Abstract
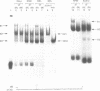
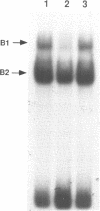
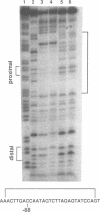
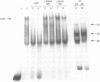
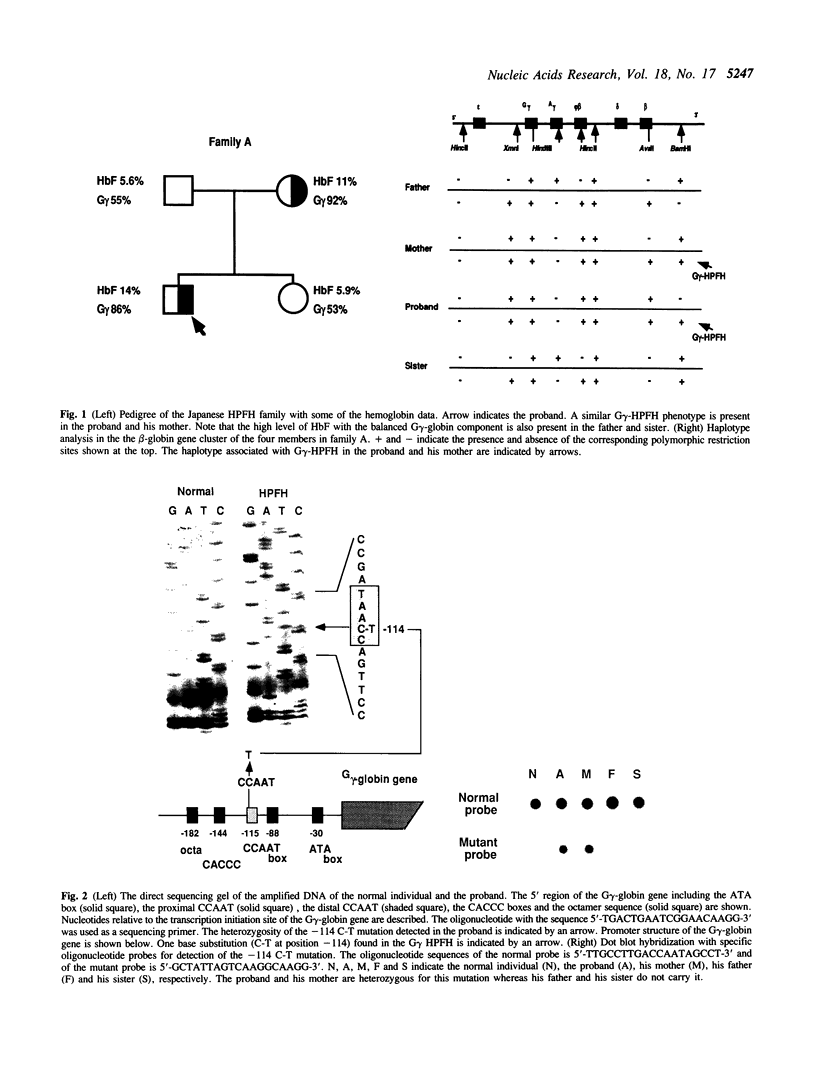
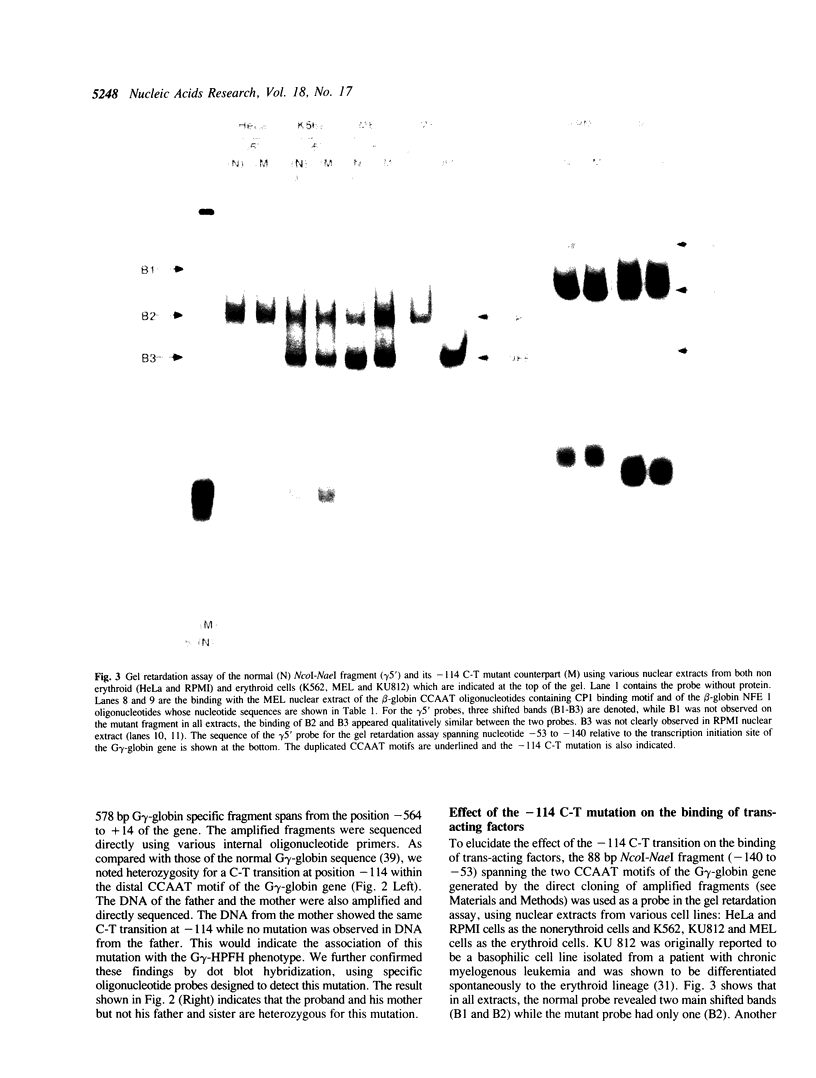
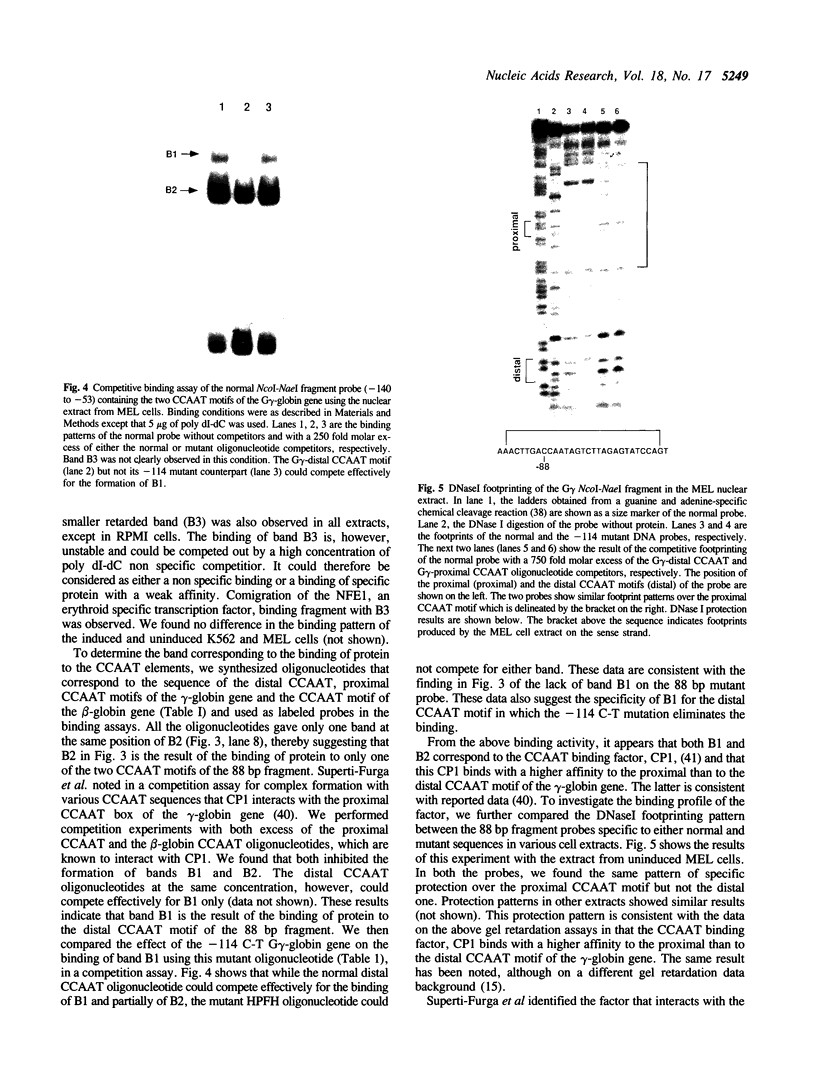
Hereditary persistence of fetal hemoglobin (HPFH) is a condition characterized by the continued expression of the fetal globin gene in adulthood. Both deletional and nondeletional forms have been described. We studied one Japanese family with two different nondeletional forms of HPFH. Analysis of polymorphic restriction sites in the beta-globin gene cluster suggested that one affecting both G gamma and A gamma globin expression in two members of the family could be associated with unknown conditions not linked to the beta-globin gene loci. Characterization by the polymerase chain reaction (PCR) of another form producing a G gamma-HPFH phenotype in two other members demonstrated a novel C-T transition at the nucleotide -114 within the distal CCAAT motif of the G gamma-globin gene. Using gel retardation assays on various nuclear extracts, we also demonstrated that this novel mutation abolishes the binding of the ubiquitous CCAAT binding factor, CP1 to the distal CCAAT motif of the gamma-globin gene but does not affect the binding of any erythroid specific factor, thereby suggesting a possible role for CP1 in the developmental regulation of fetal globin expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Reese A., Stallings M., Huisman T. H. Separation of human hemoglobins by DEAE-cellulose chromatography using glycine-KCN-NaC1 developers. Hemoglobin. 1976;1(1):27–44. doi: 10.3109/03630267609031020. [DOI] [PubMed] [Google Scholar]
- Alter B. P., Goff S. C., Efremov G. D., Gravely M. E., Huisman T. H. Globin chain electrophoresis: a new approach to the determination of the G gamma/A gamma ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980 Apr;44(4):527–534. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Anagnou N. P., Karlsson S., Moulton A. D., Keller G., Nienhuis A. W. Promoter sequences required for function of the human gamma globin gene in erythroid cells. EMBO J. 1986 Jan;5(1):121–126. doi: 10.1002/j.1460-2075.1986.tb04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Catala F., deBoer E., Habets G., Grosveld F. Nuclear protein factors and erythroid transcription of the human A gamma-globin gene. Nucleic Acids Res. 1989 May 25;17(10):3811–3827. doi: 10.1093/nar/17.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Metherall J. E., Yamakawa M., Pan J., Weissman S. M., Forget B. G. A point mutation in the A gamma-globin gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Stoeckert C. J., Jr, Serjeant G. R., Forget B. G., Weissman S. M. G gamma beta+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5' to the G gamma gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conscience J. F., Meier W. Coordinate expression of erythroid marker enzymes during dimethylsulfoxide-induced differentiation of Friend erythroleukemia cells. Exp Cell Res. 1980 Jan;125(1):111–119. doi: 10.1016/0014-4827(80)90195-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucharoen S., Fucharoen G., Fucharoen P., Fukumaki Y. A novel ochre mutation in the beta-thalassemia gene of a Thai. Identification by direct cloning of the entire beta-globin gene amplified using polymerase chain reactions. J Biol Chem. 1989 May 15;264(14):7780–7783. [PubMed] [Google Scholar]
- Fucharoen S., Fucharoen G., Sriroongrueng W., Laosombat V., Jetsrisuparb A., Prasatkaew S., Tanphaichitr V. S., Suvatte V., Tuchinda S., Fukumaki Y. Molecular basis of beta-thalassemia in Thailand: analysis of beta-thalassemia mutations using the polymerase chain reaction. Hum Genet. 1989 Dec;84(1):41–46. doi: 10.1007/BF00210668. [DOI] [PubMed] [Google Scholar]
- Fucharoen S., Katsube T., Fucharoen G., Sawada H., Oishi H., Katsuno M., Nishimura J., Motomura S., Miura Y., Fukumaki Y. Molecular heterogeneity of beta-thalassaemia in the Japanese: identification of two novel mutations. Br J Haematol. 1990 Jan;74(1):101–107. doi: 10.1111/j.1365-2141.1990.tb02545.x. [DOI] [PubMed] [Google Scholar]
- Gelinas R., Bender M., Lotshaw C., Waber P., Kazazian H., Jr, Stamatoyannopoulos G. Chinese A gamma fetal hemoglobin: C to T substitution at position-196 of the A gamma gene promoter. Blood. 1986 Jun;67(6):1777–1779. [PubMed] [Google Scholar]
- Gelinas R., Endlich B., Pfeiffer C., Yagi M., Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Bregni M., Cappellini M. D., Fiorelli G., Taramelli R., Giglioni B., Comi P., Ottolenghi S. A gene controlling fetal hemoglobin expression in adults is not linked to the non-alpha globin cluster. EMBO J. 1983;2(6):921–925. doi: 10.1002/j.1460-2075.1983.tb01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglioni B., Casini C., Mantovani R., Merli S., Comi P., Ottolenghi S., Saglio G., Camaschella C., Mazza U. A molecular study of a family with Greek hereditary persistence of fetal hemoglobin and beta-thalassemia. EMBO J. 1984 Nov;3(11):2641–2645. doi: 10.1002/j.1460-2075.1984.tb02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H. DNA sequence variation associated with elevated fetal G gamma globin production. Blood. 1985 Oct;66(4):783–787. [PubMed] [Google Scholar]
- Gilman J. G., Mishima N., Wen X. J., Kutlar F., Huisman T. H. Upstream promoter mutation associated with a modest elevation of fetal hemoglobin expression in human adults. Blood. 1988 Jul;72(1):78–81. [PubMed] [Google Scholar]
- Gilman J. G., Mishima N., Wen X. J., Stoming T. A., Lobel J., Huisman T. H. Distal CCAAT box deletion in the A gamma globin gene of two black adolescents with elevated fetal A gamma globin. Nucleic Acids Res. 1988 Nov 25;16(22):10635–10642. doi: 10.1093/nar/16.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Gray T. A., Riordan M. F., Sartor C. I., Collins F. S. Nuclear proteins that bind the human gamma-globin gene promoter: alterations in binding produced by point mutations associated with hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1988 Dec;8(12):5310–5322. doi: 10.1128/mcb.8.12.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Ronchi A., Giglioni B., Ottolenghi S. The effects of HPFH mutations in the human gamma-globin promoter on binding of ubiquitous and erythroid specific nuclear factors. Nucleic Acids Res. 1988 Aug 25;16(16):7783–7797. doi: 10.1093/nar/16.16.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Superti-Furga G., Gilman J., Ottolenghi S. The deletion of the distal CCAAT box region of the A gamma-globin gene in black HPFH abolishes the binding of the erythroid specific protein NFE3 and of the CCAAT displacement protein. Nucleic Acids Res. 1989 Aug 25;17(16):6681–6691. doi: 10.1093/nar/17.16.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Martinez G., Colombo B. A new type of hereditary persistence of foetal haemoglobin: is a diffusible factor regulating gamma-chain synthesis? Nature. 1974 Dec 20;252(5485):735–736. doi: 10.1038/252735a0. [DOI] [PubMed] [Google Scholar]
- Martinez G., Novelletto A., Di Rienzo A., Felicetti L., Colombo B. A case of hereditary persistence of fetal hemoglobin caused by a gene not linked to the beta-globin cluster. Hum Genet. 1989 Jul;82(4):335–337. doi: 10.1007/BF00273993. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller B. A., Olivieri N., Salameh M., Ahmed M., Antognetti G., Huisman T. H., Nathan D. G., Orkin S. H. Molecular analysis of the high-hemoglobin-F phenotype in Saudi Arabian sickle cell anemia. N Engl J Med. 1987 Jan 29;316(5):244–250. doi: 10.1056/NEJM198701293160504. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
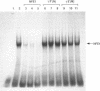
- Okano H., Endo T., Shiokawa S., Kyoden Y., Ishibashi Y., Kono A., Nishi K., Fukumaki Y. Hemoglobin synthesis of both adult and fetal types in a human CML cell line. J Biochem. 1988 Aug;104(2):162–164. doi: 10.1093/oxfordjournals.jbchem.a122433. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Nicolis S., Taramelli R., Malgaretti N., Mantovani R., Comi P., Giglioni B., Longinotti M., Dore F., Oggiano L. Sardinian G gamma-HPFH: a T----C substitution in a conserved "octamer" sequence in the G gamma-globin promoter. Blood. 1988 Mar;71(3):815–817. [PubMed] [Google Scholar]
- Plumb M., Frampton J., Wainwright H., Walker M., Macleod K., Goodwin G., Harrison P. GATAAG; a cis-control region binding an erythroid-specific nuclear factor with a role in globin and non-globin gene expression. Nucleic Acids Res. 1989 Jan 11;17(1):73–92. doi: 10.1093/nar/17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Shimizu K. Characteristics of beta A chromosome haplotypes in Japanese. Biochem Genet. 1987 Apr;25(3-4):197–203. doi: 10.1007/BF00499313. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Keino H., Terasawa T., Shichishima T., Ikuta K., Hayashi Y. Elevated haemoglobin F in juvenile and adult chronic myelogenous leukaemia. Acta Haematol. 1988;80(1):28–33. doi: 10.1159/000205594. [DOI] [PubMed] [Google Scholar]
- Shiokawa S., Fucharoen S., Fucharoen G., Tomatsu S., Fukumaki Y. Heterogeneity of the gamma-globin gene sequences in Japanese individuals: implication of gene conversion in generation of polymorphisms. J Biochem. 1989 Feb;105(2):184–189. doi: 10.1093/oxfordjournals.jbchem.a122637. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoming T. A., Stoming G. S., Lanclos K. D., Fei Y. J., Altay C., Kutlar F., Huisman T. H. An A gamma type of nondeletional hereditary persistence of fetal hemoglobin with a T----C mutation at position -175 to the cap site of the A gamma globin gene. Blood. 1989 Jan;73(1):329–333. [PubMed] [Google Scholar]
- Superti-Furga G., Barberis A., Schaffner G., Busslinger M. The -117 mutation in Greek HPFH affects the binding of three nuclear factors to the CCAAT region of the gamma-globin gene. EMBO J. 1988 Oct;7(10):3099–3107. doi: 10.1002/j.1460-2075.1988.tb03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey S., Delgrosso K., Malladi P., Schwartz E. A single-base change at position -175 in the 5'-flanking region of the G gamma-globin gene from a black with G gamma-beta+ HPFH. Blood. 1988 Mar;71(3):807–810. [PubMed] [Google Scholar]
- Tate V. E., Wood W. G., Weatherall D. J. The British form of hereditary persistence of fetal hemoglobin results from a single base mutation adjacent to an S1 hypersensitive site 5' to the A gamma globin gene. Blood. 1986 Dec;68(6):1389–1393. [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- deBoer E., Antoniou M., Mignotte V., Wall L., Grosveld F. The human beta-globin promoter; nuclear protein factors and erythroid specific induction of transcription. EMBO J. 1988 Dec 20;7(13):4203–4212. doi: 10.1002/j.1460-2075.1988.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]