Abstract
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that are involved in regulating glucose and lipid homeostasis, inflammation, proliferation and differentiation. Although all of these functions might contribute to the influence of PPARs in carcinogenesis, there is a distinct need for a balanced review of the literature and additional experimentation to determine the potential for targeting PPARs for cancer therapy and cancer chemoprevention. As PPAR agonists include drugs used for the treatment of metabolic diseases, a more complete understanding of the roles of PPARs in cancer will aid in determining any increased cancer risk for patients undergoing therapy with PPAR agonists.
At a glance
PPARs have central roles in the regulation of glucose and lipid homeostasis through their functions as molecular sensors responsive to endogenous ligands leading to modulation of gene expression. PPARs also regulate cell proliferation, differentiation and inflammation.
PPARα mediates hepatocarcinogenesis induced by long-term administration of PPARα agonists in rodent models, an effect not found in humans. The mechanism underlying species-specific hepatocarcinogenesis is through mouse PPARα-dependent regulation of the let-7c miRNA leading to increased expression of the oncoprotein MYC. The current interest in targeting PPARα for the prevention of certain cancers including colon and leukemia is based on studies showing that PPARα agonists inhibit proliferation of endothelial cells, increase synthesis of PPARγ agonists and potentially interfere with the Warburg effect.
The role of PPARβ/δ in carcinogenesis is controversial. Several studies have shown that PPARβ/δ is upregulated in cancer cells by the adenomatous polyposis coli (APC)–β-catenin–TCF4 pathway and has a pro-tumorigenic effect in many cancer types. However, other studies have shown that PPARβ/δ agonists can induce terminal differentiation and inhibit innate inflammation, suggesting anti-cancer effects. In addition, a retrospective study has shown that low expression levels of PPARβ/δ are associated with decreased survival of colorectal cancer patients. Therefore, there remains a need to further examine the PPARβ/δ protein expression patterns quantitatively in tumor models and the putative mechanisms mediated by PPARβ/δ agonists associated with anti-apoptotic or growth stimulatory effects.
PPARγ agonists can induce terminal differentiation, inhibit cell proliferation, promote apoptosis and inhibit innate inflammation in many cancer models. This has led to a number of clinical trials with PPARγ agonists, but these have generated mixed results. Moreover, some PPARγ agonists have been associated with pro-tumorigenic effects. Emerging evidence indicates that targeting PPARγ in combination with other chemopreventive or chemotherapeutic agents might increase the efficacy of the effects induced by monotherapies.
Due to similarities in the abilities of the three PPARs to improve different metabolic disorders known to be associated with increased cancer risk (such as diabetes, obesity, dyslipidemias and chronic inflammation), modulating activities of the PPARs remains an attractive approach for the treatment and prevention of cancer. The challenge is to advance the discovery of molecular mechanisms of action in order to identify and characterize effective PPAR agonists with acceptable safety profiles.
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors. The three PPAR isoforms, PPARα, PPARβ/δ (also referred to as PPARβ or PPARδ) and PPARγ, are found in all mammalian species examined to date. Since the identification of the PPAR family more than twenty years ago, numerous studies have revealed that PPARs influence many important biological functions including inflammation, cell survival and differentiation. PPARs are activated by endogenous ligands derived from the metabolism of fatty acids and other compounds found in the diet, consistent with the fact that PPARs regulate the expression of many genes involved in glucose and lipid metabolism 1. Through this mechanism, cellular homeostasis is maintained during periods of feeding and starvation. Drugs and other xenobiotics can also differentially modulate PPAR regulatory activities.
Whether PPARs function as tumor suppressors or oncogenes in cancer is still unclear. The complexity of the pathways regulated by PPARs and the propensity of these pathways to be altered in cancer offers some explanations for the disparate functions of PPARs in different tumor types. However, as targeting PPARs can improve the clinical consequences of metabolic disorders known to be associated with increased cancer risk (such as diabetes, obesity, dyslipidemias and chronic inflammation), modulating activities of the PPARs is an attractive approach for the treatment and prevention of cancer. The challenge is to elucidate the molecular mechanisms of action of PPAR agonists in different tissues and tumor types, and to identify and characterize effective PPAR agonists with acceptable safety profiles. The progress in understanding PPAR function and translating this to the clinic is discussed below. In this Review, we pay particular attention to the controversial function of PPARβ/δ in colorectal cancer.
PPAR-mediated gene expression
Substantial progress has been made in delineating the molecular mechanisms that mediate PPAR regulated gene expression and the associated cellular functions (FIG. 1). Following ligand binding, PPARs undergo a conformational change that causes the release of histone deacetylase (HDAC) co-repressors enabling PPARs to heterodimerize with retinoid X receptor (RXR). RNA polymerase II and co-activators with histone acetyl transferase (HAT) activity are then recruited to this complex, which binds to response elements in target genes leading to chromatin remodeling and ultimately increased transcription (FIG 1a). PPARβ/δ has also been shown to repress the transcription of some target genes through binding to DNA response elements in association with co-repressors, independent of ligand binding 2, 3 (FIG. 1d). Data from reporter gene assays in cultured cells indicates that PPARβ/δ might repress PPARα and PPARγ-dependent gene expression 2. However, follow-up studies examining this mechanism have largely been negative to date 4–7. PPARs can also down-regulate gene expression by interfering with other proteins and transcription factors through a “trans-repression” mechanism (FIG. 1b). For example, PPARα and PPARβ/δ can sequester the p65 subunit of the nuclear factor kappa beta (NFκB) complex and prevent NFκB-dependent regulation of genes involved in pro-inflammatory responses (reviewed in 8–13). Alternatively, trans-repression by PPARγ can involve its SUMOylation (FIG. 1c), where ligand activation leads to conjugation of PPARγ with SUMO, which binds with a nuclear co-repressor complex, causing repression of pro-inflammatory gene expression 14. SUMOylation-dependent trans-repression might also be relevant for PPARα and PPARβ/δ because the amino acid that is SUMOylated is conserved between all three PPARs 15. Trans-repression of pro-inflammatory signaling pathways is thought to be central to the well-documented anti-inflammatory activities associated with PPAR ligands and PPARs 8, 15. More recently, it was shown that the beneficial effects of PPARγ activation in diabetics can be modulated by “non-agonist” PPARγ ligands that inhibit the phosphorylation of PPARγ and so are independent of the classic receptor-mediated modulation of gene transcription 16. Thus, there are multiple levels of regulation that can be targeted to selectively alter PPAR-dependent activities.
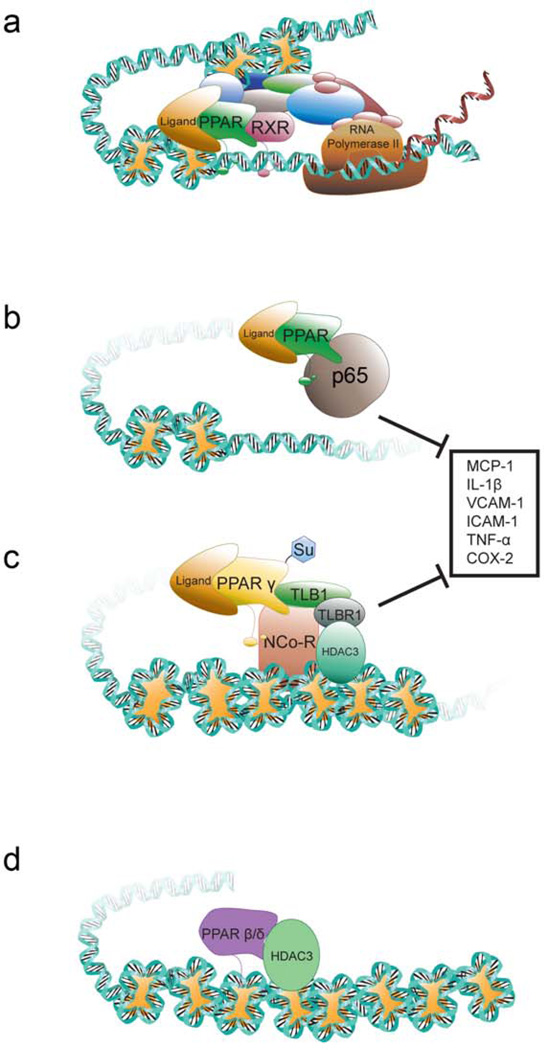
Figure 1. Molecular regulation of transcription by PPARs.
a | Transcriptional up-regulation of target gene expression. Following ligand activation, PPARs heterodimerize with RXR, recruit transcriptional machinery including RNA polymerase and co-activators with histone acetyl transferase activity causing remodeling of chromatin and increased transcription. b | Repression of pro-inflammatory gene expression. PPARs can bind to proteins including the p65 subunit of NFκB and attenuate NFκB-dependent signaling. c | Repression of pro-inflammatory gene expression by PPARγ. In the presence of a toll-like receptor agonist and a PPARγ agonist, PPARγ is SUMOylated (Su) and then binds to a nuclear receptor corepressor (NCOR)-containing complex bound to a pro-inflammatory target gene (such as TNF, IL6). This prevents degradation of the NCOR complex thereby maintaining active repression of pro-inflammatory gene expression. The lysine residue that is SUMOylated is conserved in PPARα, PPARβ/δ and PPARγ. Negative regulation of pro-inflammatory gene expression as shown in b and c underlies many of the anti-inflammatory activities associated with PPARs. d | Repression of gene expression by PPARβ/δ. PPARβ/δ can interact with histone deacetylases (HDAC) and maintain chromatin in a compact structure preventing gene expression. TBLX1, transducin-β-like 1, X-linked; TBLX1R1, TBLX1 receptor 1.
The physiological functions of the PPARs
PPARα
PPARα, the first PPAR to be identified 17, is expressed in many tissues, particularly those that require fatty acid oxidation as a source of energy 18. PPARα is central for maintenance of lipid homeostasis: a primary role of PPARα is to increase the cellular capacity to mobilize and catabolize fatty acids, particularly in the liver during starvation where oxidation of fatty acids is essential for energy production (FIG. 2, reviewed in 19). Under these conditions PPARα is probably activated by endogenous fatty acids and fatty acid derivatives (reviewed in 19). PPARα is also the molecular target of fibrates, widely used drugs that reduce serum lipids through the increased oxidation of lipids (reviewed in 19). The number of direct PPARα target genes is large and reviewed elsewhere 20, but includes many that encode enzymes involved in glucose, lipid and amino acid metabolism 21. PPARα can also improve insulin resistance in high fat and genetic models of diabetes through pleiotropic changes in gene expression that prevent weight gain and adiposity 22.
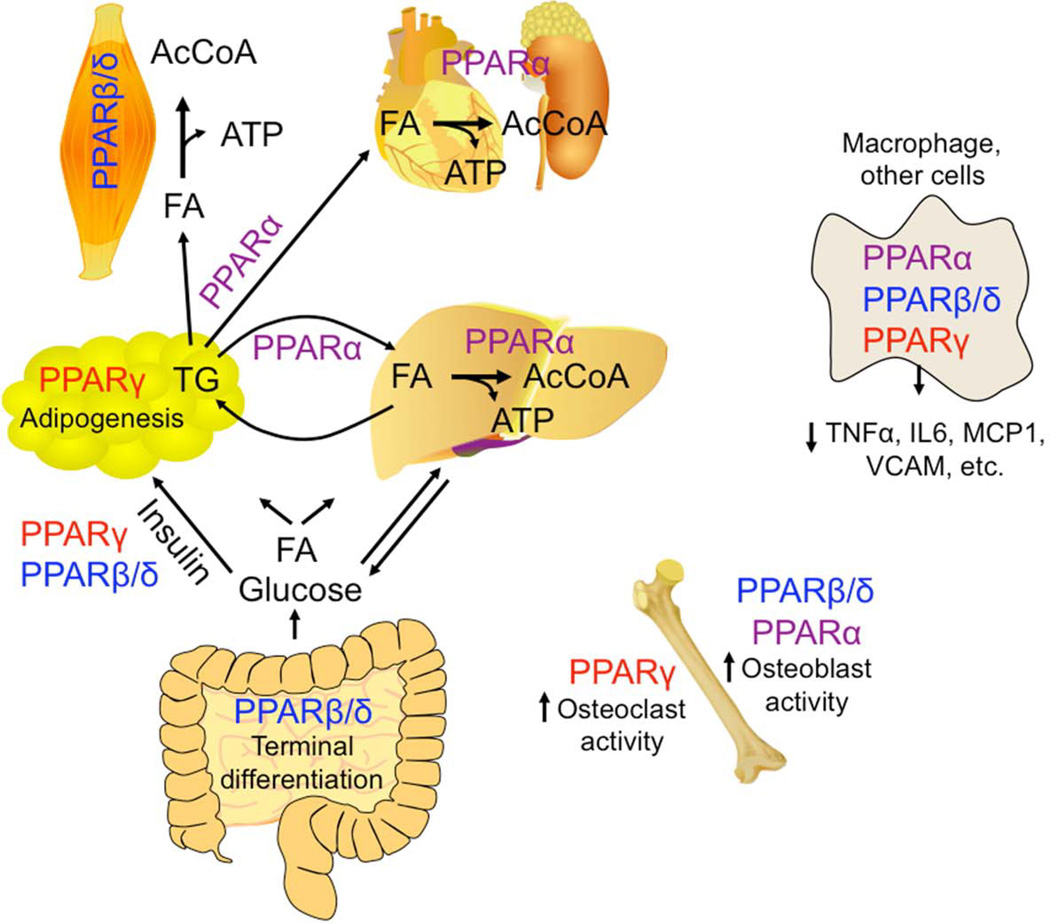
Figure 2. Physiological roles of PPARs.
a | PPARα regulates expression of enzymes that lead to mobilization of stored fatty acids (FA) in adipose tissue. PPARα also regulates expression of fatty acid catabolizing enzymes in liver, heart and kidney. Released fatty acids are then oxidized in these tissues to ultimately generate ATP. PPARβ/δ is expressed at high levels in the intestine where it mediates induction of terminal differentiation of epithelium (also important for skin). Activating PPARβ/δ or PPARγ can increase insulin sensitivity causing improved glucose uptake in diabetic models. PPARβ/δ regulates expression of fatty acid catabolizing enzymes in skeletal muscle where released fatty acids are oxidized to generate ATP. PPARγ promotes differentiation of adipocytes. b | PPARα, PPARβ/δ and PPARγ can interfere with NFκB and AP1 in tissues including macrophages, endothelial cells, epithelial cells and others causing attenuation of pro-inflammatory signaling by decreasing expression of pro-inflammatory cytokines, chemokines and cell adhesion molecules, for example, in addition to other trans-repressive mechanisms (FIG. 1). c | Activation of PPARα and PPARβ/δ promote osteoblast activity in bone whereas activation of PPARγ promotes osteoclast activity in bone. AcCoA, acetylCoA.
PPARβ/δ
PPARβ/δ also regulates glucose and lipid homeostasis (FIG. 2). PPARβ/δ is expressed in most tissues in rodents and humans 18, 23 and expression of PPARβ/δ seems to be highest in epithelia of the intestine, colon and skin 23, 24 where one study has shown that it co-localizes with RXR in the nucleus 24. Ligands that activate PPARβ/δ increase serum high-density lipoprotein cholesterol levels in rats, non-human primates and humans 25–27. This is probably mediated by PPARβ/δ-dependent expression of the reverse cholesterol transporter ATP-binding cassette A1 and increased apolipoprotein A1-specific cholesterol efflux 26. Ligand activation of PPARβ/δ can also decrease serum triglycerides, prevent high fat diet-induced obesity, increase insulin sensitivity, and improve symptoms associated with metabolic syndrome 26, 28–30 through the regulation of genes encoding fatty acid metabolizing enzymes in skeletal muscle 28, 29 and genes encoding lipogenic proteins in the liver. PPARβ/δ also inhibits hepatic inflammation caused by genetic, dietary and chemical stimuli 31–35 in part by the trans-repression of NFκB-dependent signaling, resulting in reduced expression of cytokines such as tumor necrosis factor-α (TNFα), interleukin–1β (IL1β) and IL6 (FIG. 1). Activating PPARβ/δ can also promote terminal differentiation in keratinocytes, intestinal epithelium, oligodendrocytes and osteoblasts (reviewed in 9–11, 36) and this function might have important consequences for tumor development.
PPARγ
The physiological effects of PPARγ activation are mediated primarily by PPARγ1 and PPARγ2 derived from four different mRNA species (PPARG1, PPARG2, PPARG3 and PPARG4) 37, 38. Comprehensive, quantitative expression patterns of PPARγ at the protein level have not been determined to date in any species, but expression of PPARγ protein has been demonstrated in many cell types. Significant non-specific immunoreactivity is found with some anti-PPARγ antibodies 39, 40, which probably impacts the interpretation of results from studies examining PPARγ expression. Polyunsaturated fatty acids, fatty acid derivatives such as 15-deoxy-delta-12,14-prostaglandin J2 (15d-PGJ2), 9-hydroxyoctadecadienoic acid (9-HODE), 13-HODE and nitrated fatty acids can activate PPARγ and may be endogenous ligands (reviewed in 1). PPARγ is critical for development, in particular the placenta and heart 41, and is also essential for adipogenesis and fat storage (FIG. 2) 42, 43. White adipose tissue is the primary target of the PPARγ agonists, the thiazolidinediones, which decrease serum lipids by increasing adipogenesis and lipid storage, and increase the expression of various adipokines, such as adiponectin and resistin 44, which collectively increase insulin sensitivity.
PPARs and cancer development
PPARα and liver cancer
Long-term administration of PPARα agonists causes liver cancer in rodents 45, an effect that is dependent on PPARα, as Pparα-null mice are resistant to the hepatocarcinogenic effects of PPARα agonists 46, 47. The mode of action for the hepatocarcinogenic effect of PPARα agonists has been determined and interestingly, this mechanism is not evident in humans (reviewed in 48). Recent data from studies using PPARα humanized mice (mice expressing a human PPARA gene on a Ppara-null background) offers an explanation for this difference. Although the administration of PPARα agonists causes increased expression of target genes that modulate lipid catabolism in both wild-type and PPARα humanized mice 49, hepatocarcinogenesis and the down-regulation of the let-7c micro RNA cluster is only evident in wild-type mice 50, 51. Let7c targets the mRNA encoding MYC and in its absence, the stability of MYC mRNA is increased, which might contribute to increased mitogenic signaling that causes hepatocyte proliferation 51.
A controversial role of PPARβ/δ in cancer
There is no broad consensus on the role of PPARβ/δ in cancer, due to contradictory studies in the literature (reviewed in 9–12). However, two hypotheses have emerged (FIG. 3): that PPARβ/δ is over-expressed in tumors and promotes anti-apoptotic activities and increased cell proliferation and that PPARβ/δ promotes terminal differentiation and inhibits pro-inflammatory signaling, thereby attenuating tumorigenesis.
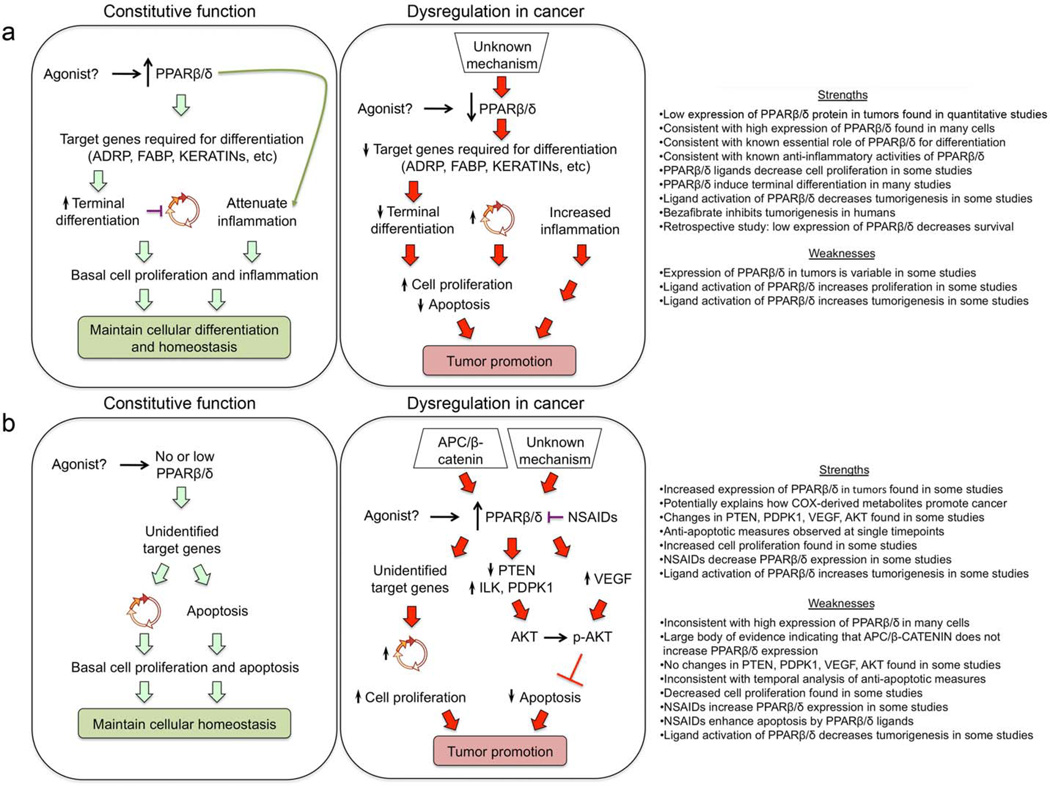
Figure 3. Contrasting mechanisms of PPARβ/δ in cancer.
a | Expression of PPARβ/δ is high in epithelial cells and many other cell types. Unidentified endogenous ligands activate PPARβ/δ, increasing the expression of proteins required for promoting terminal differentiation and causing cell cycle withdrawal. Constitutive expression of PPARβ/δ also attenuates inflammation. Both of these effects explain how PPARβ/δ maintains cellular differentiation and homeostasis. Expression of PPARβ/δ in tumor cells is decreased through undefined mechanisms. This causes deregulation of terminal differentiation and inflammatory signaling that collectively causes increased cell proliferation and reduced apoptosis causing tumor promotion. In this model, ligand activation of PPARβ/δ prevents tumorigenesis and is most consistent with recent findings. The strengths of this model include that low expression of PPARβ/δ has been quantified in some tumor types and higher expression levels have been shown in normal cells and tissues. This is consistent both with data showing that PPARβ/δ is involved in cell differentiation and that some PPARβ/δ ligands inhibit cell proliferation. In addition, activation of PPARβ/δ is anti-inflammatory and this might in part explain why ligand activated PPARβ/δ decreases tumorigenesis in some studies, as does the pan PPAR agonist bezafibrate. This model is also consistent with a recent retrospective study showing reduced survival of colorectal cancer patients exhibiting relatively low expression of PPARβ/δ in primary tumors. The weaknesses of this model include that the expression of PPARβ/δ is variable in tumor types and normal tissues and that several studies have shown that ligand activation of PPARβ/δ increases cell proliferation and tumorigenesis. b | A model based on the reduced expression of PPARβ/δ in normal cells. Unidentified endogenous ligands activate PPARβ/δ increasing expression of unidentified target genes that promote cell cycle progression and inhibit apoptosis. Expression of PPARβ/δ in tumor cells is increased through undefined mechanisms or by direct up-regulation mediated by APC–β-catenin-dependent signaling found in several tumor types. Unidentified endogenous ligands activate PPARβ/δ modulating expression of: PTEN, ILK and PDPDK1 or VEGF that collectively increase phosphorylation of AKT causing inhibition of apoptosis. Alternatively unidentified target genes that increase cell cycle progression could also be important. This model could explain how non-steroidal anti-inflammatory drugs (NSAIDs) inhibit tumorigenesis, although the reduced expression of PPARβ/δ is inconsistent both with the anti-inflammatory role of PPARβ/δ and data from other studies that NSAIDs increase PPARβ/δ and induce apoptosis. The strengths of this model include that increased expression of PPARβ/δ is found in some tumor types, consistent with studies that show that ligand activation of PPARβ/δ induces tumor development. In addition, this model might explain how COX-derived metabolites promote cancer. The weaknesses of this model include that other studies have shown that: PTEN, PDPK1, VEGF and AKT are not affected by PPARβ/δ, PPARβ/δ expression is not up-regulated by β-catenin and TCF4, PPARβ/δ does not promote anti-apoptotic activities, and ligand activation of PPARβ/δ decreases cell proliferation and tumorigenesis. This model is also inconsistent with studies showing relatively high constitutive expression of PPARβ/δ in epithelia.
An initial finding that expression of PPARB/D mRNA was higher in four colon tumors compared with non-transformed tissue was taken to indicate a role for PPARβ/δ in colon cancer progression 52. However, in this study the expression of PPARB/D mRNA was essentially absent in non-transformed colon tissue 52, a finding that is not in agreement with more recent studies from our laboratory and others in both mouse and human tissue showing that PPARβ/δ is constitutively expressed at high levels in normal colonic epithelium 23, 24, 53, 54. The increased expression of PPARB/D mRNA in colon tumors has been attributed to APC–β-catenin–TCF4-mediated transcription, similar to the known β-catenin–TCF4 target gene CCND1, which encodes cyclin D1. This led to the provocative hypothesis that PPARβ/δ regulates genes that increase cell proliferation and promote colon carcinogenesis 52 and provided the rationale for many follow-up studies. Although some of these studies support this hypothesis others do not (reviewed in 10, 11). One of the fundamental issues of uncertainty is whether PPARB/D expression is increased or decreased in tumors. Indeed, since the original report suggesting that PPARB/D expression is increased by an APC-dependent pathway some studies have found that PPARB/D expression is higher in colon tumors compared with non-transformed tissue 55–62. Studies using other tissues also indicate that expression of PPARB/D is higher in tumor tissue than non-transformed tissue, including ovarian carcinomas, squamous cell carcinomas, breast tumors and endometrial carcinomas63–67. By contrast, studies have also found that expression of PPARB/D is either unchanged or lower in colorectal tumors compared with non-transformed tissue 5, 53, 54, 58, 60, 68–76, (reviewed in 11), and in ovarian or bladder carcinomas compared with normal tissue 77, 78. However, there are important limitations to most, but not all 54, of these studies: they typically measure only mRNA expression and not protein expression; they often lack positive and negative controls; the number of samples examined is typically small; and protein expression is analyzed by immunohistochemistry. The sole use of immunohistochemical analysis of PPARβ/δ is particularly problematic because any non-specific immunoreactivity associated with anti-PPARβ/δ antibodies can produce misleading results 79, 80, (reviewed in 10). More extensive studies examining whether PPARβ/δ expression is increased by the APC–β-catenin–TCF4 signaling pathway, including microarray analysis and quantitative analysis of cells or tissues with activating mutations in the β-catenin pathway, have not reported increased PPARβ/δ expression 54, 68, 72, 73, 79, 81, 82. In addition, expression of PPARβ/δ is fairly high in normal human and mouse colon 23, 24, 53, 54 where it may function to maintain differentiation in response to an endogenous ligand. Although some data showing high expression of PPARβ/δ in human colon compared with other tissues are limited to analysis from two samples from a publically available database 23, the strength of this database lies in the ability to make comparison of relative expression with many different human tissues. These data are consistent with recent studies showing robust expression of PPARβ/δ in human samples of untransformed colon 53, 54 and one study in mice showing relatively high expression of PPARβ/δ in colon and intestine as compared to ten other tissue types 24. However, it is important to note that expression of the PPARβ/δ protein does not necessarily indicate that it is active, as the protein could be modified by endogenous ligands that may or may not be present. It also remains possible that the biological outcome (promotion or inhibition of carcinogenesis) of PPARβ/δ expression depends on the presence or absence of other gene products (oncogenes or tumor suppressors, for example).
A recent retrospective study in humans showed that higher expression of PPARβ/δ in primary tumors was associated with lower expression of Ki-67 (a surrogate marker of proliferation), increased frequency of stage I cases, a lower frequency of later stage cases and a lower rate of lymph node metastasis 60. Interestingly, PPARβ/δ was differentially expressed, with some primary tumors exhibiting relatively high expression while other primary tumors and lymph node metastases exhibiting relatively lower expression 60. Importantly, patients with colorectal cancer with relatively low expression of PPARβ/δ were ~4 times more likely to die of colorectal cancer than those with relatively higher expression of PPARβ/δ in primary tumors 60. Given the more precise quantification of PPARβ/δ in this study where immunohistochemical analysis was supported by western blot analysis, a large number of patients (141), and many years of follow-up (~ 15 years), this is the best evidence to date that supports the hypothesis that PPARβ/δ has a protective role in human colorectal cancer. Interestingly, a recent study has shown that the survival of patients with colorectal cancer whose tumor samples stained positive for both PPARβ/δ and cyclooxygenase-2 (COX2) expression was reduced compared with patients with tumors that stained only for PPARβ/δ, COX2, or were not immunoreactive for either of these proteins 62. This suggests that increased expression of PPARβ/δ in the presence of relatively high COX2 expression could cooperatively promote colorectal cancer. However, it is important to note that this study relies on immunohistochemistry only for estimating PPARβ/δ protein expression; there is no comparison of patient survival for those with lower versus higher expression of PPARβ/δ alone (the differences in PPARβ/δ expression between tumor samples is not described); and there is no comparison of survival for patients with different stage disease whose tumors were positive for COX2 only, as patients exhibiting this phenotype with early stage I tumors should survive longer than those exhibiting this phenotype with stage II–IV tumors 83.
Much like the conflicting human data, elucidating the function of PPARβ/δ in mouse cancer models is confounded by conflicting results (reviewed in 9–12). For example, some studies indicate that colon carcinogenesis is exacerbated in the absence of PPARβ/δ expression and/or that ligand activation of PPARβ/δ attenuates tumorigenesis 5, 70, 74, 84. Other studies found that colon carcinogenesis is inhibited in the absence of PPARβ/δ expression and that ligand activation of PPARβ/δ promotes tumorigenesis 85–87. Similar paradigms exist for other tumor types (reviewed in 9–12), but not all. For example, there is good evidence that PPARβ/δ protects against, and that ligand activation of PPARβ/δ attenuates chemically-induced skin carcinogenesis 88–92. Some studies show that activating PPARβ/δ increases proliferation and/or inhibits apoptosis in a variety of human lung, breast, liver, prostate cancer cell lines, and in some cases correlative studies in animal models support these findings (reviewed in 9, 10). However, studies from other laboratories show that activating PPARβ/δ either inhibits or has no effect on proliferation, and has no effect or promotes apoptosis, in human lung, breast and liver cancer cell lines; correlative studies in animal models also support some of these in vitro studies (reviewed in 9, 10). Thus, more work is needed in mouse models to try and understand the complexities of PPARβ/δ in tumorigenesis. One possible factor that might influence the role of PPARβ/δ in cancer development or suppression is its effect on angiogenesis (Box1). However, the function of PPARβ/δ and PPARγ in angiogenesis is also controversial.
Box 1. Controversial role of PPARs in angiogenesis.
The role of PPARβ/δ and PPARγ in angiogenesis remains uncertain. Angiogenesis is a complex process that provides a blood supply to growing tumors and involves production of growth factors such as vascular endothelial growth factor (VEGF) to stimulate proliferation of endothelial cells, release of proteases and expression of cell adhesion molecules to allow proliferating endothelial cells to form new blood vessels. Studies show that PPARβ/δ ligands can either increase or decrease VEGF expression in cancer cells and endothelial cells 221, 222, and increase or decrease proliferation of endothelial cells: endothelial cells from Pparβ/δ-null mice proliferate faster than those from wild-type mice (reviewed in 10). Functional studies revealed that activating PPARβ/δ promotes angiogenesis in both in vitro and in vivo models 223–225, and could explain some of the pro-tumorigenic effects associated with PPARβ/δ expression. While the mechanism remains uncertain, PPARβ/δ may regulate angiogenesis by inhibiting proliferation of endothelial cells 224. PPARγ can either promote or inhibit angiogenesis in both in vitro and in vivo models depending on the context 226–230. PPARγ agonists can also increase expression of VEGF in cancer cells 221, 231, but decrease endothelial cell viability 227. The anti-angiogenic effects of PPARγ activation might be mediated by down-regulation of the VEGF receptor, whereas the pro-angiogenic effects might be due to increased endothelial nitric oxide synthase activity. Because PPARβ/δ and PPARγ cause differential effects on regulatory pathways that modulate angiogenesis (such as VEGF expression and endothelial cell proliferation), and there can be differences in the outcome of functional analyses, there is currently no consensus for the role of these receptors in angiogenesis and whether they are involved in enhancing or inhibiting metastasis.
Several mechanisms have been proposed to explain the pro-carcinogenic effect of PPARβ/δ. Three of these mechanisms are based in part on data from cells resembling normal mouse primary keratinocytes 93, 94. Analyses of these cells suggested that ligand activation of PPARβ/δ increases expression of 3-phosphoinositide-dependent-protein kinase 1 (PDPK1) and integrin linked kinase (ILK), and decreases expression of phosphatase and tensin homolog deleted on chromosome ten (PTEN) causing increased phosphorylation of AKT leading to anti-apoptotic signaling and enhanced cell survival (FIG. 3) 93. Since this initial report, some studies in cancer models have supported these findings, but others have not (reviewed in 9–12). Issues of contention include whether true keratinocytes were studied in the models that were used to suggest this pathway was functional 93, 94. Our studies have shown that in human N/TERT-1 and HaCaT keratinocytes and mouse primary keratinocytes that express keratin 6 and normal patterns of keratinocyte differentiation markers, PTEN is not decreased, expression of PDPK1 and ILK is not increased, and/or phosphorylation of AKT is not increased by ligand activation of PPARβ/δ, despite clear up-regulation of known PPARβ/δ target genes 95, 96. Indeed, we have also found that ligand activation of PPARβ/δ inhibits proliferation of mouse keratinocytes, mouse neoplastic keratinocytes, human HaCaT keratinocytes and N/TERT-1 human keratinocytes and does not promote survival 88, 89, 95–97. Microarray analyses also show that expression of PDPK1, ILK and PTEN mRNA is unaffected by ligand activation of PPARβ/δ 98,99,100,101. In our hands, ligand activation of PPARβ/δ does not promote survival of human cancer cell lines or HaCaT keratinocytes following induction of apoptosis by a variety of stimuli 4, 54, 95, 102. Thus, we think that there are inherent limitations in establishing the putative ILK–PDPK1–PTEN–AKT pro-survival signaling as a mechanism mediated by PPARβ/δ.
A related mechanism proposed to explain the pro-carcinogenic effects of PPARβ/δ is also based on the idea that PPARβ/δ promotes cell survival by regulation of ILK–PDPK1–PTEN–AKT. It was suggested that the high ratio of intracellular fatty acid binding protein 5 to cellular retinoic acid binding protein II found in these cells diverts all trans retinoic acid to PPARβ/δ rather than the retinoic acid receptor which is thought to cause increased expression of PDPK1 leading to anti-apoptotic activities and increased cell survival 103. However, follow-up studies do not concur with these findings (4, reviewed in 9).
Another related mechanism is based on the analysis of human colon cancer cell lines and Apcmin/+ mice. Ligand activation of PPARβ/δ increases expression of vascular endothelial growth factor (VEGF) through a PPARβ/δ-dependent mechanism causing increased phosphorylation of AKT, which promotes cell survival by blocking apoptosis 86. Several studies have also found evidence supporting this mechanism, primarily by showing increased expression of VEGF in colon tumors or colon cancer cell lines following treatment with a PPARβ/δ ligand 87, 104, 105. However, we have not found altered expression of either VEGF or phosphorylation of AKT in similar models in response to activation of PPARβ/δ 102.
It has also been shown that PPARβ/δ confers resistance to PPARγ-induced apoptosis in some cancer cells based on the expression levels of both proteins in HCT116 and LS174T cells 59. However, we and others have shown that the ratio of PPARβ/δ/PPARγ is low in HCT116 cells, that expression of PPARβ/δ is actually similar between HCT116 and LS174T cells, and that expression of PPARγ is much lower in HCT116 cells than LS174T cells 39, 52, 79. This suggests that the observed resistance to PPARγ-induced apoptosis in HCT116 cells could reflect differences in expression of PPARγ rather than PPARβ/δ.
Two mechanisms have been proposed to explain the chemopreventive effects of PPARβ/δ (FIG. 3). The hypothesis that PPARβ/δ promotes the induction of terminal differentiation is supported by evidence from multiple models including keratinocytes, intestinal epithelium, osteoblasts, oligodendrocytes, monocytes and in a variety of cancer models including colon, breast and neuroblastoma cells (reviewed in 9–11, 36). This mechanism involves the increased expression of gene products required for terminal differentiation, altered expression of gene products that inhibit cell proliferation, and inhibition of cell proliferation, effects that are not seen in cells lacking expression of PPARβ/δ (reviewed in 9–11, 36). Abundant evidence also supports the idea that PPARβ/δ can inhibit pro-inflammatory signaling. For example, more than fifty studies show that PPARβ/δ can inhibit expression of pro-inflammatory signaling by decreasing the expression of TNFα, IL1β, IL6 and MCP1 (reviewed in 8–13). Many of these changes in the expression of pro-inflammatory signaling proteins are thought to be mediated by direct inhibition of NFκB-dependent signaling (reviewed in 8–13) (FIG. 1), but PPARβ/δ-dependent inhibition of AP1 and STAT3 phosphorylation has also been described (reviewed in 8–13). As inflammation is associated with the development of many cancers 106 and anti-inflammatory drugs are known to effectively prevent some cancers, it is curious that no studies to date have specifically examined whether activating PPARβ/δ could prevent tumorigenesis by inhibiting inflammation. Given the strength of evidence that PPARβ/δ can mediate potent anti-inflammatory activities, this hypothesis warrants detailed examination.
PPARγ and cancer
The function of PPARγ in tumor development is also controversial. There are many published studies showing that activating PPARγ prevents cancer in tissues such as colon, breast, prostate, lung and many others (reviewed in 107, 108). Indeed, the majority of studies to date show that PPARγ agonists can promote terminal differentiation, inhibit cell growth and increase apoptosis of human cancer cell lines; inhibit tumorigenesis in animal models of cancer; and in some cases PPARγ agonists have shown modest efficacy for chemoprevention in clinical trials (reviewed in 107, 108). Overall survival of patients with colorectal cancer is markedly better when PPARγ expression is detectable in primary tumors compared with the survival of patients with colorectal cancer with no detectable PPARγ expression in their primary tumors 109, similar to the retrospective study examining a relationship between survival of colorectal cancer patients and expression of PPARβ/δ 60. This is consistent with results showing that colon tumorigenesis is exacerbated in APCmin/+ mice with genetic ablation of Pparg compared with control APCmin/+ mice 110.
Ligand activation of PPARγ in cancer cell lines is associated with induction of cell cycle arrest, increased expression of mRNAs and proteins required for terminal differentiation including keratins, carcinoembryonic antigen, E-cadherin, alkaline phosphatase and differentiation-related gene-1 (DRG1), as well as changes in cell morphology consistent with a differentiated phenotype 111–115. One mechanism that may mediate PPARγ-dependent induction of terminal differentiation is through an interaction with HIC5, which may serve as a PPARγ co-activator 116. In this model, HIC5 and PPARγ cooperatively increase expression of fatty acid binding protein, kruppel-like factor 4 (KLF4) and keratin 20; proteins known to be required for epithelial differentiation 116. Through this mechanism, cells differentiate and in doing so, undergo obligate cell cycle arrest (FIG. 4).
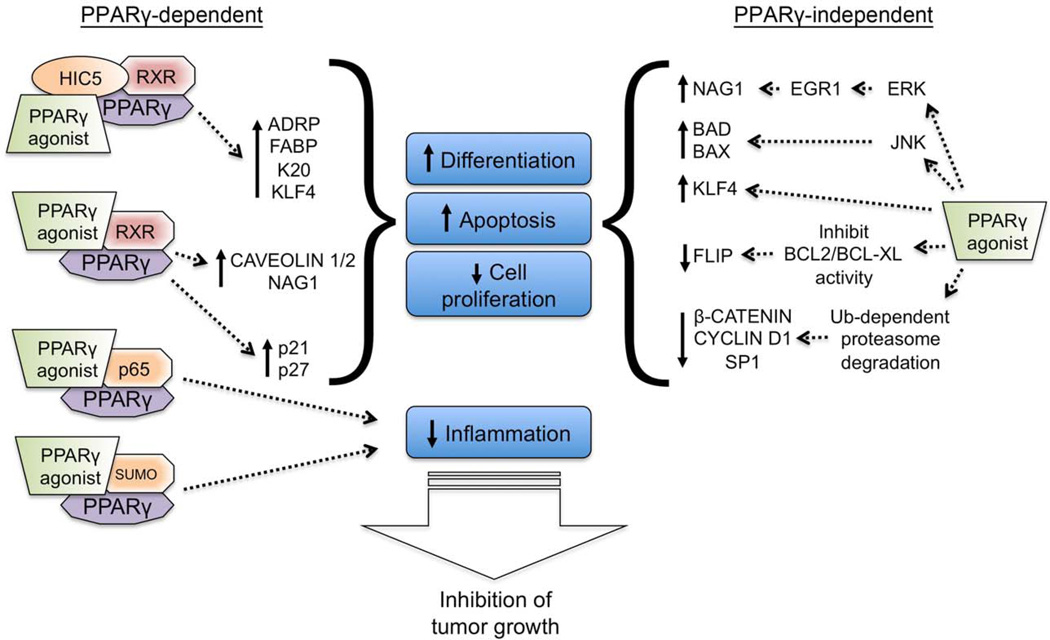
Figure 4. Targeting PPARγ for prevention and treatment of cancer.
PPARγ agonists can cause both PPARγ-dependent and PPARγ-independent alterations that inhibit tumor growth. Following ligand activation, HIC5 (also known as TGFB1I1) can act as a transcriptional co-activator of the receptor complex and increase expression of genes required for induction of terminal differentiation. Ligand activation of PPARγ can also cause increased expression of caveloin, non-steroidal anti-inflammatory drug activated gene-1 (NAG1), p21 and p27 through an undefined mechanism that requires PPARγ. Ligand activation of PPARγ can attenuate inflammation by interfering with NFκB signaling and through a SUMO-dependent mechanism. In addition to these PPARγ-dependent signaling pathways, some PPARγ agonists also cause PPARγ-independent effects (off-target effects) that include induced terminal differentiation, increased apoptosis, decreased cell proliferation and inhibition of inflammation, all of which combine to inhibit tumor growth. The extent to which these changes are induced is unique for each PPARγ agonist and probably reflects differences in functional chemical groups present in the PPARγ agonists. ADRP, adipose differentiation related protein; EGFR, epidermal growth factor receptor; FABP, fatty acid binding protein; FLIP, FLICE inhibitory protein; JNK, JUN N-terminal kinase; K20, keratin 20; KLF4, Kruppel-like factor 4; RXR, retinoic X receptor; Ub, ubiquitin.
PPARγ agonists modulate expression of different cell cycle regulators, including decreasing the expression of cyclin D1 117–121, increasing expression of the cyclin dependent kinase inhibitors p21 111, 122 and p27 122–127, and increasing turnover of β-catenin 128, 129. PPARγ agonists can also inhibit cell proliferation by inactivating eukaryotic initiation factor 2 leading to the inhibition of translation initiation 130. Although it is known that these changes contribute to the mechanisms through which PPARγ agonists inhibit cell cycle progression, the precise involvement of PPARγ in causing these changes remains uncertain.
Increased apoptotic signaling is another mechanism that mediates the growth inhibitory effects of PPARγ agonists. PPARγ agonists can increase the expression of pro-apoptotic BAX and BAD 131, 132, inhibit Bcl-XL and Bcl-2 function 131, 133, increase expression of PTEN 134–138, inhibit phosphatidylinositol-3 kinase activity and AKT phosphorylation 134, 139, 140, inhibit activation of Jun N-terminal protein kinase 131 and increase turnover of the anti-apoptotic protein FLIP 141, 142. Many of these changes increase caspase activity and apoptosis. Although there is some evidence that PPARγ may be required for regulating expression of some of these proteins such as PTEN 136, 137, many changes are independent of PPARγ and likely represent off target effects of the individual PPARγ agonists (reviewed in 143).
Chronic inflammation associated with many cancers including colorectal, liver and lung is typically associated with increased NFκB activity and is causally linked with tumor promotion 106. PPARγ agonists can inhibit the production of pro-inflammatory signaling proteins such as TNFα, IL6 and MCP1 and these changes are mediated through trans-repression mechanisms including directly interfering with NFκB activity and/or through receptor SUMOylation (FIG. 1). PPARγ is expressed in tumor cells and infiltrating immune cells, and there is evidence that anti-inflammatory activities are mediated by PPARγ in many cell types 15, 144. Indeed, PPARγ expressed in intestinal epithelial cells 145 and macrophages 146 inhibits inflammation associated with experimentally-induced colitis and inflammation is known to be required for colon carcinogenesis 147.
Despite this evidence suggesting that activating PPARγ inhibits tumorigenesis, doubts persist because some studies indicate that activating PPARγ promotes tumorigenesis 148, 149,150, 151. Indeed, increased bladder cancer incidence is reported to be associated with clinical use of rosiglitazone or pioglitazone, but there is evidence that this might reflect off-target effects of these PPARγ agonists 152,153. Additionally, despite a large body of in vitro and preclinical data showing that PPARγ inhibits breast cancer 154, over-expression of a constitutively active PPARγ fusion protein caused earlier lethality compared with controls in a breast cancer model 155. However, it is worth noting that there are substantial differences in gene expression observed between the PPARγ fusion protein and that typically found in response to ligand activation of PPARγ 156. No definitive mechanisms have been elucidated to date that explain these pro-carcinogenic effects.
PPARs and cancer treatment and prevention
Activation of PPARs causes physiological changes that in theory should make these receptors good targets for the treatment and prevention of cancer. For example, ligand activation of both PPARβ/δ and PPARγ promotes terminal differentiation (reviewed in 10, 11, 36, 107, 108). Agonists for all three PPARs are also known to exhibit potent anti-inflammatory activities 8, 15.
PPARα
There are studies suggesting that activating PPARα could be useful for the prevention or treatment of different cancers. Oral administration of different PPARα agonists inhibited the growth of tumors derived from melanoma, Lewis lung carcinoma, glioblastoma, and fibrosarcoma cell lines 157, and xenografts from A549 human lung cancer cells 158. PPARα agonists also inhibited angiogenesis in these models 157, 158. These inhibitory effects are mediated by the PPARα-dependent inhibition of endothelial cell proliferation, and PPARα-dependent down-regulation of cytochrome P450 CYP2c, an enzyme that catalyzes epoxidation of arachidonic acid to epoxyeicosatrienoic acids 158 that promote angiogenesis. As these effects are not evident in Pparα-null mice 157, 158, they are PPARα-dependent and thus PPARα agonists could be used to prevent multiple tumor types (FIG. 5).
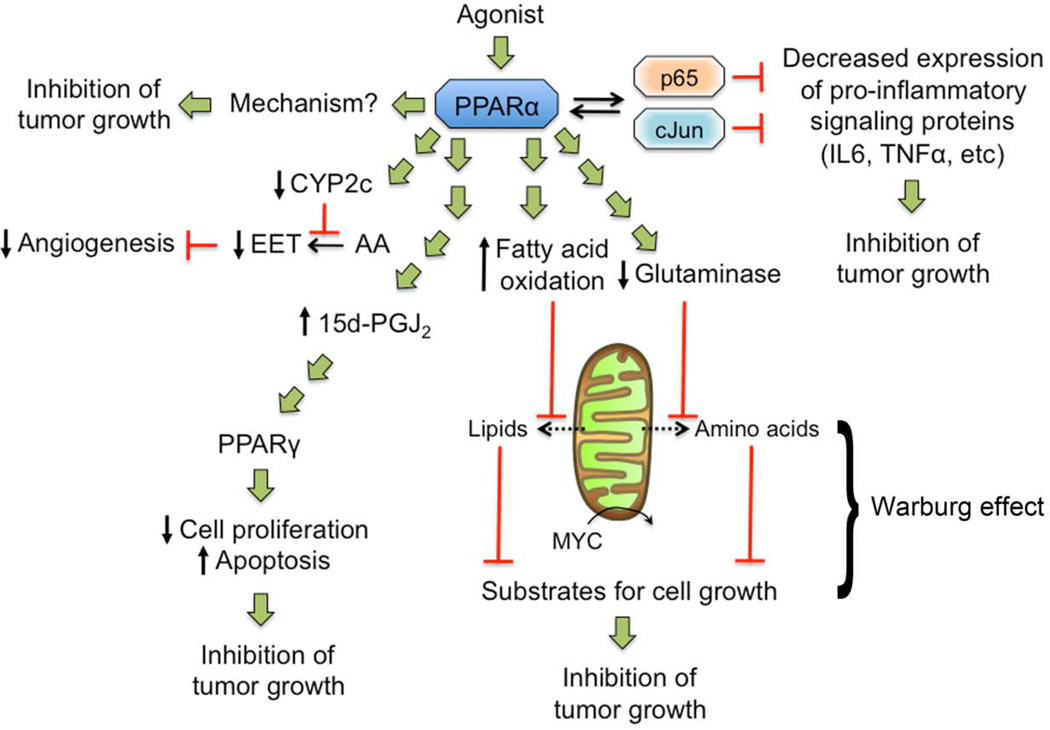
Figure 5. Potential targeting of PPARα for prevention and treatment of cancer.
Activation of PPARα can inhibit cancer cell proliferation through an undetermined mechanism and interfere with pro-inflammatory signaling through trans-repression mechanism leading to reduced expression of anti-apoptotic proteins and tumor promoting molecules. PPARα ligands can increase the synthesis of the PPARγ agonist 15-Deoxy-Delta-12,14-prostaglandin J2 (15d-PGJ2), which can cause inhibition of cell proliferation and increased apoptosis in tumor cells. Activating PPARα could target tumor cells exhibiting the Warburg effect by decreasing lipids (due to increased catabolism) and inhibiting MYC-induced increases in glutaminolysis that generate amino acids (due to reduced glutaminase), substrates that are required for cell division. Ligand activation of PPARα can decrease expression of CYP2c causing reduced conversion of arachidonic acid (AA) to epoxyeicosatrienoic acids (EET), causing inhibition of endothelial cell proliferation and angiogenesis.
There are two other potential PPARα-dependent pathways that could inhibit tumorigenesis or tumor growth (FIG. 5). First, PPARα inhibits inflammatory signaling through repressive mechanisms mediated by interacting with the p65 subunit of NFκB (reviewed in 8–13). Because inhibiting NFκB-dependent signals, such as TNFα, can effectively inhibit the growth of multiple tumor types 159, targeting this PPARα-dependent activity may be useful. Second, PPARα agonists also negatively influence the Warburg effect by interfering with metabolic pathways. Ligand activation of PPARα can increase mitochondrial oxidation of fatty acids 160, and inhibit expression of glutaminase 21, which decreases glutamine levels and limits cancer cell growth. As fatty acids and glutamine are enzymatically produced by the Warburg effect and are substrates required for cell proliferation161, targeting PPARα to inhibit tumor cell proliferation by interfering with the Warburg effect should be examined (FIG. 5).
PPARβ/δ
The potential for developing chemical agonists or antagonists of PPARβ/δ for chemoprevention remains uncertain. Given the observations that ligand activation of PPARβ/δ can inhibit or prevent metabolic syndrome, obesity, dyslipidemias, glucose intolerance and chronic inflammation, and all of these diseases are associated with cancer development 106, 162, 163, it is somewhat surprising that PPARβ/δ may promote carcinogenesis. Despite significant progress made in the past ten years, it is still not possible to unequivocally indicate whether an agonist would promote or attenuate most types of cancer (FIG. 3), with the exception of non-melanoma skin cancer where the use of PPARβ/δ agonists looks promising 88–90, 92.
Several PPARβ/δ antagonists have been developed 164–168 and the effect of two of these has been specifically examined in human cancer cell lines. The bioavailable PPARβ/δ antagonist GSK3787 inhibits PPARβ/δ-dependent activity in vivo and in vitro, despite weak PPARγ agonist activity 169. However, antagonism of PPARβ/δ in human cancer cell lines has no effect on cell proliferation 167, 169. While one study suggested that another PPARβ/δ antagonist inhibits proliferation of the A549 human lung cancer cell line, the concentration required to cause this effect also interfered with PPARγ 168. Given the central role of PPARβ/δ in many important biological functions (FIG. 2) ranging from regulation of glucose and lipid homeostasis, the maintenance of terminal differentiation, modulation of innate inflammation, and possibly cancer suppression, the development and use of a compound that specifically and exclusively antagonizes PPARβ/δ for the purpose of chemoprevention, may not be feasible.
PPARγ
As studies in mouse models and cultured cells indicate that PPARγ has potential for preventing or treating cancers, clinical trials have been undertaken to determine whether PPARγ agonists can inhibit tumorigenesis and tumor progression in patients with liposarcoma, colon cancer, breast cancer or prostate cancer. Increased differentiation in liposarcoma was observed in patients treated with troglitazone 170 and another clinical trial indicated that treatment with rosiglitazone increased the mean time to progression (defined as a doubling in tumor volume) 171. However, no effect of rosiglitazone was found in a larger cohort of patients with prostate cancer 172. Troglitazone has been tested in patients with prostate cancer with variable results on prostate-specific antigen (PSA) levels 173, 174 and administration of LY293111 to patients with prostate cancer had no clinical effect 175. In two phase II studies, troglitazone had no effect in either patients with breast cancer or colorectal cancer176, 177. Some clinical trials examining the effect of PPARγ ligands combined with other therapeutics revealed no effect for some studies 178, 179, but positive results for patients with thyroid carcinoma and glioma 180–182. It is also worth noting that a chromosomal translocation that fuses the paired box 8 gene (PAX8) with the PPARG gene is found in some cases of thyroid cancer 183. The function of this PAX8–PPARγ fusion protein remains unclear as some studies show that it acts as a dominant negative against PPARγ activity whereas other studies indicate it retains more classic PPARγ transcriptional activity 184, 185. Thus, the clinical trials to date have yielded evidence suggesting that PPARγ may be suitable for targeting in pre-cancerous and cancer cells in select tumor types.
Clinical studies show that administration of PPARγ agonists is associated with increased risk of heart failure 186, bone fractures 187–190 and possibly bladder cancer 153. Whether these negative side effects are mediated by PPARγ, and whether they represent thiazolidinedione-specific or off-target effects remains uncertain. Because PPARγ ligands can elicit different transcriptional effects due to differential recruitment of co-activators 191, it is possible that unique PPARγ ligands could be developed that retain chemopreventive activities but do not lead to negative side effects. Indeed, troglitazone was removed from the market because of idiosyncratic liver toxicity, a side effect not observed with rosiglitazone or pioglitazone. The screening and identification of natural compounds that retain PPARγ-dependent and/or PPARγ-independent anti-cancer activities could be a useful approach 143, 192. Alternatively, development of “non-agonist” modulators of PPARγ that exhibit improved safety profiles might be a suitable strategy 16. This suggests that PPARγ remains a viable target for the treatment and prevention of cancer.
Interestingly, chemicals that antagonize PPARγ can also inhibit the proliferation or invasiveness of human cancer cell lines 193–196. Studies show that some of these effects are due to PPARγ-independent mechanisms 197, but in one study, knocking down the expression of PPARγ mitigated the anti-proliferative effect of a PPARγ antagonist in a human cancer cell line 195. This paradoxically suggests that PPARγ antagonists might be useful for inhibiting tumorigenesis. However, there are several limitations with suggesting that antagonizing PPARγ will inhibit tumorigenesis including that many of the effects induced by current PPARγ antagonists do not require PPARγ, suggesting that other off-target mechanisms underlie these effects; the nature of the putative endogenous ligand that promotes tumorigenesis remains unclear; and chemicals that antagonize a nuclear receptor can also act as agonists and whether this is true for the current PPARγ antagonists has not been examined extensively to date. This last point indicates that PPARγ antagonists could function similarly to tamoxifen, which retains both agonist and antagonist activities for the estrogen receptor in a cell and tissue-specific manner 198. Thus, whether chemicals that target PPARγ as antagonists are useful for cancer chemoprevention remains to be determined.
Pan and dual PPAR agonists
It is conceivable that agonists that target more than a single PPAR might be suitable for treating or preventing cancer. Bezafibrate is a pan PPAR agonist but some of its effects are mediated by PPARα 7. A number of studies suggest that bezafibrate can inhibit colon tumorigenesis in both rodent 199–201 and human cancer models 202. Support for the idea that this is mediated by PPARα comes from data showing that a specific PPARα agonist, methylclofenopate, also inhibits intestinal tumorigenesis 203. The molecular mechanisms underlying the effects of bezafibrate and methylclofenopate on colon tumorigenesis remain elusive. Bezafibrate can also cause growth arrest, induce terminal differentiation and apoptosis in Burkitt’s lymphoma cells and these effects are enhanced by co-treatment with medroxyprogesterone acetate (MPA) 204. These changes are mediated in part by an increase in the production of 15-deoxy-Δ12,14 prostaglandin J2 (15-dPGJ2), a natural ligand of PPARγ 204. Moreover, bezafibrate induces similar changes in growth, differentiation and apoptosis in B-cell chronic lymphocytic leukemia cells, and co-treatment with MPA enhances these effects through a similar mechanism mediated by increased production of 15-dPGJ2 and apparent activation of PPARγ 205. These observations suggest that the pan PPAR agonist bezafibrate might target myeloid cancers through a mechanism that increases PPARγ activity. As bezafibrate activates PPARα, it remains a possibility that PPARα is required for these effects but this has not been determined to date (FIG. 5). The recent clinical trial demonstrating that bezafibrate is chemopreventive for colon cancer in humans 202, supports the hypothesis that development of pan PPAR agonists with relatively lower affinity for the PPARs (that is µM versus nM) could be suitable for future chemopreventive approaches. Indeed, studies suggest that high affinity dual PPAR agonists can cause tumors, including bladder cancer, liposarcomas and hemangiosarcomas, in long-term bioassays 206, indicating that the use of low affinity agents might be a more suitable approach. Identification of new dual or pan PPAR agonists could be feasible because PPAR ligands can lead to unique alterations in gene expression based in part on differential recruitment of co-activators 191. This could lead to characterization of chemicals that do not exhibit negative side effects associated with PPAR ligands including pro-carcinogenic effects in preclinical models 206, 207. In fact, dual and pan PPAR agonists might also help offset side effects observed with more selective PPAR agonists. For example, weight gain or bone fractures observed in response to administration of PPARγ agonists 187–190, 206 might be offset by agonist activity for PPARα or PPARβ/δ, which can increase lipid catabolism and stimulate osteoblast activity in bone 208. As there is also good evidence that combining PPAR activation with other chemopreventive or chemotherapeutic agents can significantly increase anti-cancer activities 92, 209–220, it remains possible that dual or pan PPAR agonists could lead to even greater improvement in efficacy.
Conclusions
Agonists for all three PPARs induce many physiological changes including increased oxidation of fatty acids that contributes to reducing serum lipids and decreasing body weight, improved insulin resistance, and inhibition of inflammatory signaling. As metabolic syndrome, obesity, dyslipidemias, glucose intolerance and chronic inflammation are associated with increased cancer risk 106, 162, 163, there is good reason to suggest that PPAR agonists should be potential candidates for treating and preventing cancer. PPARα remains a viable target for the treatment and prevention of cancer because of evidence indicating that humans are refractory to the hepatocarcinogenic effects of PPARα agonists, and because PPARα agonists can exhibit anti-inflammatory and anti-carcinogenic effects. PPARγ also remains a potential target for the treatment and prevention of cancer, in particular for PPARγ agonists with good safety profiles. By contrast, whether PPARβ/δ is suitable for targeting for the treatment and prevention of cancer is uncertain because of numerous conflicting studies. It is of interest to note that there is overlap in target genes regulated by each PPAR, but the physiological effects induced by selective PPAR agonists are unique due to the complexity of PPAR-dependent and PPAR-independent effects each agonist induces. This also illustrates the complexity of PPAR regulation and the effects resulting from receptor activation, and why considerable research and drug discovery efforts are necessary to fully delineate the potential of targeting PPARs for the treatment and prevention of cancer.
Acknowledgements
The authors gratefully acknowledge Jared Corell for technical assistance with the figures and Dr. Pallavi Devchand for critical review and suggestions for the manuscript. Our research is supported by the National Institutes of Health (CA124533, CA126826, CA141029, CA140369, AA018863) J.M.P., (R01CA148828) Y.M.S., and the National Cancer Institute Intramural Research Program (ZIABC005561, ZIABC005562, ZIABC005708) F.J.G..
Glossary terms
- PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR
This class of nuclear receptor acquired their name because the first receptor of this class identified (PPARα) mediates the phenomenon of the proliferation of peroxisomes observed in rodents given fibrates and other chemicals.
- AGONIST
A compound that binds to a receptor that invokes a biological response that is most often transcriptionally mediated. The specificity of an agonist is often defined by its ability to bind with the receptor at a given concentration and whether it is able to interact with a single receptor.
- ANTAGONIST
A compound that binds to a receptor and blocks all known receptor activities induced by activation by an agonist. The potency of an antagonist is often defined by the concentration required to inhibit activation by an agonist.
- CHEMOPREVENTION
The inhibition or prevention of disease by use of a drug or natural compound. Many chemopreventive agents show anti-inflammatory activities.
Biographies
Jeffrey M. Peters received his Ph.D. degree from the University of California at Davis and completed postdoctoral training at the National Cancer Institute. He is presently a Distinguished Professor in the Department of Veterinary and Biomedical Sciences at The Pennsylvania State University. His research program focuses on receptor-mediated regulatory mechanisms of carcinogenesis and homeostasis.
Yatrik M. Shah obtained his Ph.D. from the University of Toledo Health Science Campus and completed postdoctoral training at the National Cancer Institute. He is presently an Assistant Professor in the Department of Molecular and Integrative Physiology at the University of Michigan. His research program focuses on the molecular mechanisms by which oxygen sensing transcription factors regulate gastrointestinal homeostasis, inflammation and cancer.
Frank J. Gonzalez received his Ph.D. from the University of Wisconsin at Madison. He is currently the Chief of the Laboratory of Metabolism at the National Cancer Institute. His research program focuses on delineating the mechanisms by which mammals respond to insult by foreign chemicals or xenobiotics including dietary compounds such as food mutagens, phytochemicals, clinically used drugs, toxins and carcinogens with extensive application of transgenic and metabolomic approaches.
References
- 1.Schupp M, Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem. 2010;285:40409–40415. doi: 10.1074/jbc.R110.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y, Hon M, Evans RM. The peroxisome proliferator-activated receptor δ, an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci U S A. 2002;99:2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhikary T, et al. Genomewide analyses define different modes of transcriptional regulation by peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) PLoS One. 2011;6:e16344. doi: 10.1371/journal.pone.0016344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borland MG, et al. Stable over-expression of PPARβ/δ and PPARγ to examine receptor signaling in human HaCaT keratinocytes. Cell Signal. 2011;23:2039–2050. doi: 10.1016/j.cellsig.2011.07.020. This study critically examined possible mechanisms of PPARβ/δ-dependent regulation including whether retinoic acid activates PPARβ/δ, whether PPARβ/δ represses PPARγ activity and how PPARβ/δ regulates apoptosis and inflammatory cytokine expression following exposure to ultraviolet light.
- 5.Marin HE, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits colon carcinogenesis. Cancer Res. 2006;66:4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- 6.Matsusue K, Peters JM, Gonzalez FJ. PPARβ/δ potentiates PPARγ-stimulated adipocyte differentiation. The FASEB Journal. 2004;18:1477–1479. doi: 10.1096/fj.04-1944fje. [DOI] [PubMed] [Google Scholar]
- 7.Peters JM, Aoyama T, Burns AM, Gonzalez FJ. Bezafibrate is a dual ligand for PPARα and PPARβ: studies using null mice. Biochim Biophys Acta. 2003;1632:80–89. doi: 10.1016/s1388-1981(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 8.Kilgore KS, Billin AN. PPARβ/δ ligands as modulators of the inflammatory response. Curr Opin Investig Drugs. 2008;9:463–469. [PubMed] [Google Scholar]
- 9.Peters JM, Foreman JE, Gonzalez FJ. Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast and lung carcinogenesis. Cancer Metastasis Rev. 2011;30:619–640. doi: 10.1007/s10555-011-9320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796:230–241. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) in gastrointestinal tract function and disease. Clin Sci (Lond) 2008;115:107–127. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters JM, Morales JL, Gonzales FJ. Modulation of gastrointestinal inflammation and colorectal tumorigenesis by peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) Drug Discovery Today: Disease Mechanisms. 2011 doi: 10.1016/j.ddmec.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual G, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. Good review of literature describing mechanisms of trans-repression by PPARs.
- 16. Choi JH, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. This study demonstrated the feasibility of targeting PPARγ with a non-agonist to elicit anti-diabetic activity without causing negative side effects associated with some PPARγ agonists.
- 17. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. This study describes the cloning and characterization of PPARα, the first of the three PPARs to be identified.
- 18.Escher P, et al. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- 19.Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARα: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010;8:e002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor α target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. A useful resource listing PPARα target genes and corresponding references.
- 21.Kersten S, et al. The peroxisome proliferator-activated receptor α regulates amino acid metabolism. Faseb J. 2001;15:1971–1978. doi: 10.1096/fj.01-0147com. [DOI] [PubMed] [Google Scholar]
- 22.Guerre-Millo M, et al. PPARα activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000 doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- 23.Uhlen M, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 24.Girroir EE, et al. Quantitative expression patterns of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) protein in mice. Biochem Biophys Res Commun. 2008;371:456–461. doi: 10.1016/j.bbrc.2008.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leibowitz MD, et al. Activation of PPARδ alters lipid metabolism in db/db mice. FEBS Lett. 2000;473:333–336. doi: 10.1016/s0014-5793(00)01554-4. This study was one of the first to demonstrate a functional phenotype resulting from activating PPARβ/δ.
- 26.Oliver WR, Jr, et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprecher DL, et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor δ agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YX, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang YX, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim HJ, et al. PPARδ ligand L-165041 ameliorates Western diet-induced hepatic lipid accumulation and inflammation in LDLR−/− mice. Eur J Pharmacol. 2009;622:45–51. doi: 10.1016/j.ejphar.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, et al. Role of peroxisome proliferator-activated receptor δ/β in hepatic metabolic regulation. J Biol Chem. 2011;286:1237–1247. doi: 10.1074/jbc.M110.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagasawa T, et al. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARδ agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Shan W, et al. Peroxisome proliferator-activated receptor-β/δ protects against chemically induced liver toxicity in mice. Hepatology. 2008;47:225–235. doi: 10.1002/hep.21925. [DOI] [PubMed] [Google Scholar]
- 35.Shan W, et al. Ligand activation of peroxisome proliferator-activated receptor β/δ (PPARβ/δ) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol Sci. 2008;105:418–428. doi: 10.1093/toxsci/kfn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-β/δ in epithelial cell growth and differentiation. Cell Signal. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, et al. Structural organization of mouse peroxisome proliferator-activated receptor γ (mPPARγ) gene: alternative promoter use and different splicing yield two mPPARγ isoforms. Proc Natl Acad Sci U S A. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fajas L, Fruchart JC, Auwerx J. PPARγ3 mRNA: a distinct PPARγ mRNA subtype transcribed from an independent promoter. FEBS Lett. 1998;438:55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- 39.Foreman JE, et al. Erratum: Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal antiinflammatory drugs. Mol Carcinog. 2011;50:652–653. doi: 10.1002/mc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbott BD, Wood CR, Watkins AM, Das KP, Lau CS. Erratum to: Peroxisome proliferator-activated receptors α, β, and γ mRNA and protein expression in human fetal tissues. PPAR Res. 2010;2010 doi: 10.1155/2010/690907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barak Y, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 42.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 43.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 44.Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reddy JK, Azarnoff DL, Hignite CE. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980;283:397–398. doi: 10.1038/283397a0. This study was one of the first to demonstrate that long-term administration of PPARα agonists causes liver cancer in rodents.
- 46. Peters JM, Cattley RC, Gonzalez FJ. Role of PPARα in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 1997;18:2029–2033. doi: 10.1093/carcin/18.11.2029. This study established that PPARα is required to mediate hepatocarcinogenesis caused by long-term administration of PPARα agonists in mice.
- 47.Hays T, et al. Role of peroxisome proliferator-activated receptor-α (PPARα) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis. 2005;26:219–227. doi: 10.1093/carcin/bgh285. [DOI] [PubMed] [Google Scholar]
- 48.Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-α and liver cancer: where do we stand? J Mol Med. 2005;83:774–785. doi: 10.1007/s00109-005-0678-9. [DOI] [PubMed] [Google Scholar]
- 49.Cheung C, et al. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor-α. Cancer Research. 2004;64:3849–3854. doi: 10.1158/0008-5472.CAN-04-0322. [DOI] [PubMed] [Google Scholar]
- 50. Morimura K, Cheung C, Ward JM, Reddy JK, Gonzalez FJ. Differential susceptibility of mice humanized for peroxisome proliferator-activated receptor α to Wy-14,643-induced liver tumorigenesis. Carcinogenesis. 2006;27:1074–1080. doi: 10.1093/carcin/bgi329. This study demonstrated that PPARα humanized transgenic do not develop liver tumors after long-term administration of PPARα agonists suggesting a species difference in activities between human and rodent PPARα.
- 51. Shah YM, et al. Peroxisome proliferator-activated receptor α regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol Cell Biol. 2007;27:4238–4247. doi: 10.1128/MCB.00317-07. This study helped to elucidate the mechanism that explains why human PPARα does not mediate hepatocarcinogenesis while the mouse PPARα does by showing differential regulation of let7c miRNA.
- 52.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Modica S, et al. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 2010;138:636–648. doi: 10.1053/j.gastro.2009.09.060. 648 e1-12. [DOI] [PubMed] [Google Scholar]
- 54. Foreman JE, et al. Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer. Mol Carcinog. 2011;50:884–900. doi: 10.1002/mc.20757. This is the most quantitative study to date showing that expression of PPARβ/δ protein is lower in human and rodent colon tumors as compared to non-transformed tissue and also includes functional characterization of over-expression of PPARβ/δ in human colon cancer cell lines.
- 55.Delage B, Rullier A, Capdepont M, Rullier E, Cassand P. The effect of body weight on altered expression of nuclear receptors and cyclooxygenase-2 in human colorectal cancers. Nutr J. 2007;6:20. doi: 10.1186/1475-2891-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta RA, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci U S A. 2000;97:13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy HK, Karolski WJ, Ratashak A. Distal bowel selectivity in the chemoprevention of experimental colon carcinogenesis by the non-steroidal anti-inflammatory drug nabumetone. Int J Cancer. 2001;92:609–615. doi: 10.1002/ijc.1226. [DOI] [PubMed] [Google Scholar]
- 58.Takayama O, et al. Expression of PPARδ in multistage carcinogenesis of the colorectum: implications of malignant cancer morphology. Br J Cancer. 2006;95:889–895. doi: 10.1038/sj.bjc.6603343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D, Ning W, Xie D, Guo L, Dubois RN. Peroxisome proliferator-activated receptor δ confers resistance to peroxisome proliferator-activated receptor γ-induced apoptosis in colorectal cancer cells. Oncogene. 2011 doi: 10.1038/onc.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang L, et al. Biological Function and Prognostic Significance of Peroxisome Proliferator-Activated Receptor δ in Rectal Cancer. Clin Cancer Res. 2011;17:3760–3770. doi: 10.1158/1078-0432.CCR-10-2779. This study provides the strongest clinical evidence to date showing that PPARβ/δ protects against colorectal cancer in humans.
- 61.Yoshinaga M, et al. The simultaneous expression of peroxisome proliferator-activated receptor delta and cyclooxygenase-2 may enhance angiogenesis and tumor venous invasion in tissues of colorectal cancers. Dig Dis Sci. 2009;54:1108–1114. doi: 10.1007/s10620-008-0465-x. [DOI] [PubMed] [Google Scholar]
- 62.Yoshinaga M, et al. The Expression of Both Peroxisome Proliferator-Activated Receptor δ and Cyclooxygenase-2 in Tissues Is Associated with Poor Prognosis in Colorectal Cancer Patients. Dig Dis Sci. 2011;56:1194–1200. doi: 10.1007/s10620-010-1389-9. [DOI] [PubMed] [Google Scholar]
- 63.Davidson B, Hadar R, Stavnes HT, Trope CG, Reich R. Expression of the peroxisome proliferator-activated receptors-α, -β, and -γ in ovarian carcinoma effusions is associated with poor chemoresponse and shorter survival. Hum Pathol. 2009;40:705–713. doi: 10.1016/j.humpath.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 64.Glazer RI, Yuan H, Xie Z, Yin Y. PPARγ and PPARδ as Modulators of Neoplasia and Cell Fate. PPAR Res. 2008;2008 doi: 10.1155/2008/247379. 247379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaeckel EC, et al. Correlation of expression of cyclooxygenase-2, vascular endothelial growth factor, and peroxisome proliferator-activated receptor δ with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2001;127:1253–1259. doi: 10.1001/archotol.127.10.1253. [DOI] [PubMed] [Google Scholar]
- 66.Nijsten T, Geluyckens E, Colpaert C, Lambert J. Peroxisome proliferator-activated receptors in squamous cell carcinoma and its precursors. J Cutan Pathol. 2005;32:340–347. doi: 10.1111/j.0303-6987.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 67.Tong BJ, et al. Heightened expression of cyclooxygenase-2 and peroxisome proliferator- activated receptor-δ in human endometrial adenocarcinoma. Neoplasia. 2000;2:483–490. doi: 10.1038/sj.neo.7900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen LC, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64:3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 69.Hao CY, et al. Alteration of gene expression in macroscopically normal colonic mucosa from individuals with a family history of sporadic colon cancer. Clin Cancer Res. 2005;11:1400–1407. doi: 10.1158/1078-0432.CCR-04-1942. [DOI] [PubMed] [Google Scholar]
- 70.Harman FS, et al. Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nat Med. 2004;10:481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 71.Knutsen HK, et al. Increased levels of PPARβ/δ and cyclin D1 in flat dysplastic ACF and adenomas in Apc(Min/+) mice. Anticancer Res. 2005;25:3781–3789. [PubMed] [Google Scholar]
- 72.Notterman DA, Alon U, Sierk AJ, Levine AJ. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001;61:3124–3130. [PubMed] [Google Scholar]
- 73.Orner GA, et al. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of β-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24:263–267. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reed KR, et al. PPARδ status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene. 2004;23:8992–8996. doi: 10.1038/sj.onc.1208143. [DOI] [PubMed] [Google Scholar]
- 75.Feilchenfeldt J, Brundler MA, Soravia C, Totsch M, Meier CA. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARγ-coactivator 1 (PGC-1) Cancer Lett. 2004;203:25–33. doi: 10.1016/j.canlet.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 76.Yang L, et al. Quantitative analysis of PPARδ mRNA by real-time RT-PCR in 86 rectal cancer tissues. Eur J Surg Oncol. 2006;32:181–185. doi: 10.1016/j.ejso.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed N, Riley C, Quinn MA. An immunohistochemical perspective of PPARβ and one of its putative targets PDK1 in normal ovaries, benign and malignant ovarian tumours. Br J Cancer. 2008;98:1415–1424. doi: 10.1038/sj.bjc.6604306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshimura R, et al. Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int J Cancer. 2003;104:597–602. doi: 10.1002/ijc.10980. [DOI] [PubMed] [Google Scholar]
- 79.Foreman JE, et al. Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal antiinflammatory drugs. Mol Carcinog. 2009;48:942–952. doi: 10.1002/mc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ouyang N, Williams JL, Rigas B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR)δ expression in APC(min/+) mice proportionally to their tumor inhibitory effect: Implications for the role of PPARδ in carcinogenesis. Carcinogenesis. 2006;27:232–239. doi: 10.1093/carcin/bgi221. [DOI] [PubMed] [Google Scholar]
- 81.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sansom OJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheehan KM, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282:1254–1257. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 84.Hollingshead HE, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) and inhibition of cyclooxygenase 2 (COX2) attenuate colon carcinogenesis through independent signaling mechanisms. Carcinogenesis. 2008;29:169–176. doi: 10.1093/carcin/bgm209. [DOI] [PubMed] [Google Scholar]
- 85.Gupta RA, et al. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nat Med. 2004;10:245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 86.Wang D, et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proc Natl Acad Sci U S A. 2006;103:19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuo X, et al. Targeted genetic disruption of peroxisome proliferator-activated receptor-δ and colonic tumorigenesis. J Natl Cancer Inst. 2009;101:762–767. doi: 10.1093/jnci/djp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bility MT, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits chemically-induced skin tumorigenesis. Carcinogenesis. 2008;29:2406–2414. doi: 10.1093/carcin/bgn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bility MT, Zhu B, Kang BH, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-β/δ and inhibition of cyclooxygenase-2 enhances inhibition of skin tumorigenesis. Toxicol Sci. 2010;113:27–36. doi: 10.1093/toxsci/kfp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim DJ, et al. Peroxisome proliferator-activated receptor β (δ)-dependent regulation of ubiquitin C expression contributes to attenuation of skin carcinogenesis. J Biol Chem. 2004;279:23719–23727. doi: 10.1074/jbc.M312063200. [DOI] [PubMed] [Google Scholar]
- 91.Kim DJ, Prabhu KS, Gonzalez FJ, Peters JM. Inhibition of chemically-induced skin carcinogenicity by sulindac is independent of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) Carcinogenesis. 2006;27:1105–1112. doi: 10.1093/carcin/bgi346. [DOI] [PubMed] [Google Scholar]
- 92.Zhu B, et al. Chemoprevention of chemically induced skin tumorigenesis by ligand activation of peroxisome proliferator-activated receptor-β/δ and inhibition of cyclooxygenase 2. Mol Cancer Ther. 2011;9:3267–3277. doi: 10.1158/1535-7163.MCT-10-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Molecular Cell. 2002;10:721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 94.Tan NS, et al. Critical roles of PPARβ/δ in keratinocyte response to inflammation. Genes Dev. 2001;15:3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borland MG, et al. Ligand Activation of Peroxisome Proliferator-Activated Receptor-β/δ (PPARβ/δ) Inhibits Cell Proliferation in Human HaCaT Keratinocytes. Mol Pharmacol. 2008;74:1429–1442. doi: 10.1124/mol.108.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burdick AD, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007;19:1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim DJ, et al. PPARβ/δ selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- 98.Narkar VA, et al. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pollock CB, et al. PPARδ activation acts cooperatively with 3-phosphoinositide-dependent protein kinase-1 to enhance mammary tumorigenesis. PLoS One. 2011;6:e16215. doi: 10.1371/journal.pone.0016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tachibana K, et al. Gene expression profiling of potential peroxisome proliferator-activated receptor (PPAR) target genes in human hepatoblastoma cell lines inducibly expressing different PPAR isoforms. Nucl Recept. 2005;3:3. doi: 10.1186/1478-1336-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Szeles L, et al. Research resource: transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Mol Endocrinol. 2010;24:2218–2231. doi: 10.1210/me.2010-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hollingshead HE, et al. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007;28:2641–2649. doi: 10.1093/carcin/bgm183. [DOI] [PubMed] [Google Scholar]
- 103.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rohrl C, et al. Peroxisome-proliferator-activated receptors γ and β/δ mediate vascular endothelial growth factor production in colorectal tumor cells. J Cancer Res Clin Oncol. 2011;137:29–39. doi: 10.1007/s00432-010-0856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stephen RL, et al. Activation of peroxisome proliferator-activated receptor δ stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004;64:3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- 106.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 107.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. Lancet Oncol. 2004;5:419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 108.Koeffler HP. Peroxisome proliferator-activated receptor γ and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 109. Ogino S, et al. Colorectal cancer expression of peroxisome proliferator-activated receptor γ (PPARG, PPARγ) is associated with good prognosis. Gastroenterology. 2009;136:1242–1250. doi: 10.1053/j.gastro.2008.12.048. This study provided clinical evidence showing that PPARγ protects against colorectal cancer in humans.
- 110.McAlpine CA, Barak Y, Matise I, Cormier RT. Intestinal-specific PPARγ deficiency enhances tumorigenesis in ApcMin/+ mice. Int J Cancer. 2006;119:2339–2346. doi: 10.1002/ijc.22115. [DOI] [PubMed] [Google Scholar]
- 111.Elnemr A, et al. PPARγ ligand (thiazolidinedione) induces growth arrest and differentiation markers of human pancreatic cancer cells. Int J Oncol. 2000;17:1157–1164. doi: 10.3892/ijo.17.6.1157. [DOI] [PubMed] [Google Scholar]
- 112.Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN. Target genes of peroxisome proliferator-activated receptor γ in colorectal cancer cells. J Biol Chem. 2001;276:29681–29687. doi: 10.1074/jbc.M103779200. [DOI] [PubMed] [Google Scholar]
- 113.Sarraf P, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 114. Tontonoz P, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proc Natl Acad Sci U S A. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. This study was one of the first to establish that PPARγ ligands can induce differentiation in human cancer cells providing support for the hypothesis that targeting PPARγ may be suitable for human cancers.
- 115.Yoshizumi T, et al. Thiazolidinedione, a peroxisome proliferator-activated receptor-γ ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int J Oncol. 2004;25:631–639. [PubMed] [Google Scholar]
- 116.Drori S, et al. Hic-5 regulates an epithelial program mediated by PPARγ. Genes Dev. 2005;19:362–375. doi: 10.1101/gad.1240705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang JW, et al. Peroxisome proliferator-activated receptor γ-independent ablation of cyclin D1 by thiazolidinediones and their derivatives in breast cancer cells. Mol Pharmacol. 2005;67:1342–1348. doi: 10.1124/mol.104.007732. [DOI] [PubMed] [Google Scholar]
- 118.Lapillonne H, et al. Activation of peroxisome proliferator-activated receptor γ by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003;63:5926–5939. [PubMed] [Google Scholar]
- 119.Qin C, et al. Peroxisome proliferator-activated receptor γ agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor α in MCF-7 breast cancer cells. Cancer Res. 2003;63:958–964. [PubMed] [Google Scholar]
- 120.Wang C, et al. Inhibition of cellular proliferation through IκB kinase-independent and peroxisome proliferator-activated receptor γ-dependent repression of cyclin D1. Mol Cell Biol. 2001;21:3057–3070. doi: 10.1128/MCB.21.9.3057-3070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yin F, et al. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochem Biophys Res Commun. 2001;286:916–922. doi: 10.1006/bbrc.2001.5491. [DOI] [PubMed] [Google Scholar]
- 122.Koga H, et al. Involvement of p21(WAF1/Cip1), p27(Kip1), and p18(INK4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001;33:1087–1097. doi: 10.1053/jhep.2001.24024. [DOI] [PubMed] [Google Scholar]
- 123.Chen F, Harrison LE. Ciglitazone-induced cellular anti-proliferation increases p27kip1 protein levels through both increased transcriptional activity and inhibition of proteasome degradation. Cell Signal. 2005;17:809–816. doi: 10.1016/j.cellsig.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 124.Chen F, Kim E, Wang CC, Harrison LE. Ciglitazone-induced p27 gene transcriptional activity is mediated through Sp1 and is negatively regulated by the MAPK signaling pathway. Cell Signal. 2005;17:1572–1577. doi: 10.1016/j.cellsig.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 125.Itami A, et al. Ligands for peroxisome proliferator-activated receptor γ inhibit growth of pancreatic cancers both in vitro and in vivo. Int J Cancer. 2001;94:370–376. doi: 10.1002/ijc.1488. [DOI] [PubMed] [Google Scholar]
- 126.Koga H, et al. Troglitazone induces p27Kip1-associated cell-cycle arrest through down-regulating Skp2 in human hepatoma cells. Hepatology. 2003;37:1086–1096. doi: 10.1053/jhep.2003.50186. [DOI] [PubMed] [Google Scholar]
- 127.Motomura W, Okumura T, Takahashi N, Obara T, Kohgo Y. Activation of peroxisome proliferator-activated receptor γ by troglitazone inhibits cell growth through the increase of p27KiP1 in human. Pancreatic carcinoma cells. Cancer Res. 2000;60:5558–5564. [PubMed] [Google Scholar]
- 128.Sharma C, Pradeep A, Wong L, Rana A, Rana B. Peroxisome proliferator-activated receptor γ activation can regulate β-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. J Biol Chem. 2004;279:35583–35594. doi: 10.1074/jbc.M403143200. [DOI] [PubMed] [Google Scholar]
- 129.Wei S, et al. Thiazolidinediones modulate the expression of β-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor γ. Mol Pharmacol. 2007;72:725–733. doi: 10.1124/mol.107.035287. [DOI] [PubMed] [Google Scholar]
- 130.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Res. 2001;61:6213–6218. [PubMed] [Google Scholar]
- 131.Bae MA, Song BJ. Critical role of c-Jun N-terminal protein kinase activation in troglitazone-induced apoptosis of human HepG2 hepatoma cells. Mol Pharmacol. 2003;63:401–408. doi: 10.1124/mol.63.2.401. [DOI] [PubMed] [Google Scholar]
- 132.Zander T, et al. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARγ. J Neurochem. 2002;81:1052–1060. doi: 10.1046/j.1471-4159.2002.00899.x. [DOI] [PubMed] [Google Scholar]
- 133.Shiau CW, et al. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARγ. Cancer Res. 2005;65:1561–1569. doi: 10.1158/0008-5472.CAN-04-1677. [DOI] [PubMed] [Google Scholar]
- 134.Farrow B, Evers BM. Activation of PPARγ increases PTEN expression in pancreatic cancer cells. Biochem Biophys Res Commun. 2003;301:50–53. doi: 10.1016/s0006-291x(02)02983-2. [DOI] [PubMed] [Google Scholar]
- 135.Lee SY, et al. PPAR-γ agonist increase gefitinib's antitumor activity through PTEN expression. Lung Cancer. 2006;51:297–301. doi: 10.1016/j.lungcan.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 136.Patel L, et al. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11:764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 137.Teresi RE, et al. Increased PTEN expression due to transcriptional activation of PPARγ by Lovastatin and Rosiglitazone. Int J Cancer. 2006;118:2390–2398. doi: 10.1002/ijc.21799. [DOI] [PubMed] [Google Scholar]
- 138.Zhang W, et al. PPARγ activator rosiglitazone inhibits cell migration via upregulation of PTEN in human hepatocarcinoma cell line BEL-7404. Cancer Biol Ther. 2006;5:1008–1014. doi: 10.4161/cbt.5.8.2887. [DOI] [PubMed] [Google Scholar]
- 139.Kim KY, Kim SS, Cheon HG. Differential anti-proliferative actions of peroxisome proliferator-activated receptor-γ agonists in MCF-7 breast cancer cells. Biochem Pharmacol. 2006;72:530–540. doi: 10.1016/j.bcp.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 140.Yan KH, et al. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog. 2010;49:235–246. doi: 10.1002/mc.20593. [DOI] [PubMed] [Google Scholar]
- 141.Kim Y, Suh N, Sporn M, Reed JC. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem. 2002;277:22320–22329. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- 142.Schultze K, et al. Troglitazone sensitizes tumor cells to TRAIL-induced apoptosis via down-regulation of FLIP and Survivin. Apoptosis. 2006;11:1503–1512. doi: 10.1007/s10495-006-8896-3. [DOI] [PubMed] [Google Scholar]
- 143.Wei S, Yang J, Lee SL, Kulp SK, Chen CS. PPARγ-independent antitumor effects of thiazolidinediones. Cancer Lett. 2009;276:119–124. doi: 10.1016/j.canlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 145. Adachi M, et al. Peroxisome proliferator activated receptor γ in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104–1113. doi: 10.1136/gut.2005.081745. This study demonstrated that PPARγ inhibits inflammation in the gut and protects against inflammatory bowel, which may explain in part the protective nature of PPARγ in colonrectal cancer.
- 146.Shah YM, Morimura K, Gonzalez FJ. Expression of peroxisome proliferator-activated receptor-γ in macrophage suppresses experimentally induced colitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G657–G666. doi: 10.1152/ajpgi.00381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Greten FR, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 148.Lefebvre AM, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 149.Saez E, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nat Med. 1998;4:1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 150.Pino MV, Kelley MF, Jayyosi Z. Promotion of colon tumors in C57BL/6J-APC( min)/+ mice by thiazolidinedione PPARγ agonists and a structurally unrelated PPARγ agonist. Toxicol Pathol. 2004;32:58–63. doi: 10.1080/01926230490261320. [DOI] [PubMed] [Google Scholar]
- 151.Yang K, et al. Peroxisome proliferator-activated receptor γ agonist troglitazone induces colon tumors in normal C57BL/6J mice and enhances colonic carcinogenesis in Apc1638 N/+ Mlh1+/− double mutant mice. Int J Cancer. 2005;116:495–499. doi: 10.1002/ijc.21018. [DOI] [PubMed] [Google Scholar]
- 152.Lubet RA, et al. Rosiglitazone, a PPARγ agonist: potent promoter of hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers. Int J Cancer. 2008;123:2254–2259. doi: 10.1002/ijc.23765. [DOI] [PubMed] [Google Scholar]
- 153.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369–1371. doi: 10.2337/dc10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-γ ligands for the treatment of breast cancer. Expert Opin Investig Drugs. 2005;14:557–568. doi: 10.1517/13543784.14.6.557. [DOI] [PubMed] [Google Scholar]
- 155.Saez E, et al. PPARγ signaling exacerbates mammary gland tumor development. Genes Dev. 2004;18:528–540. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Y, Lazar MA. Differential gene regulation by PPARγ agonist and constitutively active PPARγ2. Mol Endocrinol. 2002;16:1040–1048. doi: 10.1210/mend.16.5.0825. [DOI] [PubMed] [Google Scholar]
- 157.Panigrahy D, et al. PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci U S A. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pozzi A, et al. Peroxisomal proliferator-activated receptor-α-dependent inhibition of endothelial cell proliferation and tumorigenesis. J Biol Chem. 2007;282:17685–17695. doi: 10.1074/jbc.M701429200. [DOI] [PubMed] [Google Scholar]
- 159.Balkwill F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 160.Aoyama A, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 161.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 162.Tsugane S, Inoue M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci. 2010;101:1073–1079. doi: 10.1111/j.1349-7006.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2011;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kasuga J, et al. Novel biphenylcarboxylic acid peroxisome proliferator-activated receptor (PPAR) δ selective antagonists. Bioorg Med Chem Lett. 2009;19:6595–6599. doi: 10.1016/j.bmcl.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 165.Naruhn S, et al. High Affinity Peroxisome Proliferator-Activated Recepto-β/δ-Specific Ligands with Pure Antagonistic or Inverse Agonistic Properties. Mol Pharmacol. 2011;80:828–838. doi: 10.1124/mol.111.074039. [DOI] [PubMed] [Google Scholar]
- 166.Shearer BG, et al. Identification and characterization of a selective peroxisome proliferator-activated receptor β/δ (NR1C2) antagonist. Mol Endocrinol. 2008;22:523–529. doi: 10.1210/me.2007-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shearer BG, et al. Identification and Characterization of 4-Chloro-N-(2-{[5-trifluoromethyl)-2-pyridyl]sulfonyl}ethyl)benzamide (GSK3787), a Selective and Irreversible Peroxisome Proliferator-Activated Receptor δ (PPARδ) Antagonist. J Med Chem. 2010;53:1857–1861. doi: 10.1021/jm900464j. [DOI] [PubMed] [Google Scholar]
- 168.Zaveri NT, et al. A novel peroxisome proliferator-activated receptor δ antagonist, SR13904, has anti-proliferative activity in human cancer cells. Cancer Biol Ther. 2009;8:1252–1261. doi: 10.4161/cbt.8.13.8691. [DOI] [PubMed] [Google Scholar]
- 169.Palkar PS, et al. Cellular and Pharmacological Selectivity of the PPARβ/δ Antagonist GSK3787. Mol Pharmacol. 2010;78:419–430. doi: 10.1124/mol.110.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Demetri GD, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999;96:3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Debrock G, et al. A phase II trial with rosiglitazone in liposarcoma patients. Br J Cancer. 2003;89:1409–1412. doi: 10.1038/sj.bjc.6601306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Smith MR, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101:1569–1574. doi: 10.1002/cncr.20493. [DOI] [PubMed] [Google Scholar]
- 173.Hisatake JI, et al. Down-Regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor γ in human prostate cancer. Cancer Res. 2000;60:5494–5498. [PubMed] [Google Scholar]
- 174.Mueller E, et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci U S A. 2000;97:10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schwartz GK, et al. Phase I and pharmacokinetic study of LY293111, an orally bioavailable LTB4 receptor antagonist, in patients with advanced solid tumors. J Clin Oncol. 2005;23:5365–5373. doi: 10.1200/JCO.2005.02.766. [DOI] [PubMed] [Google Scholar]
- 176.Burstein HJ, et al. Use of the peroxisome proliferator-activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat. 2003;79:391–397. doi: 10.1023/a:1024038127156. [DOI] [PubMed] [Google Scholar]
- 177.Kulke MH, et al. A phase II study of troglitazone, an activator of the PPARγ receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J. 2002;8:395–399. doi: 10.1097/00130404-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 178.Baetz T, et al. A phase I study of oral LY293111 given daily in combination with irinotecan in patients with solid tumours. Invest New Drugs. 2007;25:217–225. doi: 10.1007/s10637-006-9021-8. [DOI] [PubMed] [Google Scholar]
- 179.Read WL, Baggstrom MQ, Fracasso PM, Govindan R. A phase I study of bexarotene and rosiglitazone in patients with refractory cancers. Chemotherapy. 2007;54:236–241. doi: 10.1159/000140468. [DOI] [PubMed] [Google Scholar]
- 180.Hau P, et al. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-γ agonists in recurrent high-grade gliomas - a phase II study. Oncology. 2007;73:21–25. doi: 10.1159/000120028. [DOI] [PubMed] [Google Scholar]
- 181.Kebebew E, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006;140:960–966. doi: 10.1016/j.surg.2006.07.038. discussion 966-7. [DOI] [PubMed] [Google Scholar]
- 182.Tepmongkol S, Keelawat S, Honsawek S, Ruangvejvorachai P. Rosiglitazone effect on radioiodine uptake in thyroid carcinoma patients with high thyroglobulin but negative total body scan: a correlation with the expression of peroxisome proliferator-activated receptor-γ. Thyroid. 2008;18:697–704. doi: 10.1089/thy.2008.0056. [DOI] [PubMed] [Google Scholar]
- 183.Kroll TG, et al. PAX8-PPARγ1 fusion oncogene in human thyroid carcinoma. Science. 2000;289:1357–1360. doi: 10.1126/science.289.5483.1357. [DOI] [PubMed] [Google Scholar]
- 184.Giordano TJ, et al. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARG translocation. Clin Cancer Res. 2006;12:1983–1993. doi: 10.1158/1078-0432.CCR-05-2039. [DOI] [PubMed] [Google Scholar]
- 185.Lacroix L, et al. Follicular thyroid tumors with the PAX8-PPARγ1 rearrangement display characteristic genetic alterations. Am J Pathol. 2005;167:223–231. doi: 10.1016/s0002-9440(10)62967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Erdmann E, Charbonnel B, Wilcox R. Thiazolidinediones and cardiovascular risk - a question of balance. Curr Cardiol Rev. 2009;5:155–165. doi: 10.2174/157340309788970333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Grey A, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 188.Kahn SE, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 189.Schwartz AV, Sellmeyer DE. Thiazolidinedione therapy gets complicated: is bone loss the price of improved insulin resistance? Diabetes Care. 2007;30:1670–1671. doi: 10.2337/dc07-0554. [DOI] [PubMed] [Google Scholar]
- 190.Schwartz AV, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kodera Y, et al. Ligand type-specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. J Biol Chem. 2000;275:33201–33204. doi: 10.1074/jbc.C000517200. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Q, Zhou H, Zhai S, Yan B. Natural product-inspired synthesis of thiazolidine and thiazolidinone compounds and their anticancer activities. Curr Pharm Des. 2010;16:1826–1842. doi: 10.2174/138161210791208983. [DOI] [PubMed] [Google Scholar]
- 193.Burton JD, Castillo ME, Goldenberg DM, Blumenthal RD. Peroxisome proliferator-activated receptor-γ antagonists exhibit potent antiproliferative effects versus many hematopoietic and epithelial cancer cell lines. Anticancer Drugs. 2007;18:525–534. doi: 10.1097/CAD.0b013e3280200414. [DOI] [PubMed] [Google Scholar]
- 194.Lea MA, Sura M, Desbordes C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) γ agonists and antagonists. Anticancer Res. 2004;24:2765–2771. [PubMed] [Google Scholar]
- 195.Schaefer KL, et al. Peroxisome proliferator-activated receptor γ inhibition prevents adhesion to the extracellular matrix and induces anoikis in hepatocellular carcinoma cells. Cancer Res. 2005;65:2251–2259. doi: 10.1158/0008-5472.CAN-04-3037. [DOI] [PubMed] [Google Scholar]
- 196.Takahashi H, et al. Inhibition of peroxisome proliferator-activated receptor γ activity in esophageal carcinoma cells results in a drastic decrease of invasive properties. Cancer Sci. 2006;97:854–860. doi: 10.1111/j.1349-7006.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Burton JD, Goldenberg DM, Blumenthal RD. Potential of peroxisome proliferator-activated receptor γ antagonist compounds as therapeutic agents for a wide range of cancer types. PPAR Res. 2008;2008 doi: 10.1155/2008/494161. 494161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Osborne CK, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 199.Kohno H, Suzuki R, Sugie S, Tanaka T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5:46. doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Niho N, et al. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090–6095. [PubMed] [Google Scholar]
- 201.Tanaka T, et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 2001;61:2424–2428. [PubMed] [Google Scholar]
- 202. Tenenbaum A, et al. Does the lipid-lowering peroxisome proliferator-activated receptors ligand bezafibrate prevent colon cancer in patients with coronary artery disease? Cardiovasc Diabetol. 2008;7:18. doi: 10.1186/1475-2840-7-18. This study suggests that the pan PPAR agonist bezafibrate may prevent colon cancer in humans supporting the hypothesis that targeting all three PPARs may be suitable for chemoprevention.
- 203.Jackson L, et al. Potential role for peroxisome proliferator activated receptor (PPAR) in preventing colon cancer. Gut. 2003;52:1317–1322. doi: 10.1136/gut.52.9.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Khanim FL, et al. Combined bezafibrate and medroxyprogesterone acetate: potential novel therapy for acute myeloid leukaemia. PLoS One. 2009;4:e8147. doi: 10.1371/journal.pone.0008147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hayden RE, et al. Treatment of primary CLL cells with bezafibrate and medroxyprogesterone acetate induces apoptosis and represses the pro-proliferative signal of CD40-ligand, in part through increased 15dδ12,14,PGJ2. Leukemia. 2009;23:292–304. doi: 10.1038/leu.2008.283. [DOI] [PubMed] [Google Scholar]
- 206.Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 207.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90:269–295. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- 208.Still K, Grabowski P, Mackie I, Perry M, Bishop N. The peroxisome proliferator activator receptor α/δ agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif Tissue Int. 2008;83:285–292. doi: 10.1007/s00223-008-9175-9. [DOI] [PubMed] [Google Scholar]
- 209.Brautigam K, et al. Combined treatment with TRAIL and PPARgamma ligands overcomes chemoresistance of ovarian cancer cell lines. J Cancer Res Clin Oncol. 2011;137:875–886. doi: 10.1007/s00432-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cesario RM, Stone J, Yen WC, Bissonnette RP, Lamph WW. Differentiation and growth inhibition mediated via the RXR:PPARγ heterodimer in colon cancer. Cancer Lett. 2006;240:225–233. doi: 10.1016/j.canlet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 211.Crowe DL, Chandraratna RA. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-alpha is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 2004;6:R546–R555. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Desreumaux P, et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med. 2001;193:827–838. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fu H, et al. Chemoprevention of lung carcinogenesis by the combination of aerosolized budesonide and oral pioglitazone in A/J mice. Mol Carcinog. 2011 doi: 10.1002/mc.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Girnun GD, et al. Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hamaguchi N, et al. In vitro and in vivo therapeutic efficacy of the PPAR-γ agonist troglitazone in combination with cisplatin against malignant pleural mesothelioma cell growth. Cancer Sci. 2010;101:1955–1964. doi: 10.1111/j.1349-7006.2010.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Park BH, Lee SB, Stolz DB, Lee YJ, Lee BC. Synergistic interactions between heregulin and peroxisome proliferator-activated receptor-gamma (PPARγ) agonist in breast cancer cells. J Biol Chem. 2011;286:20087–20099. doi: 10.1074/jbc.M110.191718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Reddy RC, et al. Chemotherapeutic drugs induce PPARγ expression and show sequence-specific synergy with PPARγ ligands in inhibition of non-small cell lung cancer. Neoplasia. 2008;10:597–603. doi: 10.1593/neo.08134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tikoo K, Kumar P, Gupta J. Rosiglitazone synergizes anticancer activity of cisplatin and reduces its nephrotoxicity in 7, 12-dimethyl benz{a}anthracene (DMBA) induced breast cancer rats. BMC Cancer. 2009;9:107. doi: 10.1186/1471-2407-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yamazaki K, et al. Synergistic effects of RXR α and PPAR γ ligands to inhibit growth in human colon cancer cells--phosphorylated RXR α is a critical target for colon cancer management. Gut. 2007;56:1557–1563. doi: 10.1136/gut.2007.129858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yokoyama Y, Xin B, Shigeto T, Mizunuma H. Combination of ciglitazone, a peroxisome proliferator-activated receptor γ ligand, and cisplatin enhances the inhibition of growth of human ovarian cancers. J Cancer Res Clin Oncol. 2011;137:1219–1228. doi: 10.1007/s00432-011-0993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fauconnet S, et al. Differential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cells. J Biol Chem. 2002;277:23534–23543. doi: 10.1074/jbc.M200172200. [DOI] [PubMed] [Google Scholar]
- 222.Meissner M, Hrgovic I, Doll M, Kaufmann R. PPARδ agonists suppress angiogenesis in a VEGFR2-dependent manner. Arch Dermatol Res. 2011;303:41–47. doi: 10.1007/s00403-010-1091-y. [DOI] [PubMed] [Google Scholar]
- 223.Abdollahi A, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci U S A. 2007;104:12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Müller-Brüsselbach S, et al. Deregulation of tumor angiogenesis and blockade of tumor growth in PPARβ-deficient mice. Embo J. 2007;26:3686–3698. doi: 10.1038/sj.emboj.7601803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Piqueras L, et al. Activation of PPARβ/δ induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 226.Biscetti F, et al. Selective activation of peroxisome proliferator-activated receptor (PPAR)α and PPARγ induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes. 2008;57:1394–1404. doi: 10.2337/db07-0765. [DOI] [PubMed] [Google Scholar]
- 227.Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999;274:17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 228.Chu K, et al. Peroxisome proliferator-activated receptor-γ-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res. 2006;1093:208–218. doi: 10.1016/j.brainres.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 229.Huang PH, et al. Pioglitazone ameliorates endothelial dysfunction and restores ischemia-induced angiogenesis in diabetic mice. Biomed Pharmacother. 2008;62:46–52. doi: 10.1016/j.biopha.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 230.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 231.Chintalgattu V, Harris GS, Akula SM, Katwa LC. PPAR-γ agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovasc Res. 2007;74:140–150. doi: 10.1016/j.cardiores.2007.01.010. [DOI] [PubMed] [Google Scholar]