Abstract
Despite the frequent utilization of biomarkers in medical practice, there is a relative paucity of information regarding validated pediatric biomarkers. Frequently, biomarkers found to be efficacious in adults are extrapolated to the pediatric clinical setting without considering that the pathogenesis of many diseases is distinctly different in children, and ontogeny directly influences disease evolution and therapeutic response in children. New and innovative approaches are necessary to provide reliable, validated biomarkers that can be used to improve and advance pediatric medical care.
Keywords: biomarker, children, development, genomics, ontogeny, pediatrics, pharmacogenomics
Use of biomarkers in children
In clinical practice, validated biomarkers serve as invaluable tools to aid in understanding the etiology, diagnosis, progression, regression and treatment response of a disease [1,2]. These biologic surrogates allow for less invasive medical testing when monitoring a disease process, and can result in cost savings and improved patient care. Furthermore, the potential value of validated biomarkers in drug development and assessing pharmacodynamic response has been recognized [2,3]. The achievable benefits are readily apparent; however, currently, available data on validated biomarkers in the pediatric population are limited [4]. Despite a paucity of information regarding biomarkers specific to children, their utility is evident, as their use is integrated into everyday practice of the pediatrician. Every time blood is sampled to assess for lead toxicity [5], or white blood cell count and C-reactive protein are obtained during the initial evaluation of a bacterial infection in a febrile child [6], or HbA1c is measured to assess glycemic control in a diabetic patient [7], biologic surrogates are being utilized to assess pediatric disease processes and direct the plan of care.
There are multiple approaches when considering pediatric biomarkers. A more traditional approach utilizes well-established adult biomarkers and directly applies the data to children. This approach is analogous to the drug development process in which a drug is developed for an adult disease, studied in adult patients and then investigated for its potential use in children. In this review, we propose an alternative – a comprehensive, pediatric approach in which the pathophysiology of pediatric diseases and ontogeny of children are fundamental to the development of biomarkers specific to pediatric disease. In presenting our case, we examine the impact of ontogeny on commonly utilized biomarkers and identify potential limitations of applying adult biomarkers to children. In particular, we explore challenges related to genomic research in children and provide examples of specific challenges that arise when assessing heterogeneous disease processes in children. Finally, we offer possible solutions for the future discovery of novel biomarkers of pediatric disease.
Consideration of growth & development is critical for pediatric biomarker development & validation
From birth to 18 years of age, each healthy child's visit to the pediatrician begins with the collection of anthropometric measurements that are plotted on a growth chart [101] allowing for continuous tracking of growth over time. This seemingly mundane procedure provides a plethora of information to the practicing physician, including a rapid evaluation of the growth of a single child throughout his/her childhood. These nonlinear plotted growth curves represent the complex and nonlinear changes that occur during pediatric development.
Human development is recognized as a complex process directly impacting the care required by children, as ontogeny influences everything from organ function to drug disposition and clinical response to therapy [8]. Although obvious when examining a 34-week-old premature infant as compared with a 17-year-old high school football player, these physiologic changes that occur during a child's maturation must be accounted for when considering the utilization of biomarkers in pediatric clinical practice as one size does not fit all.
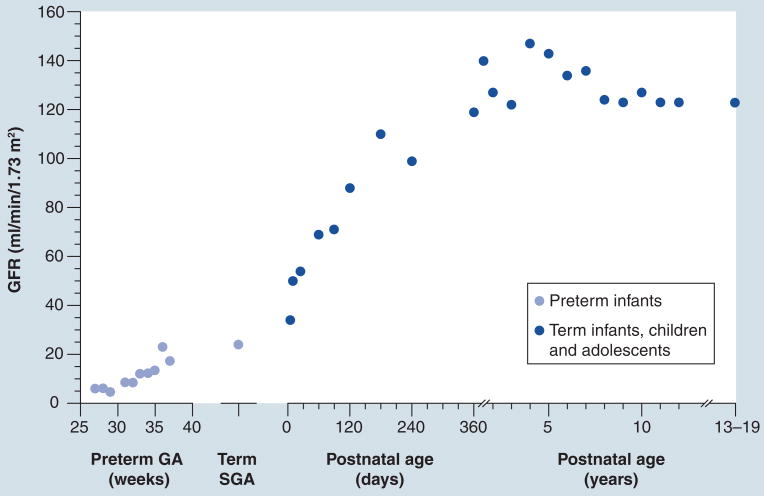
The impact of ontogeny on physiologic processes is exemplified by considering renal function in the pediatric population. Serum creatinine serves as a widely accepted biomarker for estimating glomerular filtration rate (GFR) and renal function [9,10]. Although nephrogenesis is complete by 36 weeks gestational age, renal development continues in the postnatal period with several physiologic changes occurring weeks to months following birth [11]. Renal blood flow dramatically increases in the immediate postnatal period as cardiac output increases and renal vascular resistance decreases, leading to an increase in GFR in the first week of life. The immaturity of cortical nephrons at birth results in a reduced GFR; however, as nephrons develop, GFR doubles by 1 week of age and achieves adult values by 1 year of age. Active tubular function also develops postnatally and approaches adult values by 1 year of age [12]. Prematurity further complicates the process, resulting in a more prolonged course of renal development when compared with the term infant. As these physiologic changes occur over the course of human development, the norms of serum creatinine also change [13]. At birth, serum creatinine levels are elevated, reflecting both maternal and infant concentrations, and subsequently decrease postnatally resulting in childhood normative values being significantly lower when compared with adult values. Thus, the norms for each age must be recognized when utilizing serum creatinine as a marker for renal function to accurately interpret the data (Figure 1) [14]. These well-described changes in kidney development illustrate the importance of considering human maturation when utilizing and interpreting seemingly simple biomarkers in children. Thus, as novel biomarkers such as NGAL and KIM-1 are currently being evaluated for clinical use to assess acute kidney injury in neonates and children, application of the basic understanding of renal maturation is critical [15].
Figure 1. Ontogeny of renal function: glomerular filtration rate.
GFR values (ml/min/1.73 m2) are presented for infants born preterm at various gestational ages in weeks, and for term infants, children and adolescents with postnatal ages from 0 days to 19 years. Values for GFR were obtained from [14].
GA: Gestational age; GFR: Glomerular filtration rate; SGA: Small for gestational age.
Adult biomarkers for pediatric diseases: not always the answer
All too commonly, the development of biomarkers originates in the adult population and is later applied to children. Not until then, is it recognized that the standard or reference range applied to a grown individual is not applicable to a child. Several laboratory tests obtained routinely both in the adult and pediatric patient are recognized as being age-dependent (Box 1). The extent of biological profile variation spanning the age continuum goes unrecognized if biomarker evaluation is limited to adults. For instance, γ-glutamyltransferase is an enzyme monitored in diseases of hepatobiliary dysfunction, including cholangitis, cholecysitis or biliary atresia. Although use of γ-glutamyltransferase in the adult clinical setting has occurred since the 1960s, recognition that normal levels of γ-glutamyltransferase in neonates and young children were several times that of adult levels did not occur for another decade [16].
Box 1. Examples of commonly utilized laboratory tests with age-dependent reference ranges.
Hematology
Factors V and IX
Hemoglobin
Partial thromboplastin time
Prothrombin time
Hepatology
Alkaline phosphatase
Aspartate aminotransferase
γ-glutamyl transpeptidase
Indirect bilirubin
Endocrine
IGF-1
Follicle-stimulating hormone
Luteinizing hormone
Thyroxine
Immunology/renal
White blood cell count
Immunoglobulins (IgA, IgM, IgG and IgE)
Complement C3 and C4
Creatinine
The importance of recognizing age-related changes in biologic surrogates is illustrated when considering anemia, which is a common childhood disease. Hemoglobin values are routinely obtained during the initial evaluation of anemia and results are interpreted using accepted age-dependent reference ranges. These values reveal that normative hemoglobin values in younger children are lower as compared with adolescent and adult values [17]. Without these defined age-related hemoglobin values, the inaccurate diagnosis of anemia in a young child would be commonplace. Thus, recognizing these age-associated changes is critical for understanding pediatric disease processes. The importance of this fundamental principle is further illustrated by the results of a recent re-evaluation of serum alanine aminotransferase levels in children <5 years of age. A total of 5011 alanine aminotransferase measurements were made in 1293 hepatitis C virus-uninfected children, and the data revealed that alanine aminotransferase levels decreased with increasing age, were lower in girls compared with boys, and increased with increasing weight z-scores [18], reinforcing the need for reference ranges to take into consideration not only age, but also sex and weight in pediatric populations.
The assessment of hypertension in children is a relatively new phenomenon with the first pediatric hypertension task force established in 1977 [19]. Even the relatively simplistic undertaking of interpreting blood pressure to identify hypertension is complicated in children as age, gender and height are directly associated with normative blood pressure values and must be considered when interpreting data. For example, the systolic blood pressure defining the 95th percentile among 1-year-old males is 98 mmHg for children at the 5th percentile for height and 106 mmHg for children at the 95th percentile for height. Among males aged 8 years, who are usually prepubertal, these numbers increase to 111 and 120 mmHg for males at the 5th and 95th percentile for height, respectively. Among 17-year-old males (most commonly postpubertal), these numbers increase again to 131 mmHg for males at the 5th percentile for height and to 140 mmHg for males at the 95th percentile [19]. Similar age- and height-dependent changes in blood pressure norms are observed among female children. Disturbed blood pressure homeostasis is a complication of many disease processes, including diabetes mellitus, and interpretation of disease severity must take into consideration age-related changes in norms.
As demonstrated above, an important concern regarding biomarkers discovered, characterized and validated in the context of a disease process in adults and subsequently applied to children with a nominally similar disease is the assumption that disease pathogenesis is similar unless proven otherwise. Certainly, the reality is that many diseases in children have relatively lower prevalence than the corresponding adult disease and, therefore, targeted investigations in children require collaborative efforts among multiple pediatric health centers to enroll a sufficiently large study population. Nevertheless, such efforts are essential, as absence of evidence is not evidence of absence of differences between children and adults. For example, early age of onset for asthma, inflammatory bowel disease (IBD) or juvenile idiopathic arthritis (JIA) in children should at least suggest that different pathogenic mechanisms might be operative relative to adult-onset asthma, IBD or rheumatoid arthritis. For example, childhood-onset asthma, defined as disease development before 16 years of age, occurs more frequently in boys compared with girls and is often associated with atopy, whereas adult-onset asthma develops in middle age and is more common in females compared with males; replicated genetic associations are consistent with differences in disease phenotypes as discussed further below [20]. Likewise, current evidence indicates that pancolitis is observed in approximately 60–70% of children with ulcerative colitis compared with 20–30% in adults. In addition, the younger the patient with Crohn's disease, the more likely the patient is to have colonic disease, a relationship that appears to hold true until approximately 10 years of age [21]. Similarly, uveitis is much more frequently a complication in JIA compared with adults. Whereas rheumatoid factor is positive in 80–85% of adults with rheumatoid arthritis, it is only positive in approximately 5% of JIA patients, and rarely in children less than 10 years of age [22].
The limited capabilities of pediatric patients to cooperate with physicians' commands during examination or to verbally quantify symptoms that coincide with disease severity cannot be overlooked. Asthma is one of the most common diseases in pediatrics, with a prevalence of nearly 10% in children ranging from 0–17 years of age [23]. Spirometry serves as a common method utilized in the evaluation and tracking of pulmonary function, and accepted reference ranges have been standardized that are based on sex, age, race and anthropometric data [24]. However, in children less than 8 years of age, no such standard reference is available [25], and data are often extrapolated to this younger age group with the potential for data inaccuracy and misinterpretation. Patient cooperation is imperative for accurate results, making the task more challenging and offering an area in dire need for age-appropriate biomarkers to characterize disease.
The pathogenesis of disease is often different in children
Rather than expending time and resources to test a null hypothesis that a pediatric disease is not significantly different than the corresponding adult disease, a much more efficient strategy is to focus on characterizing the mechanisms involved in the pathogenesis of pediatric disease. The intent of these investigations would be to compare processes at a molecular level to determine the suitability of biomarkers and therapeutic interventions approved for the adult disease, or for uncovering novel pathways in the pediatric disease and the potential for the development of novel therapies via repurposing strategies through programs such as the Therapeutics for Rare and Neglected Diseases (TRND) program at the NIH [102]. For example, recent investigations have begun to explore the use of gene expression profiles in accessible cells as biomarkers for the molecular classification of pediatric disease subtypes, as has been described using peripheral blood mononuclear cells obtained from patients with JIA [26,27]. In addition to the potential to refine disease diagnosis, investigations of biomarkers derived from gene expression profiling may also provide novel insights into the processes involved in drug response. In the case of JIA, available data suggest that differences in gene expression profiles between children in remission and those with active disease are minimal, implying that clinical remission in this disease is a state of homeostasis that does not involve a return of the perturbations in pro- and anti-inflammatory genes to a normal state [27]. This observation, if confirmed and validated, has clear implications for the utility of cytokine levels as biomarkers for assessing disease severity and response to treatment.
The reliance on biomarkers to inform diagnosis and monitor the response to treatment in children is further complicated by the fact that some diseases are unique to children and have no close adult correlate. For example, neonatal intensive care units must treat a variety of conditions related to premature birth or the consequences of abnormal development, such as bronchopulmonary dysplasia, persistent pulmonary hypertension of the newborn, patent ductus arteriosus and necrotizing enterocolitis. Likewise, several cancers, such as acute lymphoblastic anemia, Wilm's tumor and neuroblastoma, as well as diseases, such as Kawasaki disease, are encountered almost exclusively in children and rarely, if at all, in adults. Thus, it is extremely unlikely that a biomarker useful for the diagnosis of one of these diseases or to assess response to therapeutic intervention will arise from any initiative in adults, and will instead rely upon investigations that are targeted to a pediatric population suffering from the specific disease of interest. As is discussed in more detail below, research efforts in this area are intensifying, and although the results provide valuable insights into disease pathogenesis, they also raise new issues unique to children.
Genomic markers of disease in children
The application of pharmacogenomic markers in pediatrics can be complex and challenging, as highlighted in a recent review [28]. One of the biggest challenges facing the application of genomic tools to pediatric disease is the relatively low incidence rate of these disorders in children (e.g., hypertension, IBD and Type II diabetes). As a consequence, multicenter collaborations are necessary to enroll sufficiently large study populations of affected children to form both discovery and validation datasets to adequately power genome-wide association studies (GWAS). Kawasaki disease, an inflammatory vasculitic disease in children with a propensity for coronary artery damage that may have serious cardiovascular sequelae later in life, illustrates the inherent difficulties in studying diseases primarily restricted to children. For example, the first GWAS of Kawasaki disease consisted of a discovery cohort of 119 cases and 135 controls recruited from medical centers in one country (The Netherlands), followed by a replication cohort of 893 Kawasaki disease cases drawn from multiple centers in three countries (Australia, the UK and the USA) [29]. A second GWAS involved a discovery cohort of 250 Kawasaki disease cases recruited at a single medical center in Taiwan, followed by a validation cohort of 208 cases recruited from five medical centers in Taiwan [30]. For each of these studies, multicenter efforts resulted in study populations that can be considered rather small compared with GWAS of complex diseases in adults. On the other hand, collaboration through multicenter disease-specific networks, such as the Children's Oncology Group (COG), allows much larger sample sizes (n = 1625 cases) to be compiled for even relatively rare diseases, as recently reported for a GWAS of neuroblastoma [31]. Clearly, similar concerns do not apply to more prevalent diseases, such as childhood obesity, where it is possible to recruit adequate study populations (>1000) at a single center [32]. Childhood diseases with relatively high prevalence worldwide, such as asthma, have sufficiently large sample sizes such that the development of multinational consortia leads to data providing important insights into disease pathogenesis, and in particular, mechanisms by which the pediatric disease may differ from adult-onset disease [20]. However, even in the setting of a common pediatric disease such as asthma, phenotypic expression of disease must be considered when evaluating potential genomic markers.
An additional challenge for genomic studies in children is the availability of appropriate control samples. For GWAS, the sequence of genomic DNA is considered invariant with age, and various strategies have been applied to collect DNA samples that can be used as controls for pediatric investigations. For example, control samples for the Kawasaki disease GWAS were comprised of adult blood donors from the same geographical region as the cases in the Caucasian study [29], and randomly selected samples from the Taiwan Han Chinese Cell and Genome Bank for the Han Chinese study [30]. A preferred option is the use of pediatric samples that are matched for multiple factors (e.g., demographics such as ethnic diversity and other relevant characteristics) representative of the pediatric population from which the disease cohort is drawn. Access to pediatric control samples requires the development of large repositories, such as the pediatric repository established by the Center for Applied Genomics, at The Children's Hospital of Philadelphia (PA, USA) [32]. These pediatric repositories are accompanied by several ethical issues that are not necessarily encountered when establishing repositories of biological samples derived from adults. These issues include, but are not limited to, parental versus child consent/assent, increasing autonomy of the child with age, long-term retention of samples for future research, impact of ancillary genetic information on family members, as well as predisposition to adult-onset disease and threat of discrimination upon future application for life or disability insurance [33,34].
Although modest sample sizes for less common diseases of childhood may limit power to detect associations, such studies are critical as a preliminary step to provide more targeted treatment for children, provide important new insights into factors related to disease risk, and contribute to the identification of gene pathways and networks involved in disease pathogenesis. There have been several significant advances in this area [20,35]. A GWAS of Kawasaki disease identified a set of biologically plausible, functionally inter-related genes associated with disease susceptibility [29]. As an infectious trigger of Kawasaki disease has been implicated but remains elusive, identification of LNX1, encoding a protein ligand of the coxsackievirus and adenovirus receptor, provides a compelling hypothesis for disease pathogenesis given that development of myocarditis is associated with coxsackievirus. Conducting GWAS in patients with early onset disease has been implemented as a strategy for identifying novel genes in asthma [36] and pediatric IBD [37]. In the context of asthma, the original observation of an association between genetic variation in ORMDL3 and childhood-onset asthma [36] has been extended to an adjacent locus, GSDMB [38], and replicated in additional studies [39,40]. It is now apparent from a large consortium-based investigation involving 10,365 physician-diagnosed cases of asthma and 16,110 unaffected individuals matched for ancestry that ORDML3/GSDMB variants are only associated with childhood-onset asthma, whereas extended haplotypes encompassing HLA-DQ and -DR are associated with adult-onset disease [20]. It is reasonable to expect that drug discovery efforts targeting cellular pathways involving ORDML3 and GSDMB may lead to more effective therapeutic strategies for asthma in children.
Ontogeny & assessment of pharmacogenomic biomarkers in children
When considering the integration of pharmacogenomics to improve drug therapy in children, it is important to recognize that an individual's genotype does not change during his or her lifetime. However, phenotypes do change during growth and development, and developmental changes in the expression of genes involved in drug disposition and response are added to all the other factors – genetic and environmental, among others – governing variability in drug response in adults. This latter point is particularly relevant when attempting to apply adult experience to children. For example, genotype–phenotype relationships anticipated based on adult experience may not be fully apparent until the gene product is fully expressed. Therefore, it is reasonable to expect an increased risk of concentration-dependent toxicity early in life if the primary eliminating pathway (inferred from studies in adults) is subject to delayed maturation. Likewise, there is a risk of therapeutic failure in young children in whom the activity of clearance pathways may be increased and dosing strategies do not take ontogeny into consideration. Furthermore, variability in drug response or therapeutic failure in children may also derive from developmental changes in the expression of the cellular pathway targeted by the therapeutic intervention.
There are very few genes involved in drug absorption, distribution, metabolism and excretion for which the relative contributions of ontogeny and genetic variation have been thoroughly investigated. One of the best examples is CYP2D6, for which both in vitro [41] and in vivo [42] data indicate that genetic variation is more important than ontogeny beyond the first month of life. In neonatal settings, however, both ontogeny and genetic variation contribute to inter-individual variability in the disposition of CYP2D6 substrates, such as tramadol, while functional CYP2D6 activity is being acquired postnatally concurrent with maturation of other systems, such as renal function [43]. Data from studies investigating changes in genotype–phenotype relationships during growth and development are useful for identifying key issues related to sources of variability in drug disposition and response in children. As a recent example, we have proposed a pharmacogenomics-aided study design to efficiently characterize population variability in children of different ages for components of over-the-counter cough and cold remedies, many of which (e.g., chlorpheniramine, dextromethorphan and diphenhydramine) are substrates for CYP2D6 [44]. Obtaining similar data for other cytochrome P450 pathways is dependent upon the availability of phenotyping probes suitable for use in children of all ages. However, children present unique challenges related to availability of oral formulations, erratic drug absorption, permissible number of blood samples and ethical considerations related to nontherapeutic drug use that are not experienced in adult studies [45].
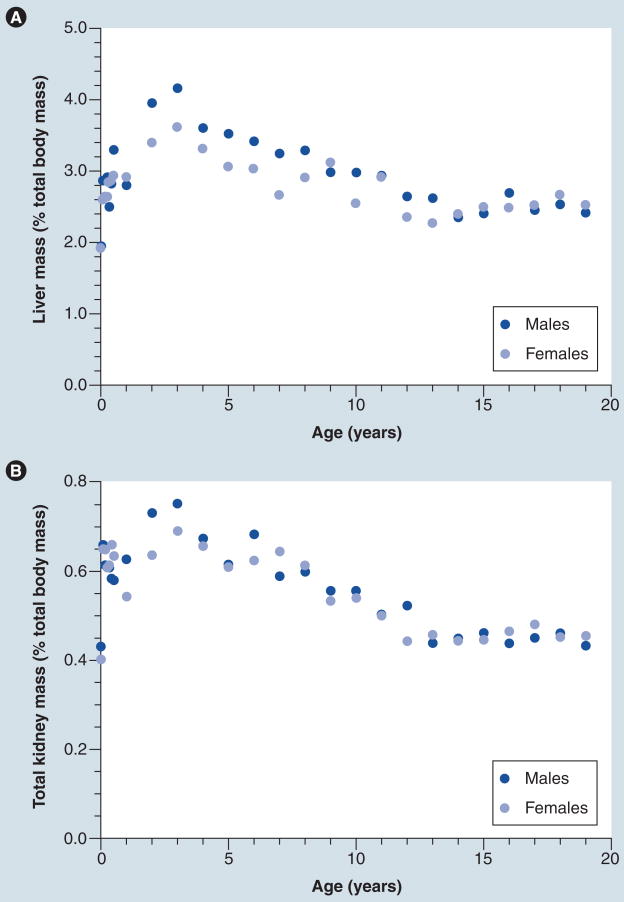
The challenges of directly extrapolating adult pharmacogenomic experience to pediatrics are illustrated by two examples of current high priority/visibility in the adult community. On 16 August 2007, the product monograph for warfarin was updated to include suggested starting doses for patients 18 years of age and older based on individual patient genotype data [103]. In adults, data from multiple studies indicate that variation in CYP2C9 and VKORC1 combined accounts for approximately 40% of the variability in warfarin dose, whereas age accounts for approximately 10% of variability [46]. By contrast, the only pediatric study to date was more limited in scope (a total of 59 patients) and reported that almost 30% of dose variation in children was accounted for by age, with the CYP2C9 and VKORC1 genotypes together accounting for <5% of variability [47]. Factors contributing to this relatively large age effect are not completely understood, but warfarin dosage requirements expressed on a mg/kg basis have been reported to decline with age between infancy and adolescence [48]. It has been proposed that weight-corrected differences in the clearance of unbound S-warfarin is a function of a larger ratio of liver mass:total body mass in prepubertal children relative to adolescents and adults [49]. The ratio of liver mass:total body mass does appear to be higher in young children, peaking between 2–4 years of age and declining to adult levels in adolescence; a similar phenomenon is observed with kidney mass (Figure 2). However, it is also important to note that developmental changes in the physiology of hemostasis are also present [50] and affect the pharmacodynamics of other anticoagulants as well [51]. The situation with warfarin is illustrative of the need to not rely too heavily on adult experience when introducing pharmacogenomics into pediatric clinical practice.
Figure 2. Relative contribution of liver and kidney mass to total body mass changes with age.
Age-dependent change in ratio between (A) liver mass and total body mass, and (B) kidney mass and total body mass for males and females aged 1 day to 19 years. Values for liver mass at a given age were obtained from [83], and kidney mass was derived from the sum of right and left kidney values in [83]. Corresponding values for body weight in kilograms represent the values for the 50th percentile obtained from the 28 November 2000 revision of growth charts for males and females developed by the National Center for Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion [101]. (A) A comprehensive analysis of developmental changes in liver volume and a model to predict liver volume in pediatric populations has been published previously [84].
A similar situation exists for CYP2C19 and response to clopidogrel. The ontogeny of CYP2C19 has been reasonably well characterized, both in vitro [52] and in vivo [53,54], and the disposition of CYP2C19 substrates, such as pantoprazole, after intravenous administration to children 2–16 years of age, appears comparable to that observed in adults [53]. However, when one considers newborns and infants, pantoprazole clearance in patients genotyped as CYP2C19 poor metabolizers is indistinguishable from the rest of the population, reflecting CYP2C19 ontogeny early in postnatal life [54]. Furthermore, platelets from young children are hyporesponsive to stimulation by physiologically relevant agonists relative to adults and, therefore, a lower clopidogrel dose is required than would be predicted from adult experience [55]. Thus, on the basis of these developmental factors related to both drug disposition and response, one can expect difficulties in applying adult clopidogrel pharmacogenomic experience to children [56].
Ethical concerns related to nontherapeutic administration of medications to children limit investigations to patient populations being treated with the compounds of interest, or who can reasonably be expected to be treated with the drug class. This is in marked contrast to the situation in adults where it is not uncommon for pharmacogenomic investigations to be conducted in healthy volunteers so that the effect of genotype on dose–exposure relationships can be assessed in the absence of confounding influences, such as disease and concurrently administered drugs. As an example, the SLCO1B1 521T>C and -11187G>A variants are associated with increased systemic exposure to pravastatin in healthy adult volunteers [57]. In children treated with pravastatin for familial hypercholesterolemia, no statistically significant differences in systemic exposure as measured by area under the curve (AUC) were observed in the presence of the variant 521T>C allele alone, and the −11187G>A variant was associated with a 74% decrease in AUC [58]. The SLCO1B1 521T>C allele was associated with a marginally significant (62%; p = 0.055) lower AUC in pediatric cardiac transplant patients, a result that is confounded in this population by concomitant administration of cyclosporine, an inhibitor of SLCO1B1 activity. Although these results have not been replicated in other pediatric studies, they imply that pharmacogenomic determinants of drug disposition and response determined in studies conducted in adults may not extrapolate directly to pediatric patients. The reasons for this are not immediately apparent, but may be related to unidentified developmental factors, the difference between healthy volunteers versus children with medical conditions, or the presence of exogenous factors such as concurrent medications. As a priori knowledge of when adult pharmacogenomic biomarkers can or cannot be applied to children, can not be predicted at the present time, there is a compelling argument for relevant studies to be conducted in the specific patient population for which the drug is to be used.
Addressing the gap in pediatric biomarkers
As described above, there are multiple challenges when considering an appropriate pediatric biomarker. Desirable pediatric biomarkers should be developed as pertaining to children while addressing ontogeny and changes in phenotypic gene expression appreciated in a cohort (Box 2). Below are two examples that address specific challenges when assessing heterogeneous disease processes in children and offer potential solutions in the discovery of future biomarkers.
Box 2. Characteristics of an ideal pediatric biomarker.
Noninvasive
Applicable to pediatric-specific diseases
Results correspond with age-dependent physiologic changes
Cost effective
Well-established pediatric normative values
Folate disruption by methotrexate in JIA
JIA, a persistent inflammatory arthritis of childhood, is one of the most common rheumatic diseases in children. JIA manifests as several clinically heterogeneous disease subtypes that make classification complex, requiring international efforts to develop an agreed upon clinical classification system [59]. The heterogeneity in disease phenotype supports variability in disease pathogenesis, which has prompted the investigation of cellular and genetic biomarkers to enhance not only an understanding of disease pathogenesis but also improve disease classification and response to therapy [26,60]. The challenges in developing clear disease phenotypes in a childhood disease with low prevalence result in expensive and logistically challenging studies to execute, which has impacted research advancements in the field.
These challenges also extend to investigations related to therapeutics in JIA. Methotrexate (MTX), the first-choice, second-line therapy in JIA was shown to be safe and effective in children with juvenile rheumatoid arthritis (previous term for JIA) resistant to NSAIDs in a double-blind, randomized, placebo-controlled study [61]. However, despite some of the most rigorous data to suggest its efficacy, there is still significant inter-individual variability in drug response and toxicity that remains unexplained. Drug dose ranges also vary considerably between adults and children, with children receiving higher absolute doses of MTX (per kilogram of body weight). Since the onset of action of the drug takes several months to take effect, there is a lag time in drug response that allows the patient to be vulnerable to drug toxicity. This shift in the risk: benefit ratio early in treatment is an opportune example of a scenario where a biomarker predictive of drug response would prove clinically meaningful.
MTX is a folate antagonist that upon entry into cells becomes polyglutamated (MTXGlun), a process that is thought to play a role in intracellular retention of the drug and enhance target enzyme inhibition [62,63]. Measurement of the polyglutamated form of the drug intracellularly, typically in erythrocytes, has been found to be more useful than serum levels of MTX, which are rapidly undetectable and lack correlation with outcome or drug dose [64,65]. Greater concentrations of long-chain MTXGlu3–5 have been shown to correlate with improved outcomes in adult rheumatoid arthritis patients [66], and increased risk of liver and gastrointestinal toxicity in children [67]. No correlations have been observed between concentrations of long-chain MTXGlun and drug efficacy in children with JIA [67,68], a finding that remains unexplained. As there is likely a continuum of drug-related antifolate effects ranging from efficacy to toxicity, one physiologic aspect that may impact this continuum is inter-individual variability in folate status at the time treatment with MTX is initiated, especially in children where the balance between folate supply and demand may change during growth and development.
Folate has been identified as a B vitamin that is involved in several important cellular activities including purine and pyrimidine synthesis [62,69], and remethylation of homocysteine to methionine [70]. Folate deficiency during pregnancy is associated with increased risk of cleft lip and palate, congenital heart defects and neural tube defects [71], and later in life a potentially increased risk of cardiovascular disease [72]. As children age, folate needs change with growth and development, and it has been shown that both serum and intracellular folate concentrations decrease with age [73]. Therefore, the increased doses of MTX (relative to weight) that are required in children for clinical efficacy may be related to increased baseline folate concentrations that must be targeted by MTX in this population.
With this in mind, it may be more appropriate to measure the antifolate effect of MTX across the age continuum, and explore how changes in folate in response to MTX correlate with drug outcome. Further investigation will then require identification and characterization of factors that modulate this variability in folate homeostasis, such as variation in an individual's genotype related to the folate pathway, clinical demographic characteristics, dose and route of drug administration, and baseline folate status before starting the drug. Taking into account how a physiologic biomarker such as folate may change with age in conjunction with changes related to the drug of interest will allow for a more accurate and clinically meaningful biomarker of MTX response.
Noninvasive technique to assess histamine response
Antihistamines are widely used in pediatrics with approximately 10% of US children in any given week using an antihistamine for a variety of allergic (e.g., seasonal allergic rhinitis and atopic dermatitis) and nonallergic (e.g., infection-associated rhinitis, nasal congestion, cough, emesis and sedation) conditions [74]. The pharmacologic activity of orally administered H1-antagonists is commonly assessed by measuring suppression of the cutaneous wheal and flare response stimulated by intradermal or epicutaneous administration of histamine [75,76]. Commonly referred to as the ‘skin prick test,’ epicutaneous histamine is considered the ‘gold standard’ for pharmacodynamic assessment of antihistamine response in both children and adults by assessing the onset, maximal activity and duration of antihistamine effect as a function of the administered dose [77]. Histamine is introduced into the skin via a small break in the skin using a ‘prick’ or ‘puncture’ device, and results in a small area of swelling (wheal) and erythema (flare) appear within minutes. The diameter of the wheal and flare response is then measured manually, and reductions in diameter following antihistamine administration is used as a pharmacodynamic assessment of response. The extent of the wheal and flare response to epicutaneous histamine correlates with dermal concentrations of H1-receptor antagonists and resolution of allergic rhinitis symptoms [76,78]. The apparent simplicity of this approach has led to relatively widespread use. However, when used as a quantitative biomarker of histamine pharmacodynamics, epicutaneous histamine has significant limitations. Major sources of variability include:
Differences in devices used to administer histamine and resultant wheal and flare response
Differences in methods for measuring the wheal and flare response
Differences inherent to inter-operator variability in placement of histamine and measurement
In addition, the epicutaneous histamine method is not amenable to continuous measurement of response, and is relatively invasive, especially for children.
An alternative approach, iontophoresis, has been widely used to administer various agents to the superficial skin surface, including lidocaine and histamine. Laser Doppler flowmetry has been used to assess microvasculature changes related to various physiologic and pathophysiologic conditions, as well as in response to cutaneous and systemic administration of various agents, including histamine [79]. Coupling histamine iontophoresis with laser Doppler flowmetry (HILD) permits continuous measurement of the dynamic changes in vasodilatation and consequent increase in blood flow that occur upon histamine challenge, leading to a more robust and accurate measurement of response to histamine compared with the prick test [80]. HILD has been used to assess cutaneous histamine response and antihistamine pharmacodynamics in adults [81]. The noninvasive delivery of a fixed dose of histamine into the skin followed by an objective and continuous measurement of blood flow also reduces variability related to inter-operator differences in histamine application and other limitations of epicutaneous testing.
In a recent evaluation of HILD at our institution, the method was validated in a cohort of adult subjects via comparison to epicutaneous histamine. A linear association was observed between HILD maximal blood flow and AUC (r2 = 0.69; p < 0.05), which is reflective of internal validity of the method. Correlation was found between AUC via epicutaneous histamine testing and HILD (r2 = 0.58; p < 0.05). Based on these findings, it appears that HILD is capable of demonstrating magnitude and response rate to histamine. It was also found that HILD is only minimally irritating as indicated by a total mean numerical itch score of 25 (scale 1–100) and a mean duration of itch of 8 min [82]. In ongoing studies being conducted in children with allergic rhinitis, preliminary results indicate that blood flow versus time curves and numerical itch ratings are similar to those observed in adults. The noninvasive nature of HILD testing makes it attractive as an objective and continuous assessment of microvasculature response to histamine, and thus an ideal technique for assessing the pharmacodynamic response to antihistamines in children and adults. Further validation in the pediatric population as well as studies to determine the intra-individual variation of the technique are needed to further validate this method for use in real world pharmacodynamic assessment.
Conclusion & future perspective
Innovative approaches are essential to adequately discover and validate biomarkers suitable for children, as extrapolation of adult biomarker data to children results in delays in clinical implementation due to the additional validation studies required to ascertain the impact of ontogeny on biomarker expression and, thus, interpretation of the results. Instead, we propose that research for pediatric-specific biomarkers should begin with children. The potential benefit of biomarker discovery efforts targeted to pediatric diseases is the identification of pathways unique to pathogenesis of the pediatric form of disease, with the possibility of uncovering novel targets for future drug development. Recent advances in genomic markers of childhood-onset asthma are prime examples of the potential in this area [20]. This effort will require a multifaceted approach that includes multicenter, collaborative programs to increase the sample size of relatively uncommon diseases; indeed, the National Institute of Child Health and Human Development has recognized the need in this area, and recently released a Funding Opportunity Announcement, PAR-11–322 ‘Biomarkers: bridging pediatric and adult therapeutics.’ Integration of research into clinical care settings can improve the categorization and phenotypic description of disease processes unique to children by standardizing clinical evaluation and documentation. Creation of biological sample repositories paired with deidentified electronic medical record data have the potential to provide rich repositories from which data can be extracted and analyzed for exploratory investigations. Sophisticated techniques including metabolomics and proteomics, which involve the measurement of complete metabolite and peptide profiles at the cellular level, have been underutilized in children to date but hold promise in providing fresh insight into disease processes.
This integrated approach is not limited to children. Senescence results in disease variability not observed in younger adults, and the elderly are a potential population under-represented when normative adult values for biomarkers are initially investigated. Adapting a comprehensive approach for biomarker discovery and validation will result in personalized biomarkers that can accommodate patient-to-patient variability and avoid the currently accepted and inadequate ‘one size fits all’ approach.
Executive summary.
Use of biomarkers in children
Biomarkers are beneficial in the assessment and management of many pediatric diseases.
Pediatricians commonly manage pediatric diseases, such as lead toxicity, infection, renal disease and juvenile diabetes, with the aid of biomarkers.
Consideration of growth & development is critical for pediatric biomarker development & validation
Growth and development (ontogeny) impacts almost all factors and systems from organ function to drug disposition and clinical response to therapy.
Commonly utilized biomarkers are directly influenced by human development.
Adult biomarkers for pediatric diseases: not always the answer
Reference ranges of many standard laboratory tests vary according to age as compared with a single standard adult reference value.
The pathogenesis of disease is often different in children compared with adults, and some diseases only occur in children. Therefore, development of pediatric-specific biomarkers is essential.
Genomic markers of disease in children
The low incidence rate of many pediatric disorders results in modest sample sizes making the identification of genetic disease associations challenging.
Future biomarker discovery and validation in children will benefit from multicenter collaborative efforts.
Ontogeny & assessment of pharmacogenomic biomarkers in children
Although an individual's genotype is invariant during one's lifetime, phenotypic expression of genes changes during growth and development.
Anticipated genotype–phenotype relationships based on adult experience are not necessarily observed in children until the gene product is fully expressed during childhood.
Addressing the gap in pediatric biomarkers
Specific challenges arise when assessing heterogeneous disease processes in children.
A potential marker of methotrexate response in children with juvenile idiopathic arthritis is folate concentration, which varies with age.
Histamine iontophoresis with laser Doppler flowmetry may serve as an objective, reliable and noninvasive test for assessing microvasculature response to histamine, making it the ideal technique for assessing the pharmacodynamic response to antihistamines in children.
Conclusion
Pediatric biomarkers should be developed specifically for children and well-recognized developmental changes in children must be considered.
Validated pediatric biomarkers are vital in caring for the pediatric patient.
Collaborative efforts are essential in developing appropriate biomarkers specific to children.
Acknowledgments
The authors would like to thank the anonymous reviewers for providing examples of contemporary biomarkers in children, such as acute kidney injury biomarkers.
J Goldman is supported by grant T32 HD069038 (GL Kearns, PI). Support for the longitudinal phenotyping studies of CYP2D6 activity in children and adolescents has been provided by grants R03 HD36783 and R01 HD058556 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (JS Leeder). B Jones is supported by grant K23 HL105783 from National Heart, Lung, and Blood Institute, and M Becker received grant support from the Kansas City Area Life Sciences Institute and a PhRMA Foundation Faculty Development Award, and currently an American College of Rheumatology Research and Education Foundation Rheumatology Investigator Award.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2001;1:182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪.Atkinson AJ, Colburn WA, Degruttola VG, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. Thoughtful consideration of issues related to biomarker discovery and validation. [DOI] [PubMed] [Google Scholar]
- 3.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx. 2004;1(2):189–195. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage WJ, Everett AD. Biomarkers in pediatrics: children as biomarker orphans. Proteomics Clin Appl. 2010;4(12):915–921. doi: 10.1002/prca.201000062. [DOI] [PubMed] [Google Scholar]
- 5.Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health. 2009;24(1):15–45. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzano S, Bailey B, Gervaix A, Cousineau J, Delvin E, Girodias JB. Markers for bacterial infection in children with fever without source. Arch Dis Child. 2011;96(5):440–446. doi: 10.1136/adc.2010.203760. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein DE, Walker B, Rawlings SS, et al. Hemoglobin A1c levels in children and adolescents with diabetes mellitus. Diabetes Care. 1980;3(4):503–507. doi: 10.2337/diacare.3.4.503. [DOI] [PubMed] [Google Scholar]
- 8▪.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. Detailed review of impact of ontogeny in drug disposition. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–263. [PubMed] [Google Scholar]
- 10.Skinner AM, Addison GM, Price DA. Changes in the urinary excretion of creatinine, albumin and N-acetyl-beta-d-glucosaminidase with increasing age and maturity in healthy schoolchildren. Eur J Pediatr. 1996;155(7):596–602. doi: 10.1007/BF01957912. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier RL. Developmental renal physiology of the low birth weight pre-term newborn. J Urol. 1996;156(2 Pt 2):714–719. doi: 10.1097/00005392-199608001-00041. [DOI] [PubMed] [Google Scholar]
- 12.Chen N, Aleksa K, Woodland C, Rieder M, Koren G. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006;21(2):160–168. doi: 10.1007/s00467-005-2105-4. [DOI] [PubMed] [Google Scholar]
- 13.Ceriotti F, Boyd JC, Klein G, et al. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem. 2008;54(3):559–566. doi: 10.1373/clinchem.2007.099648. [DOI] [PubMed] [Google Scholar]
- 14.John TR, Moore W, Jeffries JE, editors. Children Are Different: Developmental Physiology. Ross Laboratories; Columbus, OH, USA: 1978. [Google Scholar]
- 15.Al-Ismaili Z, Palijan A, Zappitelli M. Biomarkers of acute kidney injury in children: discover, evaluation, and clinical application. Pediatr Nephrol. 2011;26:29–40. doi: 10.1007/s00467-010-1576-0. [DOI] [PubMed] [Google Scholar]
- 16.Shore GM, Hoberman L, Dowdey BC, Combes B. Serum gamma-glutamyl transpeptidase activity in normal children. Am J Clin Pathol. 1975;63(2):245–250. doi: 10.1093/ajcp/63.2.245. [DOI] [PubMed] [Google Scholar]
- 17.Yip R, Johnson C, Dallman PR. Age-related changes in laboratory values used in the diagnosis of anemia and iron deficiency. Am J Clin Nutr. 1984;39(3):427–436. doi: 10.1093/ajcn/39.3.427. [DOI] [PubMed] [Google Scholar]
- 18.England K, Thorne C, Pembrey L, Tovo PA, Newell ML. Age- and sex-related reference ranges of alanine aminotransferase levels in children: European Paediatric HCV Network. J Pediatr Gastroenterol Nutr. 2009;49:71–77. doi: 10.1097/MPG.0b013e31818fc63b. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal S, Epps RP, Heavenrich R, et al. Report of the task force on blood pressure control in children. Pediatrics. 1977;59(5 Suppl. 2):I–II. 797–820. [PubMed] [Google Scholar]
- 20▪▪.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi: 10.1056/NEJMoa0906312. Large multicenter genome-wide analysis of genetic variations associated with childhood- and adult-onset asthma. Presents evidence for ORDML3/GSDMB as a genomic marker unique to childhood-onset asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine N. Pediatric inflammatory bowel disease: is it different? Dig Dis. 2009;27:212–214. doi: 10.1159/000228552. [DOI] [PubMed] [Google Scholar]
- 22.Wagner-Weiner L. Pediatric rheumatology for the adult rheumatologist. J Clin Rheumatol. 2008;14:109–119. doi: 10.1097/RHU.0b013e31816b4460. [DOI] [PubMed] [Google Scholar]
- 23.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, healthcare use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;32:1–14. [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes MG, Grom AA, Thompson SD, et al. Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2102–2112. doi: 10.1002/art.24601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowlton N, Jiang K, Frank MB, et al. The meaning of clinical remission in polyarticular juvenile idiopathic arthritis: gene expression profiling in peripheral blood mononuclear cells identifies distinct disease states. Arthritis Rheum. 2009;60(3):892–900. doi: 10.1002/art.24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piana C, Surh L, Furst-Recktenwald S, et al. Integration of pharmacogenetics and pharmacogenomics in drug development: implications for regulatory and medical decision making in pediatric diseases. J Clin Pharmacol. 2011 doi: 10.1177/0091270011401619. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29▪.Burgner D, Davila S, Breunis WB, et al. A genome-wide association study identifies novel and functionally related susceptibility loci for Kawasaki disease. PLoS Genet. 2009;5(1):e1000319. doi: 10.1371/journal.pgen.1000319. One of the first genome-wide association studies investigating a pediatric-specific disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai FJ, Lee YC, Chang JS, et al. Identification of novel susceptibility loci for Kawasaki disease in a Han Chinese population by a genome-wide association study. PLoS One. 2011;6(2):e16853. doi: 10.1371/journal.pone.0016853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen LB, Diskin SJ, Capasso M, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility loci. PLoS Genet. 2011;7(3):e1002026. doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glessner JT, Bradfield JP, Wang K, et al. A genome-wide study reveals copy number variants exclusive to childhood obesity cases. Am J Hum Genet. 2010;87(5):661–666. doi: 10.1016/j.ajhg.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hens K, Cassiman JJ, Nys H, Dierickx K. Children, biobanks and the scope of parental consent. Eur J Hum Genet. 2011;19:735–739. doi: 10.1038/ejhg.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Moran C, Thornburg CD, Barfield RC. Ethical considerations for pharmacogenomic testing in pediatric clinical care and research. Pharmacogenomics. 2011;12:889–895. doi: 10.2217/pgs.10.216. Excellent review of issues related to the investigation and clinical application of pharmacogenomics to children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinks A, Barton A, Shephard N, et al. Identification of a novel susceptibility locus for juvenile idiopathic arthritis by genome-wide association analysis. Arthritis Rheum. 2009;60(1):258–263. doi: 10.1002/art.24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 37.Kugathasan S, Baldassano RN, Bradfield JP, et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nat Genet. 2008;40:1211–1215. doi: 10.1038/ng.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouzigon E, Corda E, Aschard H, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359:1985–1994. doi: 10.1056/NEJMoa0806604. [DOI] [PubMed] [Google Scholar]
- 39.Halapi E, Gudbjartsson DF, Jonsdottir GM, et al. A sequence variant on 17q21 is associated with age at onset and severity of asthma. Eur J Hum Genet. 2010;18(8):902–908. doi: 10.1038/ejhg.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung TF, Sy HY, Ng MCY, et al. Asthma and atopy are associated with chromosome 17q21 markers in Chinese children. Allergy. 2009;64(4):621–628. doi: 10.1111/j.1398-9995.2008.01873.x. [DOI] [PubMed] [Google Scholar]
- 41.Stevens JC, Marsh SA, Zaya MJ, et al. Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos. 2008;36:1587–1593. doi: 10.1124/dmd.108.021873. [DOI] [PubMed] [Google Scholar]
- 42.Blake MJ, Gaedigk A, Pearce RE, et al. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther. 2007;81:510–516. doi: 10.1038/sj.clpt.6100101. [DOI] [PubMed] [Google Scholar]
- 43.Allegaert K, Rochette A, Veyckemans F. Developmental pharmacology of tramadol during infancy: ontogeny, pharmacogenetics and elimination clearance. Paediatr Anaesth. 2011;21:266–273. doi: 10.1111/j.1460-9592.2010.03389.x. [DOI] [PubMed] [Google Scholar]
- 44.Leeder JS, Kearns GL, Spielberg SP, Van Den Anker J. Understanding the relative roles of pharmacogenetics and ontogeny in pediatric drug development and regulatory science. J Clin Pharmacol. 2010;50:1377–1387. doi: 10.1177/0091270009360533. [DOI] [PubMed] [Google Scholar]
- 45▪▪.De Wildt SN, Ito S, Koren G. Challenges for drug studies in children: CYP3A phenotyping as example. Drug Discov Today. 2009;14:6–15. doi: 10.1016/j.drudis.2008.07.007. Thorough analysis of factors related to the application of phenotyping strategies to children. [DOI] [PubMed] [Google Scholar]
- 46.Jonas DE, McLeod HL. Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol Sci. 2009;30(7):375–386. doi: 10.1016/j.tips.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 47▪▪.Nowak-Gottl U, Dietrich K, Schaffranek D, et al. In pediatric patients, age has more impact on dosing of vitamin K antagonists than VKORC1 or CYP2C9 genotypes. Blood. 2010;116(26):6101–6105. doi: 10.1182/blood-2010-05-283861. First publication of warfarin pharmacogenetics in a pediatric population. Data indicate that age, rather than genetic variation in CYP2C9 and VKORC1, is the primary factor associated with anticoagulant dose in children. Replication of these results is required. [DOI] [PubMed] [Google Scholar]
- 48.Streif W, Andrew M, Marzinotto V, et al. Analysis of warfarin therapy in pediatric patients: a prospective cohort study of 319 patients. Blood. 1999;94(9):3007–3014. [PubMed] [Google Scholar]
- 49.Takahashi H, Ishikawa S, Nomoto S, et al. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther. 2000;68(5):541–555. doi: 10.1067/mcp.2000.110977. [DOI] [PubMed] [Google Scholar]
- 50.Monagle P. Anticoagulation in the young. Heart. 2004;90(7):808–812. doi: 10.1136/hrt.2003.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payne JH. Aspects of anticoagulation in children. Br J Haematol. 2010;150(3):259–277. doi: 10.1111/j.1365-2141.2010.08225.x. [DOI] [PubMed] [Google Scholar]
- 52.Koukouritaki SB, Manro JR, Marsh SA, et al. Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther. 2004;308(3):965–974. doi: 10.1124/jpet.103.060137. [DOI] [PubMed] [Google Scholar]
- 53.Ward RM, Kearns GL, Tammara B, et al. A multicenter, randomized, open-label, pharmacokinetics and safety study of pantoprazole tablets in children and adolescents aged 6 through 16 years with gastroesophageal reflux disease. J Clin Pharmacol. 2011;51(6):876–887. doi: 10.1177/0091270010377501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward RM, Tammara B, Sullivan SE, et al. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD) Eur J Clin Pharmacol. 2010;66(6):555–561. doi: 10.1007/s00228-010-0811-8. [DOI] [PubMed] [Google Scholar]
- 55.Li JS, Yow E, Berezny KY, et al. Dosing of clopidogrel for platelet inhibition in infants and young children: primary results of the Platelet Inhibition in Children On cLOpidogrel (PICOLO) trial. Circulation. 2008;117(4):553–559. doi: 10.1161/CIRCULATIONAHA.107.715821. [DOI] [PubMed] [Google Scholar]
- 56.Shuldiner AR, O'Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niemi M, Schaeffeler E, Lang T, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14(7):429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 58.Hedman M, Antikainen M, Holmberg C, et al. Pharmacokinetics and response to pravastatin in paediatric patients with familial hypercholesterolaemia and in paediatric cardiac transplant recipients in relation to polymorphisms of the SLCO1B1 and ABCB1 genes. Br J Clin Pharmacol. 2006;61(6):706–715. doi: 10.1111/j.1365-2125.2006.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. [PubMed] [Google Scholar]
- 60.Beresford MW. Juvenile idiopathic arthritis: new insights into classification, measures of outcome, and pharmacotherapy. Paediatr Drugs. 2011;13(3):161–173. doi: 10.2165/11588140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in resistant juvenile rheumatoid arthritis Results of the USA–USSR double-blind, placebo-controlled trial The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children's Study Group. N Engl J Med. 1992;326(16):1043–1049. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- 62.Allegra CJ, Chabner BA, Drake JC, Lutz R, Rodbard D, Jolivet J. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. J Biol Chem. 1985;260(17):9720–9726. [PubMed] [Google Scholar]
- 63.Chabner BA, Allegra CJ, Curt GA, et al. Polyglutamation of methotrexate Is methotrexate a prodrug? J Clin Invest. 1985;76(3):907–912. doi: 10.1172/JCI112088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lafforgue P, Monjanel-Mouterde S, Durand A, Catalin J, Acquaviva PC. Lack of correlation between pharmacokinetics and efficacy of low dose methotrexate in patients with rheumatoid arthritis. J Rheumatol. 1995;22(5):844–849. [PubMed] [Google Scholar]
- 65.Ravelli A, Di Fuccia G, Molinaro M, et al. Plasma levels after oral methotrexate in children with juvenile rheumatoid arthritis. J Rheumatol. 1993;20(9):1573–1577. [PubMed] [Google Scholar]
- 66.Dervieux T, Furst D, Lein DO, et al. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004;50(9):2766–2774. doi: 10.1002/art.20460. [DOI] [PubMed] [Google Scholar]
- 67.Becker ML, Gaedigk R, Van Haandel L, et al. The effect of genotype on methotrexate polyglutamate variability in juvenile idiopathic arthritis and association with drug response. Arthritis Rheum. 2011;63(1):276–285. doi: 10.1002/art.30080. [DOI] [PubMed] [Google Scholar]
- 68.Dolezalova P, Krijt J, Chladek J, Nemcova D, Hoza J. Adenosine and methotrexate polyglutamate concentrations in patients with juvenile arthritis. Rheumatology (Oxford) 2005;44(1):74–79. doi: 10.1093/rheumatology/keh401. [DOI] [PubMed] [Google Scholar]
- 69.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92(6):2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Refsum H, Helland S, Ueland PM. Fasting plasma homocysteine as a sensitive parameter of antifolate effect: a study of psoriasis patients receiving low-dose methotrexate treatment. Clin Pharmacol Ther. 1989;46(5):510–520. doi: 10.1038/clpt.1989.179. [DOI] [PubMed] [Google Scholar]
- 71.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 72.Miller ER, 3rd, Juraschek S, Pastor-Barriuso R, Bazzano LA, Appel LJ, Guallar E. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106(4):517–527. doi: 10.1016/j.amjcard.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 73.Kerr MA, Livingstone B, Bates CJ, et al. Folate, related B vitamins, and homocysteine in childhood and adolescence: potential implications for disease risk in later life. Pediatrics. 2009;123(2):627–635. doi: 10.1542/peds.2008-1049. [DOI] [PubMed] [Google Scholar]
- 74.Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Cough and cold medication use by US children, 1999–2006: results from the Slone survey. Pediatrics. 2008;122(2):e323–329. doi: 10.1542/peds.2008-0498. [DOI] [PubMed] [Google Scholar]
- 75.Simons FE, McMillan JL, Simons KJ. A double-blind, single-dose, crossover comparison of cetirizine, terfenadine, loratadine, astemizole, and chlorpheniramine versus placebo: suppressive effects on histamine-induced wheals and flares during 24 hours in normal subjects. J Allergy Clin Immunol. 1990;86(4 Pt 1):540–547. [PubMed] [Google Scholar]
- 76.Van Steekelenburg J, Clement PA, Beel MH. Comparison of five new antihistamines (H1-receptor antagonists) in patients with allergic rhinitis using nasal provocation studies and skin tests. Allergy. 2002;57(4):346–350. doi: 10.1034/j.1398-9995.2002.1s3426.x. [DOI] [PubMed] [Google Scholar]
- 77.Simons F. Advances in H1-antihistamines. N Engl J Med. 2004;351:2203–2217. doi: 10.1056/NEJMra033121. [DOI] [PubMed] [Google Scholar]
- 78.Watson WT, Simons KJ, Chen XY, Simons FE. Cetirizine: a pharmacokinetic and pharmacodynamic evaluation in children with seasonal allergic rhinitis. J Allergy Clin Immunol. 1989;84(4 Pt 1):457–464. doi: 10.1016/0091-6749(89)90358-8. [DOI] [PubMed] [Google Scholar]
- 79.Eun HC. Evaluation of skin blood flow by laser Doppler flowmetry. Clin Dermatol. 1995;13(4):337–347. doi: 10.1016/0738-081x(95)00080-y. [DOI] [PubMed] [Google Scholar]
- 80.Magerl W, Westerman RA, Mohner B, Handwerker HO. Properties of transdermal histamine iontophoresis: differential effects of season, gender, and body region. J Invest Dermatol. 1990;94:347–352. doi: 10.1111/1523-1747.ep12874474. [DOI] [PubMed] [Google Scholar]
- 81.Heyer G, Hornstein OP, Handwerker HO. Skin reactions and itch sensation induced by epicutaneous histamine application in atopic dermatitis and controls. J Invest Dermat. 1989;93:492–496. doi: 10.1111/1523-1747.ep12284051. [DOI] [PubMed] [Google Scholar]
- 82.Jones BL, Abdel-Rahman SM, Simon SD, Kearns GL, Neville KA. Assessment of histamine pharmacodynamics by microvasculature response of histamine using histamine iontophoresis laser Doppler flowimetry. J Clin Pharmacol. 2009;49(5):600–605. doi: 10.1177/0091270009332247. [DOI] [PubMed] [Google Scholar]
- 83.Stocker JT, Dehner LP, editors. Pediatric Pathology. 2nd. Lippincott, Williams & Wilkins; Philadelphia, PA, USA: 2001. [Google Scholar]
- 84.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transplant. 2005;12:1481–1493. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
Websites
- 101.Centers for Disease Control and Prevention. Growth Charts. [Accessed 2 August 2011];2010 September; www.cdc.gov/growthcharts.
- 102.Therapeutics for Rare & Neglected Diseases (TRND) program at NIH. [Accessed 15 August 2011]; http://trnd.nih.gov/
- 103.Coumadin Official US FDA information, side effects and uses. www.drugs.com/pro/coumadin.html.