Background: IL-6 is up-regulated by contraction in skeletal muscle.
Results: Contraction-induced IL-6 expression is blunted by JNK inhibition.
Conclusion: The JNK/AP-1 pathway regulates IL-6 expression in contracting muscle.
Significance: This highlights a novel contraction-mediated transcriptional pathway for IL-6 in skeletal muscle.
Keywords: Cytokine, Exercise, Gene Transcription, Signal Transduction, Skeletal Muscle Metabolism
Abstract
Exercise increases the expression of the prototypical myokine IL-6, but the precise mechanism by which this occurs has yet to be identified. To mimic exercise conditions, C2C12 myotubes were mechanically stimulated via electrical pulse stimulation (EPS). We compared the responses of EPS with the pharmacological Ca2+ carrier calcimycin (A23187) because contraction induces marked increases in cytosolic Ca2+ levels or the classical IκB kinase/NFκB inflammatory response elicited by H2O2. We demonstrate that, unlike H2O2-stimulated increases in IL-6 mRNA, neither calcimycin- nor EPS-induced IL-6 mRNA expression is under the transcriptional control of NFκB. Rather, we show that EPS increased the phosphorylation of JNK and the reporter activity of the downstream transcription factor AP-1. Furthermore, JNK inhibition abolished the EPS-induced increase in IL-6 mRNA and protein expression. Finally, we observed an exercise-induced increase in both JNK phosphorylation and IL-6 mRNA expression in the skeletal muscles of mice after 30 min of treadmill running. Importantly, exercise did not increase IL-6 mRNA expression in skeletal muscle-specific JNK-deficient mice. These data identify a novel contraction-mediated transcriptional regulatory pathway for IL-6 in skeletal muscle.
Introduction
Obesity is often associated with a chronic low-grade inflammation termed “meta-inflammation,” which is characterized by abnormal cytokine production and overactivation of inflammatory signaling pathways (1). Somewhat typical of this response to nutrient oversupply is the elevated expression of the proinflammatory cytokine IL-6. Although this suggests that IL-6 may play a causative role in the etiology of obesity-induced insulin resistance, data derived from exercise research is suggestive of an incompletely understood role of this cytokine in metabolic function. Indeed, skeletal muscle has recently been identified as an endocrine organ because it produces and releases cytokines such as IL-6 and other peptides termed “myokines” to exert paracrine, autocrine, or endocrine functions (for review, see Ref. 2).
Despite the fact that muscular contraction rapidly induces transcription of the IL-6 gene in skeletal myocytes, the intracellular signaling events that mediate this process are not well known. The intracellular signaling pathway for IL-6 was originally characterized in endotoxin-stimulated monocytes and macrophages (3, 4). Binding of the bacterial endotoxin to TLR4 (Toll-like receptor-4) recruits MyD88 (myeloid differentiation primary response protein 88) to its cytoplasmic domain, triggering a cascade of intracellular signaling events that includes stimulation of IκB kinase (IKK)3 and phosphorylation of IκB (inhibitory subunit of NFκB), which signals its proteasomal degradation. This allows translocation of NFκB to the nucleus and subsequent transcription of genes such as IL-6, TNF-α, and IL-1β, known effectors of the classical inflammatory response (5). Activation of the IKKβ/NFκB pathway and the subsequent expression of inflammatory cytokines are thought to contribute to the development of obesity-induced insulin resistance (6). Because exercise is thought to increase insulin sensitivity in the immediate post-exercise period, it would seem paradoxical that exercise would stimulate IL-6 via the classical inflammatory IKK/NFκB pathway in muscle, especially because IL-6 has been theorized as a possible mediator of this improved insulin sensitivity (2). Furthermore, although resting plasma IL-6 concentrations are elevated in patients with type 2 diabetes, gene expression in skeletal muscle is not (7), suggesting that an inflammatory pathway may not necessarily mediate skeletal muscle IL-6 expression, particularly during exercise.
Although the specific pathways mediating muscle IL-6 transcription have not been well characterized, various factors induced by exercise have been shown to stimulate IL-6 expression. For example, NO is generated in contracting skeletal muscle (8), and data from experiments manipulating NO with the inhibitor N-nitro-l-arginine methyl ester or the donor nitroglycerin are suggestive of a possible role in regulating IL-6 expression (9). Interestingly, NO-associated changes in IL-6 expression are independent of NFκB translocation. Given that contraction-induced IL-6 expression is related to the intensity and duration of exercise (2) and that a similar relationship exists between mechanical load and intracellular calcium concentration, a role of the calcium-sensitive phosphatase calcineurin in IL-6 transcription has also been postulated (10). Indeed, in cultured human myotubes, IL-6 mRNA expression responds to manipulation of intracellular calcium in a time- and dose-dependent manner (11). Furthermore, cotransfection of murine myotubes with a constitutively active form of calcineurin increases the activity of the IL-6 promoter (12). Because NFAT (nuclear factor of activated T cells) is a target of calcineurin and because NFAT activity is dependent on intracellular calcium in other cell types (13), it is possible that NFAT activity completes the signaling axis from muscle contraction to calcium release to IL-6 expression. However, we have previously shown that there is no apparent affect of 60 min of concentric exercise on the nuclear abundance of NFAT in human muscle biopsy samples that express increases in IL-6 mRNA (14). Interestingly, we also showed an exacerbation of IL-6 mRNA expression in muscle possessing low glycogen concentrations, an effect perhaps mediated by the stress-activated kinase p38 MAPK (14). Although this transcriptional pathway might increase IL-6 expression in glycogen-depleted states, IL-6 transcription is rapid and at the onset of exercise (2), suggesting that alternative signaling pathways determine basal and exercise-stimulated IL-6 expression in the fed state. With this in mind, we present here a series of experiments that highlight the role of JNK/AP-1 (activator protein-1) signaling in contraction-induced IL-6 expression independent of classical proinflammatory IKK/NFκB signaling.
EXPERIMENTAL PROCEDURES
Cell Culture
The murine skeletal myocyte cell line C2C12 and the murine macrophage cell line RAW 264.7 were grown in low-glucose DMEM and 10% FBS maintained at 37 °C and 5% CO2 according to the recommendations of American Type Culture Collection. To induce differentiation, the C2C12 myocytes were grown to 90% confluence before switching to a 2% FBS differentiation medium for 7 days, with a daily media change.
Ligand Stimulation
Dose-response and time course experiments were performed to determine the optimum treatment conditions for each of the ligands and inhibitors (data not shown). The differentiated myotubes were stimulated with the calcium ionophore calcimycin (1 μm; A23187), 500 ng/ml LPS, or 500 μm H2O2 (all from Sigma-Aldrich) for 3, 3, and 4 h respectively. RAW 264.7 cells were stimulated with calcimycin for 3 h, with LPS for 1 h, and with H2O2 for 3 h. For IKK inhibitor experiments, the cells were preincubated in the presence of the inhibitor BMS-345541 (EMD Chemicals, Gibbstown, NJ) for 1 h before the ligand stimulations. For the inhibition of NFAT, the myotubes were preincubated in medium containing 1 μm cell-permeable 11R-VIVIT (catalog no. 480401, Merck KGaA, Darmstadt, Germany) for 1 h as reported (15) before ligand stimulation. JNK inhibition experiments were performed with optimized final (10 μm) concentrations of JNK inhibitor VIII (catalog no. 420315, Merck KGaA) added the incubation medium immediately before electrical pulse stimulation (EPS) treatment.
EPS of Myotubes
To mimic excitation-contraction coupling in vitro, the C2C12 myotubes were grown and allowed to fully differentiate on a 4-well plate (catalog no. 167063, Nalge Nunc). Contraction was induced by subjecting myotubes to paced EPS (40 V/60 mm, 1 Hz, 10 ms) using a C-Pace cell culture stimulator (Model CLP100, IonOptix Ltd., Dublin, Ireland). Optimization experiments of EPS were carried out for durations of 90 min and 4, 6, and 12 h (data not shown). No significant IL-6 mRNA responses were observed at 90 min. Four hours of stimulation induced a robust IL-6 mRNA response, whereas no additive effects were seen when comparing 4 and 6 h of stimulation. After 12 h, significant lactate dehydrogenase concentrations were observed in the conditioned medium, suggestive of cell damage. Subsequent EPS experiments were therefore carried out over 4 h of stimulation. This protocol induces typical “exercise” responses in muscle such as AMP-activated protein kinase phosphorylation and decreases in phosphocreatine and glycogen in the absence of notable cell death (16, 17).
Quantitative Real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) according to manufacturer's instructions. Reverse transcription was carried out with 0.2 μg of RNA using TaqMan® reverse transcription reagents (Applied Biosystems, Mulgrave, Australia) according to the manufacturer's protocol. All real-time PCR probes were obtained from Applied Biosystems, with the exception of a custom-made JNK-1 probe to confirm our knock-out animal (see below), for which no commercial probe was available. Quantitative expression of IL-6 (Mm00446190_m1), JNK-1 (Mapk8, Mm00489514_m1), JNK-2 (Mapk9, Mm00444239_m1), and JNK-1_custom (AGTGTGTGCAGCTTATGATGCC; primers 5′-ATTGGAGATTCTAC and 3′-CATTCTGAAATGGCCGGCT; Eurogentec, Cologne, Germany) mRNAs was determined in duplicate and adjusted for total RNA content by the housekeeping genes Hprt1 (hypoxanthine-guanine phosphoribosyltransferase-1) and 18 S rRNA. Reverse transcription-negative controls were implemented to ensure the purity and specificity of the PCR. All reactions were performed on an ABI 7500 or 7900 real-time PCR system (Applied Biosystems).
Western Blotting
At the conclusion of experiments, cells were washed twice with ice-cold PBS before lysing in ice-cold buffer (50 mm HEPES, 150 mm NaCl, 10 mm NaF, 1 mm Na3VO4, 5 mm EDTA, 0.5% Triton X-100, 10% (v/v) glycerol, 100 μg/ml phenylmethylsulfonyl fluoride, and 5 μl/ml protease and phosphatase inhibitor mixture) and centrifuged at 4 °C for 30 min at maximum speed. The resultant protein-containing supernatants were transferred to fresh microcentrifuge tubes and kept frozen at −80 °C until analysis. For in vivo experiments, whole muscles were homogenized with a Polytron homogenizer (IKA Werke GmbH & Co. KG, Staufen, Germany) in protein lysis buffer (50 mm HEPES (pH 7.4), 1% Triton X-100, 0.1 m NaF, 10 mm EDTA, 50 mm NaCl, 0.1% SDS, and proteinase and phosphatase inhibitor mixture tablets (Roche Applied Science)).
SDS-PAGE was used to separate and identify protein extracts from various experiments. In general, 30 μg of protein from each sample was transferred by electrophoresis on 10% SDS-polyacrylamide gel at 130 V. The separated proteins were then transferred using a semidry transfer to a nitrocellulose membrane and incubated in blocking buffer (5% skim milk powder in Tris-buffered saline with 0.25% Tween) for 1 h. After an overnight exposure to primary antibodies against phosphorylated IKKβ, total and phosphorylated JNK, total NFκB p65 and p50, β-actin, and AKT (Cell Signaling Technology); JNK-1/3 (Santa Cruz Biotechnology); and calnexin (catalog no. 208880, Calbiochem) at 4 °C, the membrane was subjected to horseradish peroxidase-conjugated anti-rabbit secondary antibodies at a dilution of 1:2000 in blocking buffer (GE Healthcare) for 1 h at room temperature. After 60 min of washing, antibody binding was detected using SuperSignal enhanced chemiluminescent substrate (Pierce) and a ChemiDoc XRS system (Bio-Rad). Band intensities (arbitrary units) were measured by Quantity One 1-D analysis software (Bio-Rad). All quantifications were normalized against either the total protein or the loading controls (β-actin or calnexin).
ELISA
For assessment of IL-6 protein release into the surrounding cell culture medium, supernatant samples were analyzed using a commercially available, high-sensitivity ELISA (RayBiotech, Norcross, GA). Intra-assay coefficient of variation was measured at 2.3%.
IKK Activity
The total activity of the IKK complex was analyzed using the K-LISATM IKKβ inhibitor screening kit (EMD Chemicals). Briefly, cells were harvested and washed with ice-cold PBS before being pelleted by low-speed centrifugation. The pellet was then resuspended in 50 μl of PBS containing protease inhibitor mixture (Sigma-Aldrich). Cells were lysed by repeated freezing-thawing. A portion of the lysate was reserved for protein determination; 20 μl of the lysate was used for the activity assay; and each sample was analyzed in duplicate according to the protocol described by the manufacturer. The activity reading of the IKK complex was normalized against the protein concentration and expressed as -fold change relative to the control.
Pathway Profiling Reporter Assay
The secreted alkaline phosphatase pathway profiling system was purchased from Clontech. Reporter plasmids containing tandem repeats of a single transcription-binding element for NFκB, NFAT, or JNK/AP-1 were introduced into the C2C12 myotubes using Lipofectamine 2000® (Invitrogen) at a ratio of 4 parts of reagent to 1 part of DNA based on optimization experiments (data not shown). A plasmid containing β-galactosidase controlled by a CMV promoter was cotransfected with the respective reporter plasmids to account for transfection discrepancies. EPS or ligand stimulations were carried out 5 days following transfection, and pathway activation was assessed by collecting the cell medium 48 h post-stimulation to assess the expression level of the secreted alkaline phosphatase reporter protein using the Great EscAPeTM secreted alkaline phosphatase fluorescence detection kit (Clontech). Data are expressed as the total activity of secreted alkaline phosphatase over β-galactosidase content.
ChIP Assays
To demonstrate transcription factor interaction with the IL-6 promoter, ChIP assays were performed using an EZ-ChIPTM assay kit (Upstate Biotechnology, Kilsyth, Australia). Ligand- and EPS-treated C2C12 myotubes were cross-linked with 1.0% formaldehyde before being subjected to sonication, yielding chromatin fragments between 100 and 500 bp in size. Sonicated lysates were then diluted by adding 1.8 ml of ChIP dilution buffer (kit component) and precleared. Lysates were immunoprecipitated overnight at 4 °C with 4 μg of anti-acetylated histone H3 antibody (Lys-9/Lys-14; Upstate Biotechnology)) or ChIP-grade anti-p65 antibody (ab7970, Abcam, Cambridge, MA). Normal rabbit IgG or replicates (Santa Cruz Biotechnology and Quantum Scientific, Murarrie, Australia) were used as controls. Each immunocomplex was recovered with protein G-Sepharose beads, and each precipitate was washed serially with 1-ml aliquots of the following wash buffers: low-salt buffer, high-salt buffer, LiCl wash buffer, and Tris/EDTA buffer (twice). The complexes were eluted from the beads, and cross-linking was reversed by incubation with proteinase K at 65 °C. The samples were extracted using phenol/chloroform/isoamyl alcohol and cleaned with MinElute columns (Sigma-Aldrich). The recovered DNA was resuspended in 100 μl of Tris/EDTA for the input DNA and in 20 μl of Tris/EDTA for the ChIP DNA. Traditional PCR was performed using primer pairs 5′-TGTGTGTCGTCTGTCATGCG-3′ (sense) and 5′-GAGACTGGGGATGTCTGTAGCT-3′ (antisense), which flank the NFκB-binding elements of the murine IL-6 promoter. The amplification protocol PCR conditions were 95 °C for 1 min and 35 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s, with a final extension at 72 °C for 10 min. The amplicons were resolved on 2% agarose gel, and band intensities were quantified by densitometry.
Mice
All mice were cared for within institutional care committee guidelines. All animal procedures were conducted in compliance with protocols and approved by local government authorities (Bezirksregierung Köln, Cologne, Germany). The procedures were also in accordance with National Institutes of Health guidelines. All mice used in this study were backcrossed on a C57BL/6 background. To create JNK-1sm-KO male mice, muscle creatine kinase-Cre mice (which were on a pure C57BL/6 background) were crossed with JNK-1FL/FL mice (18). Details of the targeting vector used for generating the JNK-1FL/FL mice are included in supplemental Fig. 1A. In all experiments, littermates carrying the loxP-flanked alleles but not expressing Cre recombinase were used as control animals (WT). Littermates containing both the targeted homozygous loxP allele and the heterozygous Cre allele (JNK-1sm-KO) were used for analysis at all times. Mice were fed a normal chow diet (Teklad Global Rodent 2018, Harlan) containing 53.5% carbohydrates, 18.5% proteins, and 5.5% fat. Mice were housed in groups of three to five individuals in a virus-free facility at 22–24 °C on a 12-h light/12-h dark cycle. All mice had access to water and food ad libitum. At the end of the study period, mice were killed by lethal CO2 anesthesia. Relevant organs were dissected and stored at −80 °C after shock-freezing in liquid nitrogen until further preparation.
Treadmill Exercise
Exercise experiments were performed on a treadmill (TSE Systems GmbH, Bad Homburg, Germany). The following protocol was used. On the first 4 days, the mice were adapted and familiarized to sound and movement of the treadmill. On experimental day 5, the mice were forced to run for 30 or 60 min, respectively. The velocity was started at 0.22 m/s and reached a final pace of 0.25 m/s. Immediately after running, the mice were killed using CO2, and whole mixed hind limb muscle was dissected for analysis.
Statistics
Data were analyzed using Student's t test and one- or two-way analysis of variance where appropriate, with pairwise comparison determining specific differences in instances of significant main effects. On occasions when data failed tests of equal variance, Kruskal-Wallis nonparametric tests on ranks were performed (SigmaStat v3.5, Systat Software, Inc., Chicago, IL). Data are presented as means ± S.E. with the α-level set at 5% unless stated otherwise.
RESULTS
IL-6 mRNA Expression Is Differentially Regulated in Macrophages and Muscle Cells
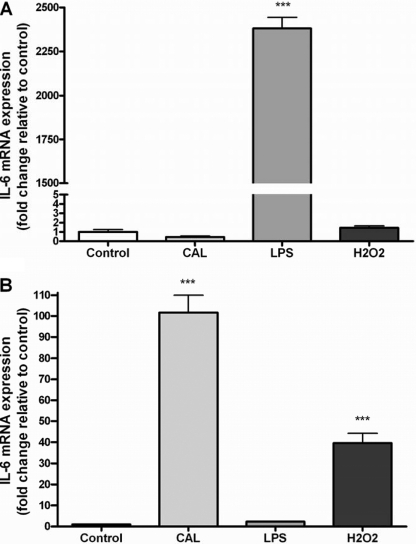
In initial experiments, we first confirmed diverse transcriptional regulation of IL-6 in macrophages and myotubes (Fig. 1). As expected, LPS induced a large elevation in IL-6 mRNA in RAW 264.7 macrophages (Fig. 1A). Interestingly, stimulation with calcimycin caused no observable increase in IL-6 gene expression in macrophages (Fig. 1A). Conversely, although no increase in IL-6 mRNA was observed in LPS-stimulated C2C12 myotubes, calcimycin induced an >100-fold elevation in IL-6 mRNA (Fig. 1B), supporting previous findings (10).
FIGURE 1.
Effect of CAL, LPS, and H2O2 treatment on RAW 264.7 macrophages (A) or C2C12 myotubes (B). Data are expressed as means ± S.E. ***, p < 0.001 versus the control (n = 6).
Neither Calcimycin (A23187) nor Contraction Increases IL-6 mRNA Expression via NFκB Signaling in Myotubes
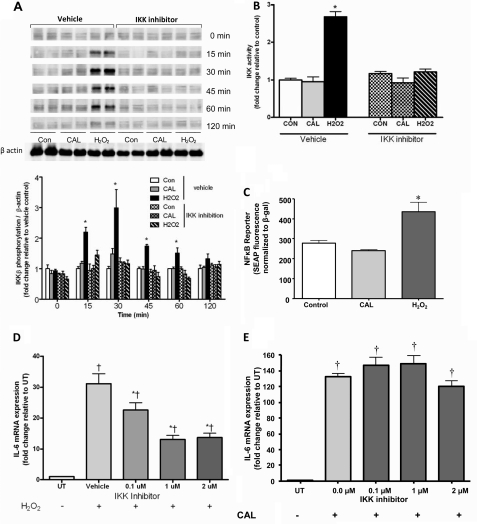
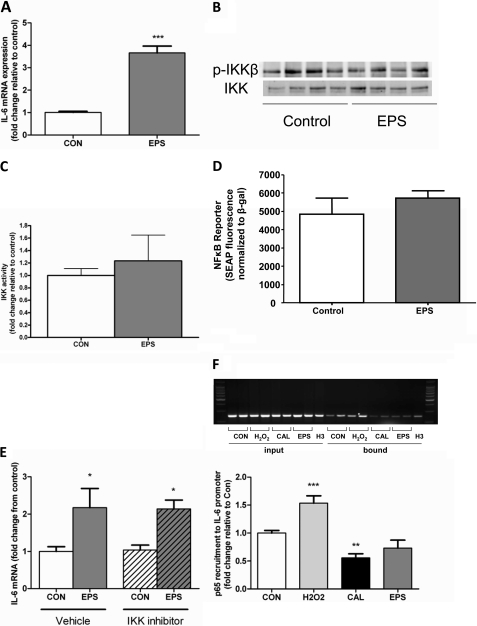
Having shown the respective IL-6 mRNA responses to the Ca2+ ionophore calcimycin (CAL; A23197), we next examined the role of IKK/NFκB signaling in transcriptional regulation in C2C12 myotubes. We observed the expected increase in IKKβ phosphorylation (Fig. 2A), activity (Fig. 2B), and NFκB reporter fluorescence (Fig. 2C) when myotubes were stimulated with our positive control, H2O2. Importantly, no such increase was observed in CAL-stimulated samples (Fig. 2, A–C). Although CAL did not induce any changes in IKKβ/NFκB activation, we nonetheless sought to confirm the lack of involvement of IKKβ in CAL-induced IL-6 mRNA expression. Accordingly, although inhibition of IKKβ with the highly selective IKKβ inhibitor BMS-345541 prevented H2O2-induced IL-6 mRNA expression, it was without effect on CAL-induced IL-6 mRNA expression (Fig. 2, D and E). Although Ca2+ ionophore stimulation clearly induces IL-6 mRNA expression, it does not provide a model of muscle contraction per se. Therefore, we continued our experiments in electrical pulse-stimulated contracting myotubes. In vivo, skeletal muscle contraction results in a significant increase in IL-6 mRNA expression. We therefore established the in vivo relevance of this in vitro model by showing an ∼4-fold increase in IL-6 mRNA in myotubes exposed to 4 h of EPS versus control samples (Fig. 3A). Consistent with our findings in CAL-treated samples, we observed no increase in IKKβ phosphorylation in contracted myotubes (Fig. 3B). Furthermore, EPS had no significant effect on IKKβ activity (Fig. 3C) or the NFκB reporter (Fig. 3D). Importantly, IKKβ inhibition failed to ablate the EPS-induced increase in IL-6 mRNA (Fig. 3E). To precisely determine the transcriptional control of the IL-6 gene, we then performed ChIP analyses. As expected, based on the data presented in Fig. 2 (A–D), the binding of the p65 subunit of NFκB to the IL-6 promoter was increased in muscle cells treated with our positive control, H2O2. Importantly, however, the binding of the p65 subunit of NFκB to the IL-6 promoter was unchanged in EPS samples and actually decreased in CAL-treated myotubes (Fig. 3F). These data provide compelling evidence that calcium- and contraction-dependent increases in muscle IL-6 mRNA expression are not under the transcriptional control of IKKβ/NFκB signaling.
FIGURE 2.
Effect of CAL or H2O2 treatment of C2C12 myotubes on IKKβ phosphorylation (A), IKK activity (B), NFκB reporter activity (C), and IL-6 mRNA expression (D and E) in the presence or absence of IKK inhibition. Data are expressed as means ± S.E. *, p < 0.05 versus the control (vehicle); †, p < 0.05 versus untreated (UT; n = 4–12 from at least two independent experiments). Con, control; SEAP, secreted alkaline phosphatase.
FIGURE 3.
Shown are IL-6 mRNA (A and E), IKK phosphorylation (B), IKK activity (C), and NFκB reporter activity (D) responses to in vitro EPS (4 h, 40 V, 1 Hz, and 10 ms) in C2C12 myotubes in the presence or absence of IKK inhibition. Myotubes were treated with H2O2, CAL, or EPS, and p65 recruitment to the IL-6 promoter was assessed by ChIP assay and resolved by gel electrophoresis (F). Data are expressed as means ± S.E. ***, p < 0.001; **, p < 0.01; *, p < 0.05 (n = 4–8 from at least two independent experiments). CON, control; SEAP, secreted alkaline phosphatase.
CAL- and EPS-induced IL-6 mRNA Expression Is Independent of NFAT Signaling
Having dismissed the involvement of IKK/NFκB signaling in the transcriptional regulation of IL-6 in contracting muscle, we next turned our attention to other possible pathways. Because calcineurin has many downstream transcription targets, including NFAT, we therefore examined whether this was a possible regulatory transcription factor in our model. Although NFAT reporter activity was elevated with CAL treatment, no such effect was seen with EPS (supplemental Fig. 2A). In addition, when NFAT was pharmacologically inhibited, CAL-induced IL-6 mRNA expression was increased (supplemental Fig. 2B), providing good evidence that NFAT does not transcriptionally regulate CAL- or EPS-induced IL-6 mRNA expression.
Contraction-induced IL-6 mRNA Expression Is Regulated by JNK/AP-1 Signaling
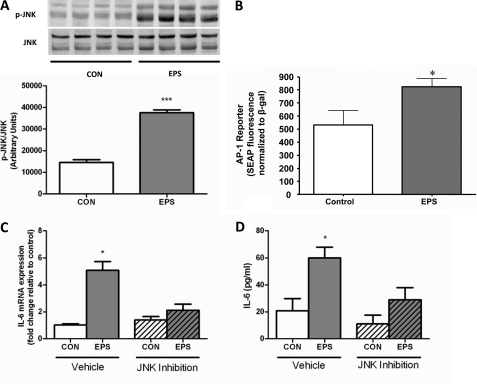
MAPKs have also been suggested to regulate IL-6 transcription. In particular, JNK has been implicated in IL-6 transcriptional regulation, albeit under conditions of bacterial and proinflammatory cytokine stimulation (19) and in adipose tissue of obese mice (20). Nedachi et al. (21) were also able to demonstrate a JNK-dependent expression of the cytokines CXCL1/KC and CXCL5/LIX in cultured myotubes stimulated with electrical pulse. In initial experiments, we observed a significant increase in Thr183/Tyr185 phosphorylation of JNK in EPS myotubes versus non-contracted controls (Fig. 4A). Because the IL-6 promoter contains a binding site for the transcription factor downstream of JNK, AP-1 (22), we next measured the effect of EPS on AP-1 activity. We observed a significant increase in AP-1 reporter activity after 4 h of contraction induced by EPS compared with non-stimulated samples (Fig. 4B). Importantly, pharmacological inhibition of JNK activity prevented the increase in IL-6 mRNA expression following EPS (Fig. 4C). Furthermore, the functional significance of this effect was emphasized by a significant reduction in IL-6 protein release from myotubes treated with the JNK inhibitor compared with the control (Fig. 4D).
FIGURE 4.
Effect of EPS (4 h, 40 V, 1 Hz, and 10 ms) on JNK phosphorylation (A), AP-1 reporter activity (B), IL-6 mRNA expression (C), and IL-6 protein measured in supernatant (D) in presence or absence or JNK inhibitor VIII. Data are expressed as means ± S.E. *, p < 0.05; ***, p < 0.001. (n = 4–8 from at least two independent experiments). CON, control; SEAP, secreted alkaline phosphatase.
IL-6 mRNA Does Not Increase in Skeletal Muscles of JNK-1 Knock-out Mice after Exercise
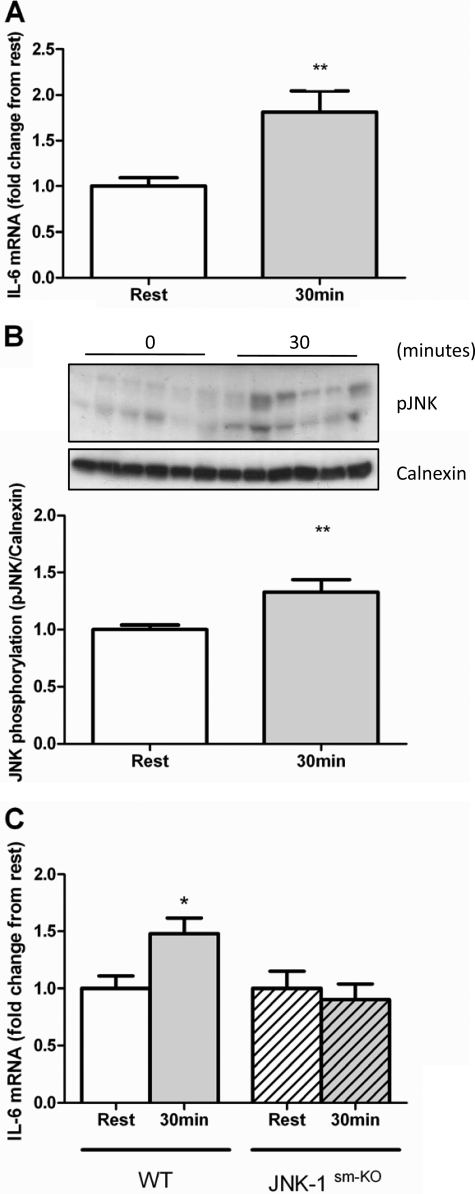
To determine the in vivo relevance of these findings, we examined skeletal muscle IL-6 mRNA expression in muscle-specific JNK-1 knock-out mice (JNK-1sm-KO). These mice exhibit a complete absence of the JNK-1-specific isoform in skeletal muscle but normal expression in other tissues (supplemental Fig. 1B), and no differences from the wild type in body weight (supplemental Fig. 1D), daily food intake (supplemental Fig. 1E), energy expenditure (supplemental Fig. 1F), respiratory exchange ratio (supplemental Fig. 1G), and exercise endurance (supplemental Fig. 1H). We first assessed IL-6 mRNA levels in muscle lysates taken from C57BL/6 mice after 30 or 60 min of treadmill exercise and compared them with those in lysates obtained from the muscles from a cohort of mice that did not undergo exercise. Treadmill running induced a robust increase in muscle IL-6 mRNA at 30 min (Fig. 5A), with no increase observed after 60 min (data not shown). We subsequently demonstrated an increased phosphorylation of JNK in muscle after 30 min of treadmill running in C57BL/6 mice (Fig. 5B). Moreover, although littermate control mice exhibited an increase in skeletal muscle IL-6 mRNA following exercise, no such effect was seen in JNK-1sm-KO mice (Fig. 5C). These data demonstrate that JNK-1 is required for exercise-induced increases in IL-6 mRNA expression in skeletal muscle.
FIGURE 5.
Effect of 30 min of treadmill running on skeletal muscle IL-6 mRNA expression (A) and JNK phosphorylation (B) in C57BL/6 mice (n = 6) and IL-6 mRNA expression in muscle-specific JNK-1 knock-out and littermate control animals (relative to their respective resting controls) before and after 30 min of treadmill running (C). Data are expressed as means ± S.E. *, p < 0.05; **, p < 0.01 (n = 6–9).
DISCUSSION
The existent thinking regarding the transcriptional regulation of contraction-induced IL-6 expression revolves around the classical inflammatory IKKβ/NFκB signaling. Herein, we have demonstrated a complete absence of any role for IKKβ/NFκB signaling in mediating calcium- and contraction-induced increases in IL-6 mRNA expression. Furthermore, we found a primary role for JNK/AP-1 signaling in the control of IL-6 expression in contracting skeletal muscle.
There is a wealth of data showing that NFκB is an important transcriptional regulator of IL-6 in macrophages and lymphocytes. Furthermore, EPS experiments similar to those detailed here have shown that inhibition of the NFκB pathway by parthenolide blocks contraction-induced expression of the cytokines CXCL1/KC and CXCL5/LIX in cultured myotubes (21). It is therefore tempting to speculate that contraction-induced IL-6 expression is under IKK/NFκB control. However, we have shown here no effect of EPS-induced contraction on NFκB activity and no effect of IKKβ inhibition on EPS-induced IL-6 expression in myotubes in vitro. Although some in vitro and in vivo experiments have demonstrated increases in muscle NFκB in response to exercise (23), others have not (9, 14). Furthermore, Tantiwong et al. (24) showed that, in both lean and obese humans, increases in skeletal muscle IL-6 mRNA preceded any changes in muscle NFκB activity, suggesting that IKKβ/NFκB signaling was not the primary mechanism of transcriptional regulation. By way of a more precise examination of IL-6 transcription during contraction, the data from ChIP assays shown here indicate no significant increases in p65 recruitment to the IL-6 promoter following either CAL or EPS treatment. These combined data provide compelling evidence that primary regulation of IL-6 transcription in contracting skeletal muscle is independent of the IKK/NFκB pathway.
Instead, we have shown here numerous lines of evidence supporting the hypothesis that JNK/AP-1 signaling controls IL-6 transcription in contracting skeletal muscle. First, we have demonstrated that myotube contraction stimulated by electric pulse induced increases in the phosphorylation of JNK. Increased JNK activity has also been demonstrated in rat muscle contracted in situ (25) and in humans following cycle exercise (14, 26, 27). The increase in JNK phosphorylation was associated with an increase in IL-6 mRNA and protein in response to EPS, an effect that was blunted by JNK inhibition. Although previous research has demonstrated an association between IL-6 expression and JNK phosphorylation in myotubes treated with lipopolysaccharide (19), this is the first depiction of the role this pathway plays in controlling IL-6 transcription in contracting skeletal muscle.
AP-1 is the name given to a family of proteins belonging to the Jun, Fos, Maf, and activating transcription factor subfamilies (28). Because JNK is upstream of AP-1 and because the IL-6 promoter contains AP-1 response elements in human fibroblasts (29) and murine monocytes (22), we examined the activity of this transcription factor following contraction induced by EPS in myotubes. Contraction caused a significant increase in AP-1 reporter activity. In support of these data, Rohrbach et al. (30) have shown that AP-1 is required for β-adrenergic-mediated IL-6 expression in cardiomyocytes.
Having further supported the role of JNK/AP-1 signaling in IL-6 transcription in the muscle of exercising mice, we propose that this pathway represents the primary mechanism by which IL-6 expression is regulated in contracting skeletal muscle. However, given the complexity of gene transcription pathways, added to the vast physiological changes that occur with exercise, it is doubtful that contraction-stimulated JNK/AP-1 signaling represents the only pathway by which IL-6 transcription can be elevated. Indeed, we have demonstrated an association between increased nuclear phosphorylation of p38 MAPK and IL-6 mRNA in human muscle after 60 min of cycle exercise (14). However, this association was seen only in muscle low in glycogen, suggesting that this mechanism is not involved at the early onset of contraction-induced IL-6 transcription. Similarly, Allen et al. (12) and others (11, 31) have demonstrated in vitro and in vivo that the calcium-activated phosphatase calcineurin stimulates IL-6 expression during exercise/contraction. Calcineurin has many downstream transcription targets, including CREB (cAMP-responsive element-binding protein), MEF2, and NFAT, and initial speculation indicated that the latter may stimulate IL-6 mRNA (11). However, we have previously shown no increase in NFAT nuclear translocation in muscle expressing IL-6 after exercise (14), and we have shown here no effect of EPS-induced contraction on NFAT reporter activity (supplemental Fig. 2A). Furthermore, Allen et al. (12) observed a 300-fold increase in calcineurin-mediated IL-6 promoter activity in C2C12 myotubes cotransfected with the dominant-negative form of NFAT. We have also shown here large increases in CAL-induced IL-6 mRNA in myotubes treated with an NFAT inhibitor (supplemental Fig. 2B). These data suggest that NFAT is in fact a repressor of IL-6 transcription and raise the question of whether calcineurin might increase IL-6 transcription via MEF2 (12) in exercising muscle in vivo (32).
To summarize, although inflammatory stimuli via IKKβ/NFκB, glycogen depletion via p38 MAPK, and calcium signaling via calcineurin might stimulate skeletal muscle IL-6 expression, we speculate that these mechanisms are secondary to the pathway that we have demonstrated here, JNK/AP-1 stimulation of IL-6 expression in contracting muscle. This adds to data describing JNK regulation of contraction-induced gene expression in skeletal muscle (25, 26) and highlights that JNK phosphorylation in muscle is of biological relevance.
Supplementary Material
Acknowledgments
We thank David Kay, Mark Hargreaves, and James Pringle for help with establishing the EPS protocol.
This work was supported by National Health and Medical Research Council of Australia (NHMRC) Grant 526606 and the Victorian Government Operational Infrastructure Support Program.

This article contains supplemental Figs. 1 and 2.
- IKK
- IκB kinase
- EPS
- electrical pulse stimulation
- CAL
- calcimycin.
REFERENCES
- 1. Hotamisligil G. S. (2006) Inflammation and metabolic disorders. Nature 444, 860–867 [DOI] [PubMed] [Google Scholar]
- 2. Pedersen B. K., Febbraio M. A. (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 [DOI] [PubMed] [Google Scholar]
- 3. de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. (1991) Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174, 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kreutz M., Ackermann U., Hauschildt S., Krause S. W., Riedel D., Bessler W., Andreesen R. (1997) A comparative analysis of cytokine production and tolerance induction by bacterial lipopeptides, lipopolysaccharides and Staphylococcus aureus in human monocytes. Immunology 92, 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guha M., Mackman N. (2001) LPS induction of gene expression in human monocytes. Cell. Signal. 13, 85–94 [DOI] [PubMed] [Google Scholar]
- 6. Donath M. Y., Shoelson S. E. (2011) Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 [DOI] [PubMed] [Google Scholar]
- 7. Carey A. L., Bruce C. R., Sacchetti M., Anderson M. J., Olsen D. B., Saltin B., Hawley J. A., Febbraio M. A. (2004) Interleukin-6 and tumor necrosis factor-α are not increased in patients with type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia 47, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 8. Balon T. W., Nadler J. L. (1994) Nitric oxide release is present from incubated skeletal muscle preparations. J. Appl. Physiol. 77, 2519–2521 [DOI] [PubMed] [Google Scholar]
- 9. Steensberg A., Keller C., Hillig T., Frøsig C., Wojtaszewski J. F., Pedersen B. K., Pilegaard H., Sander M. (2007) Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J. 21, 2683–2694 [DOI] [PubMed] [Google Scholar]
- 10. Holmes A. G., Watt M. J., Carey A. L., Febbraio M. A. (2004) Ionomycin, but not physiologic doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metabolism 53, 1492–1495 [DOI] [PubMed] [Google Scholar]
- 11. Keller C., Hellsten Y., Steensberg A., Pedersen B. K. (2006) Differential regulation of IL-6 and TNF-α via calcineurin in human skeletal muscle cells. Cytokine 36, 141–147 [DOI] [PubMed] [Google Scholar]
- 12. Allen D. L., Uyenishi J. J., Cleary A. S., Mehan R. S., Lindsay S. F., Reed J. M. (2010) Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R198–R210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dolmetsch R. E., Xu K., Lewis R. S. (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 [DOI] [PubMed] [Google Scholar]
- 14. Chan M. H., McGee S. L., Watt M. J., Hargreaves M., Febbraio M. A. (2004) Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB J. 18, 1785–1787 [DOI] [PubMed] [Google Scholar]
- 15. Noguchi H., Matsushita M., Okitsu T., Moriwaki A., Tomizawa K., Kang S., Li S. T., Kobayashi N., Matsumoto S., Tanaka K., Tanaka N., Matsui H. (2004) A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat. Med. 10, 305–309 [DOI] [PubMed] [Google Scholar]
- 16. Matthews V. B., Aström M. B., Chan M. H., Bruce C. R., Krabbe K. S., Prelovsek O., Akerström T., Yfanti C., Broholm C., Mortensen O. H., Penkowa M., Hojman P., Zankari A., Watt M. J., Bruunsgaard H., Pedersen B. K., Febbraio M. A. (2009) Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52, 1409–1418 [DOI] [PubMed] [Google Scholar]
- 17. Nedachi T., Fujita H., Kanzaki M. (2008) Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 295, E1191–E1204 [DOI] [PubMed] [Google Scholar]
- 18. Belgardt B. F., Mauer J., Wunderlich F. T., Ernst M. B., Pal M., Spohn G., Brönneke H. S., Brodesser S., Hampel B., Schauss A. C., Brüning J. C. (2010) Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc. Natl. Acad. Sci. U.S.A. 107, 6028–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frost R. A., Nystrom G. J., Lang C. H. (2003) Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R1153–R1164 [DOI] [PubMed] [Google Scholar]
- 20. Sabio G., Das M., Mora A., Zhang Z., Jun J. Y., Ko H. J., Barrett T., Kim J. K., Davis R. J. (2008) A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nedachi T., Hatakeyama H., Kono T., Sato M., Kanzaki M. (2009) Characterization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes. Am. J. Physiol. Endocrinol. Metab. 297, E866–E878 [DOI] [PubMed] [Google Scholar]
- 22. Dendorfer U., Oettgen P., Libermann T. A. (1994) Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol. Cell. Biol. 14, 4443–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kramer H. F., Goodyear L. J. (2007) Exercise, MAPK, and NFκB signaling in skeletal muscle. J. Appl. Physiol. 103, 388–395 [DOI] [PubMed] [Google Scholar]
- 24. Tantiwong P., Shanmugasundaram K., Monroy A., Ghosh S., Li M., DeFronzo R. A., Cersosimo E., Sriwijitkamol A., Mohan S., Musi N. (2010) NFκB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am. J. Physiol. Endocrinol. Metab. 299, E794–E801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aronson D., Dufresne S. D., Goodyear L. J. (1997) Contractile activity stimulates the c-Jun NH2-terminal kinase pathway in rat skeletal muscle. J. Biol. Chem. 272, 25636–25640 [DOI] [PubMed] [Google Scholar]
- 26. Aronson D., Boppart M. D., Dufresne S. D., Fielding R. A., Goodyear L. J. (1998) Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem. Biophys. Res. Commun. 251, 106–110 [DOI] [PubMed] [Google Scholar]
- 27. Petersen A. C., McKenna M. J., Medved I., Murphy K. T., Brown M. J., Della Gatta P., Cameron-Smith D. (2012) Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol. Scand. 204, 382–392 [DOI] [PubMed] [Google Scholar]
- 28. Shaulian E., Karin M. (2002) AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4, E131–E136 [DOI] [PubMed] [Google Scholar]
- 29. Eickelberg O., Pansky A., Mussmann R., Bihl M., Tamm M., Hildebrand P., Perruchoud A. P., Roth M. (1999) Transforming growth factor-β1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J. Biol. Chem. 274, 12933–12938 [DOI] [PubMed] [Google Scholar]
- 30. Rohrbach S., Engelhardt S., Lohse M. J., Werdan K., Holtz J., Muller-Werdan U. (2007) Activation of AP-1 contributes to the β-adrenoceptor-mediated myocardial induction of interleukin-6. Mol. Med. 13, 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banzet S., Koulmann N., Simler N., Birot O., Sanchez H., Chapot R., Peinnequin A., Bigard X. (2005) Fiber-type specificity of interleukin-6 gene transcription during muscle contraction in rat: association with calcineurin activity. J. Physiol. 566, 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGee S. L., Sparling D., Olson A. L., Hargreaves M. (2006) Exercise increases MEF2- and GEF DNA-binding activity in human skeletal muscle. FASEB J. 20, 348–349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.