I. OVERVIEW
Similar to many other G protein-coupled receptors (GPCRs), the functionality of α2B-adrenergic receptor (α2B-AR) is dependent on its proper transport to the cell surface. However, compared with the well-understood endocytic and recycling pathways, the molecular mechanism underlying the anterograde trafficking of newly synthesized α2B-AR from the endoplasmic reticulum (ER) through the Golgi to the plasma membrane remains poorly elucidated. Recent studies have revealed that α2B-AR targeting to the cell surface is a highly regulated process, which is coordinated by many intrinsic determinants and regulatory proteins. This chapter will review the roles of recently identified motifs and the Sar1/ARF and Rab GTPases in α2B-AR exit from intracellular organelles and transport from the ER to the cell surface.
II. INTRODUCTION
As the largest superfamily of cell surface receptors, G protein-coupled receptors (GPCRs) regulate a variety of cellular functions through coupling to heterotrimeric G proteins, which in turn modulate the activity of downstream effectors, including adenylyl cyclases, phospholipases, protein kinases, and ion channels (Hanyaloglu & von Zastrow, 2008; Pierce, Premont, & Lefkowitz, 2002; Rosenbaum, Rasmussen, & Kobilka, 2009). The life of GPCRs begins in the endoplasmic reticulum (ER) where they are synthesized. Once correctly folded and properly assembled, GPCRs are able to pass the ER quality control system and exit from the ER, beginning the journey of intracellular trafficking (Dong, Filipeanu, Duvernay, & Wu, 2007). The nascent receptors then pass many intracellular compartments, including the ER-Golgi intermediate compartment (ERGIC), the Golgi, and the trans-Golgi network (TGN), en route to the cell surface, which is the functional destination for most GPCRs. An important feature for the cell surface GPCRs is that they may undergo internalization in response to sustained agonist stimulation during which the receptors are transported from the plasma membrane to endosomes. The internalized receptors in endosomes may be sorted to different destinations, including the recycling pathway for return to the cell surface, the lysosomal compartment for degradation, and the Golgi for retrograde transport. Therefore, the balance of these dynamic intracellular trafficking events dictates the amount of the receptors at the plasma membrane, which in turn controls the magnitude of cellular response to a given extracellular signal. Over the past decades, most studies on the intracellular trafficking of GPCRs have focused on the endocytosis and recycling processes. These studies have not only greatly advanced our knowledge about the mechanisms of GPCR trafficking but also revealed physiological functions for the trafficking in regulating receptor signal propagation and in the pathogenesis of human diseases (Hanyaloglu & von Zastrow, 2008; Marchese, Chen, Kim, & Benovic, 2003; Moore, Milano, & Benovic, 2007; Tan, Brady, Nickols, Wang, & Limbird, 2004; Wu, Benovic, Hildebrandt, & Lanier, 1998; Wu, Krupnick, Benovic, & Lanier, 1997; Xia, Gray, Compton-Toth, & Roth, 2003). In contrast, the molecular mechanism underlying anterograde transport of nascent GPCRs from the ER through the Golgi apparatus to the cell surface and the role of export traffic in the functional regulation of the receptors have just begun to be elucidated.
The progress achieved over the past few years indicates that, similar to the endocytic and recycling pathways, the ER-to-cell surface movement of GPCRs is a highly regulated, dynamic process, which is orchestrated by structural features of the receptors and many regulatory proteins. First, it has been demonstrated that ER export is a rate-limiting step for the cell surface transport of GPCRs (Petaja-Repo, Hogue, Laperriere, Walker, & Bouvier, 2000). Second, a number of studies have recently identified highly conserved hydrophobic sequences, which are required for GPCR export from the ER (Bermak, Li, Bullock, & Zhou, 2001; Carrel, Hamon, & Darmon, 2006; Robert, Clauser, Petit, & Ventura, 2005; Schulein et al., 1998). These studies suggest that, similar to many other plasma membrane proteins, GPCR exit from the ER may be dictated by specific export motifs. Third, cell surface transport of GPCRs is modulated by direct interactions with multiple regulatory proteins such as the receptor activity modifying proteins (RAMPs), the ER chaperone proteins, and accessory proteins which may behave as chaperones/escort proteins, stabilizing receptor conformation and promoting their delivery to the plasma membrane (Dong et al., 2007). Fourth, dimerization (homo- and hetero-dimerization) may also participate in the regulation of GPCR export to the cell surface, likely through influencing their correct folding or assembly in the ER (Bouvier, 2001; Salahpour, Angers, Mercier, Lagace, Marullo, & Bouvier, 2004; Zhang et al., 2009; Zhou, Filipeanu, Duvernay, & Wu, 2006). Finally, GPCR transport from the ER through the Golgi to the cell surface is mediated through distinct pathways, in which the Ras-like Rab GTPases play a crucial role (Dong & Wu, 2007; Filipeanu, Zhou, Claycomb, & Wu, 2004; Filipeanu, Zhou, Fugetta, & Wu, 2006; Wu, Zhao, & He, 2003).
My laboratory has used adrenergic and angiotensin II receptors as representatives to search for the players that control the cell surface targeting of the receptors by addressing two important questions: Are there conserved structural elements in GPCRs which function as motifs dictating their exit from intracellular compartments? And could the export trafficking of GPCRs be selectively regulated by well-defined transport regulators? Over the past several years, we have identified several highly conserved residues essential for the receptors to exit from the ER and the Golgi apparatus (Dong & Wu, 2006; Duvernay et al., 2009a, 2009b; Duvernay, Zhou, & Wu, 2004; Zhou et al., 2006). We have also demonstrated that small GTPases, specifically the Rab and Sar1/ARF subfamilies, may selectively or differentially modulate the anterograde traffic of GPCRs along the secretory pathway (Dong & Wu, 2007; Dong et al., 2010a, 2010b; Dong, Zhou, Fugetta, Filipeanu, & Wu, 2008; Filipeanu et al., 2004, 2006; Wu et al., 2003; Zhang et al., 2009).
In this chapter, we will review the role of structural determinants and small GTPases, specifically the Sar1/ARF and Rab subfamilies, in the regulation of α2B-AR exit from intracellular compartments and transport from the ER to the cell surface. There are three α2-AR subtypes, designated as α2A-AR, α2B-AR, and α2C-AR, all of which play an important role in regulating sympathetic nervous system, both peripherally and centrally. All three α2-ARs have similar structural features: whereas the third intracellular loop (ICL3) is quite large with more than 170 amino acid residues, other loops and the termini are relatively short with less than 25 residues.
III. THE STRUCTURAL BASIS OF α2B-AR EXPORT FROM THE ER AND THE GOLGI
Although all three α2-ARs have a strong similarity in their structures and functions, they are markedly different in their abilities to move to the cell surface. In particular, α2C-AR transports to the cell surface in a cell type- and temperature-dependent fashion. For example, α2C-AR is able to efficiently move to the plasma membrane in some neuroendocrine cells, such as PC12 and AtT20 cells, in a temperature-independent manner, whereas the majority of α2C-AR is arrested in the intracellular compartments including the ER and the Golgi, unable to transport to the cell surface at 37°C in fibroblasts and vascular smooth muscle cells, and reducing temperature may facilitate the cell surface transport of the intracellularly accumulated receptors (Bailey, Eid, Mitra, Flavahan, & Flavahan, 2004; Daunt, Hurt, Hein, Kallio, Feng, & Kobilka, 1997; Jeyaraj, Chotani, Mitra, Gregg, Flavahan, & Morrison, 2001). Such an effect of lowering temperature on α2C-AR translocation may contribute to Raynaud syndrome which is characterized by enhanced peripheral vasoconstriction during cold exposure or emotional stress and can be ameliorated by using α2-AR antagonists. Interestingly, it has been demonstrated that the intra-cellular accumulation of α2C-AR may be under the control of multiple arginine residues in the C-terminus and hydrophobic residues in the N-terminus which may function as ER retention motifs trapping the receptor in the ER (Angelotti, Daunt, Shcherbakova, Kobilka, & Hurt, 2010; Ma et al., 2001).
In contrast to α2C-AR, both α2A-AR and α2B-AR are normally expressed at the cell surface and recent studies have demonstrated that their transport from the ER to the cell surface is controlled by multiple highly conserved specific motifs. Specifically, the F436, I443, and L444 residues [F(x)6IL motif] in the C-terminus and a single L48 residue in the first intracellular loop (ICL1) are required for α2B-AR to exit from the ER (Duvernay et al., 2004, 2009b, 2009b), whereas the Y12/S13 motif located in the N-terminus is crucial for α2B-AR export from the Golgi (Dong & Wu, 2006). In addition, the ICL3 may possess signals for the retention of the receptor in the basolateral subdomain in polarized cells (Brady, Wang, Colbran, Allen, Greengard, & Limbird, 2003; Edwards & Limbird, 1999; Keefer, Kennedy, & Limbird, 1994; Keefer & Limbird, 1993; Prezeau, Richman, Edwards, & Limbird, 1999; Saunders, Keefer, Bonner, & Limbird, 1998; Saunders & Limbird, 2000; Wozniak & Limbird, 1996).
A. The C-terminal F(x)6IL Motif in α2B-AR Export from the ER
Protein export from the ER is a selective process that may be dictated by short, linear sequences called ER export motifs (Kappeler, Klopfenstein, Foguet, Paccaud, & Hauri, 1997; Nishimura & Balch, 1997; Nishimura et al., 1999; Nishimura, Plutner, Hahn, & Balch, 2002; Nufer et al., 2002; Nufer, Kappeler, Guldbrandsen, & Hauri, 2003; Votsmeier & Gallwitz, 2001; Wendeler, Paccaud, & Hauri, 2007). Of various ER export motifs identified, the diacidic motifs have been found in the cytoplasmic C-termini of several membrane proteins such as vesicular stomatitis virus glycoprotein (VSVG), cystic fibrosis transmembrane conductance regulator, and potassium channels (KAT1, TASK-3, and Kir2.1) (Ma et al., 2001; Nishimura & Balch, 1997; Nishimura et al., 1999; Sevier, Weisz, Davis, & Machamer, 2000; Wang et al., 2004b; Zuzarte, Rinne, Schlichthorl, Schubert, Daut, & Preisig-Muller, 2007) and demonstrated to function as ER export motifs. Interestingly, export function of the diacidic motifs is mediated through their interaction with components of COPII transport vesicles, particularly Sec24 subunits. This interaction results in the concentration of cargo in ER exit sites and facilitates cargo recruitment onto the vesicles (Farhan, Reiterer, Korkhov, Schmid, Freissmuth, & Sitte, 2007).
The C-terminal tails of GPCRs consist of a putative amphipathic 8th α-helix in the membrane-proximal region and a nonstructural membrane-distal region. The function of the C-terminus, particularly the membrane-proximal 8th α-helix portion, in regulating cell surface transport of the receptors has been described for a number of GPCRs including angiotensin II type 1 receptor (AT1R), rhodopsin, vasopressin V2 receptor, dopamine D1 receptor, adenosine A1 receptor, melanin-concentrating hormone receptor 1, and luteinizing hormone/choriogonadotropin receptor (Duvernay et al., 2004; Gaborik, Mihalik, Jayadev, Jagadeesh, Catt, & Hunyady, 1998; Heymann & Subramaniam, 1997; Pankevych, Korkhov, Freissmuth, & Nanoff, 2003; Rodriguez, Xie, Wang, Collison, & Segaloff, 1992; Tetsuka, Saito, Imai, Doi, & Maruyama, 2004). We first demonstrated that deletion of the entire C-terminus almost abolished the cell surface expression of α2B-AR and subsequent mutagenesis of individual residues in the C-terminus revealed F436 and I443/L444 residues in the membrane-proximal portion essential for α2B-AR transport to the cell surface (Duvernay et al., 2004) (Fig. 1A). Consistent with the lack of cell surface expression, the mutated receptor lacking the F436 and I443/L444 was unable to initiate downstream signaling, such as activation of ERK1/2 (Duvernay et al., 2004). Further subcellular distribution analysis showed that the mutated receptors were strongly accumulated in the ER, suggestive of defective ER export. Interestingly, the function of F436 and I443/L444 in mediating α2B-AR export cannot be fully substituted by any other hydrophobic residues (Duvernay et al., 2009b). These data indicate that the F(x)6IL motif modulates α2B-AR export at the level of the ER and this function is mediated by its unique properties.
FIGURE 1.
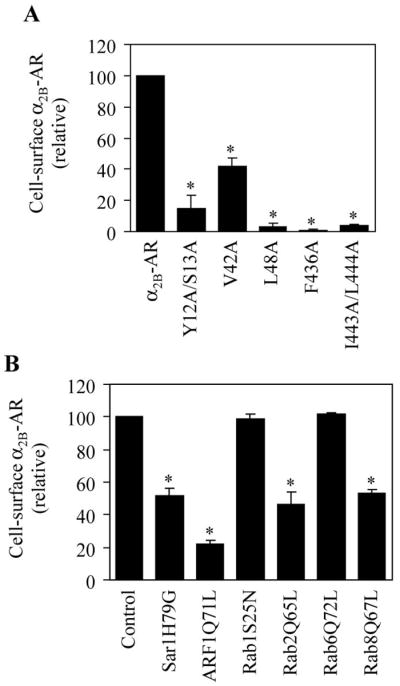
Effect of mutating specific residues (A) and expressing small GTPase mutants (B) on the cell surface expression of α2B-AR as measured by intact cell ligand binding. (A) α2B-AR and its mutants in which specific residues were mutated to alanines were transiently transfected into HEK293 cells. (B) α2B-AR was transfected with or without individual small GTPase mutants into HEK293 cells. The cell surface expression of α2B-AR was measured by intact cell ligand binding by using [3H]-RX821002 at a concentration of 20 nM. *, p < 0.05 versus wild type α2B-AR (A) or control (B). (The data are adapted from the references Dong & Wu, 2006; Duvernay et al., 2009a, 2009b).
Consistent with the role of the F(x)6IL motif in α2B-AR transport, several similar motifs, such as the E(x)3LL, FN(x)2LL(x)3L, and F(x)3F(x)3F motifs, have been identified to control the ER-to-cell surface transport of other GPCRs (Bermak et al., 2001; Robert et al., 2005; Schulein et al., 1998). Importantly, the F(x)6LL motif (where x can be any residues and L leucine or isoleucine) is highly conserved in the membrane-proximal C-termini of many family A GPCRs (Duvernay et al., 2004) and indeed, this motif is also required for ER export of several other GPCRs, including α1B-AR, β2-AR, and AT1R (Duvernay et al., 2009b).
To further provide insights into how the F(x)6IL motif controls α2B-AR transport, we analyzed the structural features of the motif by homology modeling based on the newly published crystal structure of β2-AR. F436 residue is buried within the hydrophobic core of the receptor and in close proximity to V42 in the first transmembrane domain and mutation of V42 also significantly impairs α2B-AR export to the cell surface (Fig. 1A). Furthermore, the defect in the transport of the F436A mutant can be partially rescued by a number of treatments, such as chemical chaperones and lowing temperature, and the mutant has enhanced abilities to bind to the chaperone proteins calnexin and calreticulin. These data suggest that the F436 residue is likely involved in the regulation of proper α2B-AR folding in the ER, which is mediated through intramolecular interactions with other hydrophobic residues, such as V42 in the first transmembrane domain, enabling the receptor to pass the ER quality control and to export from the ER.
How I443/L444 residues influence α2B-AR export from the ER remains unknown. The dileucine-based motifs have been demonstrated to be involved in both endocytosis and basolateral delivery. The fact that the branched carbon side chains of the I443/L444 residues are exposed to the cytosolic space suggests that they are capable of providing a docking site for other proteins (Duvernay et al., 2009b). Indeed, our recent studies have demonstrated that Rab8 GTPase modulates β2-AR transport from the TGN, which is likely mediated through its physical association with the C-terminal dileucine motif of the receptor. However, mutation of the I443/L444 residues did not alter α2B-AR interaction with Rab8 (Dong et al., 2010a). Therefore, to search for proteins interacting with the dileucine motif in the cytoplasm, particularly components of transport machinery or other trafficking-related regulatory proteins, will help to elucidate the mechanism of the I443/L444 motif in α2B-AR export from the ER.
B. The L48 Residue in α2B-AR Exit from the ER
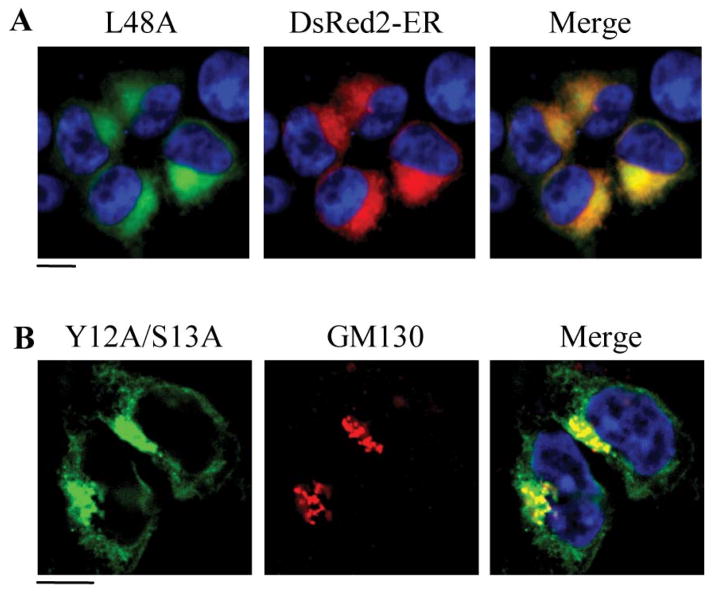
The ICL1 of α2B-AR is very short, composed of only 12 amino acid residues. Similar to the C-terminus, the ICL1 is absolutely necessary for proper transport of α2B-AR to the cell surface, as the ICL1-deleted receptor was accumulated in intracellular compartments and unable to transport to the cell surface (Duvernay et al., 2009a). Mutagenesis studies identified a single L48 residue essential for the cell surface transport of α2B-AR (Duvernay et al., 2009a) (Fig. 1A) and the mutated receptor was very well co-localized with the ER marker DsRed2-ER (Fig. 2A), suggesting that L48 residue is involved in the regulation of α2B-AR exit from the ER.
FIGURE 2.
Colocalization of the α2B-AR mutants L48A and Y12A/S13A with ER and Golgi markers, respectively. (A) Colocalization of the α2B-AR mutant L48A with the ER marker DsRed2-ER. HEK293 cells were transfected with the GFP-tagged L48A mutant together with pDsRed2-ER and the subcellular distribution and colocalization of the receptor with DsRed2-ER were revealed by fluorescence microscopy. (B) Colocalization of the α2B-AR Y12A/S13A mutant with the cis-Golgi marker GM130. HEK293 cells were transfected with Y12A/S13A and its co-localization with GM130 was revealed by fluorescence microscopy following staining with antibodies against GM130 at 1:50 dilution. Scale bars, 10 μm. (The data are adapted from the references Dong & Wu, 2006; Duvernay et al., 2009a).
An isolated leucine residue in the center of the ICL1 is remarkably conserved among the class A GPCRs. About 85% of the family A GPCRs in human and 83% in all species contain a leucine residue in the center of ICL1 (Duvernay et al., 2009a). Mutation of this conserved residue also significantly attenuated the cell surface expression of several other GPCRs, including β1-AR, AT1R, and α1B-AR (Duvernay et al., 2009a). These data suggest that the single leucine residue in the ICL1 may be a common signal mediating the ER export of a number of GPCRs.
C. The N-terminal Y12/S13 Motif in α2B-AR Export from the Golgi
Recent studies have demonstrated that, similar to exit from the ER, protein export from the Golgi/TGN is a selective process that may be dictated by specific export motifs. Newly synthesized proteins are sorted at the Golgi/TGN to be delivered to final cellular destinations, such as endosomes, lysosomes, and the plasma membrane. There are several well-defined endosomal sorting signals including tyrosine-based motifs (NPxY and YxxØ, where x can be any residue and Ø is a hydrophobic residue) and dileucine-based motifs ([D/E]xxxL[L/I] and DxxLL). Whereas YxxØ and [D/E]xxxL[L/I] motifs are recognized by the adaptor protein complexes, DxxLL is recognized by Golgi-localized γ-ear-containing ARF1-binding proteins (GGAs) (Hirst, Lui, Bright, Totty, Seaman, & Robinson, 2000; Puertollano, Aguilar, Gorshkova, Crouch, & Bonifacino, 2001; Puertollano, Randazzo, Presley, Hartnell, & Bonifacino, 2001). These motifs function to sort protein transport from the TGN to the endosomal compartment (Boucher, Larkin, Brodeur, Gagnon, Theriault, & Lavoie, 2008; Chen, Yuan, & Lobel, 1997; Hou, Suzuki, Pessin, & Watson, 2006; Johnson & Kornfeld, 1992; Lori, Florencia, & Frederick, 2007).
The fact that G protein-coupled olfactory and chemokine receptors as well as the opsin mutant E150K are released from the ER, but accumulated in the Golgi (Gimelbrant, Haley, & McClintock, 2001; Venkatesan, Petrovic, Van Ryk, Locati, Weissman, & Murphy, 2002; Zhu et al., 2006) suggests that GPCR export from the Golgi and transport from the Golgi to the cell surface is a regulated process. We found that the N-terminus, specifically Y12 and S13 residues in the membrane-proximal N-terminal region, is absolutely required for the transport of α2B-AR to the cell surface. Single and double substitution of the Y12/S13 motif significantly reduced the cell surface expression of α2B-AR (Dong & Wu, 2006) (Fig. 1A). However, unlike the F(x)6IL and L48 mutants that were accumulated in the ER, the Y12/S13 motif mutants were retained in the Golgi apparatus (Dong & Wu, 2006) (Fig. 2B), suggesting that the Y12/S13 motif mediates α2B-AR export at the level of the Golgi. The YS motif only exists in the membrane proximal N-termini of three α2-AR family members and indeed, it exerts a similar function on α2A-AR trafficking (Dong & Wu, 2006). Therefore, the YS motif may function as an export signal specifically modulating the Golgi export of the members of α2-AR subfamily.
In addition to α2B-AR, an important role for the N-terminus in the intracellular trafficking of GPCRs has been described for other GPCRs. For example, the deletion of the N-termini facilitates the cell surface export of α1D-AR and α2C-AR, suggesting that the N-termini may contain signals retaining the receptors in the ER (Angelotti et al., 2010; Hague, Chen, Pupo, Schulte, Toews, & Minneman, 2004). Taken together, these studies demonstrate that, similar to the C-termini, the N-termini may also contain signals modulating the export of GPCRs from intracellular compartments.
The Y12/S13 motif represents the first Golgi export motif identified in the GPCR superfamily. As the N-terminus is positioned towards the lumen of ER and Golgi during the export process, the YS motif is not able to directly interact with components of transport machinery in the cytoplasm. Furthermore, the fact that YS mutant receptors are able to exit from the ER to reach the Golgi compartment suggests that they are properly folded. Therefore, the defective transport is unlikely caused by misfolding. Further investigation is needed to clarify the molecular mechanism underlying the function of YS motif in the regulation of receptor export from the Golgi.
D. The ICL3 in the Basolateral Targeting of Three α2-ARs
It has been well demonstrated that the ICL3 is involved in the regulation of receptor coupling to G proteins, phosphorylation, internalization, and signal termination (DeGraff, Gurevich, & Benovic, 2002; Jewell-Motz, Small, Theiss, & Liggett, 2000; Pao & Benovic, 2005; Small, Brown, Forbes, & Liggett, 2001; Wade, Lim, Lan, Chung, Nanamori, & Neubig, 1999; Wade, Scribner, Dalman, Taylor, & Neubig, 1996; Wang & Limbird, 2002; Wang et al., 2004a; Wu et al., 1997, 1998; Wu, Bogatkevich, Mukhin, Benovic, Hildebrandt, & Lanier, 2000). The role of the ICL3 in the localization and trafficking of three α2-ARs have been extensively studied in polarized Madin–Darby canine kidney II (MDCKII) cells in the laboratory of Dr. Lee Limbird (Brady et al., 2003; Edwards & Limbird, 1999; Keefer et al., 1994; Prezeau et al., 1999; Saunders et al., 1998; Saunders & Limbird, 2000; Wozniak & Limbird, 1996). It has been demonstrated that three newly synthesized α2-ARs use different pathways to target to the basolateral domain and have distinct retention profiles in MDCKII cells. Consistent with different transport abilities of the three α2-ARs in some cell types, at steady state, both α2A-AR and α2B-ARs are almost exclusively located at the basolateral surface, while about half of α2C-AR is localized at the basolateral membrane and another half in the intracellular compartments. More interestingly, it appears that α2A-AR and α2B-AR utilize different paths for their basolateral targeting. α2B-AR is first randomly transported to both the apical and basolateral surfaces and then selectively retained at the basolateral domain, whereas α2A-AR is directly delivered to the basolateral membrane. Despite the remarkable differences in basolateral targeting, three α2-ARs exhibit comparable half-life of about 10–12 h at the basolateral domain (Wozniak & Limbird, 1996).
The ICL3 and the C-terminus are not involved in the regulation of direct basolateral delivery of α2A-AR and indeed, the basolateral targeting information for α2A-AR is identified in the membrane-embedded regions (Keefer et al., 1994; Keefer & Limbird, 1993; Saunders et al., 1998). However, removal of the ICL3 significantly facilitates the turnover of the cell surface α2A-AR, shortening its half-life to about 4 h (Edwards & Limbird, 1999). This function of the ICL3 in stabilizing α2A-AR and α2B-AR at the basolateral surface is directly linked to its ability to physically associate with spinophilin (Brady et al., 2003; Richman, Brady, Wang, Hensel, Colbran, & Limbird, 2001). Taken together, these data suggest that the stabilization/retention of α2A-AR and α2B-AR at specific membrane domains is most likely mediated through ICL3 interactions with other proteins.
IV. THE ROLE OF SMALL GTPASE IN THE EXPORT TRAFFICKING OF α2B-AR
The Ras-like small GTPase superfamily consists of more than 150 members and can be divided into Ras, Rho/Rac/Cdc42, Ran, Sar1/ARF and Rab subfamilies. The small GTPases in the Ras and Rho/Rac/Cdc42 subfamilies have been well documented to function as signaling proteins modulating gene expression, cell division, and cytoskeletal reorganization, the small GTPases in the Rab and Sar1/Arf subfamilies regulate vesicle trafficking, and the Ran GTPases regulate nucleocytoplasmic transport (Takai, Sasaki, & Matozaki, 2001). The roles of the small GTPases in the transport of newly synthesized GPCRs from the ER to the cell surface have been recently studied. Through manipulating the function of endogenous small GTPases by expressing their GDP- and GTP-bound mutants and siRNA targeting to specific GTPases, we and others have recently demonstrated that multiple small GTPases in the Sar1/ARF and Rab subfamilies modulate GPCR cell surface transport en route from the ER and the Golgi/TGN.
A. Sar1 in α2B-AR Exit from the ER
The small GTPase Sar1 and the heterodimeric Sec23/24 and Sec13/31 complexes are the components of COPII-coated transport vesicles, which exclusively mediate export of newly synthesized cargo from the ER. It has been well demonstrated that GDP/GTP exchange and GTP hydrolysis by Sar1 GTPase play a crucial role in the formation and budding of COPII-coated vesicles on the ER membrane. Assembly of the COPII coat takes place on the ER membrane at discrete locations called ER exit sites and is initiated by the exchange of GDP for GTP on Sar1 GTPase. GTP activation of Sar1 GTPase recruits the Sec23/24 and Sec13/31 complexes onto the ER membrane forming the COPII-coated vesicles. Hydrolysis of GTP to GDP by Sar1 GTPase results in the dissociation of Sar1 GTPase from the ER membrane and the release of the COPII vesicles (Gurkan, Stagg, Lapointe, & Balch, 2006; Pucadyil & Schmid, 2009).
As a first study to define the role of the ER-derived COPII transport vesicles in GPCR export from the ER, we determined the effect of transient expression of the GTP-restricted mutant Sar1H79G, which presumably blocks the release of the COPII vesicles from the ER membrane, on the cell surface expression and subcellular distribution of α2B-AR (Dong et al., 2008). Expression of Sar1H79G significantly attenuated the cell surface expression of α2B-AR (Fig. 1B) and arrested α2B-AR in ER exit sites (Dong et al., 2008). These data indicate that α2B-AR export from the ER and transport to the cell surface is dependent on the normal function of the small GTPase Sar1. These data also suggest that, similar to many other proteins, α2B-AR exit from the ER is mediated through the Sar1-dependent COPII-coated vesicles. Similar to α2B-AR, the cell surface expression of β2-AR, AT1R, and human calcium receptor (hCaR) was attenuated by Sar1H79G mutant and siRNA-mediated knockdown of Sar1 (Dong et al., 2008; Zhuang, Chowdhury, Northup, & Ray, 2010), further confirming a general role for Sar1 GTPase in the cell surface transport of the GPCR superfamily.
B. ARF GTPases in α2B-AR Exit from the ER and the Golgi
Of the five ARF GTPases (ARF1, 3, 4, 5, and 6) identified in humans, ARF1 and ARF6 are the best characterized and well understood members. ARF6 primarily engages in the regulation of endocytic trafficking and cytoskeleton remodeling, whereas ARF1 recruits different sets of coat proteins to form distinct transport vesicles that control protein transport at different intracellular organelles (Palacios, Price, Schweitzer, Collard, & D’Souza-Schorey, 2001; Spang, 2002; Stearns, Willingham, Botstein, & Kahn, 1990). For example, ARF1 recruits coatomers in the formation of COPI vesicles, which mediate protein transport from the Golgi to the ER, from the ERGIC to the Golgi, and intra-Golgi traffic, whereas ARF1-mediated recruitment of adaptor proteins and GGA, leading to the formation of the clathrin-coated vesicles on the TGN controls post-Golgi transport between the TGN, the plasma membrane and the endosomal compartment (Bonifacino, 2004). Based on the sequence homology, it is believed that ARF1 and ARF3 share the same function. In contrast, the function of ARF4 and ARF5 remains largely unknown.
We have recently determined the role of each ARF GTPase in the cell surface targeting of α2B-AR (Dong et al., 2010b). Our studies demonstrated that expression of the GDP-bound, GTP-bound, and guanine nucleotide-deficient mutants of both ARF1 and ARF3 produced a profound inhibitory effect on the cell surface expression of α2B-AR, whereas ARF4, ARF5, and ARF6 mutants produced only moderate or no effect. These data indicate that five human ARF GTPases differentially modulate α2B-AR cell surface transport and that ARF1 and ARF3 are the primary ones regulating α2B-AR export trafficking. Interestingly, we have demonstrated that ARF1 is able to physically associate with α2B-AR as measured by coimmunoprecipitation and GST fusion protein pull-down assay and the interaction domain has been mapped to the C-terminus of the receptor (Dong et al., 2010b). These studies suggest that regulation of α2B-AR transport by ARF1 may be mediated through their direct interaction.
It appears that ARF1 GTPase modulates the cell surface transport of α2B-AR at multiple transport steps as the GDP- and GTP-bound ARF1 mutants arrested the receptors in distinct intracellular compartments (Dong et al., 2010b). Whereas expression of the GDP-bound mutant ARF1T31N arrested α2B-AR in the ER, the GTP-bound mutant ARF1Q71L induced an accumulation of the receptors in the post-ER compartments, including ERGIC, Golgi, and TGN (Dong et al., 2010b). These data indicate that expression of different ARF1 mutants blocks the export of the cargo receptors from different subcellular compartments. Such differential regulation of α2B-AR export by the ARF1 GDP- and GTP mutants could be explained by their effects on the formation of transport vesicles from the different intracellular compartments. Expression of the GDP-bound mutant ARF1T31N would block the formation of COPI vesicles to disrupt the retrograde transport system, which will impair the reuse of components of transport machinery and induces defective anterograde trafficking of newly synthesized cargo. On the contrary, expression of the GTP-bound ARF1Q71L mutant would influence the release of the COPI vesicles from the ERGIC and the Golgi or the clathrin-coated vesicles from the TGN, resulting in the accumulation of α2B-AR in these compartments.
Our studies have demonstrated that ARF1 may play a general role in the anterograde trafficking of the GPCR superfamily. In addition to α2B-AR, we have also measured the effect of the ARF1 mutants on the cell surface transport and subcellular distribution of several other GPCRs including β2-AR, AT1R, and C-X-C chemokine receptor type 4. Similar to their effects on α2B-AR, expression of the ARF1 mutants markedly inhibited the cell surface expression of all three receptors examined and the GDP- and GTP-bound mutants arrested these receptors in different intracellular compartments (Dong et al., 2010b).
C. Rab GTPases in the ER–Golgi-Cell Surface Transport of α2B-AR
Consisting of more than 60 members in mammals and 11 in yeast, Rab GTPases form the largest subfamily of the Ras-related GTPases and function as traffic “cops” to coordinate almost every step of vesicle-mediated transport, particularly the targeting, tethering, and fusion of the transport vesicles. Each Rab GTPase has a distinct subcellular distribution pattern that correlates with the compartments between which it coordinates the transport (Takai et al., 2001). There are at least three Rab GTPases, Rab1, Rab2, and Rab6, which coordinate protein transport in the early secretory pathway. Rab1 is localized at the ER and the Golgi, and regulates the anterograde transport of proteins from the ER to the Golgi. Rab2 is localized to the ERGIC that works as the first station sorting cargo into anterograde or retrograde transport pathway and coordinates the early event between the ERGIC and the ER. Rab6 mainly locates in the Golgi and regulates the trafficking from the late to early Golgi cisternae and from the Golgi to the ER. In contrast, Rab8 mediates the vesicle-mediated trafficking from the Golgi/TGN to the plasma membrane.
Most studies on the roles of Rab GTPases in the intracellular trafficking of GPCRs have been focused on the events involved in the internalization (Fan, Lapierre, Goldenring, & Richmond, 2003; Murph, Scaccia, Volpicelli, & Radhakrishna, 2003; Seachrist, Anborgh, & Ferguson, 2000). In contrast, much less is known about the involvement of Rab GTPases in GPCR export to the plasma membrane. As an initial approach to investigate the anterograde transport pathways of GPCRs, we have determined the role of Rab1, Rab2, Rab6, and Rab8 GTPases in the cell surface transport of α2B-AR by transiently expressing dominant-negative mutants and siRNA-mediated depletion of individual Rab GTPases. We found that these Rab GTPases differentially modulate α2B-AR transport to the cell surface. Specifically, inhibition of Rab2 and Rab8 function significantly inhibited α2B-AR transport to the cell surface, whereas inhibition of Rab1 and Rab6 function did not produce any effect (Dong & Wu, 2007; Dong et al., 2010a; Wu et al., 2003). These data demonstrate that the cell surface transport of α2B-AR is dependent on the normal function of Rab2 and Rab8, but independent of Rab1 and Rab6, which have been well documented to function as generic regulators for protein transport between the ER and the Golgi.
As discussed above, the expression of GTP-bound mutant ARF1Q71L induced an extensive accumulation of α2B-AR in the Golgi (Dong et al., 2010b), indicating that α2B-AR actually passes the Golgi stacks en route to the cell surface. Therefore, Rab1/Rab6-independent transport of α2B-AR strongly implies that α2B-AR uses a nonconventional pathway to move from the ER to the Golgi. However, how this novel pathway operates remains unknown. Compared with other GPCRs, α2B-AR is one of a few GPCRs that do not contain N-linked glycosylation sites in the N-termini. Glycosylation of the receptors occurs during their transport through the Golgi apparatus, resulting in the formation of mature receptors competent for subsequent transport to the cell surface. Whether posttranslational modifications such as N-linked glycoyslation function as one of the determinants for the selection of transport pathways and whether the N-linked glycosylation dictates the receptors into the Rab1/Rab6-coordinated transport need further investigation. In addition, to further study the function of other Rab GTPases in the ER-to-Golgi transport of α2B-AR may provide important insights into this nonclassic transport pathway.
In contrast to α2B-AR, the cell surface transport of other GPCRs including α1- AR, β-AR, AT1R, AT2R, and hCaR was attenuated by functional inhibition of Rab1, Rab2, Rab6, and Rab8 (Dong & Wu, 2007; Dong et al., 2010a; Filipeanu et al., 2004, 2006; Li et al., 2010; Wu et al., 2003; Zhang et al., 2009; Zhuang, Adipietro, Datta, Northup, & Ray, 2010). These data demonstrate that Rab1 and Rab6 may selectively modulate the transport of distinct GPCRs. These data also suggest that distinct GPCRs that have common structural features, track to the cell surface and couple to heterotrimeric G proteins may utilize different pathways (i.e., Rab1/Rab6-dependent and Rab1/Rab6-independent) for their movement from the ER to the Golgi.
The function of Rab GTPases in regulating GPCR trafficking may be mediated through their direct interactions with the receptors. For example, Rab4, Rab5, Rab7, and Rab11 bind to AT1R to modulate its endocytic trafficking (Esseltine, Dale, & Ferguson, 2010; Seachrist et al., 2002). We recently demonstrated that both β2-AR and α2B-AR are able to associate with Rab8 as revealed by coimmunoprecipitation. Interestingly, these two adrenergic receptors use different motifs to bind Rab8. In contrast to β2-AR using the LL motif to interact with Rab8, α2B-AR uses multiple sites located in the membrane-proximal (TVFN) and distal (PW and QTGW) C-terminus to interact with Rab8 (Dong et al., 2010a). In particular, the residues N433 and P447 likely play a crucial role in mediating α2B-AR interaction with Rab8 as mutation of either one almost abolished the interaction in GST fusion protein pull down assays. These data suggest that different GPCRs (i.e., α2B-AR and β2-AR) may provide distinct docking sites for Rab8 GTPase to coordinate their export from the TGN (Dong et al., 2010a).
V. CONCLUSIONS AND PERSPECTIVES
The players involved in the cell surface targeting of GPCRs in general or α2B-AR in particular are just beginning to be revealed. Recent studies have demonstrated that export from the ER and the Golgi of α2B-AR is dictated by specific amino acid residues or motifs scattered throughout the receptor and the transport of α2B-AR from the ER through the Golgi to the cell surface along the secretory pathway is coordinated by multiple GTPases (Fig. 3). However, the mechanism underlying the regulation of α2B-AR export trafficking is still largely unknown. First, although several essential sequences for ER export of α2B-AR or many other GPCRs have been identified (Bermak et al., 2001; Duvernay et al., 2004; Oksche, Dehe, Schulein, Wiesner, & Rosenthal, 1998; Robert et al., 2005; Rodriguez et al., 1992; Schulein et al., 1998; Tai, Chuang, Bode, Wolfrum, & Sung, 1999), none of them have been shown to directly interact with components of COPII vesicles. The most interesting experiment probably is to continue to search for such motifs that are able to directly interact with the components of COPII transport vesicles and facilitate α2B-AR recruitment onto the vesicles. Second, the experiment to use different protein–protein interaction strategies to look for proteins interacting with the well-defined export motifs as discussed above will help to elucidate the possible molecular mechanism for these motifs. Third, as it is clear that α2B-AR uses a nonclassic pathway to move from the ER to the Golgi, the immediate experiments are to fully characterize this pathway.
FIGURE 3.
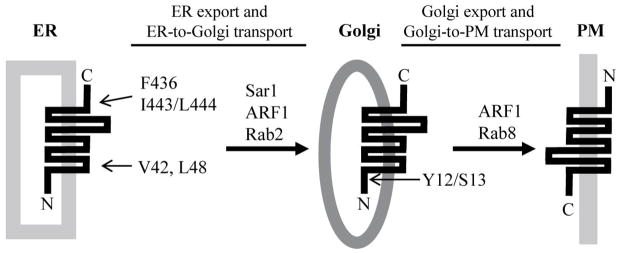
Summary of the structural basis and the roles of small GTPases in the anterograde trafficking of α2B-AR. The F436, I443/L444, V42, and L48 residues regulate the exit of α2B-AR from the ER and the Y12/S13 residues influence the exit from the Golgi. The small GTPase Sar1 controls α2B-AR export from the ER by modulating the function of the COPII vesicles, whereas ARF1 may be involved in the export of α2B-AR from multiple intracellular compartments including the ER and the Golgi. α2B-AR transport from the ER to the Golgi depends on the normal function of Rab2, but independent of Rab1 and Rab6, and its transport from the Golgi to the cell surface requires Rab8.
Cell surface targeting of GPCRs is one of the important factors determining the functionality of the receptors. Indeed, dysfunction of GPCRs caused by defective cell surface trafficking is clearly associated with the development of a number of human diseases such as nephrogenic diabetes insipidus, retinitis pigmentosa, and male pseudohermaphroditism. Therefore, to thoroughly understand the mechanism underlying export trafficking of GPCRs will provide a foundation for the development of therapeutic strategies targeting on specific components of the transport pathway.
Acknowledgments
This work was supported by National Institutes of Health grant R01GM076167 (to G. Wu).
References
- Angelotti T, Daunt D, Shcherbakova OG, Kobilka B, Hurt CM. Regulation of G-protein coupled receptor traffic by an evolutionary conserved hydrophobic signal. Traffic. 2010;11(4):560–578. doi: 10.1111/j.1600-0854.2010.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94(10):1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;3(5):492–498. doi: 10.1038/35074561. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS. The GGA proteins: Adaptors on the move. Nat Rev Mol Cell Biol. 2004;5(1):23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Boucher R, Larkin H, Brodeur J, Gagnon H, Theriault C, Lavoie C. Intracellular trafficking of LRP9 is dependent on two acidic cluster/dileucine motifs. Histochem Cell Biol. 2008;130(2):315–327. doi: 10.1007/s00418-008-0436-5. [DOI] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2(4):274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Brady AE, Wang Q, Colbran RJ, Allen PB, Greengard P, Limbird LE. Spinophilin stabilizes cell surface expression of alpha 2B-adrenergic receptors. J Biol Chem. 2003;278(34):32405–32412. doi: 10.1074/jbc.M304195200. [DOI] [PubMed] [Google Scholar]
- Carrel D, Hamon M, Darmon M. Role of the C-terminal dileucine motif of 5-HT1A and 5-HT1B serotonin receptors in plasma membrane targeting. J Cell Sci. 2006;119(Pt 20):4276–4284. doi: 10.1242/jcs.03189. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor ii receptor cytoplasmic domain. an acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J Biol Chem. 1997;272(11):7003–7012. doi: 10.1074/jbc.272.11.7003. [DOI] [PubMed] [Google Scholar]
- Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol Pharmacol. 1997;51(5):711–720. doi: 10.1124/mol.51.5.711. [DOI] [PubMed] [Google Scholar]
- DeGraff JL, Gurevich VV, Benovic JL. The third intracellular loop of alpha 2-adrenergic receptors determines subtype specificity of arrestin interaction. J Biol Chem. 2002;277(45):43247–43252. doi: 10.1074/jbc.M207495200. [DOI] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768(4):853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wu G. Regulation of anterograde transport of alpha2-adrenergic receptors by the N termini at multiple intracellular compartments. J Biol Chem. 2006;281(50):38543–38554. doi: 10.1074/jbc.M605734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wu G. Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal. 2007;19(11):2388–2399. doi: 10.1016/j.cellsig.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Yang L, Zhang X, Gu H, Lam ML, Claycomb WC, et al. Rab8 interacts with distinct motifs in {alpha}2B- and {beta}2-adrenergic receptors and differentially modulates their transport. J Biol Chem. 2010a;285(26):20369–20380. doi: 10.1074/jbc.M109.081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Zhang X, Zhou F, Dou H, Duvernay MT, Zhang P, et al. ADP-ribosylation factors modulate the cell surface transport of G protein-coupled receptors. J Pharmacol Exp Ther. 2010b;333(1):174–183. doi: 10.1124/jpet.109.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Zhou F, Fugetta EK, Filipeanu CM, Wu G. Endoplasmic reticulum export of adrenergic and angiotensin II receptors is differentially regulated by Sar1 GTPase. Cell Signal. 2008;20(6):1035–1043. doi: 10.1016/j.cellsig.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Dong C, Zhang X, Robitaille M, Hebert TE, Wu G. A single conserved leucine residue on the first intracellular loop regulates ER export of G protein-coupled receptors. Traffic. 2009a;10(5):552–566. doi: 10.1111/j.1600-0854.2009.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Dong C, Zhang X, Zhou F, Nichols CD, Wu G. Anterograde trafficking of G protein-coupled receptors: function of the C-terminal F(X)6LL motif in export from the endoplasmic reticulum. Mol Pharmacol. 2009b;75(4):751–761. doi: 10.1124/mol.108.051623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J Biol Chem. 2004;279(29):30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- Edwards SW, Limbird LE. Role for the third intracellular loop in cell surface stabilization of the alpha2A-adrenergic receptor. J Biol Chem. 1999;274(23):16331–16336. doi: 10.1074/jbc.274.23.16331. [DOI] [PubMed] [Google Scholar]
- Esseltine JL, Dale LB, Ferguson SS. Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: Evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Mol Pharmacol. 2010;79(1):175–184. doi: 10.1124/mol.110.068379. [DOI] [PubMed] [Google Scholar]
- Fan GH, Lapierre LA, Goldenring JR, Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101(6):2115–2124. doi: 10.1182/blood-2002-07-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan H, Reiterer V, Korkhov VM, Schmid JA, Freissmuth M, Sitte HH. Concentrative export from the endoplasmic reticulum of the {gamma}-aminobutyric acid transporter 1 requires binding to SEC24D. J Biol Chem. 2007;282(10):7679–7689. doi: 10.1074/jbc.M609720200. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem. 2004;279(39):41077–41084. doi: 10.1074/jbc.M405988200. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Fugetta EK, Wu G. Differential regulation of the cell-surface targeting and function of beta- and alpha1-adrenergic receptors by Rab1 GTPase in cardiac myocytes. Mol Pharmacol. 2006;69(5):1571–1578. doi: 10.1124/mol.105.019984. [DOI] [PubMed] [Google Scholar]
- Gaborik Z, Mihalik B, Jayadev S, Jagadeesh G, Catt KJ, Hunyady L. Requirement of membrane-proximal amino acids in the carboxyl-terminal tail for expression of the rat AT1a angiotensin receptor. FEBS Lett. 1998;428(3):147–151. doi: 10.1016/s0014-5793(98)00511-0. [DOI] [PubMed] [Google Scholar]
- Gimelbrant AA, Haley SL, McClintock TS. Olfactory receptor trafficking involves conserved regulatory steps. J Biol Chem. 2001;276(10):7285–7290. doi: 10.1074/jbc.M005433200. [DOI] [PubMed] [Google Scholar]
- Gurkan C, Stagg SM, Lapointe P, Balch WE. The COPII cage: Unifying principles of vesicle coat assembly. Nat Rev Mol Cell Biol. 2006;7(10):727–738. doi: 10.1038/nrm2025. [DOI] [PubMed] [Google Scholar]
- Hague C, Chen Z, Pupo AS, Schulte NA, Toews ML, Minneman KP. The N terminus of the human alpha1D-adrenergic receptor prevents cell surface expression. J Pharmacol Exp Ther. 2004;309(1):388–397. doi: 10.1124/jpet.103.060509. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Heymann JA, Subramaniam S. Expression, stability, and membrane integration of truncation mutants of bovine rhodopsin. Proc Natl Acad Sci USA. 1997;94(10):4966–4971. doi: 10.1073/pnas.94.10.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lui WWY, Bright NA, Totty N, Seaman MNJ, Robinson MS. A family of proteins with {gamma}-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol. 2000;149(1):67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JC, Suzuki N, Pessin JE, Watson RT. A specific dileucine motif is required for the GGA-dependent entry of newly synthesized insulin-responsive aminopeptidase into the insulin-responsive compartment. J Biol Chem. 2006;281(44):33457–33466. doi: 10.1074/jbc.M601583200. [DOI] [PubMed] [Google Scholar]
- Jewell-Motz EA, Small KM, Theiss CT, Liggett SB. alpha 2A/alpha 2C-adrenergic receptor third loop chimera show that agonist interaction with receptor subtype backbone establishes G protein-coupled receptor kinase phosphorylation. J Biol Chem. 2000;275(37):28989–28993. doi: 10.1074/jbc.M005381200. [DOI] [PubMed] [Google Scholar]
- Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol. 2001;60(6):1195–1200. doi: 10.1124/mol.60.6.1195. [DOI] [PubMed] [Google Scholar]
- Johnson KF, Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992;119(2):249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler F, Klopfenstein DRC, Foguet M, Paccaud J-P, Hauri H-P. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem. 1997;272(50):31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- Keefer JR, Kennedy ME, Limbird LE. Unique structural features important for stabilization versus polarization of the alpha 2A-adrenergic receptor on the basolateral membrane of Madin–Darby canine kidney cells. J Biol Chem. 1994;269(23):16425–16432. [PubMed] [Google Scholar]
- Keefer JR, Limbird LE. The alpha 2A-adrenergic receptor is targeted directly to the basolateral membrane domain of Madin–Darby canine kidney cells independent of coupling to pertussis toxin-sensitive GTP-binding proteins. J Biol Chem. 1993;268(15):11340–11347. [PubMed] [Google Scholar]
- Li Y, Wang G, Lin K, Yin H, Zhou C, Liu T, et al. Rab1 GTPase promotes expression of beta-adrenergic receptors in rat pulmonary microvascular endothelial cells. Int J Biochem Cell Biol. 2010;42(7):1201–1209. doi: 10.1016/j.biocel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori LT, Florencia BS, Frederick RM. Role of an acidic cluster/dileucine motif in cation-independent mannose 6-phosphate receptor traffic. Traffic. 2007;8(4):402–413. doi: 10.1111/j.1600-0854.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, et al. Role of ER export signals in controlling surface potassium channel numbers. Science. 2001;291(5502):316–319. doi: 10.1126/science.291.5502.316. [DOI] [PubMed] [Google Scholar]
- Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28(7):369–376. doi: 10.1016/S0968-0004(03)00134-8. [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Murph MM, Scaccia LA, Volpicelli LA, Radhakrishna H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J Cell Sci. 2003;116(Pt 10):1969–1980. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277(5325):556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, et al. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J Biol Chem. 1999;274(22):15937–15946. doi: 10.1074/jbc.274.22.15937. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Plutner H, Hahn K, Balch WE. The delta subunit of AP-3 is required for efficient transport of VSV-G from the trans-Golgi network to the cell surface. Proc Natl Acad Sci USA. 2002;99(10):6755–6760. doi: 10.1073/pnas.092150699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nufer O, Guldbrandsen S, Degen M, Kappeler F, Paccaud JP, Tani K, et al. Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J Cell Sci. 2002;115(Pt 3):619–628. doi: 10.1242/jcs.115.3.619. [DOI] [PubMed] [Google Scholar]
- Nufer O, Kappeler F, Guldbrandsen S, Hauri HP. ER export of ERGIC-53 is controlled by cooperation of targeting determinants in all three of its domains. J Cell Sci. 2003;116(Pt 21):4429–4440. doi: 10.1242/jcs.00759. [DOI] [PubMed] [Google Scholar]
- Oksche A, Dehe M, Schulein R, Wiesner B, Rosenthal W. Folding and cell surface expression of the vasopressin V2 receptor: Requirement of the intracellular C-terminus. FEBS Lett. 1998;424(1–2):57–62. doi: 10.1016/s0014-5793(98)00140-9. [DOI] [PubMed] [Google Scholar]
- Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 2001;20(17):4973–4986. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevych H, Korkhov V, Freissmuth M, Nanoff C. Truncation of the A1 adenosine receptor reveals distinct roles of the membrane-proximal carboxyl terminus in receptor folding and G protein coupling. J Biol Chem. 2003;278(32):30283–30293. doi: 10.1074/jbc.M212918200. [DOI] [PubMed] [Google Scholar]
- Pao CS, Benovic JL. Structure/function analysis of alpha2A-adrenergic receptor interaction with G protein-coupled receptor kinase 2. J Biol Chem. 2005;280(12):11052–11058. doi: 10.1074/jbc.M412996200. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem. 2000;275(18):13727–13736. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Prezeau L, Richman JG, Edwards SW, Limbird LE. The zeta isoform of 14-3-3 proteins interacts with the third intracellular loop of different alpha2-adrenergic receptor subtypes. J Biol Chem. 1999;274(19):13462–13469. doi: 10.1074/jbc.274.19.13462. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science. 2009;325(5945):1217–1220. doi: 10.1126/science.1171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001a;292(5522):1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001b;105(1):93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated Interaction between alpha2-adrenergic receptors and spinophilin. J Biol Chem. 2001;276(18):15003–15008. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- Robert J, Clauser E, Petit PX, Ventura MA. A novel C-terminal motif is necessary for the export of the vasopressin V1b/V3 receptor to the plasma membrane. J Biol Chem. 2005;280(3):2300–2308. doi: 10.1074/jbc.M410655200. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Xie YB, Wang H, Collison K, Segaloff DL. Effects of truncations of the cytoplasmic tail of the luteinizing hormone/chorionic gonadotropin receptor on receptor-mediated hormone internalization. Mol Endocrinol. 1992;6(3):327–336. doi: 10.1210/mend.6.3.1316539. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Mercier J-F, Lagace M, Marullo S, Bouvier M. Homodimerization of the {beta}2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279(32):33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- Saunders C, Keefer JR, Bonner CA, Limbird LE. Targeting of G protein-coupled receptors to the basolateral surface of polarized renal epithelial cells involves multiple, non-contiguous structural signals. J Biol Chem. 1998;273(37):24196–24206. doi: 10.1074/jbc.273.37.24196. [DOI] [PubMed] [Google Scholar]
- Saunders C, Limbird LE. Microtubule-dependent regulation of alpha(2B) adrenergic receptors in polarized MDCKII cells requires the third intracellular loop but not G protein coupling. Mol Pharmacol. 2000;57(1):44–52. [PubMed] [Google Scholar]
- Schulein R, Hermosilla R, Oksche A, Dehe M, Wiesner B, Krause G, et al. A dileucine sequence and an upstream glutamate residue in the intracellular carboxyl terminus of the vasopressin V2 receptor are essential for cell surface transport in COS.M6 cells. Mol Pharmacol. 1998;54(3):525–535. doi: 10.1124/mol.54.3.525. [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Anborgh PH, Ferguson SS. beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem. 2000;275(35):27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Laporte SA, Dale LB, Babwah AV, Caron MG, Anborgh PH, et al. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277(1):679–685. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- Sevier CS, Weisz OA, Davis M, Machamer CE. Efficient export of the vesicular stomatitis virus G protein from the endoplasmic reticulum requires a signal in the cytoplasmic tail that includes both tyrosine-based and diacidic motifs. Mol Biol Cell. 2000;11(1):13–22. doi: 10.1091/mbc.11.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Brown KM, Forbes SL, Liggett SB. Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276(7):4917–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- Spang A. ARF1 regulatory factors and COPI vesicle formation. Curr Opin Cell Biol. 2002;14(4):423–427. doi: 10.1016/s0955-0674(02)00346-0. [DOI] [PubMed] [Google Scholar]
- Stearns T, Willingham MC, Botstein D, Kahn RA. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci USA. 1990;87(3):1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97(7):877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Saito Y, Imai K, Doi H, Maruyama K. The basic residues in the membrane-proximal C-terminal tail of the rat melanin-concentrating hormone receptor 1 are required for receptor function. Endocrinology. 2004;145(8):3712–3723. doi: 10.1210/en.2003-1638. [DOI] [PubMed] [Google Scholar]
- Venkatesan S, Petrovic A, Van Ryk DI, Locati M, Weissman D, Murphy PM. Reduced cell surface expression of CCR5 in CCR5Delta 32 heterozygotes is mediated by gene dosage, rather than by receptor sequestration. J Biol Chem. 2002;277(3):2287–2301. doi: 10.1074/jbc.M108321200. [DOI] [PubMed] [Google Scholar]
- Votsmeier C, Gallwitz D. An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J. 2001;20(23):6742–6750. doi: 10.1093/emboj/20.23.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SM, Lim WK, Lan KL, Chung DA, Nanamori M, Neubig RR. G(i) activator region of alpha(2A)-adrenergic receptors: Distinct basic residues mediate G (i) versus G(s) activation. Mol Pharmacol. 1999;56(5):1005–1013. doi: 10.1124/mol.56.5.1005. [DOI] [PubMed] [Google Scholar]
- Wade SM, Scribner MK, Dalman HM, Taylor JM, Neubig RR. Structural requirements for G(o) activation by receptor-derived peptides: Activation and modulation domains of the alpha 2-adrenergic receptor i3c region. Mol Pharmacol. 1996;50(2):351–358. [PubMed] [Google Scholar]
- Wang Q, Limbird LE. Regulated interactions of the alpha 2A adrenergic receptor with spinophilin, 14-3-3zeta, and arrestin 3. J Biol Chem. 2002;277(52):50589–50596. doi: 10.1074/jbc.M208503200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, et al. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004a;304(5679):1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Wang X, Matteson J, An Y, Moyer B, Yoo JS, Bannykh S, et al. COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a diacidic exit code. J Cell Biol. 2004b;167(1):65–74. doi: 10.1083/jcb.200401035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeler MW, Paccaud JP, Hauri HP. Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep. 2007;8(3):258–264. doi: 10.1038/sj.embor.7400893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak M, Limbird LE. The three alpha 2-adrenergic receptor subtypes achieve basolateral localization in Madin–Darby canine kidney II cells via different targeting mechanisms. J Biol Chem. 1996;271(9):5017–5024. doi: 10.1074/jbc.271.9.5017. [DOI] [PubMed] [Google Scholar]
- Wu G, Benovic JL, Hildebrandt JD, Lanier SM. Receptor docking sites for G-protein betagamma subunits. Implications for signal regulation. J Biol Chem. 1998;273(13):7197–7200. doi: 10.1074/jbc.273.13.7197. [DOI] [PubMed] [Google Scholar]
- Wu G, Bogatkevich GS, Mukhin YV, Benovic JL, Hildebrandt JD, Lanier SM. Identification of Gbetagamma binding sites in the third intracellular loop of the M(3)-muscarinic receptor and their role in receptor regulation. J Biol Chem. 2000;275(12):9026–9034. doi: 10.1074/jbc.275.12.9026. [DOI] [PubMed] [Google Scholar]
- Wu G, Krupnick JG, Benovic JL, Lanier SM. Interaction of arrestins with intracellular domains of muscarinic and alpha2-adrenergic receptors. J Biol Chem. 1997;272(28):17836–17842. doi: 10.1074/jbc.272.28.17836. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J Biol Chem. 2003;278(47):47062–47069. doi: 10.1074/jbc.M305707200. [DOI] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278(24):21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang G, Dupre DJ, Feng Y, Robitaille M, Lazartigues E, et al. Rab1 GTPase and dimerization in the cell surface expression of angiotensin II type 2 receptor. J Pharmacol Exp Ther. 2009;330(1):109–117. doi: 10.1124/jpet.109.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Filipeanu CM, Duvernay MT, Wu G. Cell-surface targeting of alpha2-adrenergic receptors – inhibition by a transport deficient mutant through dimerization. Cell Signal. 2006;18(3):318–327. doi: 10.1016/j.cellsig.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Imanishi Y, Filipek S, Alekseev A, Jastrzebska B, Sun W, et al. Autosomal recessive retinitis pigmentosa and E150K mutation in the opsin gene. J Biol Chem. 2006;281(31):22289–22298. doi: 10.1074/jbc.M602664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Adipietro KA, Datta S, Northup JK, Ray K. Rab1 small GTP-binding protein regulates cell surface trafficking of the human calcium-sensing receptor. Endocrinology. 2010a;151(11):5114–5123. doi: 10.1210/en.2010-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Chowdhury S, Northup JK, Ray K. Sar1-dependent trafficking of the human calcium receptor to the cell surface. Biochem Biophys Res Commun. 2010b;396(4):874–880. doi: 10.1016/j.bbrc.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuzarte M, Rinne S, Schlichthorl G, Schubert A, Daut J, Preisig-Muller R. A diacidic sequence motif enhances the surface expression of the potassium channel TASK-3. Traffic. 2007;8(8):1093–1100. doi: 10.1111/j.1600-0854.2007.00593.x. [DOI] [PubMed] [Google Scholar]