Abstract
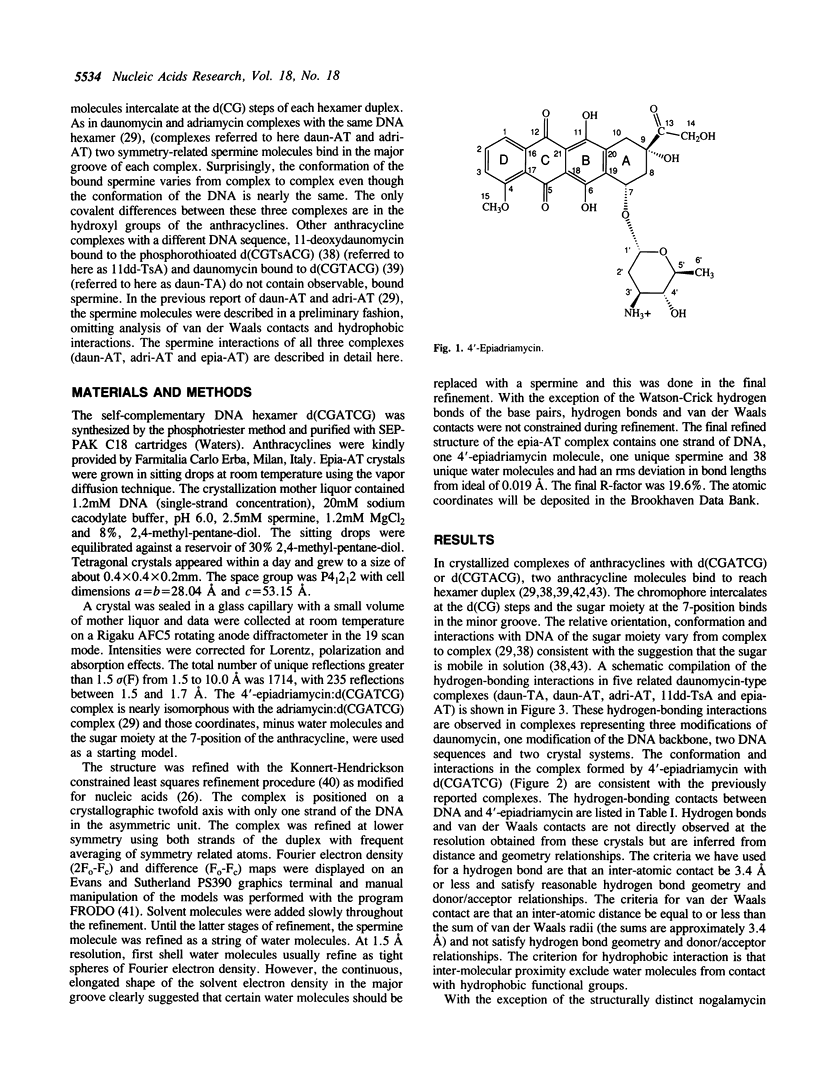
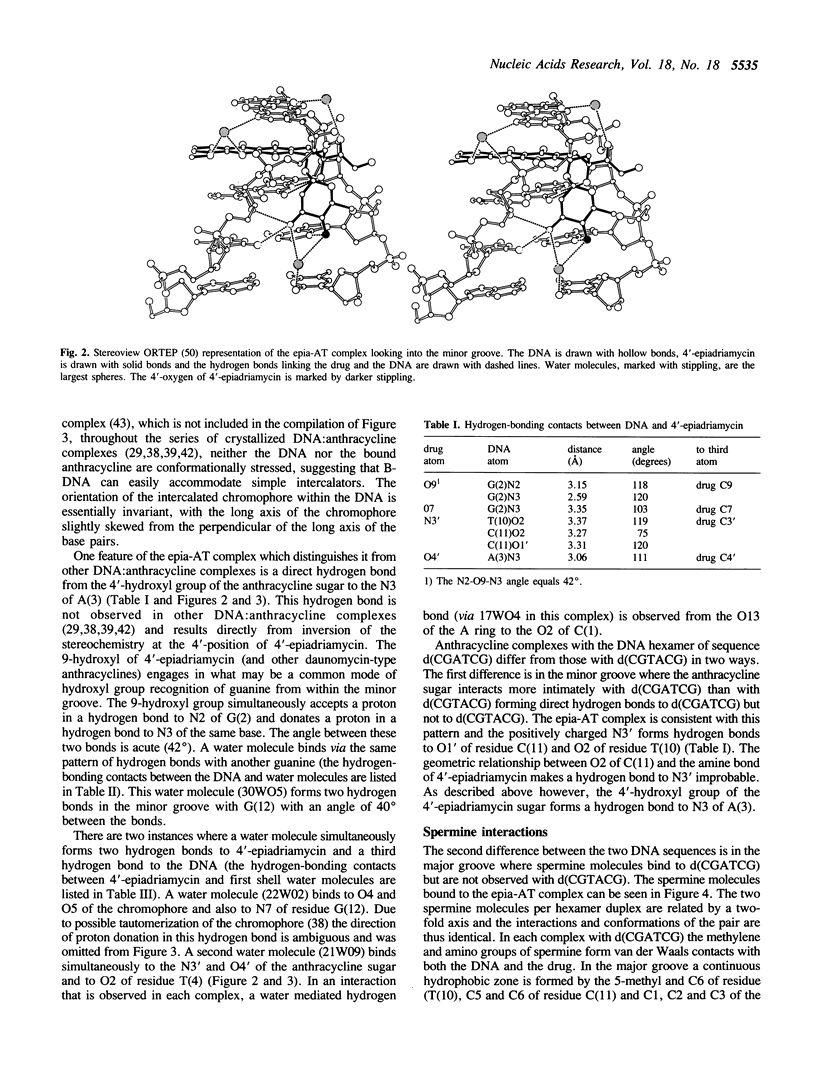
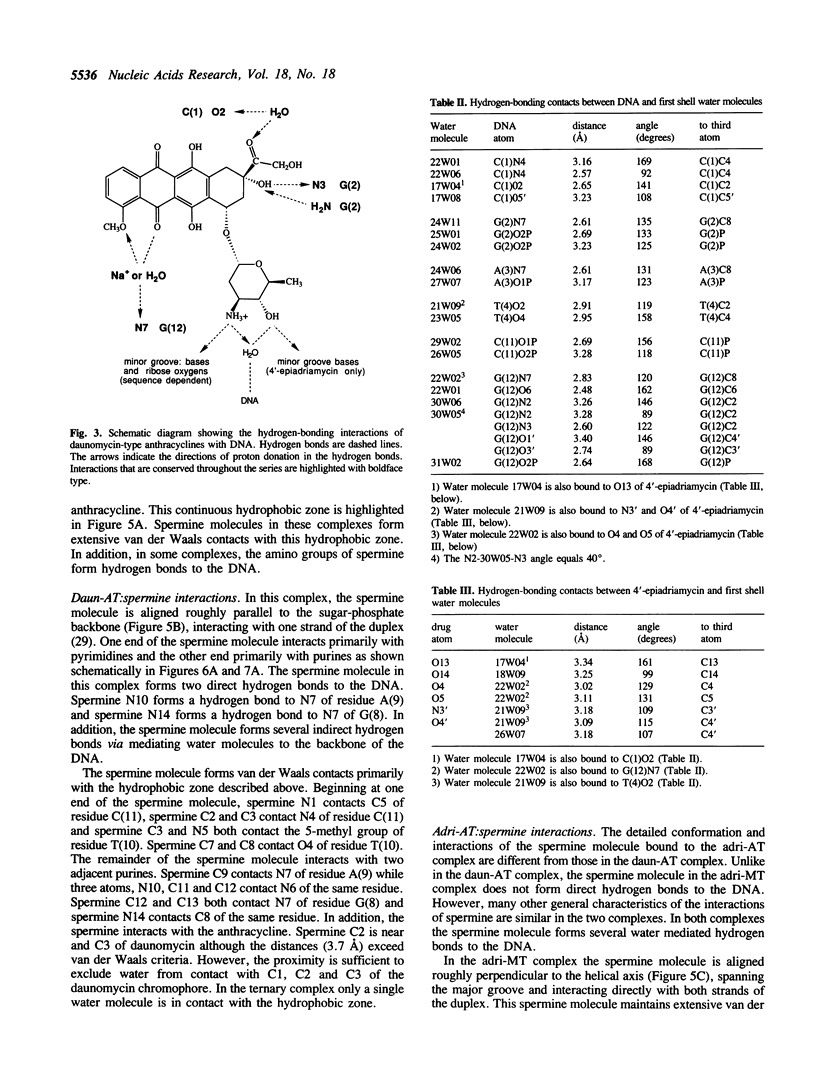
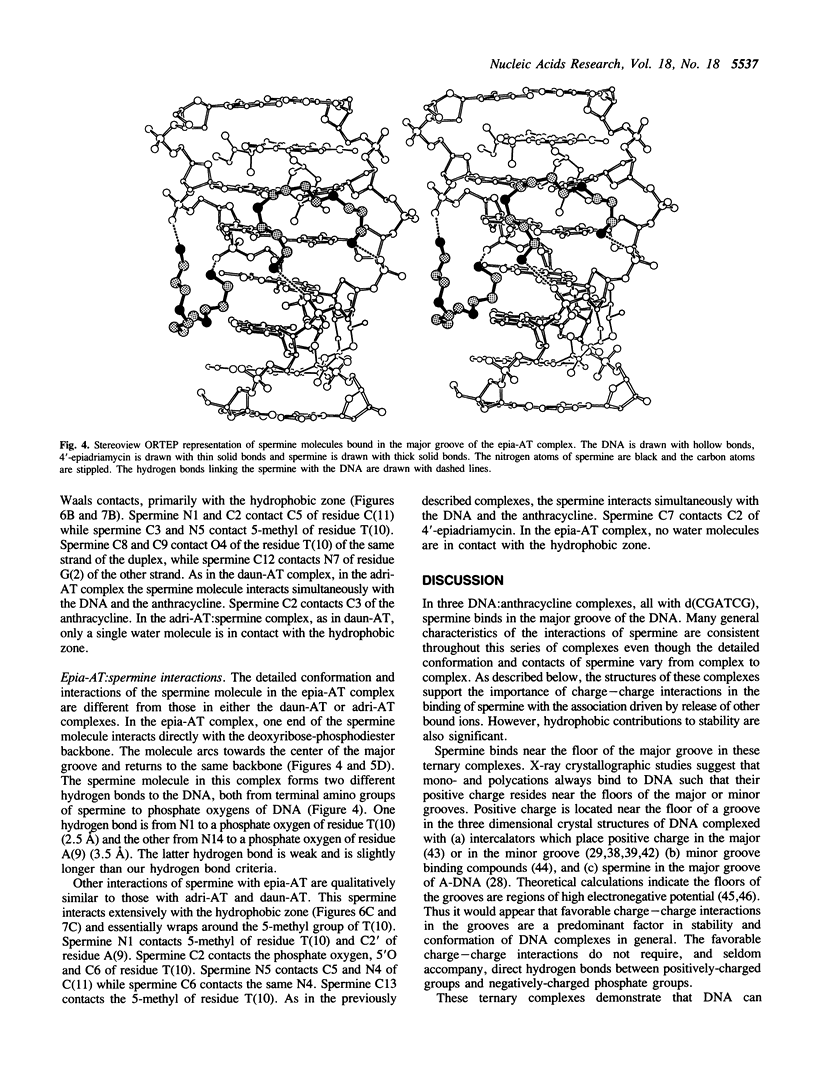
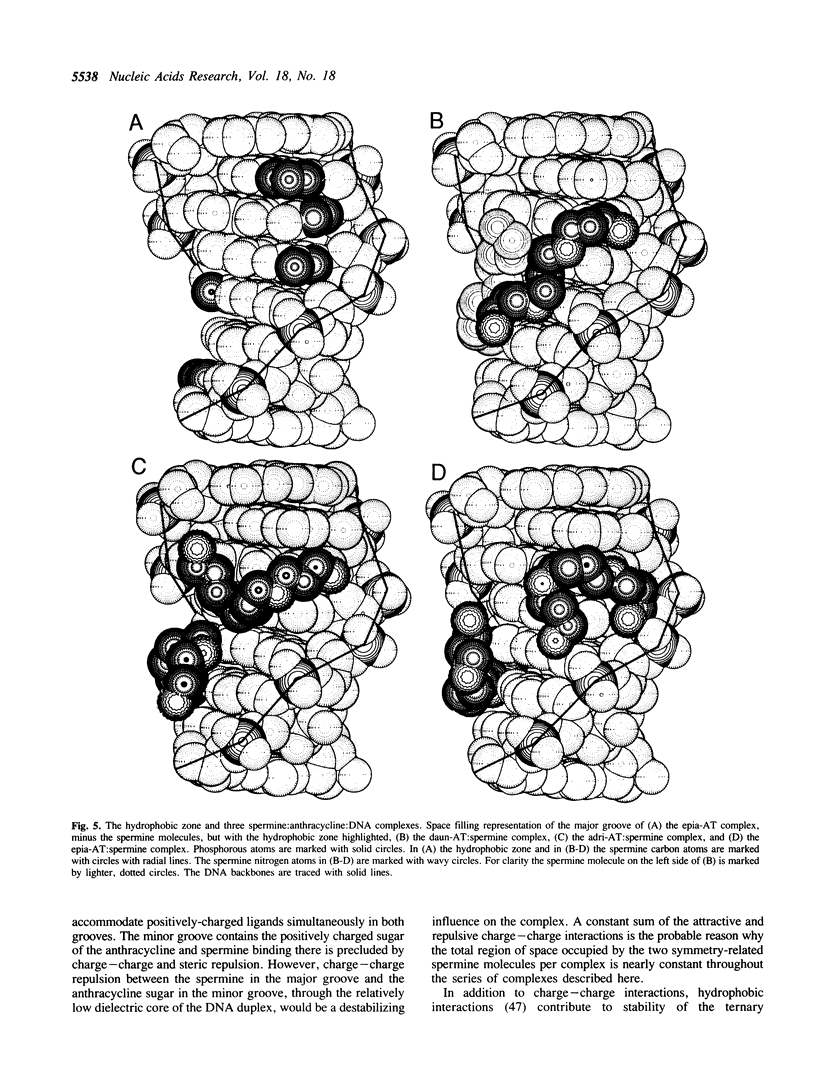
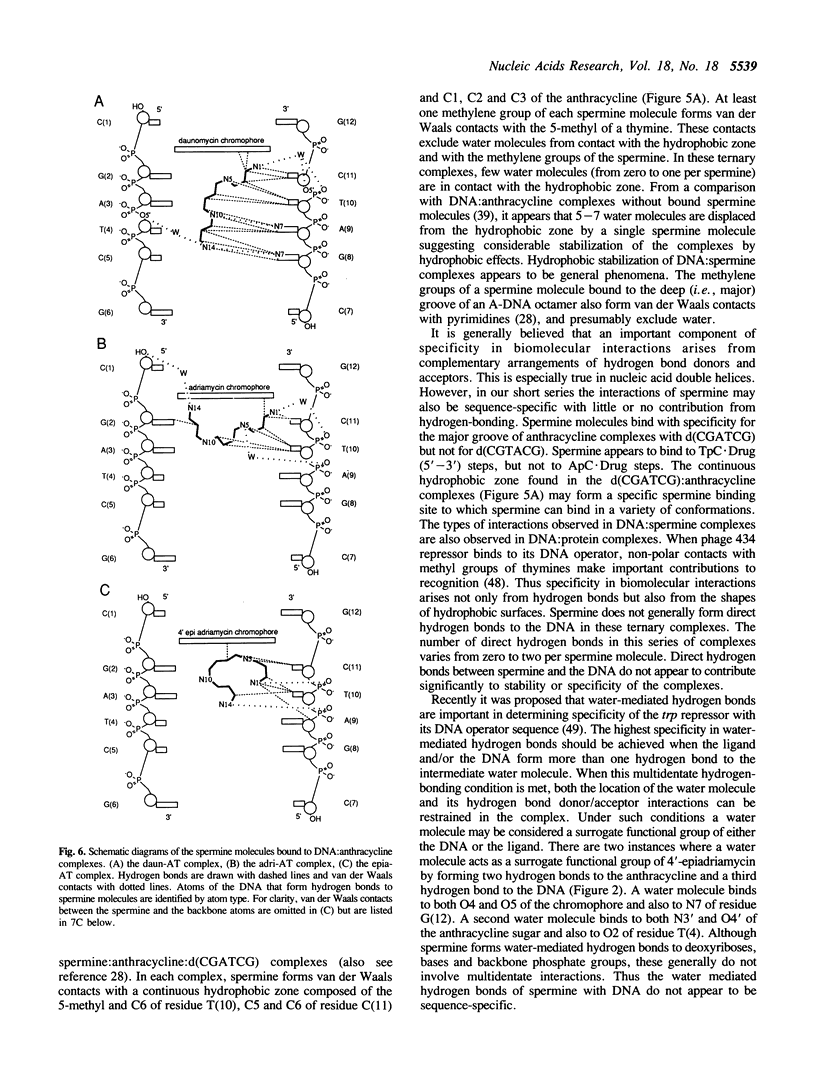
The recently developed anthracycline 4'-epiadriamycin, an anti-cancer drug with improved activity, differs from adriamycin by inversion of the stereochemistry at the 4'-position. We have cocrystallized 4'-epiadriamycin with the DNA hexamer d(CGATCG) and solved the structure to 1.5 A resolution using x-ray crystallography. One drug molecule binds at each d(CG) step of the hexamer duplex. The anthracycline sugar binds in the minor groove. A feature of this complex which distinguishes it from the earlier DNA:adriamycin complex is a direct hydrogen bond from the 4'-hydroxyl group of the anthracycline sugar to the adenine N3 on the floor of the DNA minor groove. This hydrogen bond results directly from inversion of the stereochemistry at the 4'-position. Spermine molecules bind in the major groove of this complex. In anthracycline complexes with d(CGATCG) a spermine molecule binds to a continuous hydrophobic zone formed by the 5-methyl and C6 of a thymidine, C5 and C6 of a cytidine and the chromophore of the anthracycline. This report discusses three anthracycline complexes with d(CGATCG) in which the spermine molecules have different conformations yet form extensive van der Waals contacts with the same hydrophobic zone. Our results suggest that these hydrophobic interactions of spermine are DNA sequence specific and provide insight into the question of whether DNA:spermine complexes are delocalized and dynamic or site-specific and static.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A. K., Rodgers D. W., Drottar M., Ptashne M., Harrison S. C. Recognition of a DNA operator by the repressor of phage 434: a view at high resolution. Science. 1988 Nov 11;242(4880):899–907. doi: 10.1126/science.3187531. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunlin W. H., Strick T. J., Record M. T., Jr Equilibrium dialysis studies of polyamine binding to DNA. Biopolymers. 1982 Jul;21(7):1301–1314. doi: 10.1002/bip.360210704. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Chen H. H., Behe M. J., Rau D. C. Critical amount of oligovalent ion binding required for the B-Z transition of poly (dG-m5dC). Nucleic Acids Res. 1984 Mar 12;12(5):2381–2389. doi: 10.1093/nar/12.5.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orazi D., Fracassini D. S., Bagni N. Polyamine effects on the stability of DNA-actinomycin D complex. Biochem Biophys Res Commun. 1979 Sep 12;90(1):362–367. doi: 10.1016/0006-291x(79)91633-4. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Spermine-DNA interactions: a theoretical study. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5948–5952. doi: 10.1073/pnas.83.16.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick C. A., Williams L. D., Ughetto G., van der Marel G. A., van Boom J. H., Rich A., Wang A. H. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry. 1990 Mar 13;29(10):2538–2549. [PubMed] [Google Scholar]
- Geiger L. E., Morris D. R. Stimulation of deoxyribonucleic acid replication fork movement by spermidine analogs in polyamine-deficient Escherichia coli. J Bacteriol. 1980 Mar;141(3):1192–1198. doi: 10.1128/jb.141.3.1192-1198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner R. V., Frederick C. A., Quigley G. J., Rich A., Wang A. H. The molecular structure of the left-handed Z-DNA double helix at 1.0-A atomic resolution. Geometry, conformation, and ionic interactions of d(CGCGCG). J Biol Chem. 1989 May 15;264(14):7921–7935. doi: 10.2210/pdb1dcg/pdb. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988 Apr 1;48(7):1722–1726. [PubMed] [Google Scholar]
- Igarashi K., Sakamoto I., Goto N., Kashiwagi K., Honma R., Hirose S. Interaction between polyamines and nucleic acids or phospholipids. Arch Biochem Biophys. 1982 Dec;219(2):438–443. doi: 10.1016/0003-9861(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Jain S., Zon G., Sundaralingam M. Base only binding of spermine in the deep groove of the A-DNA octamer d(GTGTACAC). Biochemistry. 1989 Mar 21;28(6):2360–2364. doi: 10.1021/bi00432a002. [DOI] [PubMed] [Google Scholar]
- Lavery R., Pullman B. The dependence of the surface electrostatic potential of B-DNA on environmental factors. J Biomol Struct Dyn. 1985 Feb;2(5):1021–1032. doi: 10.1080/07391102.1985.10507618. [DOI] [PubMed] [Google Scholar]
- Lavery R., Pullman B. The molecular electrostatic potential and steric accessibility of A-DNA. Nucleic Acids Res. 1981 Sep 25;9(18):4677–4688. doi: 10.1093/nar/9.18.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Meers P., Hong K., Bentz J., Papahadjopoulos D. Spermine as a modulator of membrane fusion: interactions with acidic phospholipids. Biochemistry. 1986 Jun 3;25(11):3109–3118. doi: 10.1021/bi00359a007. [DOI] [PubMed] [Google Scholar]
- Moore M. H., Hunter W. N., d'Estaintot B. L., Kennard O. DNA-drug interactions. The crystal structure of d(CGATCG) complexed with daunomycin. J Mol Biol. 1989 Apr 20;206(4):693–705. doi: 10.1016/0022-2836(89)90577-9. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pingoud A. Spermidine increases the accuracy of type II restriction endonucleases. Suppression of cleavage at degenerate, non-symmetrical sites. Eur J Biochem. 1985 Feb 15;147(1):105–109. doi: 10.1111/j.1432-1033.1985.tb08725.x. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Zwelling L. A., Kao-Shan C. S., Whang-Peng J., Bradley M. O. Correlations between intercalator-induced DNA strand breaks and sister chromatid exchanges, mutations, and cytotoxicity in Chinese hamster cells. Cancer Res. 1985 Jul;45(7):3143–3149. [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Crothers D. M. Condensation of chromatin: role of multivalent cations. Biochemistry. 1986 Apr 8;25(7):1495–1503. doi: 10.1021/bi00355a004. [DOI] [PubMed] [Google Scholar]
- TABOR H. The protective effect of spermine and other polyamines against heat denaturation of deoxyribonucleic acid. Biochemistry. 1962 May 25;1:496–501. doi: 10.1021/bi00909a021. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Ionic and structural effects on the thermal helix-coil transition of DNA complexed with natural and synthetic polyamines. Biopolymers. 1984 Jul;23(7):1295–1306. doi: 10.1002/bip.360230713. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Srivenugopal K. S., Reid B. R., Morris D. R. Nuclear magnetic resonance studies of polyamine binding to a defined DNA sequence. J Mol Biol. 1985 Sep 20;185(2):457–459. doi: 10.1016/0022-2836(85)90418-8. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Williams L. D., Egli M., Qi G., Bash P., van der Marel G. A., van Boom J. H., Rich A., Frederick C. A. Structure of nogalamycin bound to a DNA hexamer. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2225–2229. doi: 10.1073/pnas.87.6.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]
- Zakrzewska K., Pullman B. Spermine-nucleic acid interactions: a theoretical study. Biopolymers. 1986 Mar;25(3):375–392. doi: 10.1002/bip.360250302. [DOI] [PubMed] [Google Scholar]


