPMI. Forty-eight hours later, supernatant was collected, filtered, and added to 58−/− T cell hybridoma cells with lipofectamine at a final volume of 8 µl/mL. T cells were centrifuged in retroviral supernatant at 1200 × g for 45 min at room temperature, cultured at 37°C for 3 days, and stained for cell surface mouse Cβ, mouse Vβ8, or human Vβ5 (868 scTv) with antibodies and analyzed on an Accuri C6 flow cytometer. To enrich for the transduced, positive population, cells were sorted with either anti-mouse Vβ8 (F23.2) for 2C and m33 or anti-human Vβ5.2 for 868, using a FACS Aria (BD Biosciences).
The 3D high-affinity TCR contained mouse Vα3 (TRAV9-1 subfamily, distinct from TRAV9-4 of 2C TCR, also called Vα3.1), mouse Vβ10 (TRBV4), and CDR3 mutations that conferred higher affinity for WT-1/Db (unpublished data), after isolation by T cell display 33. The 3D receptor contained unmodified TCR constant domains (Cα48:Thr, Cβ57:Ser), and was introduced into the 58−/− line as described for scTv and three-domain constructs. The 3D TCR+ population was sorted with anti-Cβ antibodies prior to transduction of m33 scTv or m33 threedomain.
pepMHC multimer binding
Transduced cells were stained with SIY/Kb pepMHC tetramer on ice for 2 hours. SIY/Kb tetramer was produced as has been described 40. After washing, cells were resuspended in cold PBS/BSA and analyzed for bound fluorescent tetramers by flow cytometry. Non-transduced T cell hybridoma 58−/− line was used as a control. Alternatively, WT-1/Db pepMHC Ig-dimers (DimerX, BD Biosciences) were prepared by incubation overnight at 37°C with WT-1 peptide at 120-molar excess. Transduced cells were stained with WT-1/DimerX for 2 hours on ice in the dark, washed twice with 10-fold excess volume PBS/BSA (0.5%), and incubated on ice for 1 h in the dark with goat anti-mouse IgG APC Fab’ Alexa Fluor 647 at 10 µg/mL (Molecular Probes).
T cell activation assays
T cells (7.5 × 104) were incubated with SIY or OVA peptide loaded T2-Kb cells (7.5 × 104), plate bound antibodies (anti-CD3ε or anti-mouse Vβ8 (F23.2)), or plate bound pepMHC tetramer (SIY/Kb or OVA/Kb) as described 40. For the 868 scTv cell line, cells were incubated with plate-bound antigenic SL9 (HIV Gag)/HLA.A2 or null Tax/HLA.A2 streptavidin tetramers or SL9 or WT-1 peptide-loaded T2 cells (7.5 × 104). T cells were incubated with the various stimuli for 24 h at 37°C in a final volume of 200 µL, and supernatants were collected. For IL-2 detection, 96-well plates were coated with 2.5 µg/mL anti-murine IL-2 (BD Pharmingen) in 0.1 M Na2HPO4 (pH=9.0), and IL-2 in supernatants was detected with 6.7 µg/mL biotinylated anti-murine IL-2 (BD Pharmingen), followed by a 1/10,000 dilution of streptavidin-HRP (BD Pharmingen), and finally, TMB substrate (Kirkegaard & Perry Laboratories). Absorbance at 450 nm was measured with an ELx800 universal plate reader (Bio-Tek Instruments).
Primary T cell transductions
Primary T cells were isolated from C57/BL6 splenocytes and treated with ACK lysis buffer to remove RBCs. Subsequently, cells were separated into CD4 or CD8 T cell populations using magnetic beads (MACS, Miltenyi Biotec). 1 × 106 purified cells were stimulated with anti-CD3/anti-CD28 Dynabeads (Invitrogen) per manufacturer’s instructions and 30 U/mL of IL-2 24 hours prior to transduction. Retroviral supernatant was prepared as described for 58−/− cells. Primary cells were centrifuged at 1200 xg, 30 °C for 1 hour (spinfection) with full-length TCR, scTv fusion (CD28, 4-1BB, CD3ζ) or mock (no vector DNA) retroviral supernatants, placed at 37 °C, 5% CO2 overnight followed by a second spinfection the next morning.
For human T cell transductions, CD8+ T cells were purified from total donor PBMCs by MACs purification using CD8 beads (Miltenyi Biotec). Purified CD8+ T cells were stimulated by incubation at a 1:1 ratio with CD3/CD28 Dynabeads (Dynal Biotech) and then transduced at 6 hours and 24 hours post-stimulation with lentiviral supernatants containing virus encoding the TCR alpha and beta chains from the WT1-specific TCR, or supernatants containing the retrovirus encoding the 868 scTv. Transductions were performed by spinfection for 90 minutes at 2000 xg in a 32 °C centrifuge in the presence of 5µg/ml Polybrene. Transduced T cells were cultured in 100 units of IL-2 for 7 days and then sorted for human Vbeta5.2 and SL9 tetramer expression.
Cytotoxicity Assays
For the m33 mouse T cell assays, transduced polyclonal CD4+ and CD8+ T cells were cultured in the presence of IL-2 (20 U/mL, day 3 post-activation) and on day 6 cells were incubated with 51Cr labeled T2-Kb cells pulsed with either 1 µM SIY or 1 µM OVA for four hours at 37 °C at an effector to target ratio of 20:1. For human PBMCs, activated T cells transduced with WT-1 specific αβTCR (mock) or 868 scTv were cultured with SL9 peptide pulsed T2 cells. Supernatants were isolated and 51Cr was quantified for each sample using a scintillation counter. Percent specific lysis was determined relative to spontaneous and maximal 51Cr release.
Acknowledgements
We thank the staff of University of Illinois Biotechnology Center for assistance in fluorescence activated cell sorting. We also thank Phil Holler for original engineering of high-affinity TCRs, including m33, and Carolina Soto for assistance with the m33 T cell cytotoxicity assays
Footnotes
This work was supported by grants from the NIH, GM55767 (to DMK), CA097296 (to HS and DMK), CA033084 and CA18029 (to PDG), a grant from the James S. McDonnell Foundation (to DMK), and grant 7040 from the Leukemia and Lymphoma Society (to PDG). BE was supported by a Research Fellowship of the DFG; JDS was supported by the Samuel and Ruth Engelberg/Irvington Institute Fellowship of the Cancer Research Institute.
Conflict of interest
The authors do not have a conflict of interest.
References
- 1.Bendle GM, Haanen JB, Schumacher TN. Preclinical development of T cell receptor gene therapy. Curr Opin Immunol. 2009;21:209–214. doi: 10.1016/j.coi.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–570. doi: 10.1038/nm.2128. 561p following 570. [DOI] [PubMed] [Google Scholar]
- 5.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 6.Sarukhan A, Garcia C, Lanoue A, von Boehmer H. Allelic inclusion of T cell receptor alpha genes poses an autoimmune hazard due to low-level expression of autospecific receptors. Immunity. 1998;8:563–570. doi: 10.1016/s1074-7613(00)80561-0. [DOI] [PubMed] [Google Scholar]
- 7.Willemsen RA, Weijtens ME, Ronteltap C, Eshhar Z, Gratama JW, Chames P, et al. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Ther. 2000;7:1369–1377. doi: 10.1038/sj.gt.3301253. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt TM, Ragnarsson GB, Greenberg PD. T cell receptor gene therapy for cancer. Hum Gene Ther. 2009;20:1240–1248. doi: 10.1089/hum.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci U S A. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engels B, Uckert W. Redirecting T lymphocyte specificity by T cell receptor gene transfer - a new era for immunotherapy. Mol Aspects Med. 2007;28:115–142. doi: 10.1016/j.mam.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Heemskerk MH, Hagedoorn RS, van der Hoorn MA, van der Veken LT, Hoogeboom M, Kester MG, et al. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood. 2007;109:235–243. doi: 10.1182/blood-2006-03-013318. [DOI] [PubMed] [Google Scholar]
- 13.Sommermeyer D, Neudorfer J, Weinhold M, Leisegang M, Engels B, Noessner E, et al. Designer T cells by T cell receptor replacement. Eur J Immunol. 2006;36:3052–3059. doi: 10.1002/eji.200636539. [DOI] [PubMed] [Google Scholar]
- 14.Govers C, Sebestyen Z, Coccoris M, Willemsen RA, Debets R. T cell receptor gene therapy: strategies for optimizing transgenic TCR pairing. Trends Mol Med. 2010;16:77–87. doi: 10.1016/j.molmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced Antitumor Activity of Murine-Human Hybrid T-Cell Receptor (TCR) in Human Lymphocytes Is Associated with Improved Pairing and TCR/CD3 Stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebestyen Z, Schooten E, Sals T, Zaldivar I, San Jose E, Alarcon B, et al. Human TCR that incorporate CD3zeta induce highly preferred pairing between TCRalpha and beta chains following gene transfer. J Immunol. 2008;180:7736–7746. doi: 10.4049/jimmunol.180.11.7736. [DOI] [PubMed] [Google Scholar]
- 17.Boulter JM, Glick M, Todorov PT, Baston E, Sami M, Rizkallah P, et al. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng. 2003;16:707–711. doi: 10.1093/protein/gzg087. [DOI] [PubMed] [Google Scholar]
- 18.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas S, Xue SA, Cesco-Gaspere M, San Jose E, Hart DP, Wong V, et al. Targeting the Wilms tumor antigen 1 by TCR gene transfer: TCR variants improve tetramer binding but not the function of gene modified human T cells. J Immunol. 2007;179:5803–5810. doi: 10.4049/jimmunol.179.9.5803. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi PS, Mak TW, Van den Elsen P, Yanagi Y, Yoshikai Y, Calman AF, et al. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985;316:606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- 22.Minami Y, Weissman AM, Samelson LE, Klausner RD. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1987;84:2688–2692. doi: 10.1073/pnas.84.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schodin BA, Tsomides TJ, Kranz DM. Correlation between the number of T cell receptors required for T cell activation and TCR-ligand affinity. Immunity. 1996;5:137–146. doi: 10.1016/s1074-7613(00)80490-2. [DOI] [PubMed] [Google Scholar]
- 24.Jorritsma A, Gomez-Eerland R, Dokter M, van de Kasteele W, Zoet YM, Doxiadis II, et al. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood. 2007;110:3564–3572. doi: 10.1182/blood-2007-02-075010. [DOI] [PubMed] [Google Scholar]
- 25.de Witte MA, Jorritsma A, Kaiser A, van den Boom MD, Dokter M, Bendle GM, et al. Requirements for effective antitumor responses of TCR transduced T cells. J Immunol. 2008;181:5128–5136. doi: 10.4049/jimmunol.181.7.5128. [DOI] [PubMed] [Google Scholar]
- 26.Sommermeyer D, Uckert W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J Immunol. 2010;184:6223–6231. doi: 10.4049/jimmunol.0902055. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, He X, Tsang TC, Harris DT. Transgenic TCR expression: comparison of single chain with full-length receptor constructs for T-cell function. Cancer Gene Ther. 2004;11:487–496. doi: 10.1038/sj.cgt.7700703. [DOI] [PubMed] [Google Scholar]
- 28.Schaft N, Lankiewicz B, Drexhage J, Berrevoets C, Moss DJ, Levitsky V, et al. T cell retargeting to EBV antigens following TCR gene transfer: CD28-containing receptors mediate enhanced antigen-specific IFNgamma production. Int Immunol. 2006;18:591–601. doi: 10.1093/intimm/dxh401. [DOI] [PubMed] [Google Scholar]
- 29.Voss RH, Thomas S, Pfirschke C, Hauptrock B, Klobuch S, Kuball J, et al. Coexpression of the T-cell receptor constant alpha domain triggers tumor reactivity of single chain TCR transduced human T cells. Blood. 2010 doi: 10.1182/blood-2009-11-254078. [DOI] [PubMed] [Google Scholar]
- 30.Richman SA, Aggen DH, Dossett ML, Donermeyer DL, Allen PM, Greenberg PD, et al. Structural features of T cell receptor variable regions that enhance domain stability and enable expression as single-chain ValphaVbeta fragments. Mol Immunol. 2009;46:902–916. doi: 10.1016/j.molimm.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schodin BA, Kranz DM. Binding affinity and inhibitory properties of a single-chain anti-T cell receptor antibody. J Biol Chem. 1993;268:25722–25727. [PubMed] [Google Scholar]
- 32.Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4:55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- 33.Chervin AS, Aggen DH, Raseman JM, Kranz DM. Engineering higher affinity T cell receptors using a T cell display system. J Immunol Methods. 2008;339:175–184. doi: 10.1016/j.jim.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho BK, Lian KC, Lee P, Brunmark A, McKinley C, Chen J, et al. Differences in antigen recognition and cytolytic activity of CD8(+) and CD8(−) T cells that express the same antigen-specific receptor. Proc Natl Acad Sci U S A. 2001;98:1723–1727. doi: 10.1073/pnas.98.4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kieke MC, Shusta EV, Boder ET, Teyton L, Wittrup KD, Kranz DM. Selection of functional T cell receptor mutants from a yeast surface- display library. Proc Natl Acad Sci U S A. 1999;96:5651–5656. doi: 10.1073/pnas.96.10.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber KS, Donermeyer DL, Allen PM, Kranz DM. Class II-restricted T cell receptor engineered in vitro for higher affinity retains peptide specificity and function. Proc Natl Acad Sci U S A. 2005;102:19033–19038. doi: 10.1073/pnas.0507554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiger TL, Nguyen P, Leitenberg D, Flavell RA. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood. 2001;98:2364–2371. doi: 10.1182/blood.v98.8.2364. [DOI] [PubMed] [Google Scholar]
- 39.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by Peptide/Major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chervin AS, Stone JD, Holler PD, Bai A, Chen J, Eisen HN, et al. The impact of TCR-binding properties and antigen presentation format on T cell responsiveness. J Immunol. 2009;183:1166–1178. doi: 10.4049/jimmunol.0900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letourneur F, Malissen B. Derivation of a T cell hybridoma variant deprived of functional T cell receptor alpha and beta chain transcripts reveals a nonfunctional alpha-mRNA of BW5147 origin. Eur J Immunol. 1989;19:2269–2274. doi: 10.1002/eji.1830191214. [DOI] [PubMed] [Google Scholar]
- 42.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 43.Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aggen DH, Chervin AS, Insaidoo FK, Piepenbrink KH, Baker BM, Kranz DM. Identification and engineering of human variable regions that allow expression of stable single-chain T cell receptors. Protein Engineering, Design, & Selection. 2011;24:361–372. doi: 10.1093/protein/gzq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badowski MS, Zhang T, Tsang TC, Harris DT. Chimeric antigen receptors for stem cell based immunotherapy. J Exp Ther Oncol. 2009;8:53–63. [PubMed] [Google Scholar]
- 47.Ji Q, Perchellet A, Goverman JM. Viral infection triggers central nervous system autoimmunity via activation of CD8+ T cells expressing dual TCRs. Nat Immunol. 2010;11:628–634. doi: 10.1038/ni.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholten KB, Kramer D, Kueter EW, Graf M, Schoedl T, Meijer CJ, et al. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin Immunol. 2006;119:135–145. doi: 10.1016/j.clim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Holst J, Vignali KM, Burton AR, Vignali DA. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods. 2006;3:191–197. doi: 10.1038/nmeth858. [DOI] [PubMed] [Google Scholar]
- 50.Leisegang M, Engels B, Meyerhuber P, Kieback E, Sommermeyer D, Xue SA, et al. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. J Mol Med. 2008;86:573–583. doi: 10.1007/s00109-008-0317-3. [DOI] [PubMed] [Google Scholar]
- 51.Voss RH, Willemsen RA, Kuball J, Grabowski M, Engel R, Intan RS, et al. Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J Immunol. 2008;180:391–401. doi: 10.4049/jimmunol.180.1.391. [DOI] [PubMed] [Google Scholar]
- 52.Bialer G, Horovitz-Fried M, Ya'acobi S, Morgan RA, Cohen CJ. Selected murine residues endow human TCR with enhanced tumor recognition. J Immunol. 2010;184:6232–6241. doi: 10.4049/jimmunol.0902047. [DOI] [PubMed] [Google Scholar]
- 53.Frankel TL, Burns WR, Peng PD, Yu Z, Chinnasamy D, Wargo JA, et al. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J Immunol. 2010;184:5988–5998. doi: 10.4049/jimmunol.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goff SL, Johnson LA, Black MA, Xu H, Zheng Z, Cohen CJ, et al. Enhanced receptor expression and in vitro effector function of a murine-human hybrid MART-1-reactive T cell receptor following a rapid expansion. Cancer Immunol Immunother. 2010;59:1551–1560. doi: 10.1007/s00262-010-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Veken LT, Hagedoorn RS, van Loenen MM, Willemze R, Falkenburg JH, Heemskerk MH. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res. 2006;66:3331–3337. doi: 10.1158/0008-5472.CAN-05-4190. [DOI] [PubMed] [Google Scholar]
- 56.van der Veken LT, Coccoris M, Swart E, Falkenburg JH, Schumacher TN, Heemskerk MH. Alpha beta T cell receptor transfer to gamma delta T cells generates functional effector cells without mixed TCR dimers in vivo. J Immunol. 2009;182:164–170. doi: 10.4049/jimmunol.182.1.164. [DOI] [PubMed] [Google Scholar]
- 57.Hiasa A, Nishikawa H, Hirayama M, Kitano S, Okamoto S, Chono H, et al. Rapid alphabeta TCR-mediated responses in gammadelta T cells transduced with cancer-specific TCR genes. Gene Ther. 2009;16:620–628. doi: 10.1038/gt.2009.6. [DOI] [PubMed] [Google Scholar]
- 58.Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A. 2008;105:17481–17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heemskerk MH, Hoogeboom M, Hagedoorn R, Kester MG, Willemze R, Falkenburg JH. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med. 2004;199:885–894. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto S, Mineno J, Ikeda H, Fujiwara H, Yasukawa M, Shiku H, et al. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 2009;69:9003–9011. doi: 10.1158/0008-5472.CAN-09-1450. [DOI] [PubMed] [Google Scholar]
- 61.Wucherpfennig KW, Gagnon E, Call MJ, Huseby ES, Call ME. Structural biology of the T-cell receptor: insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb Perspect Biol. 2010;2:a005140. doi: 10.1101/cshperspect.a005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartok I, Holland SJ, Kessels HW, Silk JD, Alkhinji M, Dyson J. T cell receptor CDR3 loops influence alphabeta pairing. Mol Immunol. 2010;47:1613–1618. doi: 10.1016/j.molimm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Hart DP, Xue SA, Thomas S, Cesco-Gaspere M, Tranter A, Willcox B, et al. Retroviral transfer of a dominant TCR prevents surface expression of a large proportion of the endogenous TCR repertoire in human T cells. Gene Ther. 2008;15:625–631. doi: 10.1038/sj.gt.3303078. [DOI] [PubMed] [Google Scholar]
- 64.Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, et al. Single-chain antigen-binding proteins. Science. 1988;242:423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 65.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 66.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willemsen RA, Debets R, Hart E, Hoogenboom HR, Bolhuis RL, Chames P. A phage display selected fab fragment with MHC class I-restricted specificity for MAGE-A1 allows for retargeting of primary human T lymphocytes. Gene Ther. 2001;8:1601–1608. doi: 10.1038/sj.gt.3301570. [DOI] [PubMed] [Google Scholar]
- 68.Stewart-Jones G, Wadle A, Hombach A, Shenderov E, Held G, Fischer E, et al. Rational development of high-affinity T-cell receptor-like antibodies. Proc Natl Acad Sci U S A. 2009;106:5784–5788. doi: 10.1073/pnas.0901425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chames P, Willemsen RA, Rojas G, Dieckmann D, Rem L, Schuler G, et al. TCR-like human antibodies expressed on human CTLs mediate antibody affinity-dependent cytolytic activity. J Immunol. 2002;169:1110–1118. doi: 10.4049/jimmunol.169.2.1110. [DOI] [PubMed] [Google Scholar]
- 70.Verma B, Neethling FA, Caseltine S, Fabrizio G, Largo S, Duty JA, et al. TCR mimic monoclonal antibody targets a specific peptide/HLA class I complex and significantly impedes tumor growth in vivo using breast cancer models. J Immunol. 2010;184:2156–2165. doi: 10.4049/jimmunol.0902414. [DOI] [PubMed] [Google Scholar]
- 71.Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, et al. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung AF, Dupage MJ, Dong HK, Chen J, Jacks T. Regulated expression of a tumor-associated antigen reveals multiple levels of T-cell tolerance in a mouse model of lung cancer. Cancer Res. 2008;68:9459–9468. doi: 10.1158/0008-5472.CAN-08-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richman SA, Kranz DM. Display, engineering, and applications of antigen-specific T cell receptors. Biomol Eng. 2007;24:361–373. doi: 10.1016/j.bioeng.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179:5845–5854. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chervin AS, Stone JD, Bowerman NA, Kranz DM. Cutting edge: inhibitory effects of CD4 and CD8 on T cell activation induced by high-affinity noncognate ligands. J Immunol. 2009;183:7639–7643. doi: 10.4049/jimmunol.0901664. [DOI] [PubMed] [Google Scholar]
- 76.Dossett ML, Teague RM, Schmitt TM, Tan X, Cooper LJ, Pinzon C, et al. Adoptive immunotherapy of disseminated leukemia with TCR-transduced, CD8+ T cells expressing a known endogenous TCR. Mol Ther. 2009;17:742–749. doi: 10.1038/mt.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kranz DM, Tonegawa S, Eisen HN. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shusta EV, Holler PD, Kieke MC, Kranz DM, Wittrup KD. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat Biotechnol. 2000;18:754–759. doi: 10.1038/77325. [DOI] [PubMed] [Google Scholar]
- 80.Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]